The present section is divided into three main subsections. Hence, the first subsection will discuss the alumina nanofluid properties and how they are affected by the concentration of the nanoparticles. Emphasis is given to the effect of nanoparticles deposition on wettability. The second section discusses the overall effect of the nanofluids on the pool boiling performance. Boiling processes are studied on a hydrophilic stainless-steel surface with a 1.5 mm circular superhydrophobic region. This surface configuration was chosen as previous work [
22] showed significantly improved control on the basic nucleation phenomena, isolating influencing parameters and being able to focus on the impact of the nanoparticles’ concentration. Once this effect is well defined, the final section will discuss the combination of both effects (surface modification, using the biphilic pattern suggested in [
17] with the nanofluids.
3.1. Thermophysical Properties of the Alumina Nanofluids
The effective density (
), specific heat (
), viscosity (
), and thermal conductivity (
), were calculated using Equations (1)–(4). The results are depicted in
Table 4.
Looking at the table, it can be noticed that the largest variations, relative to water, occur for the nanofluid with the highest nanoparticle concentration. Density, viscosity, and thermal conductivity increased with nanoparticle concentration increment, while the specific heat decreased. However, the differences were quite small, and even for the highest nanoparticle concentration, they were only around 2%.
During the experimental test, particles got deposited in several parts of the tank. In particular, tank walls, heat resistance, and most of all, the tank base, which was included in the test surface. The deposition of nanoparticles will lead to a decrease in concentration, so the percentage increase that was previously estimated will actually be much lower due to this phenomenon. In fact, nanoparticle deposition will change not only the concentration but even more relevant aspects, which are the surface-related parameters, including wettability. The modification of surface appearance is visible after the experimental test, as shown in
Figure 2.
Table 5 depicts the mean surface tension values, measured before and after the experimental tests, for all working fluids.
Analyzing the values in the table it is not possible to establish a relation between nanoparticle concentration and surface tension variation. Additionally, the differences were small, and there were errors associated with the measurement, easily noticed by the standard deviation (SD) values. Such discrepancy was reported for the same nanofluid, due to different nanoparticle concentrations in each measure, especially after the experiment, in which the fluid loses some stability. Furthermore, according to the literature, the surface tension is much more susceptible to change with temperature rather than nanoparticle concentration. Hence, one can conclude that the surface tension is not significantly altered by nanoparticle concentration. This is in agreement with previous works, e.g., Malý et al. [
31].
The measured contact angles for both the hydrophilic and superhydrophobic regions before the experimental test are summarized in
Table 6.
The contact angle for the hydrophilic region varies between 60° and 90°, which is expected for this type of surface. There was no apparent relation between the concentration of the nanoparticles and the variation of the contact angle. Nevertheless, higher contact angles were obtained when using nanofluids, which indicates that the existence of nanoparticles constitutes an obstacle for liquid spreading over the surface. Notwithstanding, all fluids exhibited a large SD, which indicated that the contact angle varied a lot, depending on the spot where it was measured. This is a known factor due to hysteresis [
25].
Regarding the static contact angle for the superhydrophobic regions, it can be noticed that all fluids had values above 150° and hysteresis less than 10°; therefore, these regions were indeed superhydrophobic. The static and quasi-static angles were comprised between 155° and 161°; hence, the use of nanofluids does not affect these contact angles.
The measured nanofluid contact angles, for both hydrophilic and superhydrophobic regions, after the experimental test are shown in
Table 7.
Comparing with the values measured before the experiment, it can be seen that the values of the superhydrophobic contact angle remained practically unchanged. There was only a small decrease as a result of the deterioration of the spot throughout the experiment. On the other hand, the values of the hydrophilic contact angle decrease substantially. After the test, the contact angle was comprised between 30° and 60°. On average, there was a 30° decrement as a result of the nanoparticles deposited during the experiment, which formed a porous layer above the surface that promotes liquid spreading. This reduction is in accordance with the literature [
22], and as described before, surfaces with higher wettability promote bubble detachment, as the balance of forces in the triple line is altered.
3.2. Thermophysical Properties of the Alumina Nanofluids
The lack of synchronization between the high-speed camera and the infrared camera, due to nanofluids opacity, observed for higher nanoparticles concentrations, makes it impossible to distinguish and individualize each nucleation. However, this problem can be overcome by establishing a relation between bubble formation and thermographic parameters, such as the dissipated heat flux and the mean surface temperature for a certain area. It is worth reminding that, for this part of the investigation, the surface in the study is a stainless-steel surface with a 1.5 mm circular superhydrophobic region. To do this, an experiment was conducted using water, with both cameras synchronized. Then, the dissipated heat flux and the mean surface temperature were determined for a circular area with twice the diameter of the superhydrophobic spot.
The temporal evolution of the dissipated heat flux and mean surface temperature for one video recorded with an imposed heat flux of 1290 W/m
2 is depicted in
Figure 3. The plots also include the instant when a bubble detaches from the surface, and consequently, a new nucleation beginning (red lines).
Looking at
Figure 3, both dissipated heat flux and mean surface temperature exhibited a cyclical variation, marked by bubble detachment; that is, each nucleation presented a similar behavior throughout the stages of bubble formation. It is important to notice that these parameters show completely opposite behaviors. This trend observed was also observed for the experiments with nanofluids.
In order to analyze the heat transfer promoted by the formation of bubbles in the superhydrophobic region, the dissipated heat flux was evaluated for a nucleation time frame for different imposed fluxes. The obtained results for water and the nanofluid with the maximum concentration, as a function of dimensionless time, are shown in
Figure 4. Examining the three graphs, it can be concluded that the dissipated heat flux curve was not altered when using nanofluids.
The results suggest that the behavior of the dissipated heat flux curve was independent of the imposed current intensity and the working fluid. The dissipated heat flux increased in the early stages of bubble nucleation, promoted by previously bubble detachment, as a result of fresh liquid renewal near the superhydrophobic region. A maximum value was reached somewhere between the hemisphere formation phase and the beginning of vertical elongation (for a non-dimensional time of t* = 0.1 and t* = 0.4, where t*/ttotal, being t the time instant of the frame in analysis, counting from the instant when bubble nucleation starts, and the total time that the bubble takes to form and detach from the surface).
As the bubble growth progresses, the layer of superheated vapor inside the bubble increases significantly, reducing the ability to dissipate heat at its base. Consequently, the dissipated heat flux will decrease until the final stages of the vertical elongation phase (t* = 0.8 to t* = 0.9). Finally, in the necking phase, the dissipated heat flux undergoes a new and substantial increment, caused by the decrease in the vapor mass directly above the spot, which is now occupied by liquid—allied to the fact that necking also promotes vapor movement inside the bubble, increasing the convective heat transfer coefficient. This rise continues until bubble separation.
Averaging the temperature profiles of all nucleations along a horizontal line that goes over the center of the superhydrophobic region, with twice its diameter, it is possible to draw a mean temperature profile. This procedure was done for the imposed heat fluxes of 1290 W/m
2, 2132 W/m
2, and 3790 W/m
2. Those temperature profiles are shown in
Figure 5. The highest temperature occurs in the center of the superhydrophobic region, substantially decreasing in the hydrophilic region. All working fluids presented a similar behavior when rising the imposed heat flux. A substantial increment in temperature was observed in the center of the superhydrophobic region and not that significant in the hydrophilic region. This shows that heat dissipation is much more efficient in the hydrophilic region, given that the convective heat transfer coefficient of water in this region is much higher than the one of vapors inside the bubble.
The comparison between the different fluids studied did not show any evident relation between the nanoparticles concentration augmentation and temperature variations. Moreover, the temperature differences were small and were within the range of uncertainty. However, the temperature differences are more significant for the superhydrophobic region, at higher imposed heat fluxes, where there was a temperature increase for 1 wt% concentration (worse results) and a decrement for 2.5 wt% and 3 wt% concentration (better results).
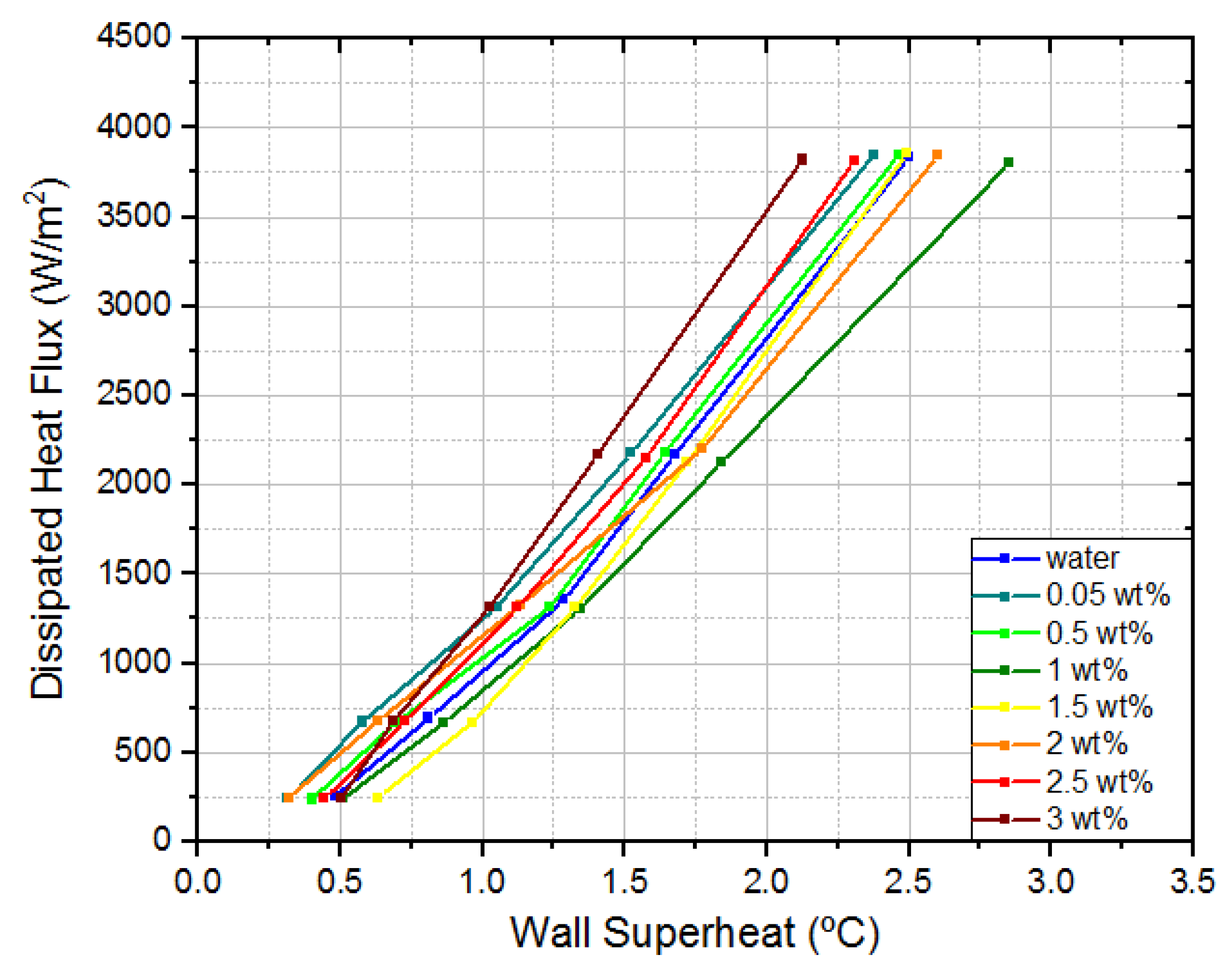
Once completed the analysis of the dissipated heat flux and temperature profiles, it was possible to draw the typical pool boiling curves: wall superheat versus dissipated heat flux (
Figure 6) and dissipated heat flux versus heat transfer coefficient (
Figure 7).
Looking at
Figure 6, it can be easily concluded that nanofluids only mildly influenced the dissipated heat flux. Additionally, it could be verified that nanofluids do not significantly affect wall superheat. The largest variations occur at the highest dissipated heat flux for 1 wt% and 3 wt% concentration. In the first case, the wall superheat was 0.35 °C higher than water. In the second case, the wall superheat was 0.38 °C lower than water. This means that, for the same dissipated heat flux, the temperature in the interest region was 0.38 °C lower than for water, which represents a 15% decrease.
To assess boiling performance, it is important to analyze heat transfer coefficient variations. As illustrated in
Figure 7, heat transfer coefficient increased progressively with heat flux increment as a result of higher bubble frequency. The worst case is for 1 wt% concentration, which exhibited a 202 W/(m
2K) decrement in the heat transfer coefficient, compared with water. Hence, for the same imposed heat flux, higher temperatures are obtained. On the other hand, using a 3 wt% concentration was verified a 265 W/(m
2K) heat transfer coefficient increment, which corresponds to a 17% increase. This shows a higher ability to dissipate heat; therefore, lower surface temperatures were reported (even though absolute temperature differences are small).
3.3. Combining Nanofluids with Biphilic Surfaces
Once the impact of the alumina nanofluids was analyzed, the best performing nanofluid was then combined with a biphilic surface, using a pattern of superhydrophobic regions with 1.5 mm in diameter, 1.5 mm apart, following the recommendation of [
17]. The existence of multiple superhydrophobic regions promotes the onset of boiling for very low superheat degrees (1–3 K) and the circulation of the cold flow on the hydrophilic region. Additionally, the bubble coalescence also promoted fluid motion, resulting in greater heat transfer coefficient. In order to evaluate the impact of bubble coalescence in boiling heat transfer, the dissipated heat flux was analyzed for a nucleation time frame, for the different imposed heat fluxes. The analysis performed here covers a circular area around the central superhydrophobic spot with twice its diameter. The dissipated heat flux obtained for water and 3 wt% concentration nanofluid, as a function of the dimensionless time, are depicted in
Figure 8.
Looking at the three different graphs, it is clear that the dissipated heat flux was not altered when using nanofluids. Additionally, the curves depict a similar behavior, regardless of the imposed current intensity. Initially, there was a small increase (up to 50 W/m2) in the dissipated heat flux, as a result of previously bubble detachment, that promotes fresh liquid renewal near the superhydrophobic regions. Then, it stabilized somewhere between hemisphere formation and bubble vertical elongation beginning (t* = 0.2 and t* = 0.4). Posteriorly, unlike what happens for the single bubble case, in the final phase of bubble growth (between t* = 0.8 and t* = 0.9), the dissipated heat flux started to rise abruptly as a result of bubble interaction. At this stage of bubble formation, bubbles came close to each other, touching in their maximum diameter point. This phenomenon induced great fluid motion between bubbles, and the dissipated heat flux reaches its maximum value at t* = 0.9. However, as bubbles continued to grow, they fully joined with each other, at t* = 0.95, in what is called bubble coalescence. In this phase, a single and larger bubble is formed. The space between bubbles that was occupied by liquid is then fulfilled by vapor. The dissipated heat flux drops again quickly as a result of vapor lower convective heat transfer coefficient and remains that way until bubble detachment.
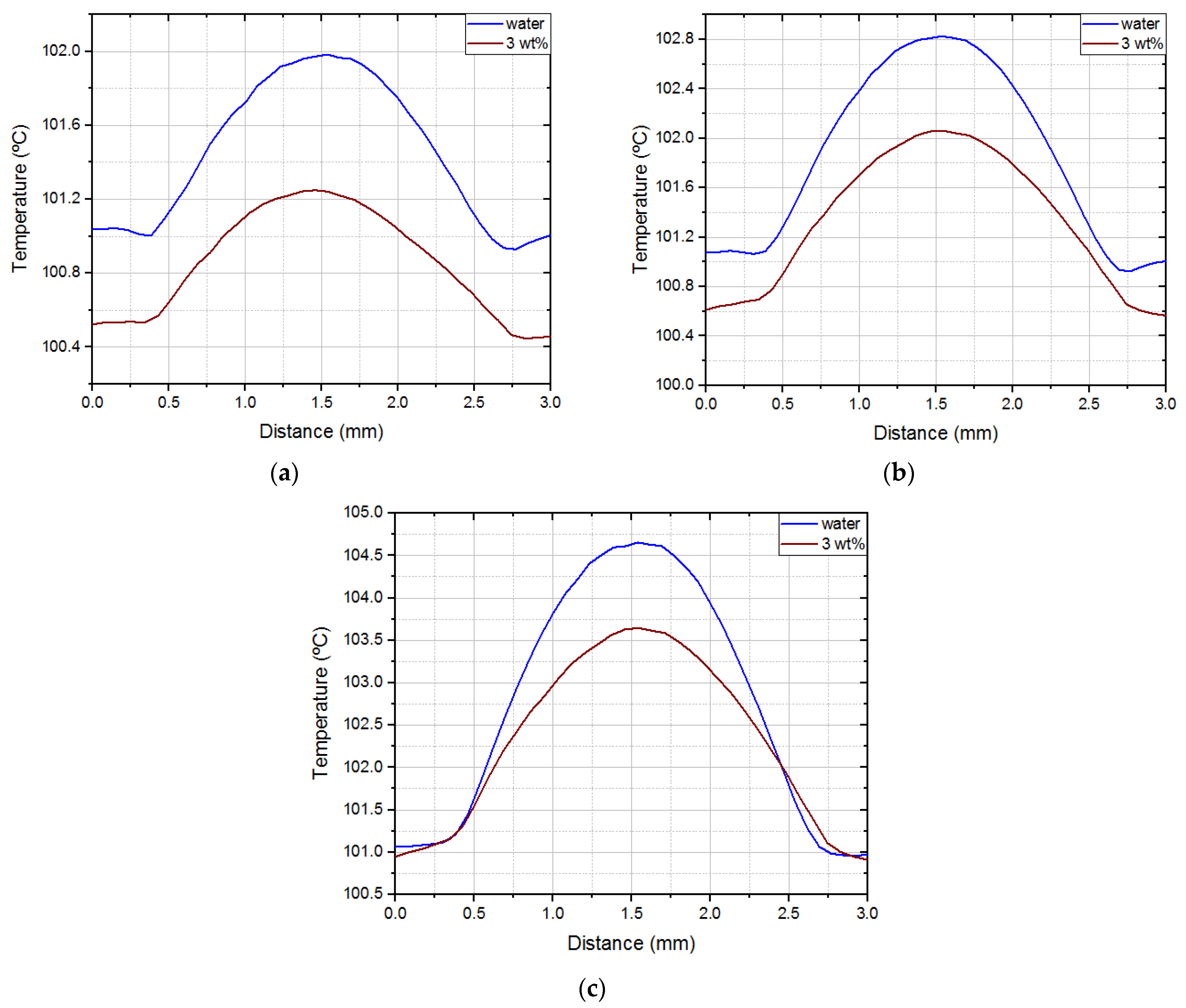
Averaging the temperature profiles of all nucleations and videos, it is possible to draw a mean surface temperature profile for both fluids. The results are shown in
Figure 9.
For all imposed heat fluxes, there was a temperature decrement relative to water when using the 3 wt% concentration nanofluid. Notwithstanding, the temperature differences were small (no more than 5 °C), so further testing is necessary to make definite conclusions. Still, the constant temperature reduction in the center of the superhydrophobic spot suggests that the use of nanoparticles can lead to smaller bubbles. Either suspended or deposited nanoparticles can contribute to changes in the balance of forces in the triple line. As a result, bubble volume can decrease, and bubble frequency increase. On the other hand, the temperature decrement in the hydrophilic region, for lower imposed heat fluxes, may be due to deposited nanoparticles that improve heat transfer by conduction.
Averaging the temperature and dissipated heat flux, the typical wall superheat versus heat flux graph can be drawn, as depicted in
Figure 10.
Looking at the figure, lower temperatures are obtained at the surface when using a 3 wt% concentration nanofluid. The largest difference occurred for 1290 W/m
2, for which the surface temperature was 0.6 °C lower when using the nanofluid. This corresponds to a 52% reduction. Temperature differences tended to attenuate as the imposed heat flux was increased. Heat transfer coefficient evolution, as a function of the dissipated heat flux, is represented in
Figure 11.
Comparing the two fluids is clear that the 3 wt% concentration nanofluid shows better boiling performance throughout the curve. This was particularly evident at lower imposed heat fluxes. The maximum difference was obtained for 1290 W/m2, where an 1108 W/(m2K) increase was observed relative to water. This represents an impressive 98% increment. Part of this substantial gain may be due to increased conductive and convective heat transfer as a result of deposited and suspended nanoparticles. Nevertheless, the prominent factor for heat transfer coefficient enhancement shall be the formation of smaller and more frequent bubbles, as a consequence of the deposited nanoparticles that improve wettability and lead to changes in the balance of forces in the triple line. Despite the large improvement for lower imposed heat fluxes, further studies are now required to extend these conclusions drawn to the basic conditions that allow studying the fundamental processes of nucleation, e.g., to extend the boiling curve to elevated heat fluxes.
So, in agreement with our previous studies, e.g., [
17,
22], biphilic surfaces show a better performance than hydrophilic or superhydrophobic surface alone since, as they combine extreme wetting characteristics, they promote the boiling to be triggered at very low superheat values in the superhydrophobic regions, while promoting fluid flow within these regions (in the remaining hydrophilic surface). Furthermore, the derived pattern allows for a controlled bubble coalescence, which enhances the induced convection during bubble detachment.