Optimisation of Propane Production from Hydrothermal Decarboxylation of Butyric Acid Using Pt/C Catalyst: Influence of Gaseous Reaction Atmospheres
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Method
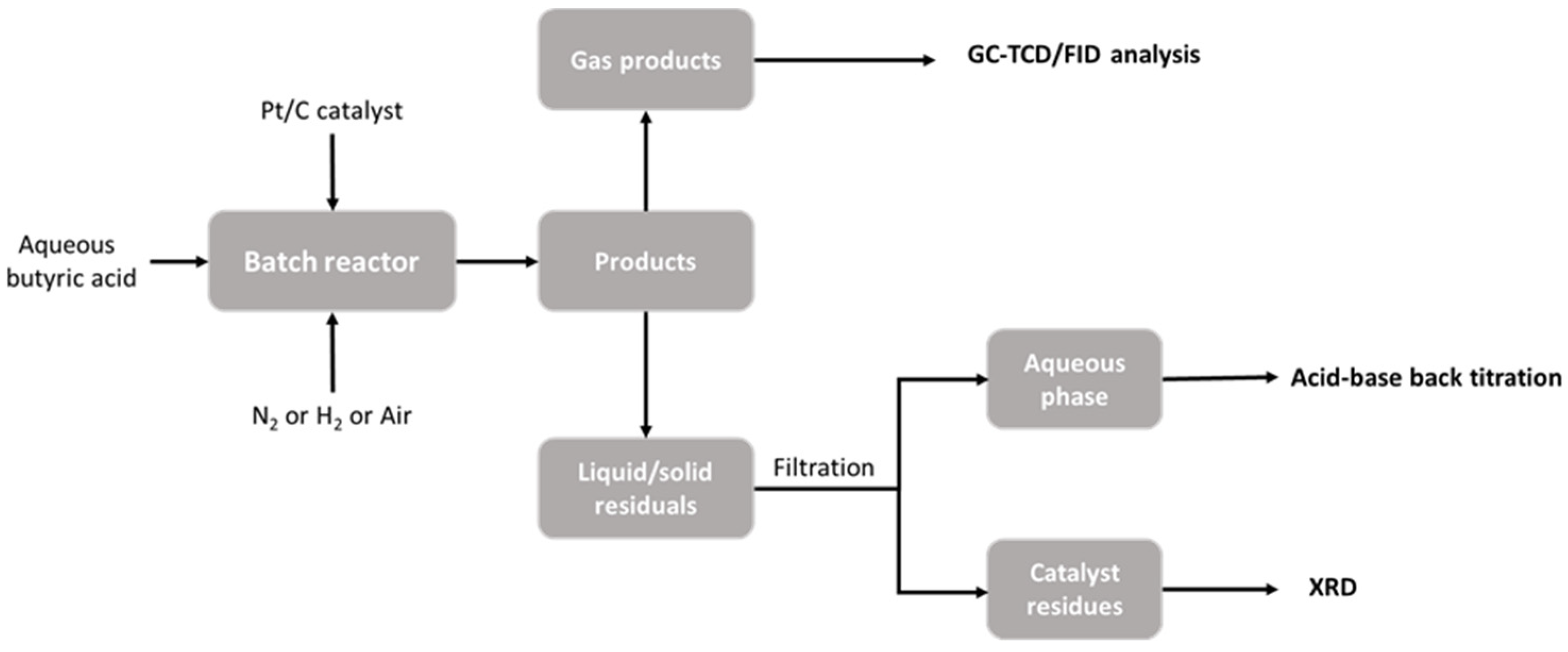
2.2.1. Batch Reactor Procedure
2.2.2. Product Collection
2.2.3. Analysis of Gaseous Phase
2.2.4. Analysis of Aqueous Phase
- S = volume of HCl used in titration (mL)
- B = volume of HCl used in blank titration (mL)
- C = concentration of HCl (mol/L)
- V = volume of aqueous phase collected (mL)
- m = mass of butyric acid feed
- 88.11 is the mol. wt% of butyric acid (g/mol)
2.2.5. Analysis of Pt/C Catalyst and Solid Residue
2.2.6. Statistical Analysis
3. Results and Discussions
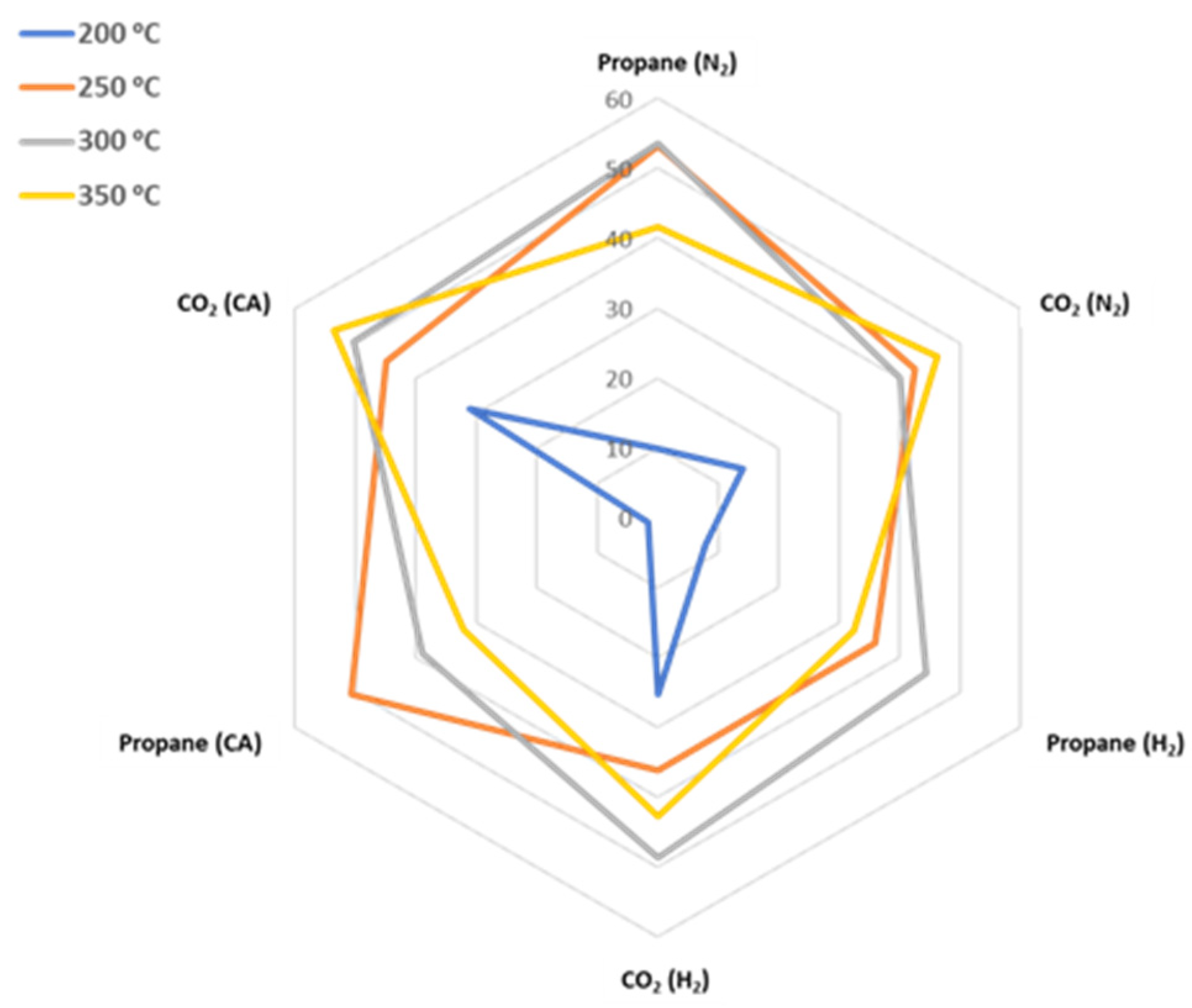
3.1. Product Yields
3.1.1. Effect of Nitrogen Atmosphere at Different Temperatures
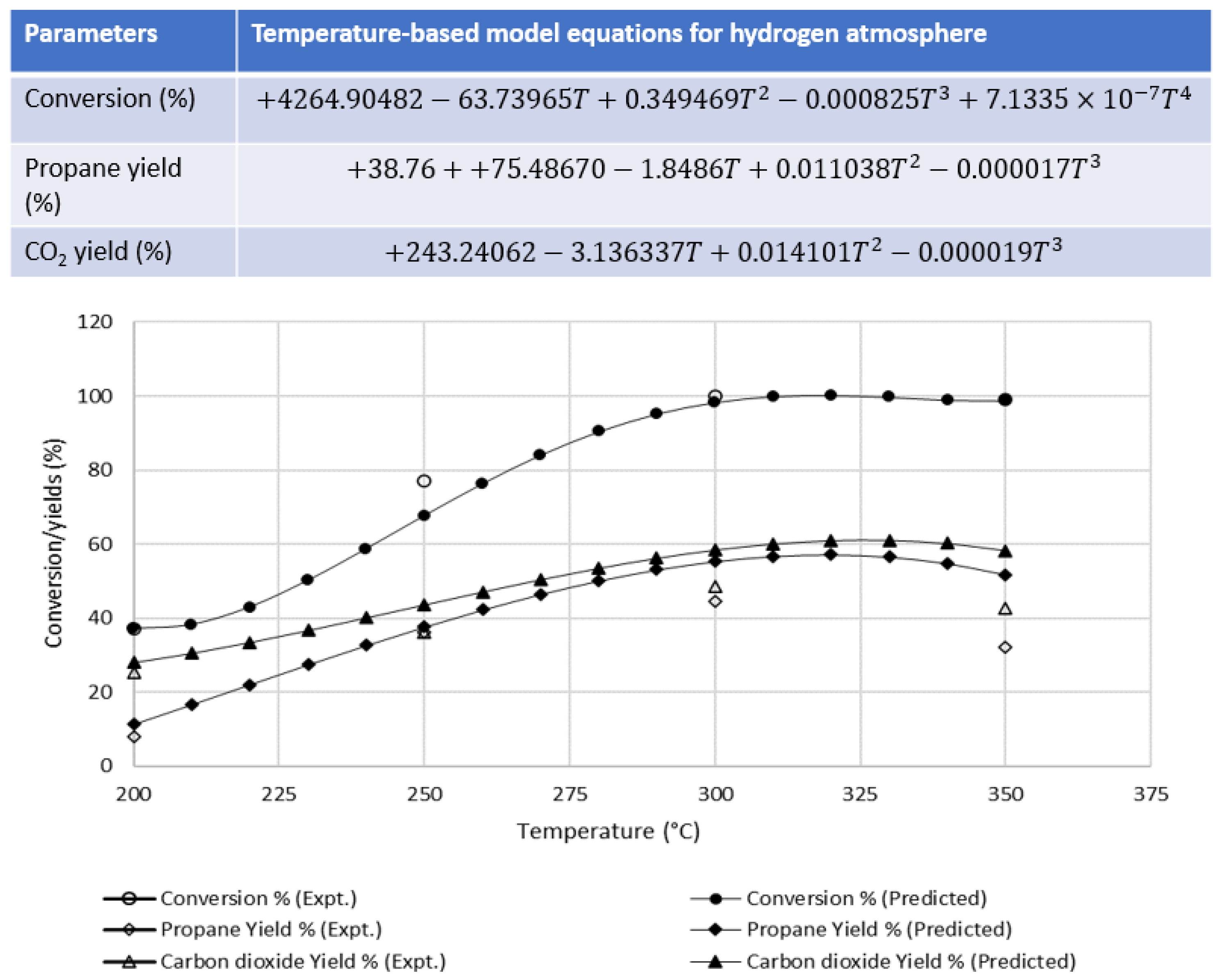
3.1.2. Effect of Hydrogen Atmosphere at Different Temperatures
3.1.3. Effect of Hydrogen Pressure at 300 °C
3.1.4. Effect of Compressed Air with Varying Temperature
3.2. Statistical Analysis Results
3.2.1. Optimisation of Reaction Conditions
3.2.2. Fitting the Surface Response Models
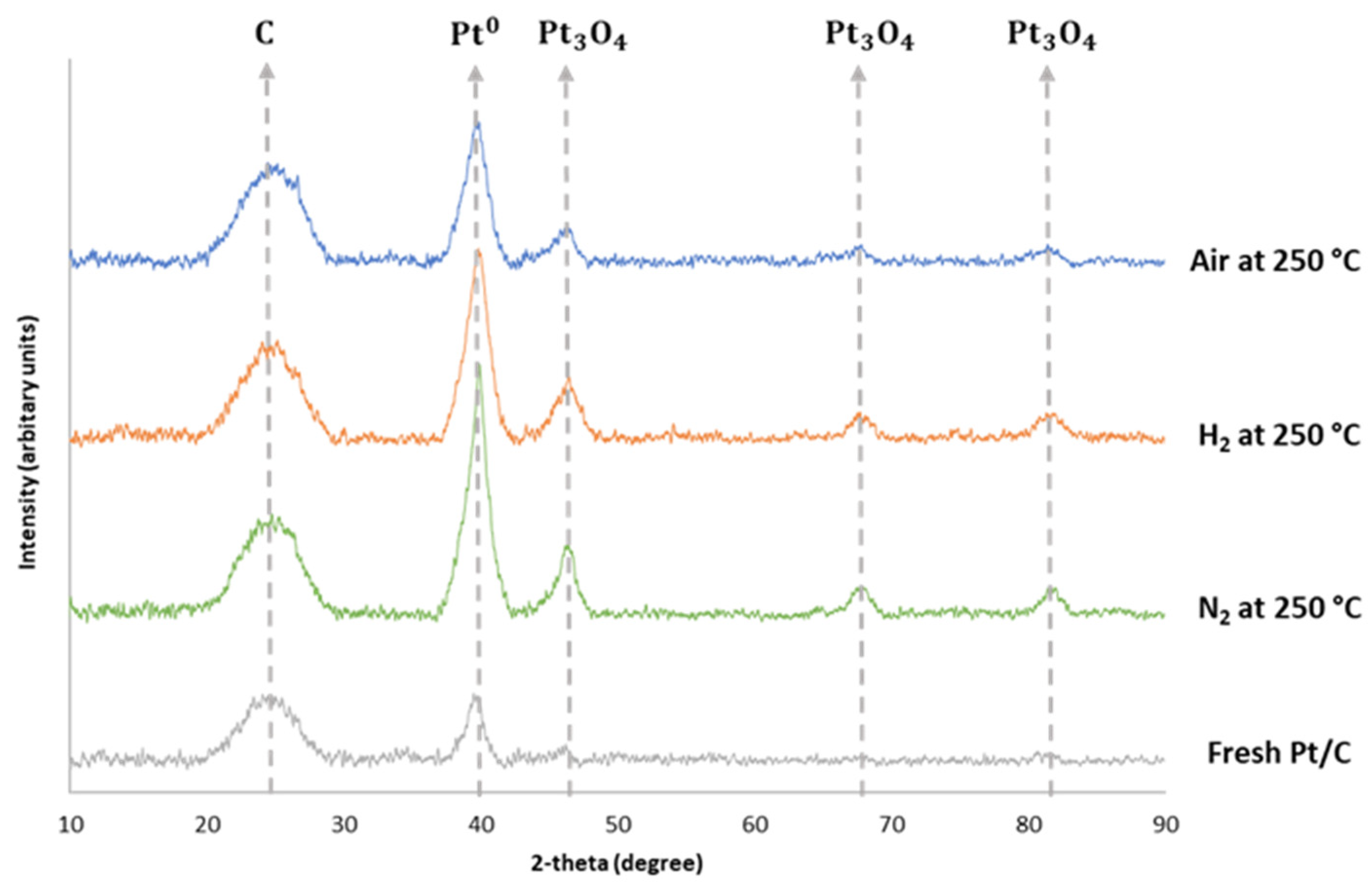
3.3. Catalyst and Solid Residue Characterisation by XRD
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- UK Committee on Climate Change. Net Zero. The UK’s Contribution to Stopping Global Warming. 2019. Available online: https://www.theccc.org.uk/wp-content/uploads/2019/05/Net-Zero-The-UKscontribution-to-stopping-global-warming.pdf (accessed on 1 October 2021).
- WLPGA. The Role of LPG and bioLPG in Europe. 2019. Available online: https://www.wlpga.org/wpcontent/uploads/2019/09/The-Role-of-LPG-Bio-LPG-in-Europe-The-2019-Report.pdf (accessed on 2 October 2021).
- Liquid Gas UK. A Practical Approach—Analysis of Off-Grid Heat Decarbonisation Pathways. 2019. Available online: https://www.liquidgasuk.org/uploads/DOC5DA5B347CF3A7.pdf (accessed on 2 October 2021).
- NNFCC. A Business Case for an Indigenous BioLPG Supply Chain in the UK. Prepared for Liquid Gas UK. 2020. Available online: https://www.liquidgasuk.org/uploads/DOC5FC77254A1388.pdf (accessed on 10 October 2021).
- NNFCC. Biopropane: Feedstocks, Feasibility and Our Future Pathway. Prepared for Liquid Gas UK. 2019. Available online: https://www.liquidgasuk.org/uploads/DOC5DA5B52BBA49F.pdf (accessed on 5 August 2021).
- Johnson, E. A carbon footprint of HVO biopropane. Biofuels. Bioprod. Bioref. 2017, 11, 887–896. [Google Scholar] [CrossRef]
- Immer, J.G.; Lamb, H.H. Fed-batch catalytic deoxygenation of free fatty acids. Energy Fuels 2010, 24, 5291–5299. [Google Scholar] [CrossRef]
- Fu, J.; Lu, X.; Savage, P.E. Hydrothermal decarboxylation and hydrogenation of fatty acids over Pt/C. ChemSusChem 2011, 4, 481–486. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.Z.; Chowdhury, M.B.I.; Jhawar, A.K.; Xu, W.Z.; Charpentier, P.A. Continuous low-pressure decarboxylation of fatty acids to fuel-range hydrocarbons with in situ hydrogen production. Fuel 2018, 212, 470–478. [Google Scholar] [CrossRef]
- Deng, F.; Huang, J.; Ember, E.E.; Achterhold, K.; Dierolf, M.; Jentys, A.; Liu, Y.; Pfeiffer, F.; Lercher, J.A. On the mechanism of catalytic decarboxylation of carboxylic acids on carbon-supported palladium hydride. ACS Catal. 2021, 11, 14625–14634. [Google Scholar] [CrossRef]
- Yeh, T.; Linic, S.; Savage, P.E. Deactivation of Pt catalysts during hydrothermal decarboxylation of butyric acid. ACS Sustain. Chem. Eng. 2014, 2, 2399–2406. [Google Scholar] [CrossRef]
- Lu, M.; Lepore, A.W.; Choi, J.; Li, Z.; Wu, Z.; Polo-Garzon, F.; Hu, M.Z. Acetic acid/propionic acid conversion on metal doped molybdenum carbide catalyst beads for catalytic hot gas filtration. Catalysts 2018, 8, 643. [Google Scholar] [CrossRef]
- Lopez-Ruiz, J.A.; Davis, R.J. Decarbonylation of heptanoic acid over carbon-supported platinum nanoparticles. Green Chem. 2014, 16, 683–694. [Google Scholar] [CrossRef]
- Clark, J.M.; Pilath, H.M.; Mittal, A.; Michener, W.E.; Robichaud, D.J.; Johnson, D.K. Direct production of propene from the thermolysis of poly(β-hydroxybutyrate) (PHB). An experimental and DFT investigation. J. Phys. Chem. A 2016, 120, 332–345. [Google Scholar] [CrossRef]
- Yeh, T.M.; Hockstad, R.L.; Linic, S.; Savage, P.E. Hydrothermal decarboxylation of unsaturated fatty acids over PtSnx/C catalysts. Fuel 2015, 156, 219–224. [Google Scholar] [CrossRef]
- Goshima, T.; Isoda, Y.; Sakaguchi, M.; Fukudome, K.; Mizuta, K.; Nii, S. Feasibility of zeolites in converting butyric acid into propylene for biorefineries. Energy Sources Part A Recovery Util. Environ. Eff. 2018, 40, 306–311. [Google Scholar] [CrossRef]
- Razaq, I.; Simons, K.E.; Onwudili, J.A. Parametric study of Pt/C-catalysed hydrothermal decarboxylation of butyric acid as a potential route for biopropane production. Energies 2021, 14, 3316. [Google Scholar] [CrossRef]
- National Renewable Energy Laboratory, USA. NREL Process for Renewable Butyric Acid Good for Environment, Cheaper Biofuels. 2021. Available online: https://www.nrel.gov/news/program/2021/nrel-process-for-renewable-butyric-acid-good-for-environment-cheaper-biofuels.html (accessed on 25 October 2021).
- Salvachu, D.; Saboe, P.O.; Nelson, R.S.; Singer, C.; McNamara, I.; del Cerro, C.; Chou, Y.-C.; Mohagheghi, A.; Peterson, D.J.; Haugen, S.; et al. Process intensification for the biological production of the fuel precursor butyric acid from biomass. Cell Rep. Phys. Sci. 2021, 2, 100587–100606. [Google Scholar] [CrossRef]
- European Commission Regulation. European Commission Regulation 2568/91 on the characteristics of olive oil and olive residue oil and on the relevant methods of analysis, and subsequent amendments. Off. J. Eur. Community 1991, L248, 1–102. [Google Scholar]
- Taylor, M.J.; Beaumont, S.K.; Islam, M.J.; Tsatsos, S.; Parlett, C.A.M.; Issacs, M.A.; Kyriakou, G. Atom efficient PtCu bimetallic catalysts and ultra-dilute alloys for the selective hydrogenation of furfural. Appl. Catal. B Environ. 2021, 284, 19737. [Google Scholar] [CrossRef]
- Sampaio, F.C.; De Faveri, D.; Mantovani, H.C.; Passos, F.M.L.M.; Perego, P.; Converti, A. Use of response surface methodology for optimization of xylitol production by the new yeast strain Debaryomyces hansenii UFV-170. J. Food Eng. 2006, 76, 376–386. [Google Scholar] [CrossRef]
- Wang, H.; Cui, X.; Wang, R.; Li, C. Response surface optimization of the operating parameters for a complex distillation column based on process simulation. Energy Procedia 2012, 16, 571–578. [Google Scholar] [CrossRef][Green Version]
- Asadi, N.; Zilouei, H. Optimization of organosolv pretreatment of rice straw for enhanced biohydrogen production using Enterobacter aerogenes. Bioresour. Technol. 2017, 227, 335–344. [Google Scholar] [CrossRef]
- Ghorbannezhad, P.; Park, S.; Onwudili, J.A. Co-pyrolysis of biomass and plastic waste over zeolite- and sodium-based catalysts for enhanced yields of hydrocarbon products. Waste Manag. 2020, 102, 909–918. [Google Scholar] [CrossRef]
- Fu, J.; Lu, X.; Savage, P.E. Catalytic hydrothermal deoxygenation of palmitic acid. Energy Environ Sci. 2010, 3, 311–317. [Google Scholar] [CrossRef]
- Sermon, P.A.; Keryou, K.M.; Ahmed, F. Products and intermediates in propane hydrogenolysis on supported Pt. Phys. Chem. Chem. Phys. 2000, 2, 5723–5729. [Google Scholar] [CrossRef]
- Chan, F.L.; Altinkaya, G.; Fung, N.; Tanksale, A. Low temperature hydrogenation of carbon dioxide into formaldehyde in liquid media. Catal. Today 2018, 309, 242–247. [Google Scholar] [CrossRef]
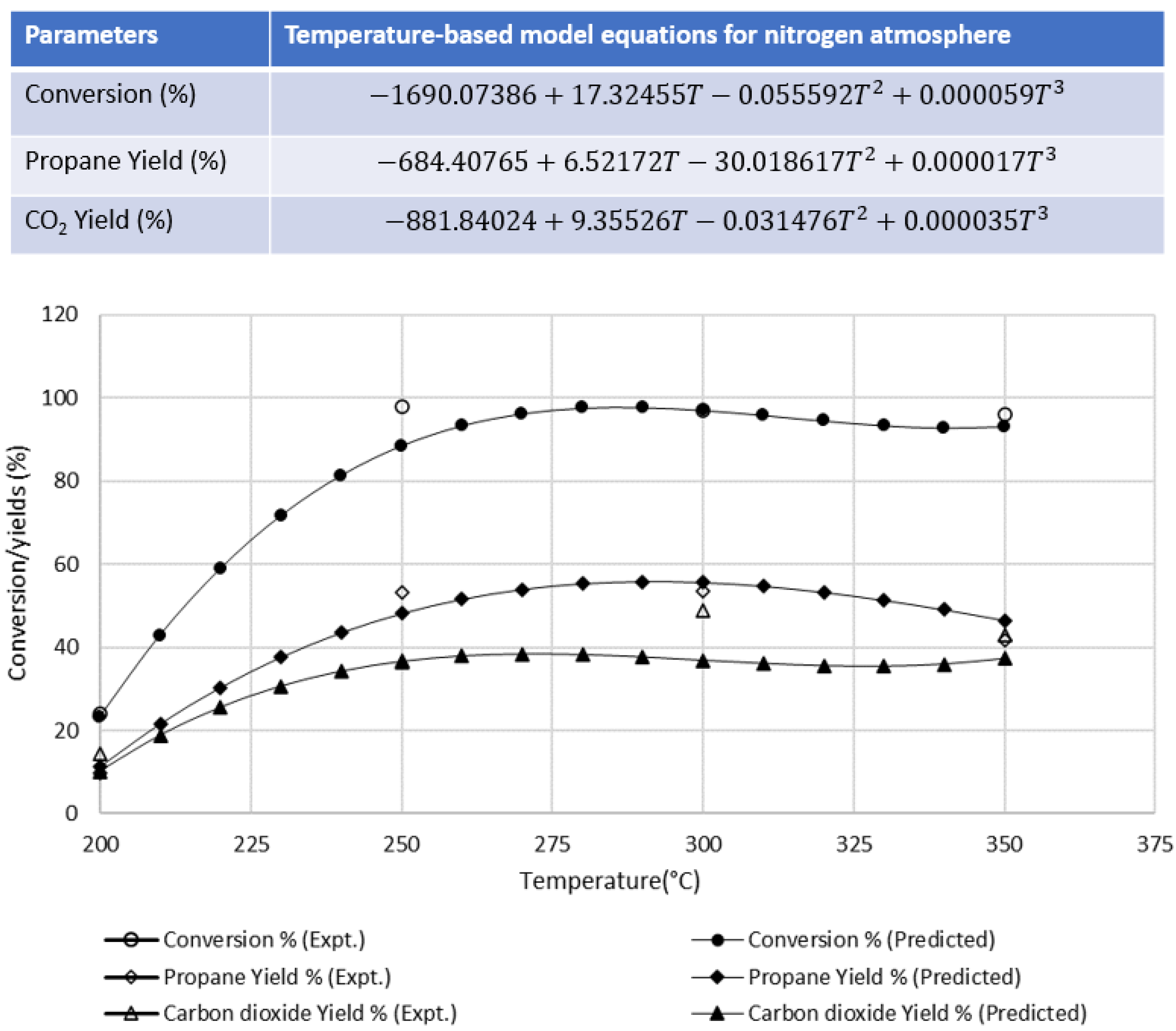

| Wt % | Temperature (°C) | |||
|---|---|---|---|---|
| 200 | 250 | 300 | 350 | |
| Conversion | 24 | 98 | 97 | 96 |
| Hydrogen | - | 0.3 | 0.7 | 1.3 |
| Methane | - | 0.2 | 1.0 | 4.7 |
| Ethane | 0.5 | 1.7 | 3.6 | 5.3 |
| Propane | 9.8 | 53.2 | 53.5 | 41.6 |
| Butane | - | 0.1 | 0.1 | 0.1 |
| CO2 | 14.5 | 41.5 | 43.1 | 46.1 |
| * Propane selectivity | 94 | 96 | 92 | 80 |
| Wt % | Temperature (°C) | |||
|---|---|---|---|---|
| 200 | 250 | 300 | 350 | |
| Conversion | 37 | 77 | 100 | 99 |
| Hydrogen | 3.3 | 3.7 | 4.3 | 5.7 |
| Methane | 0.0 | 0.1 | 0.5 | 12.8 |
| Ethane | 0.4 | 0.7 | 1.9 | 5.0 |
| Propane | 7.8 | 36.0 | 44.4 | 32.3 |
| Butane | 0.1 | 0.4 | 0.1 | 0.1 |
| CO2 | 25.4 | 36.2 | 48.7 | 42.8 |
| * Propane selectivity | 94 | 97 | 94 | 64 |
| Wt % | Pressure (bar) | ||
|---|---|---|---|
| 2.5 | 5.0 | 10.0 | |
| Conversion | 96 | 100 | 100 |
| Hydrogen | 3.1 | 4.3 | 7.3 |
| Methane | 2.5 | 0.5 | 0.4 |
| Ethane | 3.5 | 1.9 | 2.1 |
| Propane | 45.8 | 44.4 | 48.5 |
| Formaldehyde | - | - | 12 |
| Butane | 0.1 | 0.1 | 0.1 |
| CO2 | 40.6 | 48.7 | 29.3 |
| * Propane Selectivity | 88.3 | 94.7 | 95 |
| Wt % | Temperature (°C) | |||
|---|---|---|---|---|
| 200 | 250 | 300 | 350 | |
| Conversion | 33 | 98 | 97 | 98 |
| Hydrogen | 0.0 | 0.0 | 0.4 | 1.5 |
| Methane | 0.2 | 0.4 | 4.6 | 7.4 |
| Ethane | 0.4 | 2.9 | 4.7 | 3.2 |
| Propane | 1.5 | 50.6 | 38.9 | 32.0 |
| Butane | 0.0 | 0.1 | 0.1 | 0.1 |
| CO2 | 31.0 | 44.8 | 50.3 | 53.6 |
| * Propane selectivity | 60 | 94 | 79 | 74 |
| Parameters | Model Equations Based on Coded Factors under Nitrogen Atmosphere | Minimum (X = −1) | Central Point (X = 0) | Maximum (X = +1) | Optimum Temperature (°C) |
|---|---|---|---|---|---|
| Conversion (%) | 23.6 (24.0) | 98.2 (97.0) | 95.5 (96.0) | 300 (290) | |
| Propane Yield (%) | 9.81 (10.2) | 50.8 (53.5) | 40.6 (41.6) | ||
| CO2 Yield (%) | 16.7 (14.5) | 42.8 (43.1) | 41.6 (46.1) | ||
| Parameters | Model Equations Based on Coded Factors under Hydrogen Atmosphere | Minimum (X = −1) | Central Point (X = 0) | Maximum (X = +1) | Optimum Temperature (°C) |
| Conversion (%) | 37.2 (37.1) | 87.6 (100) | 99.2 (99.0) | 320 (300) | |
| Propane Yield (%) | 7.61 (7.80) | 38.8 (36.0) | 32.1 (32.0) | ||
| CO2 Yield (%) | 25.1 (25.4) | 44.4 (48.7) | 42.6 (42.8) | ||
| Parameters | Model Equations Based on Based Coded Factors under Compressed Air Atmosphere | Minimum (X = −1) | Central Point (X = 0) | Maximum (X = +1) | Optimum Temperature (°C) |
| Conversion (%) | 31.8 (33.0) | 98.0 (97.0) | 98.7 (98.0) | 270 (250) | |
| Propane Yield (%) | 0.32 (1.50) | 46.0 (50.6) | 30.4 (32.0) | ||
| CO2 Yield (%) | 29.9 (31.0) | 46.4 (44.8) | 54.0 (53.6) |
| Models | Statistics | Response | ||
|---|---|---|---|---|
| Conversion | Propane | CO2 | ||
| Linear | R2 | 0.68 | 0.28 | 0.81 |
| R2-adj. | 0.65 | 0.22 | 0.79 | |
| R2-pred. | 0.56 | 0.1 | 0.74 | |
| Sdv. | 15.8 | 14 | 4.01 | |
| Linear Square | R2 | 0.93 | 0.9 | 0.95 |
| R2-adj. | 0.94 | 0.88 | 0.94 | |
| R2-pred. | 0.92 | 0.85 | 0.93 | |
| Sdv. | 6.4 | 5.4 | 2.06 | |
| Full quadratic | R2 | 0.97 | 0.98 | 0.98 |
| R2-adj. | 0.96 | 0.97 | 0.97 | |
| R2-pred. | 0.93 | 0.94 | 0.94 | |
| Sdv. | 5.1 | 2.6 | 1.5 | |
| Cubic | R2 | 0.95 | 0.96 | 0.95 |
| R2-adj. | 0.93 | 0.95 | 0.94 | |
| R2-pred. | 0.9 | 0.93 | 0.91 | |
| Sdv. | 6.7 | 3.2 | 2.2 | |
| Response | Factors | DF | Mean of Square | F-Value | p-Value |
|---|---|---|---|---|---|
| Conversion | Model | 4 | 2124.9 | 81.63 | 0.0001 |
| A | 1 | 434.6 | 16.7 | 0.0035 | |
| A2 | 1 | 563.5 | 21.65 | 0.0016 | |
| A3 | 1 | 4.7 | 0.18 | 0.68 | |
| A4 | 1 | 203 | 7.80 | 0.02 | |
| Residual | 8 | 26.03 | - | - | |
| Lack of fit | 3 | 69.4 | 13013.3 | 0.0001 | |
| Pure error | 5 | 0.005 | - | - | |
| Total | 12 | - | - | - | |
| Propane | Model | 4 | 749.75 | 105 | 0.0001 |
| A | 1 | 15.16 | 2.14 | 0.18 | |
| A2 | 1 | 218.1 | 30.85 | 0.0001 | |
| A3 | 1 | 200.9 | 28.4 | 0.0007 | |
| A4 | 1 | 36.9 | 5.2 | 0.05 | |
| Residual | 8 | 7.57 | - | - | |
| Lack of fit | 3 | 8.4 | 1.3 | 0.36 | |
| Pure error | 5 | 6.29 | - | - | |
| Total | 12 | - | - | - | |
| CO2 | Model | 4 | 235.9 | 105.04 | 0.0001 |
| A | 1 | 71.1 | 31.7 | 0.0005 | |
| A2 | 1 | 51.3 | 22.9 | 0.0014 | |
| A3 | 1 | 0.12 | 0.05 | 0.8 | |
| A4 | 1 | 24.67 | 10.72 | 0.01 | |
| Residual | 8 | 2.2 | - | - | |
| Lack of fit | 3 | 5.9 | 117.3 | 0.0001 | |
| Pure error | 5 | 0.05 | - | - | |
| Total | 12 | - | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Onwudili, J.A.; Razaq, I.; Simons, K.E. Optimisation of Propane Production from Hydrothermal Decarboxylation of Butyric Acid Using Pt/C Catalyst: Influence of Gaseous Reaction Atmospheres. Energies 2022, 15, 268. https://doi.org/10.3390/en15010268
Onwudili JA, Razaq I, Simons KE. Optimisation of Propane Production from Hydrothermal Decarboxylation of Butyric Acid Using Pt/C Catalyst: Influence of Gaseous Reaction Atmospheres. Energies. 2022; 15(1):268. https://doi.org/10.3390/en15010268
Chicago/Turabian StyleOnwudili, Jude A., Iram Razaq, and Keith E. Simons. 2022. "Optimisation of Propane Production from Hydrothermal Decarboxylation of Butyric Acid Using Pt/C Catalyst: Influence of Gaseous Reaction Atmospheres" Energies 15, no. 1: 268. https://doi.org/10.3390/en15010268
APA StyleOnwudili, J. A., Razaq, I., & Simons, K. E. (2022). Optimisation of Propane Production from Hydrothermal Decarboxylation of Butyric Acid Using Pt/C Catalyst: Influence of Gaseous Reaction Atmospheres. Energies, 15(1), 268. https://doi.org/10.3390/en15010268







