Effects of Pressure and Coal Rank on the Oxy-Fuel Combustion of Pulverized Coal
Abstract
:1. Introduction
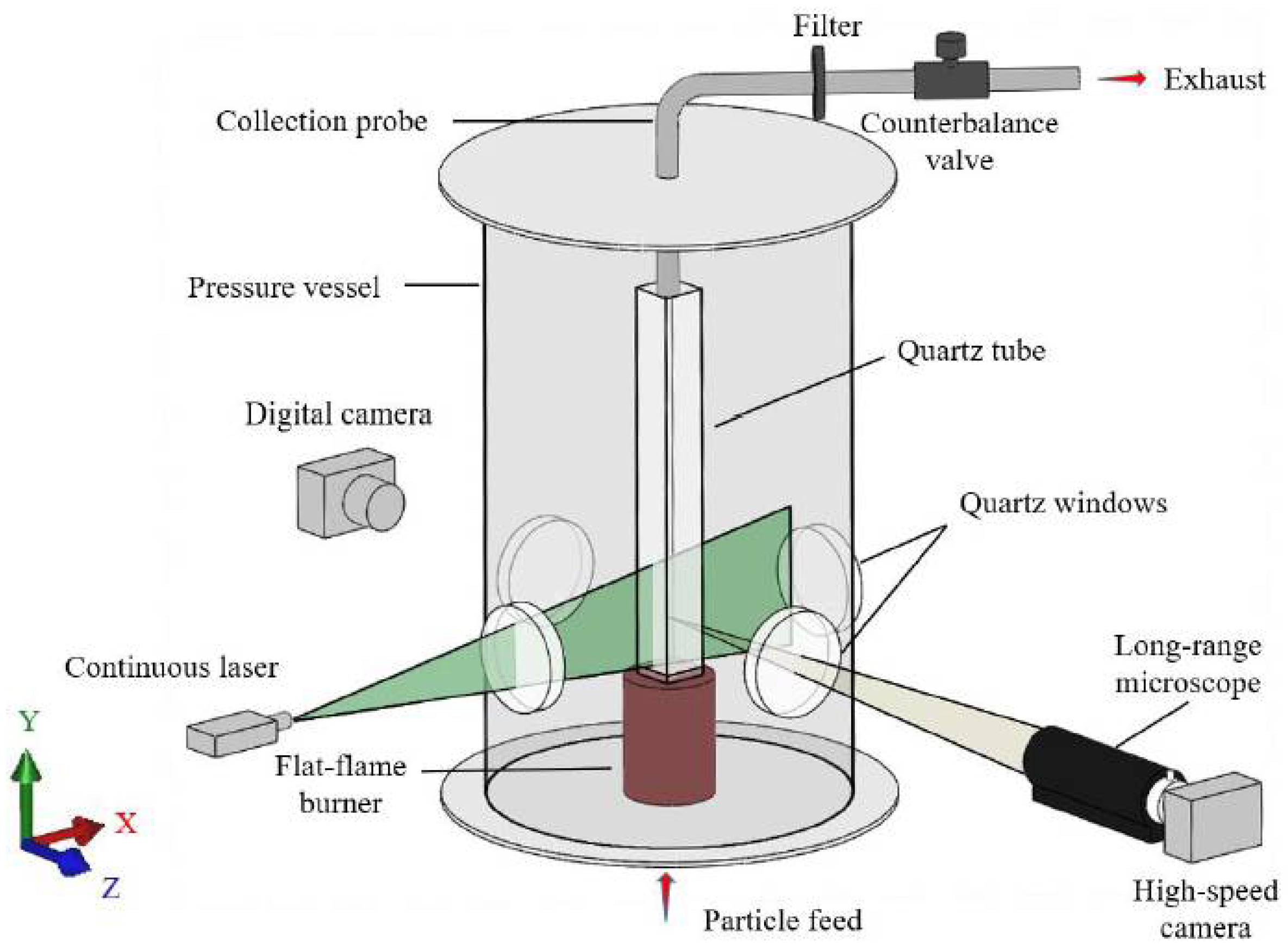
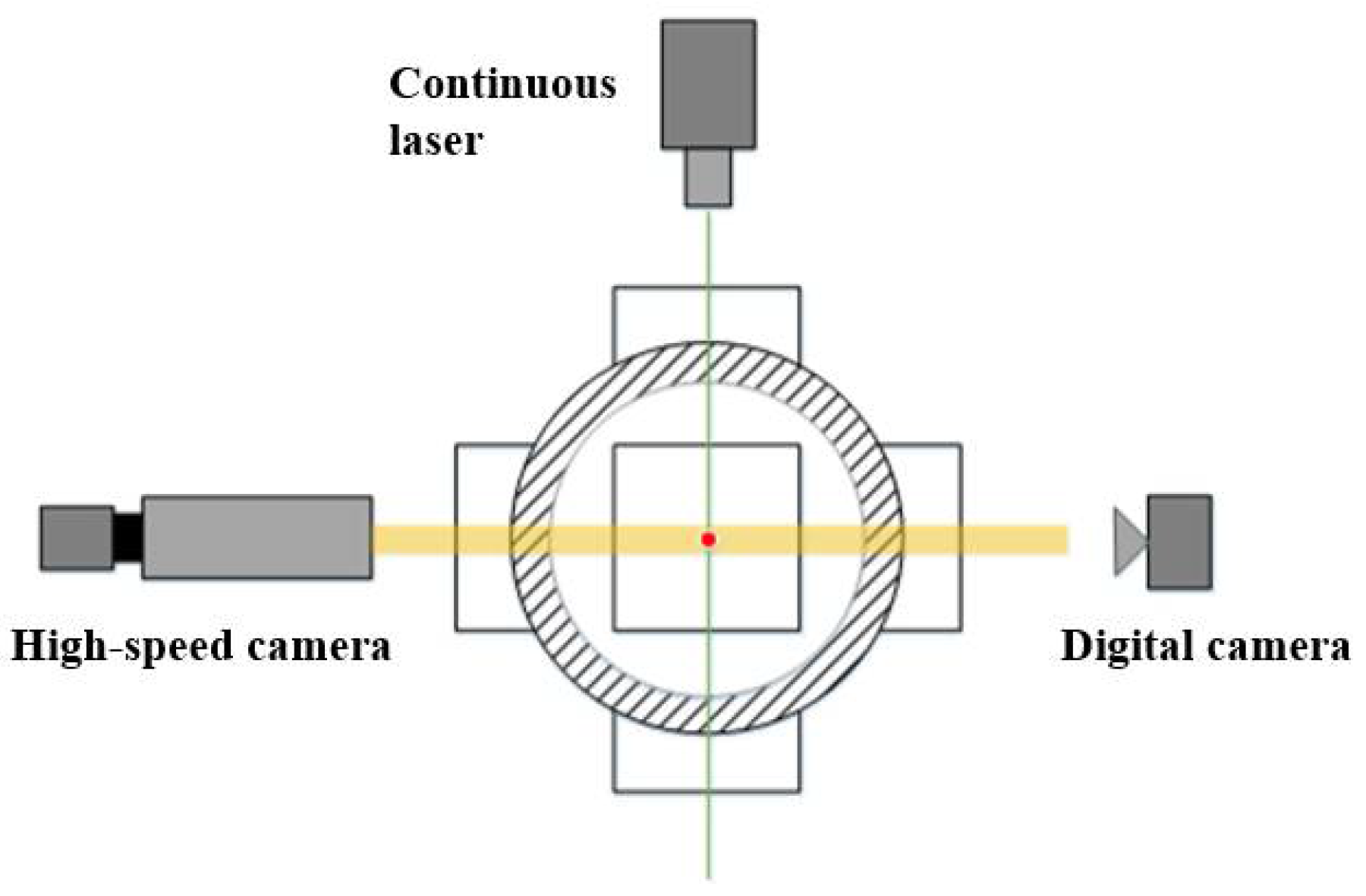
2. Experimental Methods
2.1. Experimental Setup
2.2. Methods of Data Processing
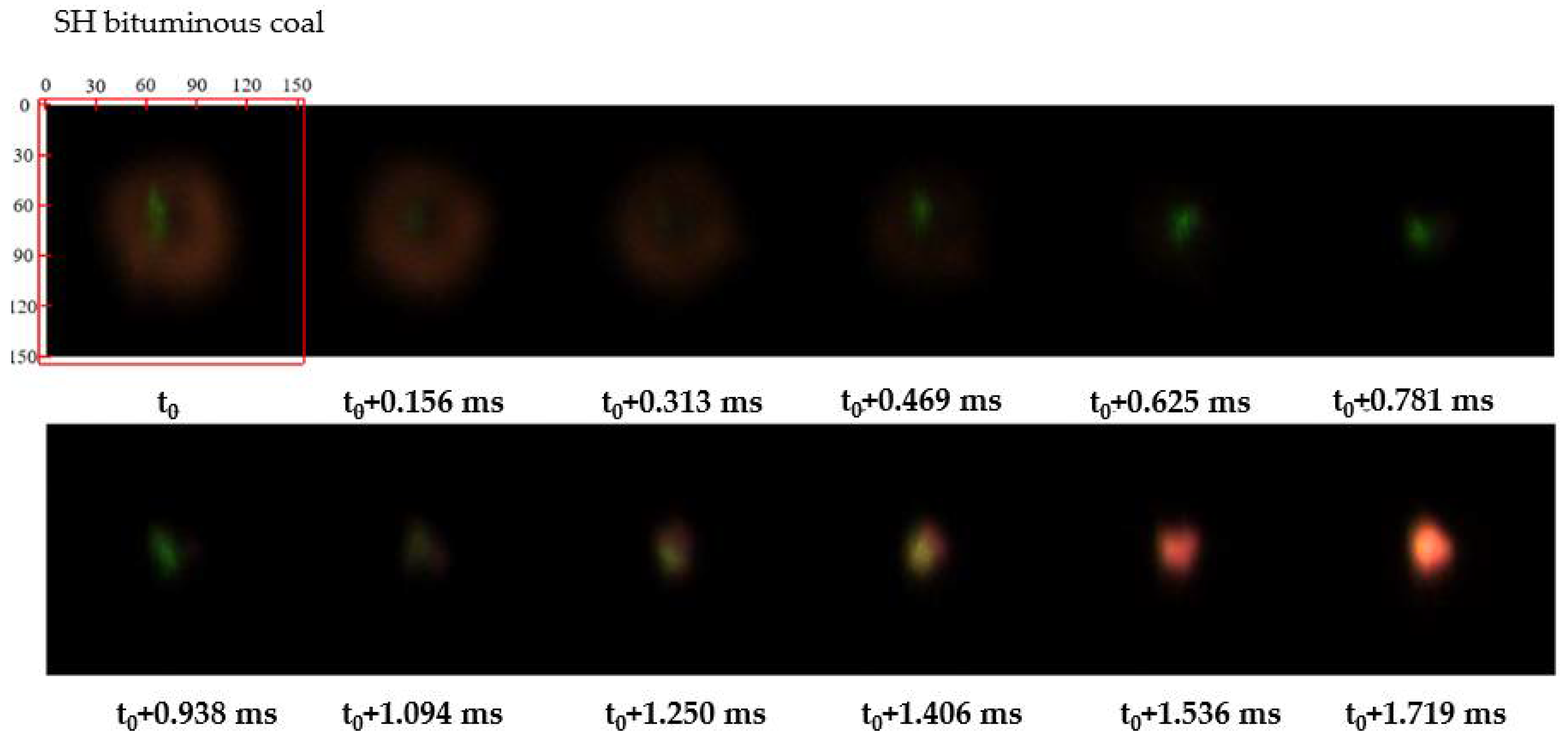
2.2.1. Identification of Devolatilizing Particles
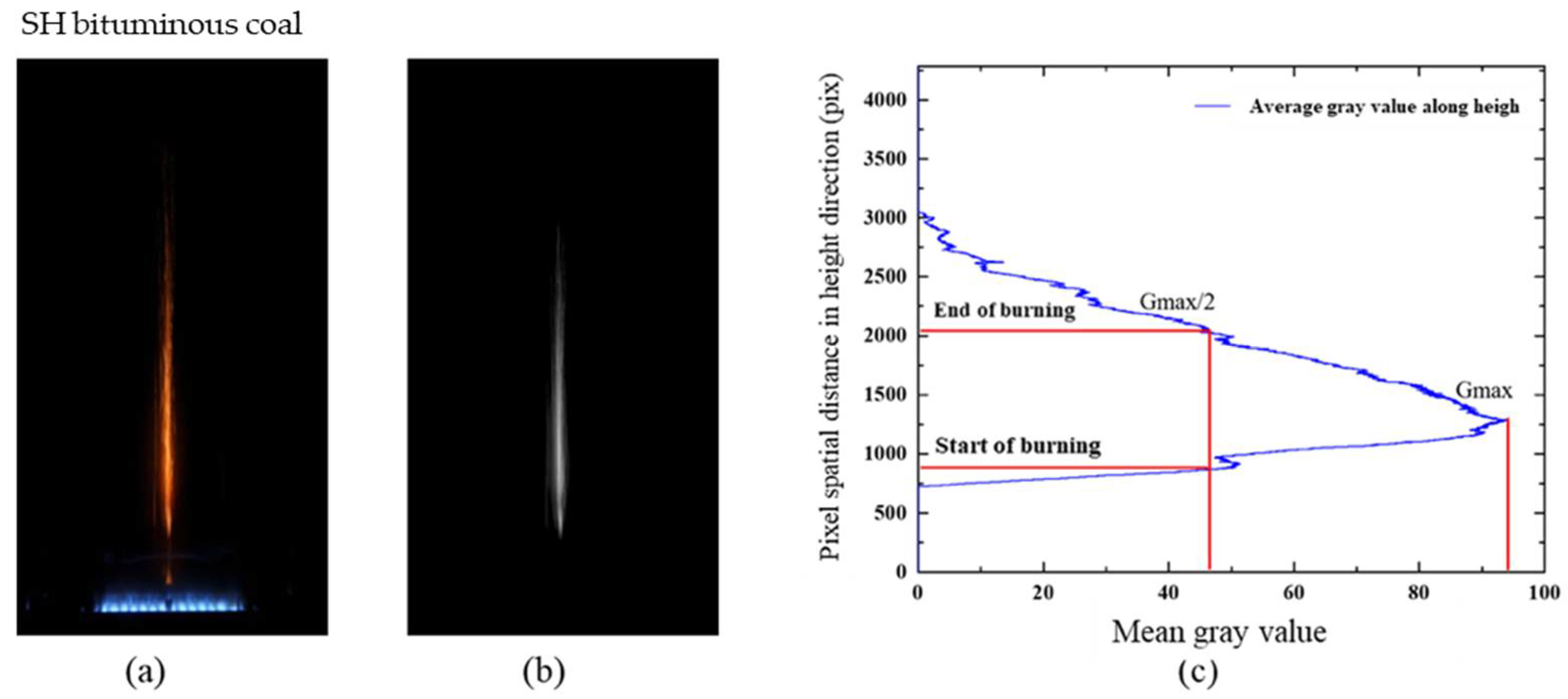
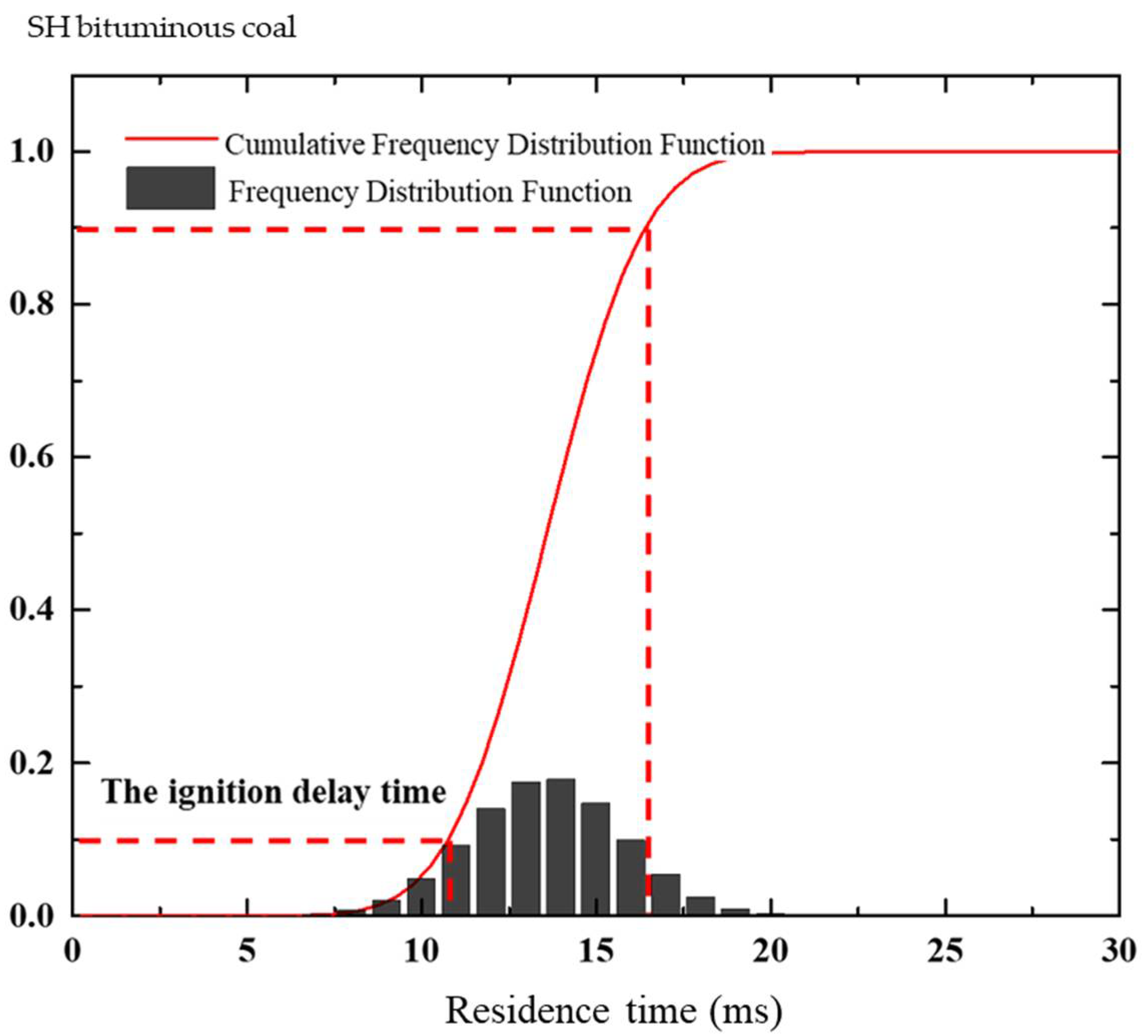
2.2.2. Determination of Ignition Delay Time
2.2.3. Two-Color Pyrometry
2.3. Fuels and Experimental Conditions
3. Results and Discussions
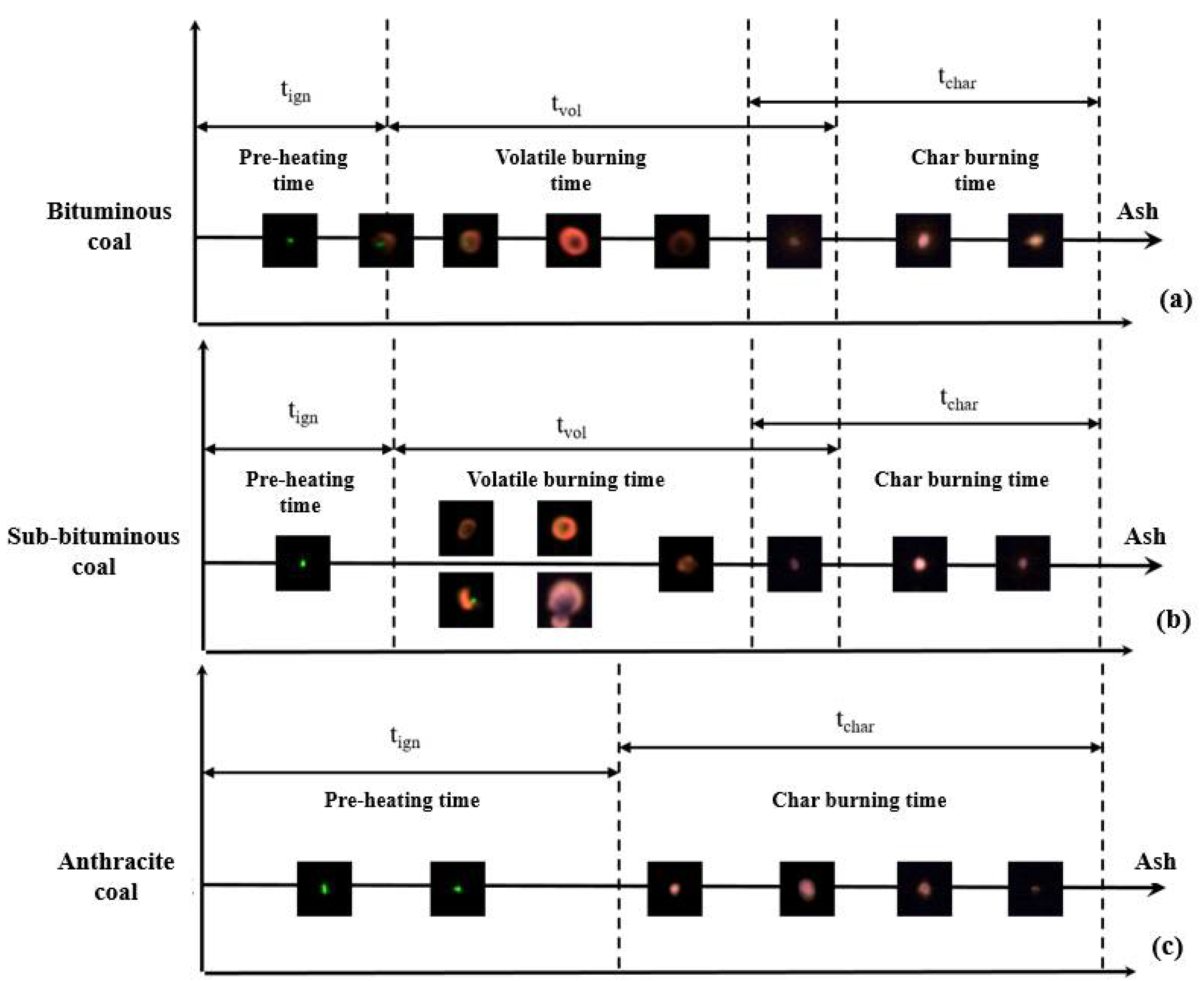
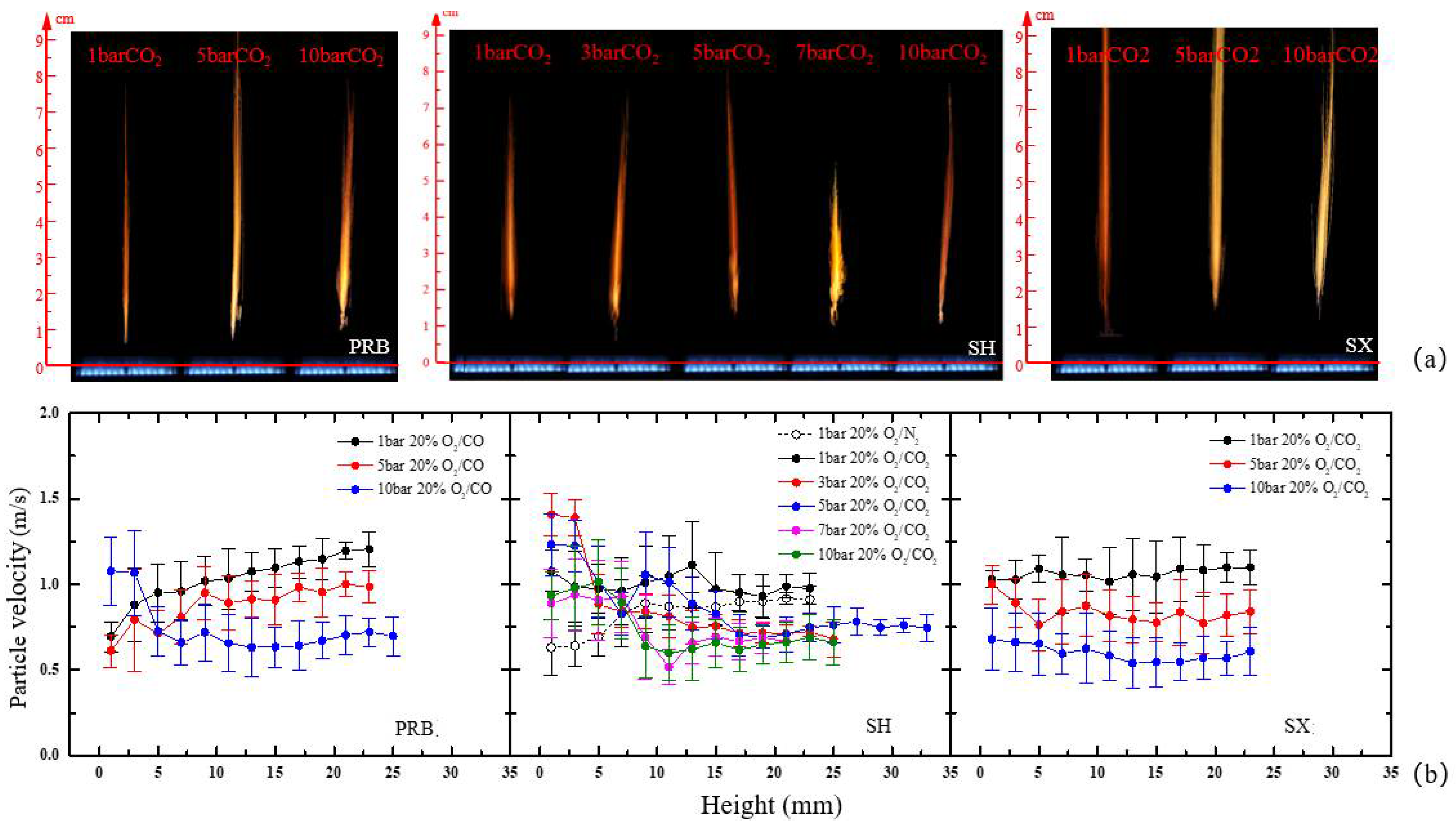
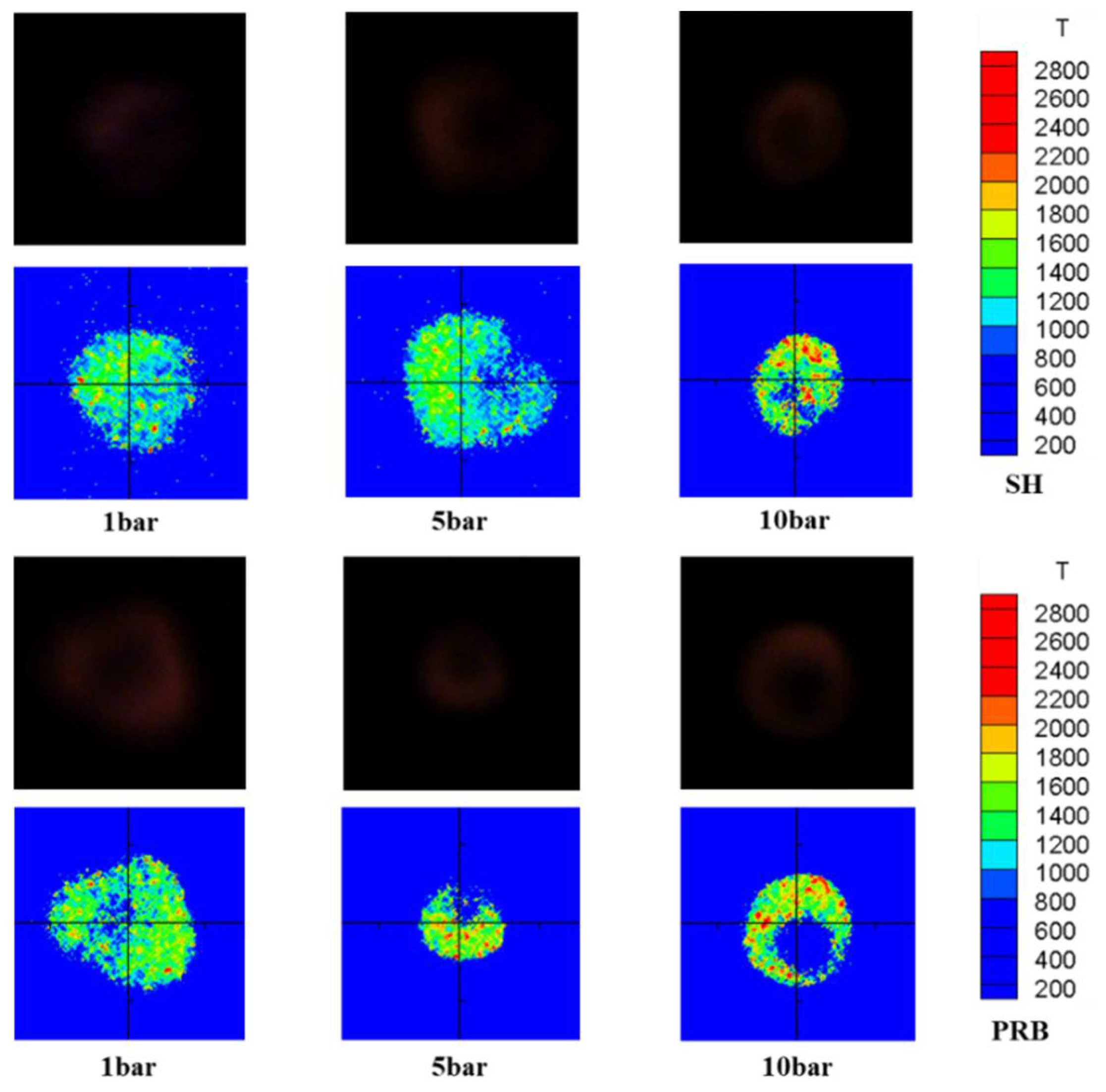
3.1. Micromorphology of Different Coals during Ignition and Combustion
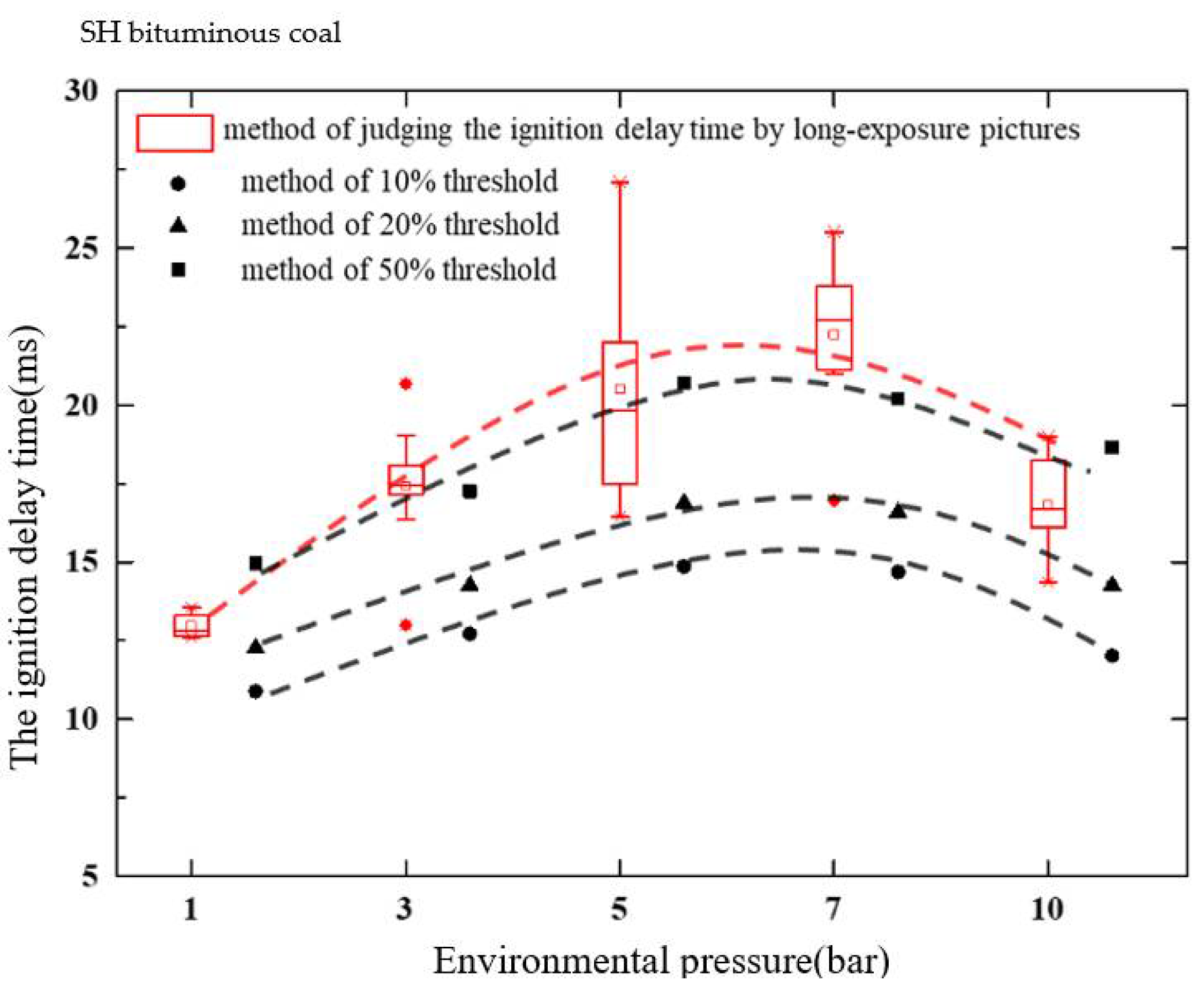
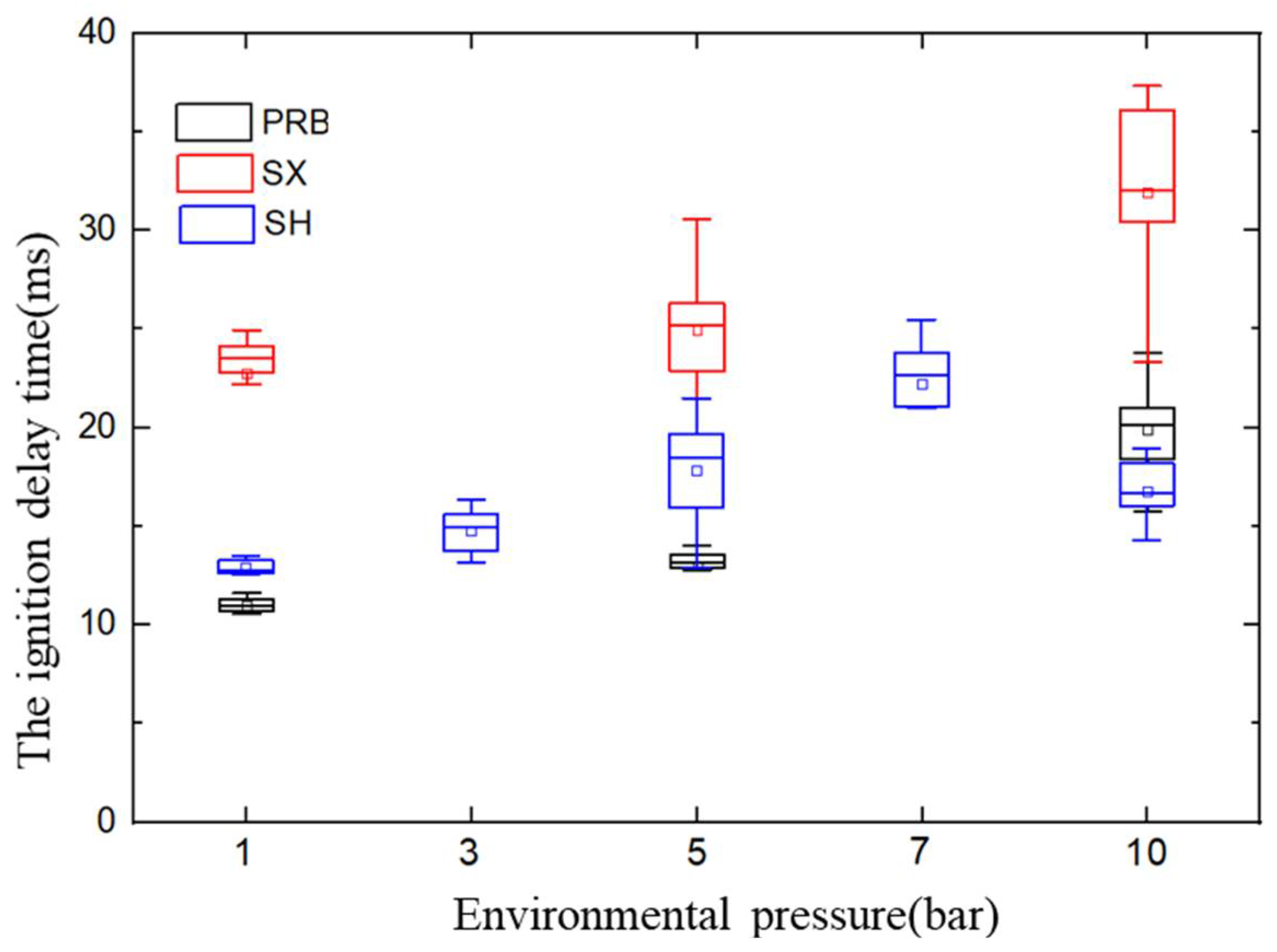
3.2. Effect of Pressure on the Ignition Delay Time
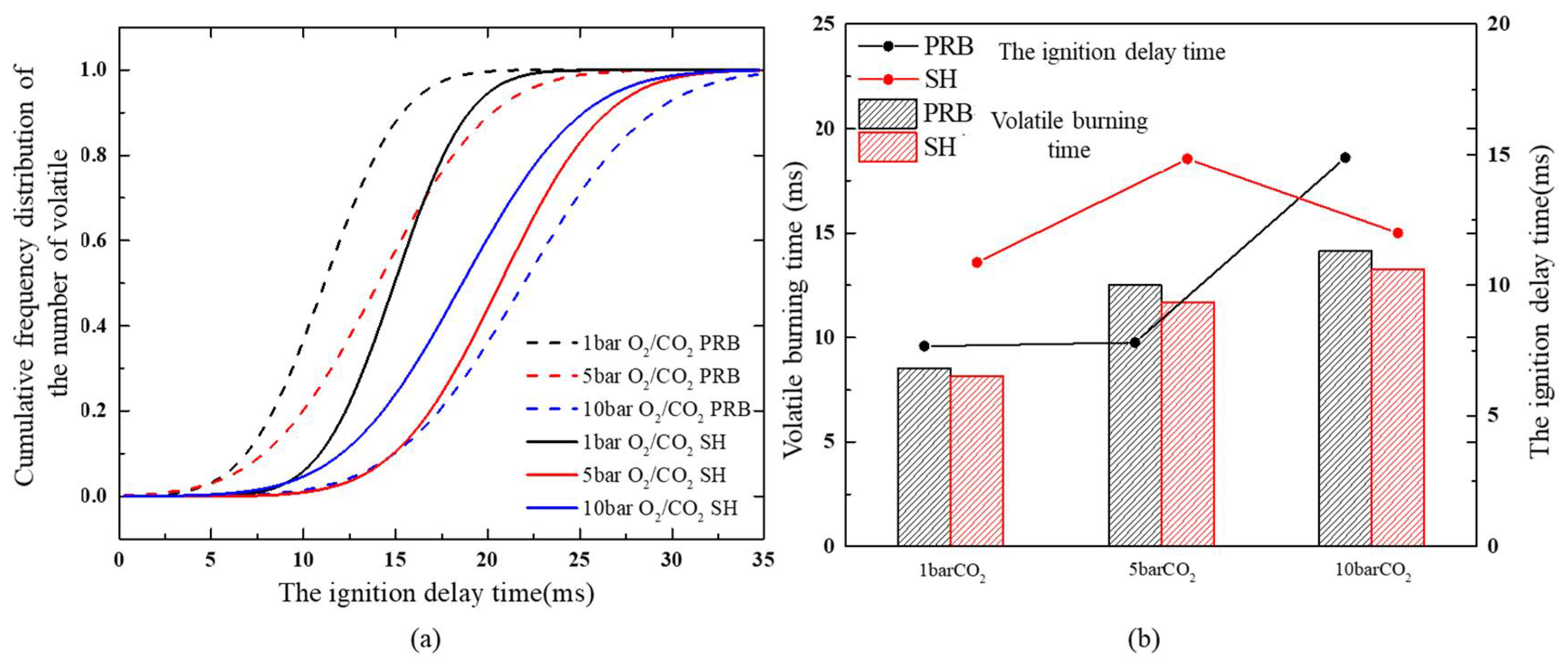
3.3. Effect of Pressure on the Duration of Volatile Combustion
4. Conclusions
- 1.
- At atmospheric pressure, the ignition delay time of SX anthracite coal was longer than that of SH bituminous coal, while the PRB sub-bituminous coal was the shortest. Under pressurized conditions, the ignition delay time of PRB sub-bituminous coal and SX anthracite coal increased with the increase in pressure. As the reduction in the pyrolysis temperature of bituminous coal was larger than that of sub-bituminous coal, the ignition delay time of bituminous coal was shorter than that of bituminous coal; moreover, the ignition delay time of SH bituminous coal firstly increased and then decreased;
- 2.
- Volatile compounds appear in the combustion process of homogeneous ignition coal. Pressure also affects the pyrolysis process of coal. As the pressure increases, it became more difficult to release the volatiles produced by coal pyrolysis, which reduced the release rate of volatiles during the ignition stage, and prolonged the release time and burning duration time of the volatiles. Since the volatile content of PRB sub-bituminous coal was higher than that of SH bituminous coal, the volatile analysis rate was lower and the process lasted longer. Therefore, the devolatilization duration time of volatiles in PRB sub-bituminous coal was longer.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- National Bureau of Statistics of People’s Republic of China. China Statistics Yearbook 2020; China Statistics Press: Beijing, China, 2020.
- Barbucci, P. Enel strategy for zero emission power generation. In Proceedings of the Beijing Symposium on Sino-Italy Cooperation on Clean Coal Technology and CCS, Beijing, China, 15 December 2008. [Google Scholar]
- Ghoniem, A.F. ENEL-MIT Clean Energy Program; MIT: Cambridge, MA, USA, October 2008; Available online: https://energy.mit.edu/search/?q=Clean%20Energy%20Program (accessed on 10 October 2021).
- Zheng, C.; Zhao, Y.; Guo, X. Research and Development of Oxy-fuel Combustion in China. In Proceedings of the CSEE, Kuala Lumpur, Malaysia, 8–9 March 2014; pp. 3856–3864. [Google Scholar]
- Pei, X. Study on Pressurized Oxy—Coal Combustion Characteristics and System Energy Consumption Analysis. Ph.D. Thesis, Beijing Jiaotong University, Beijing, China, 2017. [Google Scholar]
- Shaddix, C.R.; Molina, A. Particle Imaging Of Ignition and Devolatilization Of Pulverized Coal During Oxy-Fuel Combustion. Proc. Combust. Inst. 2009, 32, 2091–2098. [Google Scholar] [CrossRef]
- Xu, M.; Yu, D.; Yao, H.; Liu, X.; Qiao, Y. Coal combustion-generated aerosols: Formation and properties. Proc. Combust. Inst. 2011, 33, 1681–1697. [Google Scholar] [CrossRef]
- Hong, J.S.; Chaudhry, G.; Brisson, J.G.; Field, R.; Gazzino, M.; Ghoniem, A.F. Analysis of oxy-fuel combustion power cycle utilizing a pressurized coal combustor. Energy 2009, 34, 1332–1340. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.; Choi, S. An observation of combustion behavior of a single coal particle entrained into hot gas flow. Combust. Flame 2015, 162, 2610–2620. [Google Scholar] [CrossRef]
- Yuan, Y.; Li, S.; Zhao, F.; Yao, Q.; Long, M.B. Characterization on hetero-homogeneous ignition of pulverized coal particle streams using CH∗ chemiluminescence and 3 color pyrometry. Fuel 2016, 184, 1000–1006. [Google Scholar] [CrossRef]
- Xu, Y.; Li, S.; Yao, Q.; Yuan, Y. Investigation of Steam Effect on Ignition of Dispersed Coal Particles in O2/N2 and O2/CO2 Ambiences. Fuel 2018, 233, 388–395. [Google Scholar] [CrossRef]
- Liu, Y.; Geier, M.; Molina, A.; Shaddix, C.R. Pulverized coal stream ignition delay under conventional and oxy-fuel combustion conditions. Int. J. Greenh. Gas Control 2011, 5, S36–S46. [Google Scholar] [CrossRef]
- Molina, A.; Shaddix, C.R. Ignition and Devolatilization of Pulverized Bituminous Coal Particles during Oxygen/Carbon Dioxide Coal Combustion. Proc. Combust. Inst. 2007, 31, 1905–1912. [Google Scholar] [CrossRef]
- Schiemann, M.; Scherer, V.; Wirtz, S. Optical Coal Particle Temperature Measurement under Oxy-Fuel Conditions: Measurement Methodology and Initial Results. Chem. Eng. Technol. 2009, 32, 2000–2004. [Google Scholar] [CrossRef]
- Sun, C.L.; Zhang, M.Y. Ignition of Coal Particles at High Pressure in a Thermogravimetric Analyzer. Combust. Flame 1998, 115, 267–274. [Google Scholar] [CrossRef]
- Yin, C.; Yan, J. Oxy-fuel combustion of pulverized fuels: Combustion fundamentals and modeling. Appl. Energy 2016, 162, 742–762. [Google Scholar] [CrossRef]
- Ying, Z.; Zheng, X.; Cui, G. Pressurized oxy-fuel combustion performance of pulverized coal for CO2 capture. Appl. Therm. Eng. 2016, 99, 411–418. [Google Scholar] [CrossRef]
- Zheng, L.; Pomalis, R.; Clements, B. Technical feasibility study of TIPS process and comparison with other CO2 capture power generation processes. In Proceedings of the 32nd International Technical Conference on Coal Utilization and Fuel Systems, Clearwater, FL, USA, 10–15 June 2007. [Google Scholar]
- Li, L.; Duan, L.; Yang, Z.; Zhao, C. Pressurized oxy-fuel combustion characteristics of single coal particle in a visualized fluidized bed combustor. Combust. Flame 2020, 211, 218–228. [Google Scholar] [CrossRef]
- Lewis, A.D. Gasification of Biomass, Coal, and Petroleum Coke at High Heating Rates and Elevated Pressure; Brigham Young University: Provo, UT, USA, 2014. [Google Scholar]
- Ma, L. Combustion and Gasification of Chars in Oxygen and Carbon Dioxide at Elevated Pressure. Ph.D. Thesis, Stanford University, Stanford, CA, USA, 2006. [Google Scholar]
- Schiemann, M.; Vorobiev, N.; Scherer, V. Stereoscopic Pyrometer for Char Combustion Characterization. Appl. Opt. 2015, 54, 1097. [Google Scholar] [CrossRef] [PubMed]
- Köser, J.; Li, T.; Vorobiev, N.; Dreizler, A.; Schiemann, M.; Boehm, B. Multi-parameter diagnostics for high-resolution in-situ measurements of single coal particle combustion. Proc. Combust. Inst. 2019, 37, 2893–2900. [Google Scholar] [CrossRef]
- Chen, Q.; Qin, D.; Li, J.; Liu, Z. A Particle-Tracking Image Pyrometer for Characterizing Ignition of Pulverized Coal Particles. Fuel Processing Technol. 2022, 225, 107065. [Google Scholar] [CrossRef]
- Li, J. PIV Experimental Study and Direct Numerical Simulation of Gas-Solid Two-Phase Turbulence in a Horizontal Channel. Ph.D. Thesis, Huazhong University of Science and Technology, Wuhan, China, 2010. [Google Scholar]
- Levendis, Y.A.; Joshi, K.; Khatami, R.; Sarofim, A.F. Combustion Behavior in Air of Single Particles from Three Different Coal Ranks and from Sugarcane Bagasse. Combust. Flame 2011, 158, 452–465. [Google Scholar] [CrossRef]

| Coal | Pressure | Gas Temperature | Gas Composition | ||
|---|---|---|---|---|---|
| atm | K | O2 | CO2 | H2O | |
| PRB sub-bituminous | 1 | 1400 | 20% | 78.22% | 1.78% |
| 5 | 1400 | 20% | 79.65% | 0.35% | |
| 10 | 1400 | 20% | 79.65% | 0.35% | |
| Shenhua bituminous | 1 | 1400 | 20% | 78.22% | 1.78% |
| 3 | 1400 | 20% | 79.43% | 0.57% | |
| 5 | 1400 | 20% | 79.65% | 0.35% | |
| 7 | 1400 | 20% | 79.66% | 0.34% | |
| 10 | 1400 | 20% | 79.65% | 0.35% | |
| Shanxi anthracite | 1 | 1400 | 20% | 78.22% | 1.78% |
| 5 | 1400 | 20% | 79.65% | 0.35% | |
| 10 | 1400 | 20% | 79.65% | 0.35% | |
| Coal | PRB Sub-Bituminous | SH Bituminous | SX Anthracite |
|---|---|---|---|
| Elemental analysis (wt.%, ad) | |||
| Water | 1.47 | 1.26 | 0.40 |
| Ash | 7.11 | 13.89 | 23.18 |
| Volatiles | 54.72 | 48.27 | 21.60 |
| Fixed carbon | 36.7 | 36.58 | 51.22 |
| Elemental analysis (wt.%, ad) | |||
| C | 67.99 | 64.44 | 60.6 |
| H | 4.45 | 3.76 | 3.27 |
| N | 1.27 | 1.08 | 1.16 |
| S | 0.55 | 0.36 | 2.36 |
| O | 17.15 | 15.21 | 9.03 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qin, D.; Chen, Q.; Li, J.; Liu, Z. Effects of Pressure and Coal Rank on the Oxy-Fuel Combustion of Pulverized Coal. Energies 2022, 15, 265. https://doi.org/10.3390/en15010265
Qin D, Chen Q, Li J, Liu Z. Effects of Pressure and Coal Rank on the Oxy-Fuel Combustion of Pulverized Coal. Energies. 2022; 15(1):265. https://doi.org/10.3390/en15010265
Chicago/Turabian StyleQin, Dingyi, Qianyun Chen, Jing Li, and Zhaohui Liu. 2022. "Effects of Pressure and Coal Rank on the Oxy-Fuel Combustion of Pulverized Coal" Energies 15, no. 1: 265. https://doi.org/10.3390/en15010265
APA StyleQin, D., Chen, Q., Li, J., & Liu, Z. (2022). Effects of Pressure and Coal Rank on the Oxy-Fuel Combustion of Pulverized Coal. Energies, 15(1), 265. https://doi.org/10.3390/en15010265







