Heat Transfer Limitations in Supercritical Water Gasification
Abstract
1. Introduction
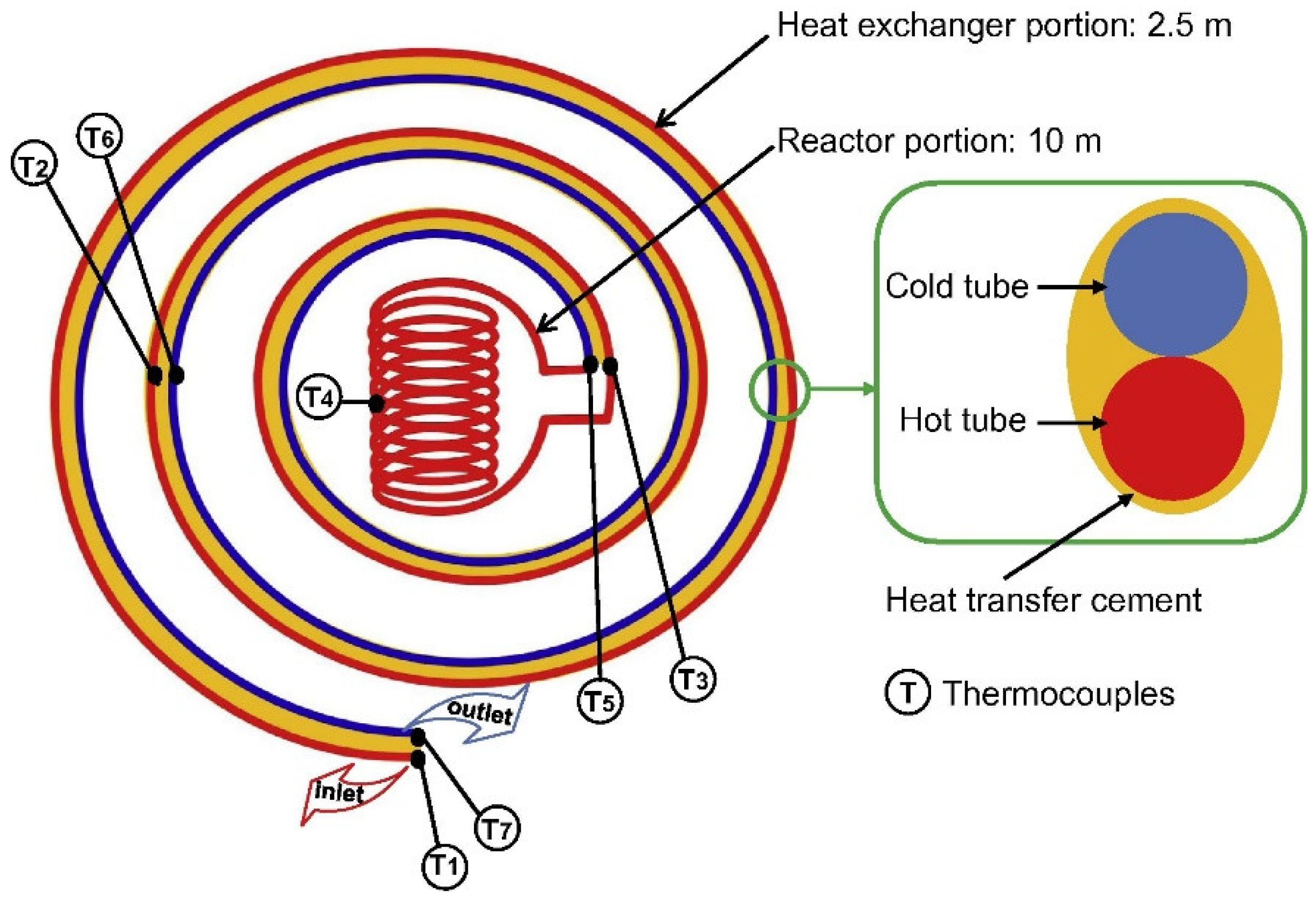
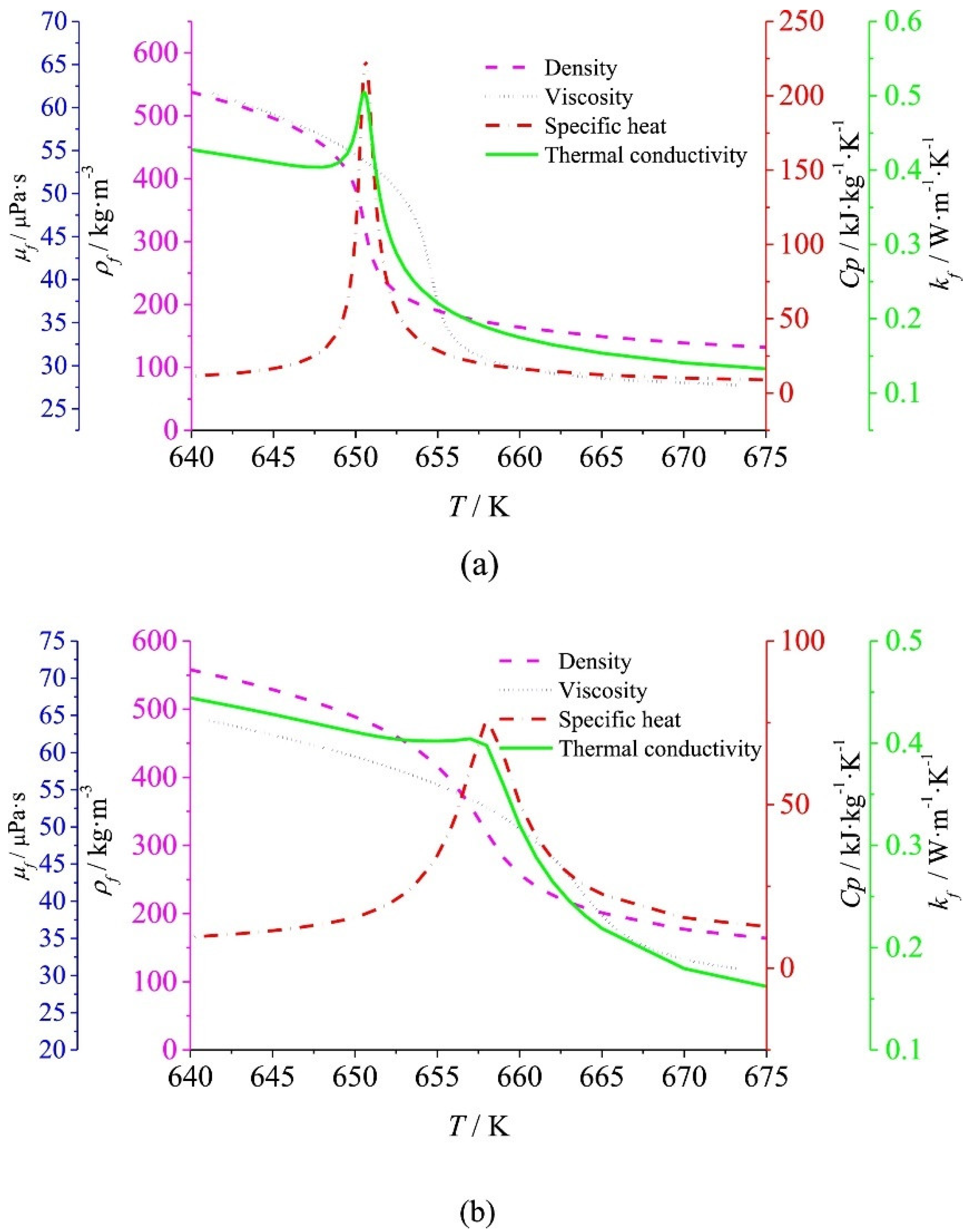
2. Heat Transfer Deterioration in SCWG Experiments
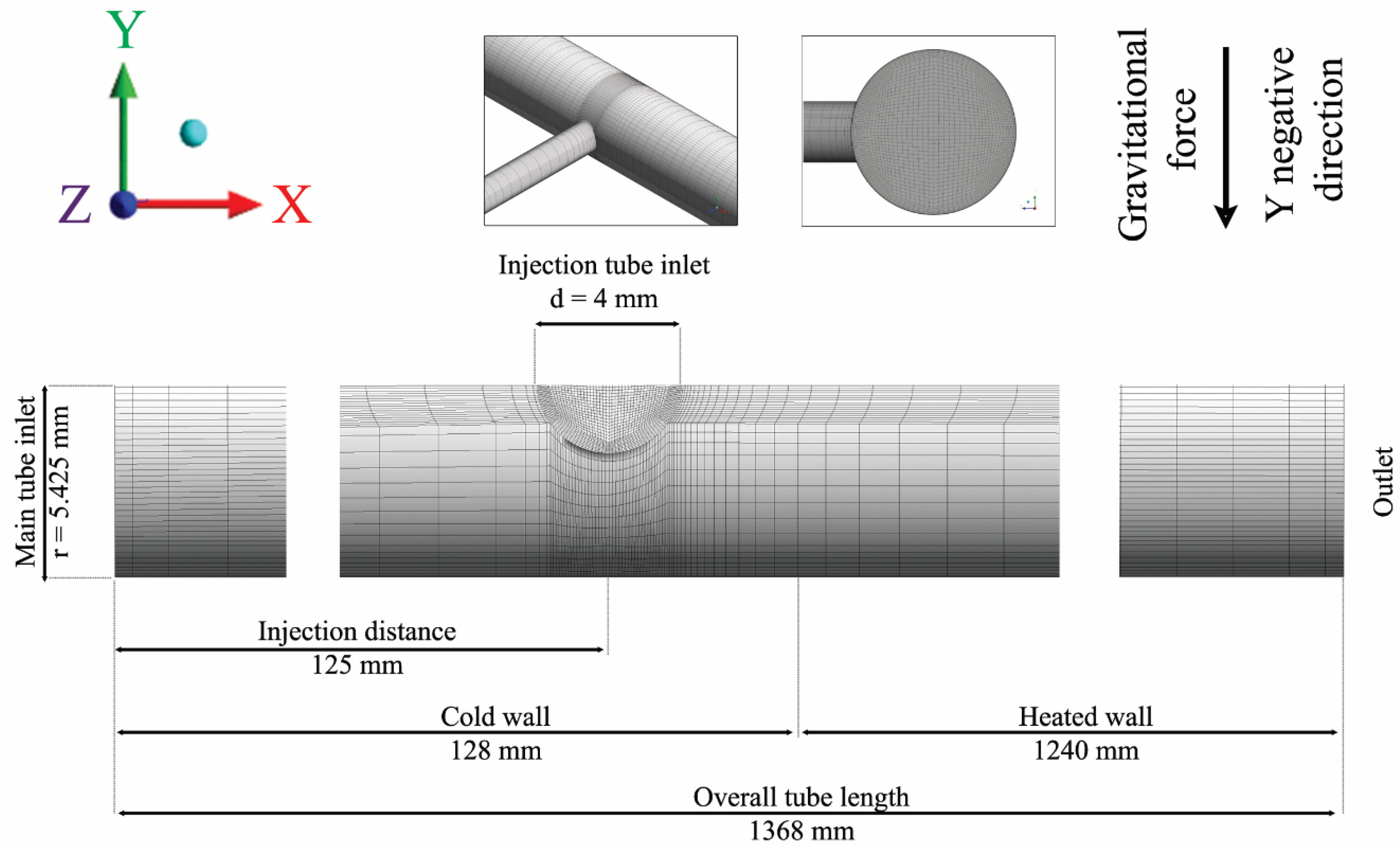
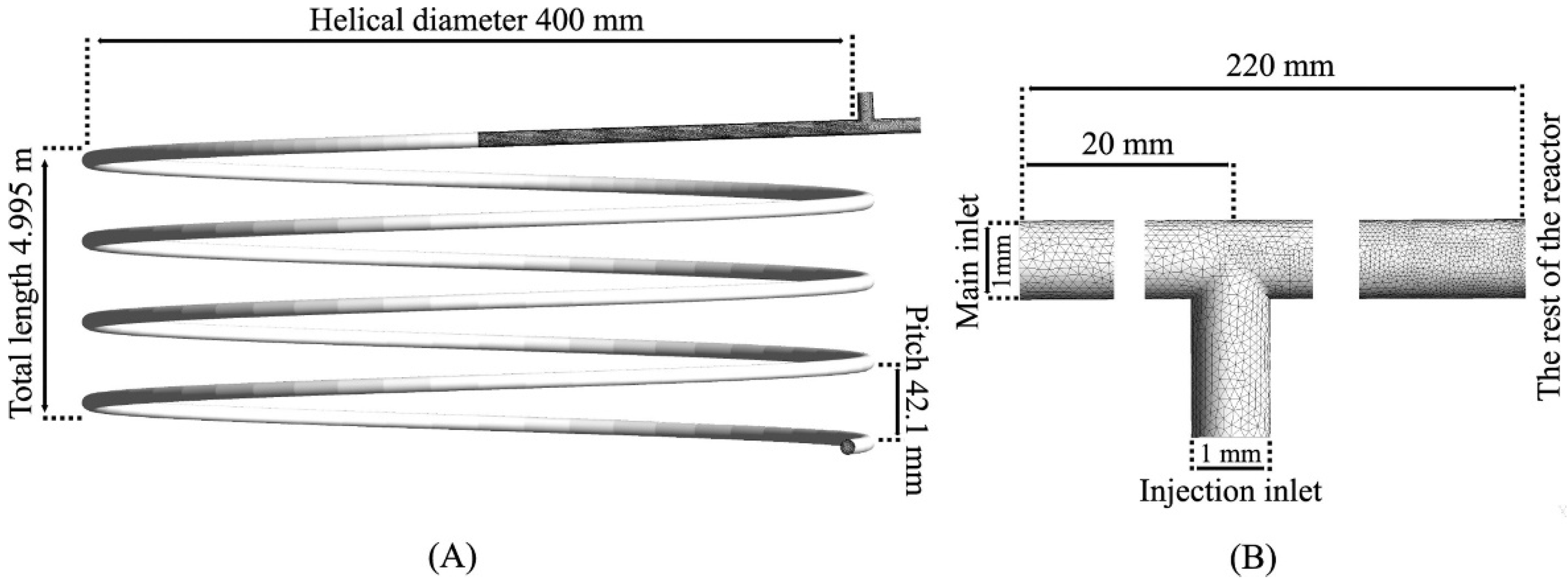
3. Experimental Deficiencies and Feasible Troubleshooting
4. Next Perspectives in Design
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Savage, P.E. Organic Chemical Reactions in Supercritical Water. Chem. Rev. 1999, 99, 603–621. [Google Scholar] [CrossRef]
- Matsumura, Y.; Minowa, T.; Potic, B.; Kersten, S.R.A.; Prins, W.; Van Swaaij, W.P.M.; Van De Beld, B.; Elliott, D.C.; Neuenschwander, G.G.; Kruse, A.; et al. Biomass gasification in near- and super-critical water: Status and prospects. Biomass Bioenergy 2005, 29, 269–292. [Google Scholar] [CrossRef]
- Kruse, A. Supercritical water gasification. Biofuels Bioprod. Biorefin. 2008, 2, 415–437. [Google Scholar] [CrossRef]
- Fan, C.; Jin, H. A numerical study on gasification of a single char particle in supercritical water for hydrogen production. Fuel 2020, 268, 117399. [Google Scholar] [CrossRef]
- Wen, Q.L.; Gu, H.Y. Numerical simulation of heat transfer deterioration phenomenon in supercritical water through vertical tube. Ann. Nucl. Energy 2010, 37, 1272–1280. [Google Scholar] [CrossRef]
- Gutiérrez Ortiz, F.J.; Kruse, A. The use of process simulation in supercritical fluids applications. React. Chem. Eng. 2020, 5, 424–451. [Google Scholar] [CrossRef]
- Pinkard, B.R.; Gorman, D.J.; Tiwari, K.; Kramlich, J.C.; Reinhall, P.G.; Novosselov, I.V. Review of Gasification of Organic Compounds in Continuous-Flow, Supercritical Water Reactors. Ind. Eng. Chem. Res. 2018, 57, 3471–3481. [Google Scholar] [CrossRef]
- Rodriguez Correa, C.; Kruse, A. Supercritical water gasification of biomass for hydrogen production—Review. J. Supercrit. Fluids 2018, 133, 573–590. [Google Scholar] [CrossRef]
- Ferreira-Pinto, L.; Silva Parizi, M.P.; de Araújo, P.C.C.S.; Zanette, A.F.; Cardozo-Filho, L. Experimental basic factors in the production of H2 via supercritical water gasification. Int. J. Hydrogen Energy 2019, 44, 25365–25383. [Google Scholar] [CrossRef]
- Ibrahim, A.B.A.; Akilli, H. Supercritical water gasification of wastewater sludge for hydrogen production. Int. J. Hydrogen Energy 2019, 44, 10328–10349. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, L.; Xu, P.; Liu, B.; Shuai, Y.; Li, B. Hydrogen production through biomass gasification in supercritical water: A review from exergy aspect. Int. J. Hydrogen Energy 2019, 44, 15727–15736. [Google Scholar] [CrossRef]
- Hu, Y.; Gong, M.; Xing, X.; Wang, H.; Zeng, Y.; Xu, C.C. Supercritical water gasification of biomass model compounds: A review. Renew. Sustain. Energy Rev. 2020, 118, 12–14. [Google Scholar] [CrossRef]
- Gutiérrez Ortiz, F.J. Techno-economic assessment of supercritical processes for biofuel production. J. Supercrit. Fluids 2020, 160, 104788. [Google Scholar] [CrossRef]
- Abdpour, S.; Santos, R.M. Recent advances in heterogeneous catalysis for supercritical water oxidation/gasification processes: Insight into catalyst development. Process Saf. Environ. Prot. 2021, 149, 169–184. [Google Scholar] [CrossRef]
- Farobie, O.; Matsumura, Y.; Syaftika, N.; Amrullah, A.; Hartulistiyoso, E.; Bayu, A.; Moheimani, N.R.; Karnjanakom, S.; Saefurahman, G. Recent advancement on hydrogen production from macroalgae via supercritical water gasification. Bioresour. Technol. Rep. 2021, 16, 100844. [Google Scholar] [CrossRef]
- Lee, C.S.; Conradie, A.V.; Lester, E. Review of supercritical water gasification with lignocellulosic real biomass as the feedstocks: Process parameters, biomass composition, catalyst development, reactor design and its challenges. Chem. Eng. J. 2021, 415, 128837. [Google Scholar] [CrossRef]
- Okolie, J.A.; Epelle, E.I.; Nanda, S.; Castello, D.; Dalai, A.K.; Kozinski, J.A. Hydrogen Production: A Comprehensive Review. J. Supercrit. Fluids 2021, 173, 105199. [Google Scholar] [CrossRef]
- Matsumura, Y.; Minowa, T. Fundamental design of a continuous biomass gasification process using a supercritical water fluidized bed. Int. J. Hydrogen Energy 2004, 29, 701–707. [Google Scholar] [CrossRef]
- Müller, J.B.; Vogel, F. Tar and coke formation during hydrothermal processing of glycerol and glucose. Influence of temperature, residence time and feed concentration. J. Supercrit. Fluids 2012, 70, 126–136. [Google Scholar] [CrossRef]
- Lu, Y.; Zhang, T.; Dong, X. Bed to wall heat transfer in supercritical water fluidized bed: Comparison with the gas-solid fluidized bed. Appl. Therm. Eng. 2014, 88, 297–305. [Google Scholar] [CrossRef]
- Zhang, T.; Lu, Y.; Yao, L. Experimental study of wall-to-bed heat transfer in a supercritical water fluidized bed. Int. J. Multiph. Flow 2018, 109, 26–34. [Google Scholar] [CrossRef]
- Wu, Z.; Ren, Y.; Ou, G.; Jin, H. Influence of special water properties variation on the heat transfer of supercritical water flow around a sphere. Chem. Eng. Sci. 2020, 222, 115698. [Google Scholar] [CrossRef]
- Liang, X.; Zhao, Q.; Dong, Y.; Guo, L.; Jin, Z.; Liu, Q. Experimental investigation on supercritical water gasification of organic-rich shale with low maturity for syngas production. Energy Fuels 2021, 35, 7657–7665. [Google Scholar] [CrossRef]
- Sinag, A.; Kruse, A.; Maniam, P. Hydrothermal conversion of biomass and different model compounds. J. Supercrit. Fluids 2012, 71, 80–85. [Google Scholar] [CrossRef]
- Chen, Y.; Guo, L.; Jin, H.; Yin, J.; Lu, Y.; Zhang, X. An experimental investigation of sewage sludge gasification in near and super-critical water using a batch reactor. Int. J. Hydrogen Energy 2013, 38, 12912–12920. [Google Scholar] [CrossRef]
- Castello, D.; Rolli, B.; Kruse, A.; Fiori, L. Supercritical water gasification of biomass in a ceramic reactor: Long-time batch experiments. Energies 2017, 10, 1734. [Google Scholar] [CrossRef]
- Graz, Y.; Bostyn, S.; Richard, T.; Bocanegra, P.E.; De Bilbao, E.; Poirier, J.; Gokalp, I. Hydrothermal conversion of Ulva macro algae in supercritical water. J. Supercrit. Fluids 2016, 107, 182–188. [Google Scholar] [CrossRef]
- Weijin, G.; Zizheng, Z.; Yue, L.; Qingyu, W.; Lina, G. Hydrogen production and phosphorus recovery via supercritical water gasification of sewage sludge in a batch reactor. Waste Manag. 2019, 96, 198–205. [Google Scholar] [CrossRef] [PubMed]
- Demirel, E.; Erkey, C.; Ayas, N. Supercritical water gasification of fruit pulp for hydrogen production: Effect of reaction parameters. J. Supercrit. Fluids 2021, 177, 105329. [Google Scholar] [CrossRef]
- Weiss-Hortala, E.; Kruse, A.; Ceccarelli, C.; Barna, R. Influence of phenol on glucose degradation during supercritical water gasification. J. Supercrit. Fluids 2010, 53, 42–47. [Google Scholar] [CrossRef]
- Goodwin, A.K.; Rorrer, G.L. Reaction rates for supercritical water gasification of xylose in a micro-tubular reactor. Chem. Eng. J. 2010, 163, 10–21. [Google Scholar] [CrossRef]
- Zhang, L.; Champagne, P.; Xu, C.C. Supercritical water gasification of an aqueous by-product from biomass hydrothermal liquefaction with novel Ru modified Ni catalysts. Bioresour. Technol. 2011, 102, 8279–8287. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez Ortiz, F.J.; Serrera, A.; Galera, S.; Ollero, P. Experimental study of the supercritical water reforming of glycerol without the addition of a catalyst. Energy 2013, 56, 193–206. [Google Scholar] [CrossRef]
- Castello, D.; Kruse, A.; Fiori, L. Low temperature supercritical water gasification of biomass constituents: Glucose/phenol mixtures. Biomass Bioenergy 2015, 73, 84–94. [Google Scholar] [CrossRef]
- Nanda, S.; Reddy, S.N.; Hunter, H.N.; Dalai, A.K.; Kozinski, J.A. Supercritical water gasification of fructose as a model compound for waste fruits and vegetables. J. Supercrit. Fluids 2015, 104, 112–121. [Google Scholar] [CrossRef]
- Ferreira-Pinto, L.; Feirhrmann, A.C.; Corazza, M.L.; Fernandes-Machado, N.R.C.; Dos Reis Coimbra, J.S.; Saldaña, M.D.A.; Cardozo-Filho, L. Hydrogen production and TOC reduction from gasification of lactose by supercritical water. Int. J. Hydrogen Energy 2015, 40, 12162–12168. [Google Scholar] [CrossRef]
- Gutiérrez Ortiz, F.J.; Campanario, F.J.; Ollero, P. Supercritical water reforming of model compounds of bio-oil aqueous phase: Acetic acid, acetol, butanol and glucose. Chem. Eng. J. 2016, 298, 243–258. [Google Scholar] [CrossRef]
- Adar, E.; Ince, M.; Bilgili, M.S. Supercritical water gasification of sewage sludge by continuous flow tubular reactor: A pilot scale study. Chem. Eng. J. 2020, 391, 123499. [Google Scholar] [CrossRef]
- Bergmann, C.M.; Ormiston, S.J.; Chatoorgoon, V. Comparative study of turbulence model predictions of upward supercritical fluid flow in vertical rod bundle subchannels. Nucl. Eng. Des. 2017, 322, 177–191. [Google Scholar] [CrossRef]
- Jaromin, M.; Anglart, H. A numerical study of heat transfer to supercritical water flowing upward in vertical tubes under normal and deteriorated conditions. Nucl. Eng. Des. 2013, 264, 61–70. [Google Scholar] [CrossRef]
- Shang, Z.; Chen, S. Numerical investigation of diameter effect on heat transfer of supercritical water flows in horizontal round tubes. Appl. Therm. Eng. 2011, 31, 573–581. [Google Scholar] [CrossRef][Green Version]
- Huang, X.; Wang, Q.; Song, Z.; Yin, Y.; Wang, H. Heat transfer characteristics of supercritical water in horizontal double-pipe. Appl. Therm. Eng. 2020, 173, 115191. [Google Scholar] [CrossRef]
- El-Morshedy, S.E.D.; Ibrahim, S.M.A.; Alyan, A.; Abdelmaksoud, A. Heat transfer deterioration mechanism for water at supercritical pressure. Int. J. Thermofluids 2020, 7–8, 100020. [Google Scholar] [CrossRef]
- Gao, Z.; Bai, J. Numerical analysis on nonuniform heat transfer of supercritical pressure water in horizontal circular tube. Appl. Therm. Eng. 2017, 120, 10–18. [Google Scholar] [CrossRef]
- Liu, L.; Xiao, Z.; Yan, X.; Zeng, X.; Huang, Y. Heat transfer deterioration to supercritical water in circular tube and annular channel. Nucl. Eng. Des. 2013, 255, 97–104. [Google Scholar] [CrossRef]
- Dongliang, M.; Tao, Z.; Bing, L.; Ali Shahzad, M.; Yanping, H. An improved correlation on the onset of heat transfer deterioration in supercritical water. Nucl. Eng. Des. 2018, 326, 290–300. [Google Scholar] [CrossRef]
- Xie, J.; Yan, H.; Sundén, B.; Xie, G. A numerical prediction on heat transfer characteristics from a circular tube in supercritical fluid crossflow. Appl. Therm. Eng. 2019, 153, 692–703. [Google Scholar] [CrossRef]
- Wang, Z.; Qi, G.; Li, M. Numerical investigation of heat transfer to supercritical water in vertical tube under semicircular heating condition. Energies 2019, 12, 3958. [Google Scholar] [CrossRef]
- Zhang, L.; Cai, B.; Weng, Y.; Gu, H.; Wang, H.; Li, H.; Chatoorgoon, V. Experimental investigations on flow characteristics of two parallel channels in a forced circulation loop with supercritical water. Appl. Therm. Eng. 2016, 106, 98–108. [Google Scholar] [CrossRef]
- Basu, P.; Mettanant, V. Biomass Gasification in Supercritical Water—A Review. Int. J. Chem. React. Eng. 2009, 7. [Google Scholar] [CrossRef]
- Odu, S.O.; Koster, P.; Van Der Ham, A.G.J.; Van Der Hoef, M.A.; Kersten, S.R.A. Heat Transfer to Sub- and Supercritical Water Flowing Upward in a Vertical Tube at Low Mass Fluxes: Numerical Analysis and Experimental Validation. Ind. Eng. Chem. Res. 2016, 55, 13120–13131. [Google Scholar] [CrossRef]
- Kawasaki, S.I.; Sue, K.; Ookawara, R.; Wakashima, Y.; Suzuki, A.; Hakuta, Y.; Arai, K. Engineering study of continuous supercritical hydrothermal method using a T-shaped mixer: Experimental synthesis of NiO nanoparticles and CFD simulation. J. Supercrit. Fluids 2010, 54, 96–102. [Google Scholar] [CrossRef]
- Qu, M.; Yang, D.; Liang, Z.; Wan, L.; Liu, D. Experimental and numerical investigation on heat transfer of ultra-supercritical water in vertical upward tube under uniform and non-uniform heating. Int. J. Heat Mass Transf. 2018, 127, 769–783. [Google Scholar] [CrossRef]
- Lee, I.G.; Kim, M.S.; Ihm, S.K. Gasification of glucose in supercritical water. Ind. Eng. Chem. Res. 2002, 41, 1182–1188. [Google Scholar] [CrossRef]
- May, A.; Salvadó, J.; Torras, C.; Montané, D. Catalytic gasification of glycerol in supercritical water. Chem. Eng. J. 2010, 160, 751–759. [Google Scholar] [CrossRef]
- Susanti, R.F.; Nugroho, A.; Lee, J.; Kim, Y.; Kim, J. Noncatalytic gasification of isooctane in supercritical water: A Strategy for high-yield hydrogen production. Int. J. Hydrogen Energy 2011, 36, 3895–3906. [Google Scholar] [CrossRef]
- Tushar, M.S.H.K.; Dutta, A.; Xu, C.C. Catalytic supercritical gasification of biocrude from hydrothermal liquefaction of cattle manure. Appl. Catal. B Environ. 2016, 189, 119–132. [Google Scholar] [CrossRef]
- Kumabe, K.; Itoh, N.; Matsumoto, K.; Hasegawa, T. Hydrothermal gasification of glucose and starch in a batch and continuous reactor. Energy Rep. 2017, 3, 70–75. [Google Scholar] [CrossRef]
- Amrullah, A.; Matsumura, Y. Supercritical water gasification of sewage sludge in continuous reactor. Bioresour. Technol. 2018, 249, 276–283. [Google Scholar] [CrossRef] [PubMed]
- Purkarová, E.; Ciahotný, K.; Šváb, M.; Skoblia, S.; Beňo, Z. Supercritical water gasification of wastes from the paper industry. J. Supercrit. Fluids 2018, 135, 130–136. [Google Scholar] [CrossRef]
- Mylapilli, S.V.P.; Reddy, S.N. Catalytic and non-catalytic degradation of acetaminophen in supercritical water. Environ. Res. 2021, 7, 112191. [Google Scholar] [CrossRef] [PubMed]
- Hendry, D.; Venkitasamy, C.; Wilkinson, N.; Jacoby, W. Exploration of the effect of process variables on the production of high-value fuel gas from glucose via supercritical water gasification. Bioresour. Technol. 2011, 102, 3480–3487. [Google Scholar] [CrossRef] [PubMed]
- Cantero, D.A.; Álvarez, A.; Bermejo, M.D.; Cocero, M.J. Transformation of glucose into added value compounds in a hydrothermal reaction media. J. Supercrit. Fluids 2015, 98, 204–210. [Google Scholar] [CrossRef]
- Sierra-Pallares, J.; Huddle, T.; Alonso, E.; Mato, F.A.; García-Serna, J.; Cocero, M.J.; Lester, E. Prediction of residence time distributions in supercritical hydrothermal reactors working at low Reynolds numbers. Chem. Eng. J. 2016, 299, 373–385. [Google Scholar] [CrossRef]
- Vaquerizo, L.; Cocero, M.J. Ultrafast heating by high efficient biomass direct mixing with supercritical water. Chem. Eng. J. 2019, 378, 122199. [Google Scholar] [CrossRef]
- Yukananto, R.; Pozarlik, A.K.; Brem, G. Computational fluid dynamic model for glycerol gasification in supercritical water in a tee junction shaped cylindrical reactor. J. Supercrit. Fluids 2018, 133, 330–342. [Google Scholar] [CrossRef]
- Guo, S.; Guo, L.; Cao, C.; Yin, J.; Lu, Y.; Zhang, X. Hydrogen production from glycerol by supercritical water gasification in a continuous flow tubular reactor. Int. J. Hydrogen Energy 2012, 37, 5559–5568. [Google Scholar] [CrossRef]
- Farobie, O.; Sasanami, K.; Matsumura, Y. A novel spiral reactor for biodiesel production in supercritical ethanol. Appl. Energy 2015, 147, 20–29. [Google Scholar] [CrossRef]
- Yi, L.; Wang, L.; Guo, L.; Cheng, K.; Jin, H.; Chen, Y.; Cao, W. Hydrogen production by supercritical water gasification of methylhydrazine in continuous system. J. Water Process Eng. 2021, 42, 102037. [Google Scholar] [CrossRef]
- Vashisth, S.; Kumar, V.; Nigam, K.D.P. A review on the potential applications of curved geometries in process industry. Ind. Eng. Chem. Res. 2008, 47, 3291–3337. [Google Scholar] [CrossRef]
- Zhao, H.; Li, X.; Wu, X. Transfer Characteristics in Vertical Helical Tubes. J. Supercrit. Fluids 2017, 127, 48–61. [Google Scholar] [CrossRef]
- Yukananto, R.; Pozarlik, A.; Bramer, E.; Brem, G. Numerical modelling of char formation during glucose gasification in supercritical water. J. Supercrit. Fluids 2018, 140, 258–269. [Google Scholar] [CrossRef]
- Li, F.; Bai, B. Flow and heat transfer of supercritical water in the vertical helically-coiled tube under half-side heating condition. Appl. Therm. Eng. 2018, 133, 512–519. [Google Scholar] [CrossRef]
- Mokry, S.; Pioro, I.; Farah, A.; King, K.; Gupta, S.; Peiman, W.; Kirillov, P. Development of supercritical water heat-transfer correlation for vertical bare tubes. Nucl. Eng. Des. 2011, 241, 1126–1136. [Google Scholar] [CrossRef]
- Lester, E.; Blood, P.; Denyer, J.; Giddings, D.; Azzopardi, B.; Poliakoff, M. Reaction engineering: The supercritical water hydrothermal synthesis of nano-particles. J. Supercrit. Fluids 2006, 37, 209–214. [Google Scholar] [CrossRef]
- Gu, J.; Zhang, Y.; Wu, Y.; Li, Z.; Tang, G.; Wang, Q.; Lyu, J. Numerical study of flow and heat transfer of supercritical water in rifled tubes heated by one side. Appl. Therm. Eng. 2018, 142, 610–621. [Google Scholar] [CrossRef]
- Miwa, Y.; Noishiki, K.; Suzuki, T.; Takatsuki, K. Research and Development Kobe Steel Engineering Reports; Kobe Steel Ltd.: Kobe, Japan, 2013; pp. 23–27. [Google Scholar]
- Mortean, M.V.V.; Cisterna, L.H.R.; Paiva, K.V.; Mantelli, M.B.H. Development of diffusion welded compact heat exchanger technology. Appl. Therm. Eng. 2016, 93, 995–1005. [Google Scholar] [CrossRef]
- Sarmiento, A.P.C.; Soares, V.H.T.; Carqueja, G.G.; Batista, J.V.C.; Milanese, F.H.; Mantelli, M.B.H. Thermal performance of diffusion-bonded compact heat exchangers. Int. J. Therm. Sci. 2020, 153, 106384. [Google Scholar] [CrossRef]
- Vacuum Process Engineering Diffusion Bonded Microchannel Heat Exchangers. Available online: https://www.vpei.com/diffusion-bonded-microchannel-heat-exchangers/ (accessed on 9 November 2021).
- Kobe Steel Ltd. Diffusion Bonded Compact Heat Exchanger. Available online: https://www.kobelco.co.jp/english/products/ecmachinery/dche/files/Microchannel_dche_smcr_brochure.pdf (accessed on 9 November 2021).
- Stankiewicz, A.; Moulijn, J. Re-Engineering the Chemical Processing Plant. Process Intensification; Marcel Dekker, Inc.: New York, NY, USA, 2004; ISBN 0824743024. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gutiérrez Ortiz, F.J.; López-Guirao, F.; Jiménez-Espadafor, F.J.; Benjumea, J.M. Heat Transfer Limitations in Supercritical Water Gasification. Energies 2022, 15, 177. https://doi.org/10.3390/en15010177
Gutiérrez Ortiz FJ, López-Guirao F, Jiménez-Espadafor FJ, Benjumea JM. Heat Transfer Limitations in Supercritical Water Gasification. Energies. 2022; 15(1):177. https://doi.org/10.3390/en15010177
Chicago/Turabian StyleGutiérrez Ortiz, Francisco Javier, Francisco López-Guirao, Francisco José Jiménez-Espadafor, and José Manuel Benjumea. 2022. "Heat Transfer Limitations in Supercritical Water Gasification" Energies 15, no. 1: 177. https://doi.org/10.3390/en15010177
APA StyleGutiérrez Ortiz, F. J., López-Guirao, F., Jiménez-Espadafor, F. J., & Benjumea, J. M. (2022). Heat Transfer Limitations in Supercritical Water Gasification. Energies, 15(1), 177. https://doi.org/10.3390/en15010177