Influencing Factors of Performance Degradation of Zinc–Air Batteries Exposed to Air
Abstract
1. Introduction
2. Materials and Methods
2.1. Assembly of Zinc–Air Unit Cell
2.2. Material Characterization
2.3. Electrochemical Characterization
3. Results and Discussion
3.1. Whole Battery Analysis
3.2. Anode Analysis
3.3. Cathode Analysis
3.4. Electrolyte Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dong, C.; Yue, X.; Li, X.; Wu, X.; Zhou, Y. Research progress of cathode materials for lithi-um-selenium batteries. Acta Phys. Chim. Sin. 2019, 35, 667–683. [Google Scholar]
- Liu, K.; Liu, Y.; Zhu, H.; Dong, X.; Wang, Y.; Wang, C.; Xia, Y. NaTiSi2O6/C composite as a novel anode material for lithium-ion batteries. Acta Phys. Chim. Sin. 2020, 36, 1912030. [Google Scholar]
- Shen, Y.; Liu, B.; Liu, X.; Liu, J.; Ding, J.; Zhong, C.; Hu, W. Water-in-salt electrolyte for safe and high-energy aqueous battery. Energy Storage Mater. 2021, 34, 461–474. [Google Scholar] [CrossRef]
- Zhong, C.; Liu, B.; Ding, J.; Liu, X.; Zhong, Y.; Li, Y.; Sun, C.; Han, X.; Deng, Y.; Zhao, N.; et al. Decoupling electrolytes towards stable and high-energy rechargeable aqueous zinc–manganese dioxide batteries. Nat. Energy 2020, 5, 440–449. [Google Scholar] [CrossRef]
- Teng, X.-L.; Sun, X.-T.; Guan, L.; Hu, H.; Wu, M.-B. Self-supported transition metal oxide electrodes for electrochemical energy storage. Tungsten 2020, 2, 337–361. [Google Scholar] [CrossRef]
- Li, G.; Mu, Y.; Huang, Z.; Wang, N.; Chen, Y.; Liu, J.; Liu, G.; Li, O.L.; Shao, M.; Shi, Z. Poly-active centric Co3O4-CeO2/Co-NC composites as superior oxygen reduction catalysts for Zn-air batteries. Sci. China Mater. 2021, 64, 73–84. [Google Scholar] [CrossRef]
- Song, Z.; Ding, J.; Liu, B.; Liu, X.; Han, X.; Deng, Y.; Hu, W.; Zhong, C. A Rechargeable Zn–Air Battery with High Energy Efficiency and Long Life Enabled by a Highly Water-Retentive Gel Electrolyte with Reaction Modifier. Adv. Mater. 2020, 32, e1908127. [Google Scholar] [CrossRef]
- Lu, Q.; Zou, X.; Liao, K.; Ran, R.; Zhou, W.; Ni, M.; Shao, Z. Direct growth of ordered N-doped carbon nanotube arrays on carbon fiber cloth as a free-standing and binder-free air electrode for flexible quasi-solid-state rechargeable Zn-Air batteries. Carbon Energy 2020, 2, 461–471. [Google Scholar] [CrossRef]
- Yang, D.; Chen, D.; Jiang, Y.; Ang, E.H.; Feng, Y.; Rui, X.; Yu, Y. Carbon-based materials for all-solid-state zinc-air batteries. Carbon Energy 2021, 3, 50–65. [Google Scholar] [CrossRef]
- Du, Q.; Wu, Q.; Wang, H.; Meng, X.; Ji, Z.; Zhao, S.; Zhu, W.; Liu, C.; Ling, M. Carbon dots modified silicon nanoparticle for lithium ion batteries. Int. J. Miner. Metall. Mater. 2021. [Google Scholar] [CrossRef]
- Guo, L.-F.; Zhang, S.-Y.; Xie, J.; Zhen, D.; Jin, Y.; Wan, K.-Y.; Zhuang, D.-G.; Zheng, W.-Q.; Zhao, X.-B. Controlled synthesis of nanosized Si by magnesiothermic reduction from diatomite as anode material for Li-ion batteries. Int. J. Min. Met. Mater. 2020, 27, 515–525. [Google Scholar] [CrossRef]
- Pei, P.; Wang, K.; Ma, Z. Technologies for extending zinc-air battery’s cyclelife: A review. Appl. Energy 2014, 128, 315–324. [Google Scholar] [CrossRef]
- Liu, X.; Yuan, Y.; Liu, J.; Liu, B.; Chen, X.; Ding, J.; Han, X.; Deng, Y.; Zhong, C.; Hu, W. Utilizing solar energy to improve the oxygen evolution reaction kinetics in zinc–air battery. Nat. Commun. 2019, 10, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Liu, B.; Fan, X.; Liu, X.; Liu, J.; Ding, J.; Han, X.; Deng, Y.; Hu, W.; Zhong, C. Long-Shelf-Life Polymer Electrolyte Based on Tetraethylammonium Hydroxide for Flexible Zinc–Air Batteries. ACS Appl. Mater. Interfaces 2019, 11, 28909–28917. [Google Scholar] [CrossRef] [PubMed]
- Perez, M.G.; O’Keefe, M.J.; O’Keefe, T.; Ludlow, D. Chemical and morphological analyses of zinc powders for alkaline batteries. J. Appl. Electrochem. 2007, 37, 225–231. [Google Scholar] [CrossRef]
- Sun, Y.; Liu, X.; Jiang, Y.; Li, J.; Ding, J.; Hu, W.; Zhong, C. Recent advances and challenges in divalent and multivalent metal electrodes for metal–air batteries. J. Mater. Chem. A 2019, 7, 18183–18208. [Google Scholar] [CrossRef]
- Zhao, Z.; Fan, X.; Ding, J.; Hu, W.; Zhong, C.; Lu, J. Challenges in Zinc Electrodes for Alkaline Zinc–Air Batteries: Obstacles to Commercialization. ACS Energy Lett. 2019, 4, 2259–2270. [Google Scholar] [CrossRef]
- Henninot, C.; Strauven, Y. Alloyed Zinc Powders with Pierced Particles for Alkaline Batteries. U.S. Patent 8,142,540 B2, 27 March 2012. [Google Scholar]
- Dongmo, S.; Stock, D.; Alexander Kreissl, J.J.; Groß, M.; Weixler, S.; Hagen, M.; Miyazaki, K.; Abe, T.; Schröder, D. Implications of testing a zinc–oxygen battery with zinc foil anode revealed by oper-ando gas analysis. ACS Omega 2020, 5, 626–633. [Google Scholar] [CrossRef]
- Li, Y.; Fan, X.; Liu, X.; Qu, S.; Liu, J.; Ding, J.; Han, X.; Deng, Y.; Hu, W.; Zhong, C. Long-battery-life flexible zinc–air battery with near-neutral polymer electrolyte and nanoporous integrated air elec-trode. J. Mater. Chem. A 2019, 7, 25449–25457. [Google Scholar] [CrossRef]
- Wu, J.; Liu, B.; Fan, X.; Ding, J.; Han, X.; Deng, Y.; Hu, W.; Zhong, C. Carbon-based cathode ma-terials for rechargeable zinc-air batteries: From current collectors to bifunctional integrated air electrodes. Carbon Energy 2020, 2, 370–386. [Google Scholar] [CrossRef]
- Min, Y.-J.; Oh, S.-J.; Kim, M.-S.; Choi, J.-H.; Eom, S. Effect of carbon properties on the electro-chemical performance of carbon-based air electrodes for rechargeable zinc–air batteries. J. Appl. Electrochem. 2018, 48, 405–413. [Google Scholar] [CrossRef]
- Drillet, J.F.; Holzer, F.; Kallis, T.; Müller, S.; Schmidt, V.M. Influence of CO2 on the stability of bi-functional oxygen electrodes for rechargeable zinc/air batteries and study of different CO2 filter materials. Phys. Chem. Chem. Phys. 2001, 3, 368–371. [Google Scholar] [CrossRef]
- Goh, F.W.T.; Liu, Z.; Hor, T.S.A.; Zhang, J.; Ge, X.; Zong, Y.; Yu, A.; Khoo, W. A Near-Neutral Chloride Electrolyte for Electrically Rechargeable Zinc-Air Batteries. J. Electrochem. Soc. 2014, 161, A2080–A2086. [Google Scholar] [CrossRef]
- Jörissen, L. Bifunctional oxygen/air electrodes. J. Power Sources 2006, 155, 23–32. [Google Scholar] [CrossRef]
- Schröder, D.; Krewer, U. Model based quantification of air-composition impact on secondary zinc air batteries. Electrochim. Acta 2014, 117, 541–553. [Google Scholar] [CrossRef]
- Stamm, J.; Varzi, A.; Latz, A.; Horstmann, B. Modeling nucleation and growth of zinc oxide during discharge of primary zinc-air batteries. J. Power Sources 2017, 360, 136–149. [Google Scholar] [CrossRef]
- Yang, S.; Kim, K. Observation of Water Consumption in Zn–air Secondary Batteries. J. Electrochem. Sci. Technol. 2019, 10, 381–386. [Google Scholar] [CrossRef]
- Zhang, J.; Zhou, Q.; Tang, Y.; Zhang, L.; Li, Y. Zinc–air batteries: Are they ready for prime time? Chem. Sci. 2019, 10, 8924–8929. [Google Scholar] [CrossRef]
- Hopkins, B.J.; Chervin, C.N.; Long, J.W.; Rolison, D.R.; Parker, J.F. Projecting the Specific Energy of Rechargeable Zinc–Air Batteries. ACS Energy Lett. 2020, 5, 3405–3408. [Google Scholar] [CrossRef]
- Stock, D.; Dongmo, S.; Janek, J.; Schröder, D. Benchmarking anode concepts: The future of elec-trically rechargeable zinc–air batteries. ACS Energy Lett. 2019, 4, 1287–1300. [Google Scholar] [CrossRef]
- Fu, J.; Lee, D.U.; Hassan, F.M.; Bai, Z.; Park, M.G.; Chen, Z. Flexible high-energy poly-mer-electrolyte-based rechargeable zinc–air batteries. Adv. Mater. 2015, 27, 5617–5622. [Google Scholar] [CrossRef] [PubMed]
- Peng, K.; Zhang, Z.J.; Zhao, Z.J.; Yang, C.; Tian, Z.L.; Lai, Y.Q. Performance of carbon-coated nano-ZnO prepared by carbonizing gel precursor as anodic material for secondary alkaline Zn batteries. Trans. Nonferr. Metal Soc. 2019, 29, 2151–2159. [Google Scholar] [CrossRef]
- Beverskog, B.; Puigdomenech, I. Revised pourbaix diagrams for zinc at 25–300 °C. Corros. Sci. 1997, 39, 107–114. [Google Scholar] [CrossRef]
- Wongrujipairoj, K.; Poolnapol, L.; Arpornwichanop, A.; Suren, S.; Kheawhom, S. Suppression of zinc anode corrosion for printed flexible zinc-air battery. Phys. Status Solidi. 2016, 254, 1600442. [Google Scholar] [CrossRef]
- Liu, M.; Cook, G.M.; Yao, N.P. Passivation of Zinc Anodes in KOH Electrolytes. J. Electrochem. Soc. 1981, 128, 1663–1668. [Google Scholar] [CrossRef]
- Lee, S.-M.; Kim, Y.-J.; Eom, S.-W.; Choi, N.-S.; Kim, K.-W.; Cho, S.-B. Improvement in self-discharge of Zn anode by applying surface modification for Zn–air batteries with high energy density. J. Power Sources 2013, 227, 177–184. [Google Scholar] [CrossRef]
- Jo, Y.N.; Prasanna, K.; Kang, S.H.; Ilango, P.R.; Kim, H.S.; Eom, S.W.; Lee, C.W. The effects of mechanical alloying on the self-discharge and corrosion behavior in Zn-air batteries. J. Ind. Eng. Chem. 2017, 53, 247–252. [Google Scholar] [CrossRef]
- Jo, Y.N.; Kang, S.H.; Prasanna, K.; Eom, S.W.; Lee, C.W. Shield effect of polyaniline between zinc active material and aqueous electrolyte in zinc-air batteries. Appl. Surf. Sci. 2017, 422, 406–412. [Google Scholar] [CrossRef]
- Xu, M.; Ivey, D.; Xie, Z.; Qu, W. Rechargeable Zn-air batteries: Progress in electrolyte development and cell configuration advancement. J. Power Sources 2015, 283, 358–371. [Google Scholar] [CrossRef]
- Schmid, M.; Schadeck, U.; Willert-Porada, M. Development of silica based coatings on zinc particles for improved oxidation behavior in battery applications. Surf. Coat. Technol. 2017, 310, 51–58. [Google Scholar] [CrossRef]
- Kimura, T.; Yamazaki, Y. Effects of CO2 Concentration and Electric Current on the Ionic Conductivity of Anion Exchange Membranes for Fuel Cells. Electrochemistry 2011, 79, 94–97. [Google Scholar] [CrossRef]
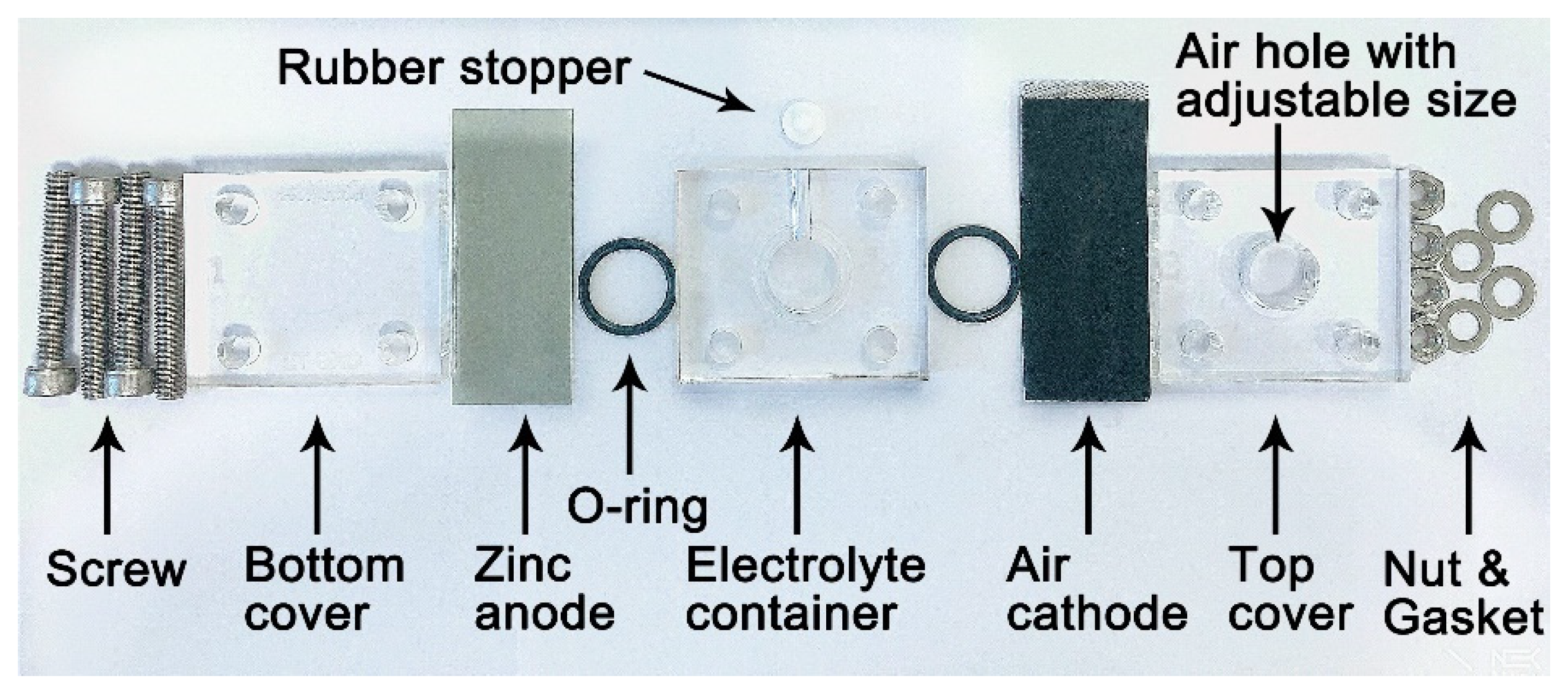

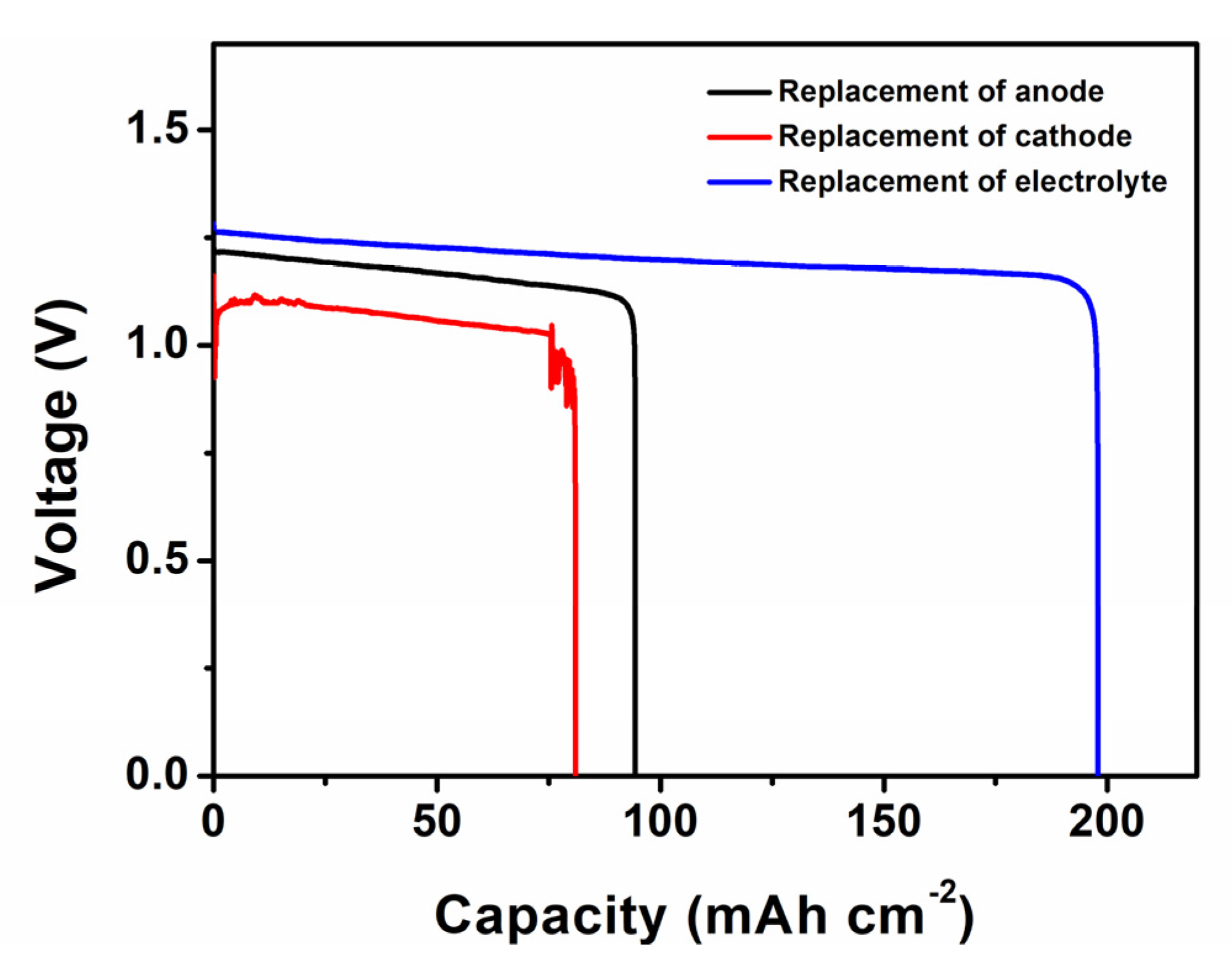

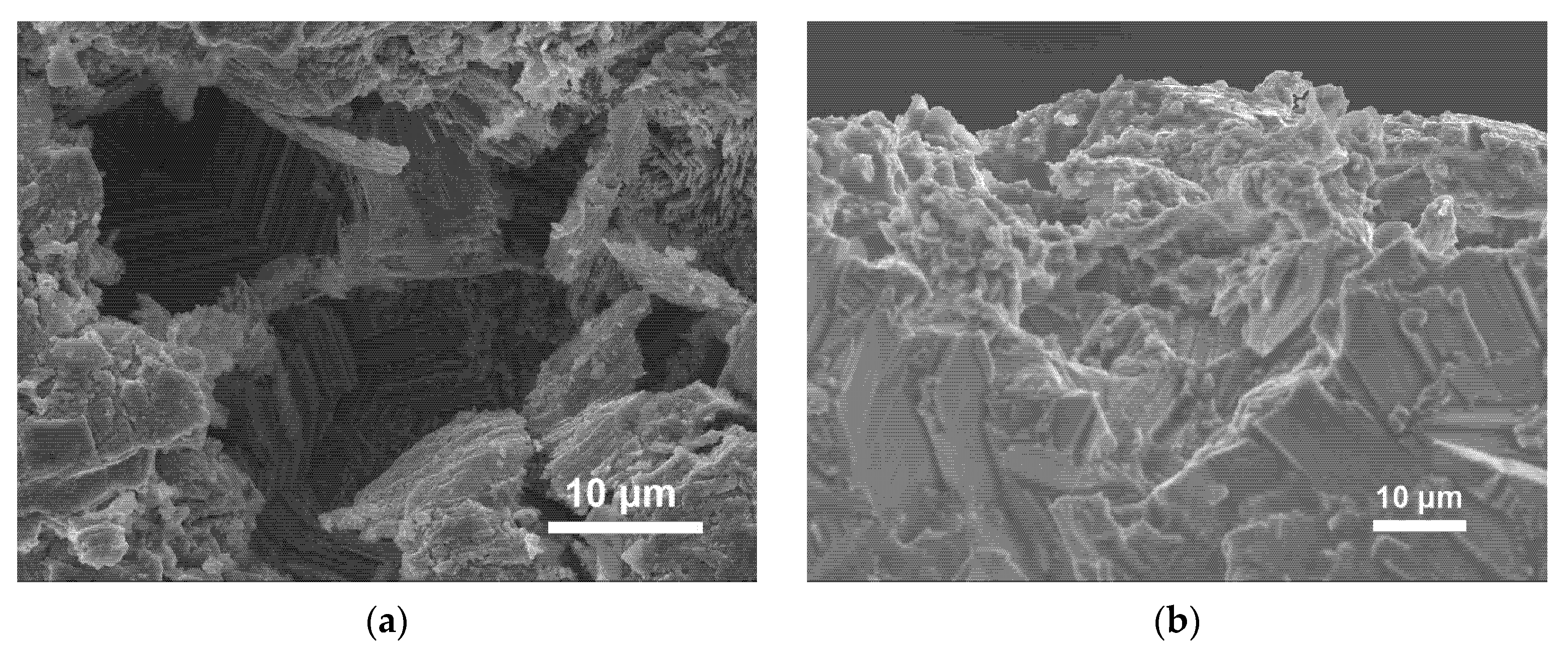




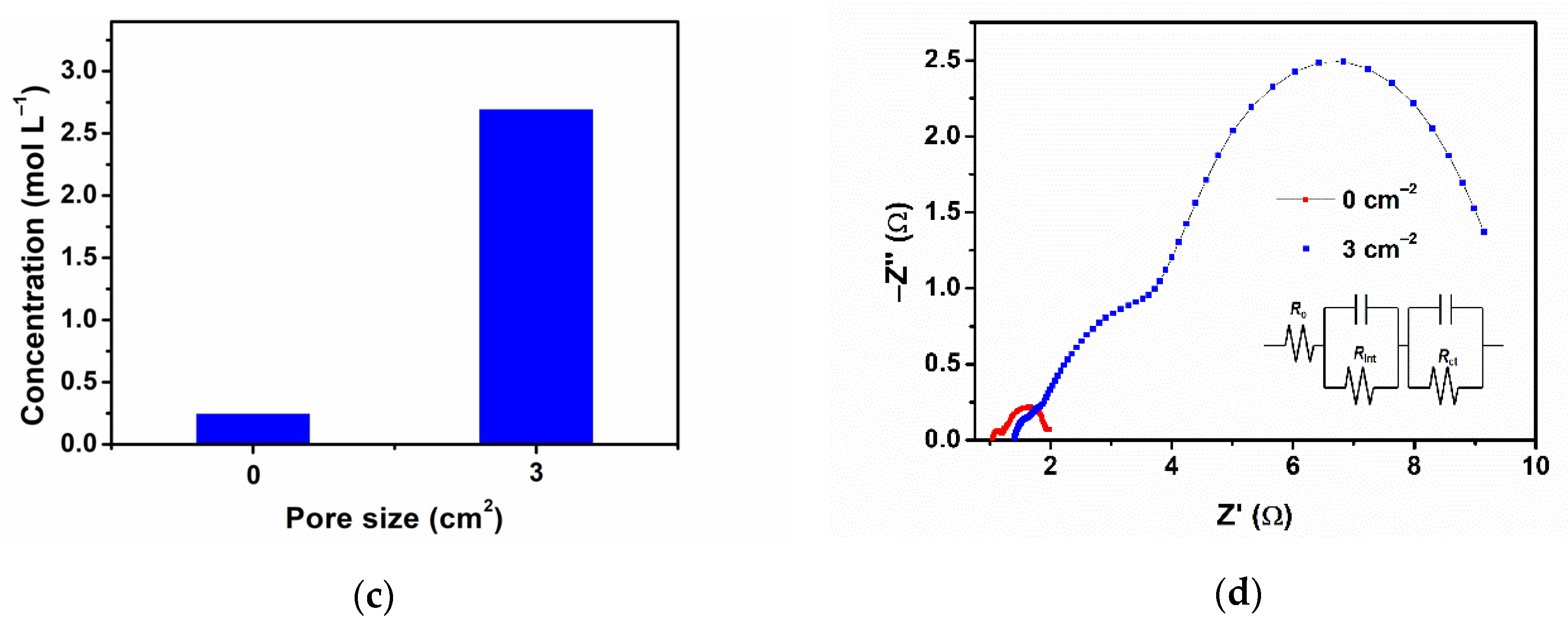
| Storage Time | Ro/Ω | Rint/Ω | Rct/Ω |
|---|---|---|---|
| before storing | 1.148 | 0.1854 | 0.635 |
| 2 days | 1.327 | 0.2109 | 0.749 |
| 4 days | 1.411 | 0.2178 | 0.876 |
| 7 days | 1.495 | 0.2531 | 1.011 |
| 10 days | 1.788 | 0.2819 | 1.200 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhong, Y.; Liu, B.; Zhao, Z.; Shen, Y.; Liu, X.; Zhong, C. Influencing Factors of Performance Degradation of Zinc–Air Batteries Exposed to Air. Energies 2021, 14, 2607. https://doi.org/10.3390/en14092607
Zhong Y, Liu B, Zhao Z, Shen Y, Liu X, Zhong C. Influencing Factors of Performance Degradation of Zinc–Air Batteries Exposed to Air. Energies. 2021; 14(9):2607. https://doi.org/10.3390/en14092607
Chicago/Turabian StyleZhong, Yuwei, Bin Liu, Zequan Zhao, Yuanhao Shen, Xiaorui Liu, and Cheng Zhong. 2021. "Influencing Factors of Performance Degradation of Zinc–Air Batteries Exposed to Air" Energies 14, no. 9: 2607. https://doi.org/10.3390/en14092607
APA StyleZhong, Y., Liu, B., Zhao, Z., Shen, Y., Liu, X., & Zhong, C. (2021). Influencing Factors of Performance Degradation of Zinc–Air Batteries Exposed to Air. Energies, 14(9), 2607. https://doi.org/10.3390/en14092607







