1. Introduction
The European Commission has adopted a circular and sustainable bioeconomy strategy requesting new insights in circular business models ideally based on local resources, as a means to drive the modernization of industries and tackle global climate and environmental challenges [
1]. Following these purposes, Portugal has implemented some instruments that contribute to the strengthening of the national bioeconomy, namely the National Plan for the Promotion of Biorefineries [
2] focusing on the valorization of low-value biomass feedstocks. In this regard, distinct processes can be considered to convert biomass into energy, biofuels, and bioproducts, some of which already with industrial application. Biomass slow pyrolysis is one of those processes that, due to its versatility and thermal/material synergies with other processes (e.g., combustion), is receiving increasing industrial interest in the context of new advanced biorefineries.
A common aspect of biomass pyrolysis processes is that they are thermally driven. Biomass particles—containing moisture, lignin, holocellulose, extractives, and ash—undergo sequential transformations as a function of temperature. These transformations start by drying as the temperature rises to 100 °C, which is followed by subsequent cleavage reactions (e.g., dehydrations and depolymerizations) at higher temperatures, all of these concurring to the volatilization of a large array of compounds [
3]. The final result is that the biomass converts into three main product fractions: bio-oil, permanent gases, and biochar (in this work referred as charcoal according to e.g., [
4]), with the proportions and composition of these being dependent on the nature of biomass, namely the relative proportions of lignin and cellulose, and the operating conditions, such as temperature and heating rate. In the case of slow pyrolysis processes where the goal is to maximize the yield of charcoal, a combination between low temperatures (<450 °C), low heating rates (<10 °C/min), and high lignin content fuels lead to better results [
5,
6,
7]. Still, the process can be fine-tuned to adjust the properties of charcoal (e.g., heating value, carbon content, volatile matter, porosity) according to the needs of distinct applications (fuels, reducing agents, cosmetics, art products, soil ameliorants, purification materials [
8,
9,
10]).
Among the products arising from biomass slow pyrolysis, only charcoal has met commercial applications worldwide, and its utilization dates back to ancient times [
11,
12]. It has been a major source of energy in Europe, namely for iron melting [
13], sometimes replacing fossil fuels in some applications during the industrial revolution [
14]. Charcoal production is still in practice in various European regions [
15], although its real contribution to the valorization of biomass feedstocks is poorly understood. In Portugal, a recent study on the mapping of charcoal production in the district of Portalegre (Alentejo Region) revealed hundreds of kilns [
16], almost all of them operating traditionally. Although similar situations are likely to be found in adjacent districts, pointing the fact that this is a relevant socio-economic activity, charcoal production is seldom addressed in studies dealing with the national bioenergy sector (e.g., [
17,
18]).
Given that charcoal production mainly relies on traditional methods and considering its distribution across rural areas, it becomes clear that this is perhaps the branch of the bioenergy sector where funding and scientific research can be more successful in pushing forward. Carbonization can be more rapidly leveraged based on existing know-how and infrastructure at lower investment costs. Moreover, it has the potential to sustain or increase the economic activity in regions of low population density, contributing to forest fire prevention in Southern European countries—especially after the dramatic death toll in the recent years [
19]—since a low risk of fire is often associated to well-managed forests. In this regard, charcoal production can be viewed as a complementary solution in well-balanced biomass valorization systems where certain types of biomass, especially low value wood—e.g., residues from agroforestry activities, wood from invasive species control, partially burnt wood from post-fire recovery actions, or waste wood from storm devastated forests—are preferably valorized by carbonization instead of combustion or pellets production, which nearly represents the present situation [
20,
21]. Large pieces of waste wood, such as tree trunks and stumps, may be especially suited for charcoal production as they are compatible with current carbonization methods and technologies where fuel chipping is not required. However, to become a regular practice, laboratory research is needed to demonstrate the yield and quality of charcoal arising from the abovesaid types of wood, including its suitability for distinct markets, so that the industrial interest for the carbonization of alternative feedstock may increase. For instance, charcoal production in the district of Portalegre (Portugal) largely relies on waste wood from cork oak and holm oak [
16], while there are vast areas of alternative feedstocks nearby (e.g., maritime pine and eucalyptus) frequently affected by large forest fires, which are not used for carbonization to the same extent. One reason for this is that charcoal production in the Portalegre district, as well as throughout the country, is mainly intended for barbecueing, both in household and professional appliances (e.g., restaurants), the latter being the preferred market for many producers. It turns out that professional consumers mainly request charcoal from holm oak and cork oak, as they have the perception that these charcoals offer greater value for the money (“it weighs more”, “it burns longer”, according to some consumers), which ends up conditioning the type of wood being processed by charcoal producers. However, the European standard EN1860-2-2005:E [
22] establishes the minimum quality requisites for barbeque charcoal, thus offering the basis to evaluate the suitability of alternative types of wood for charcoal production.
In this context, this work aims to provide insights into the properties and yield of charcoal produced from ten types of wood common in Southern Europe, under operational conditions relevant for biomass carbonization technologies. The holm oak is taken here as a reference wood according to the referred charcoal demand–supply context in Portugal. Furthermore, it extends the results and consolidates the analysis carried out in our previous work [
23], contributing to diversifying the fuel supply chain and expanding the relevance of existing charcoal production activities within the Portuguese (and South-European) bioenergy sector.
3. Results and Discussion
3.1. Evaluation of Measurement Uncertainties
The experience with the experimental facility showed that successive pyrolysis tests can be done with good repeatability in terms of heating rate (±0.1 °C/min of setpoint) and final temperature (±5 °C of setpoint). Examples of temperature–time profiles can be found in [
23]. The largest deviations between actual and setpoint temperatures always occur during the initial heating stage (below 150 °C) due to the thermal inertia of the reactor and wood particles. Concerning the yields of charcoal, results show uncertainties of ±2% of average values. For the physical–chemical analysis of wood and charcoal (proximate/elemental compositions, apparent density, and lignin content), results show deviations typically within ±2 to ±5% of average values, depending on the parameter. The exception is the ash contents with uncertainties typically of ±20%, although higher values were also obtained (see e.g.,
Table 1); this is partially due to the low ash contents of some woods/charcoals, which adds uncertainty to the measurements. Regarding apparent density, variations of ±5% of the average values were observed. A comparison between the geometrical method and the alternative method based on Archimedes’ principle showed a reasonable agreement [
23].
Despite the relatively low uncertainties arising from the analysis of individual samples, it should be noted that the properties of wood/charcoal can vary between logs or even within the same log. Although this subject is outside the scope of this work, an initial assessment of this variability was done by analyzing a limited number of wood samples for proximate composition and apparent density. The results suggested that the ash content and bulk density are the parameters with higher variability. For example, differences in densities up to 100 kg/m3 were found between samples of holm oak wood from distinct logs. These differences can lead to the production of charcoals with different properties, and one should be aware of such heterogeneity associated with biomass feedstocks.
3.2. Feedstock Properties
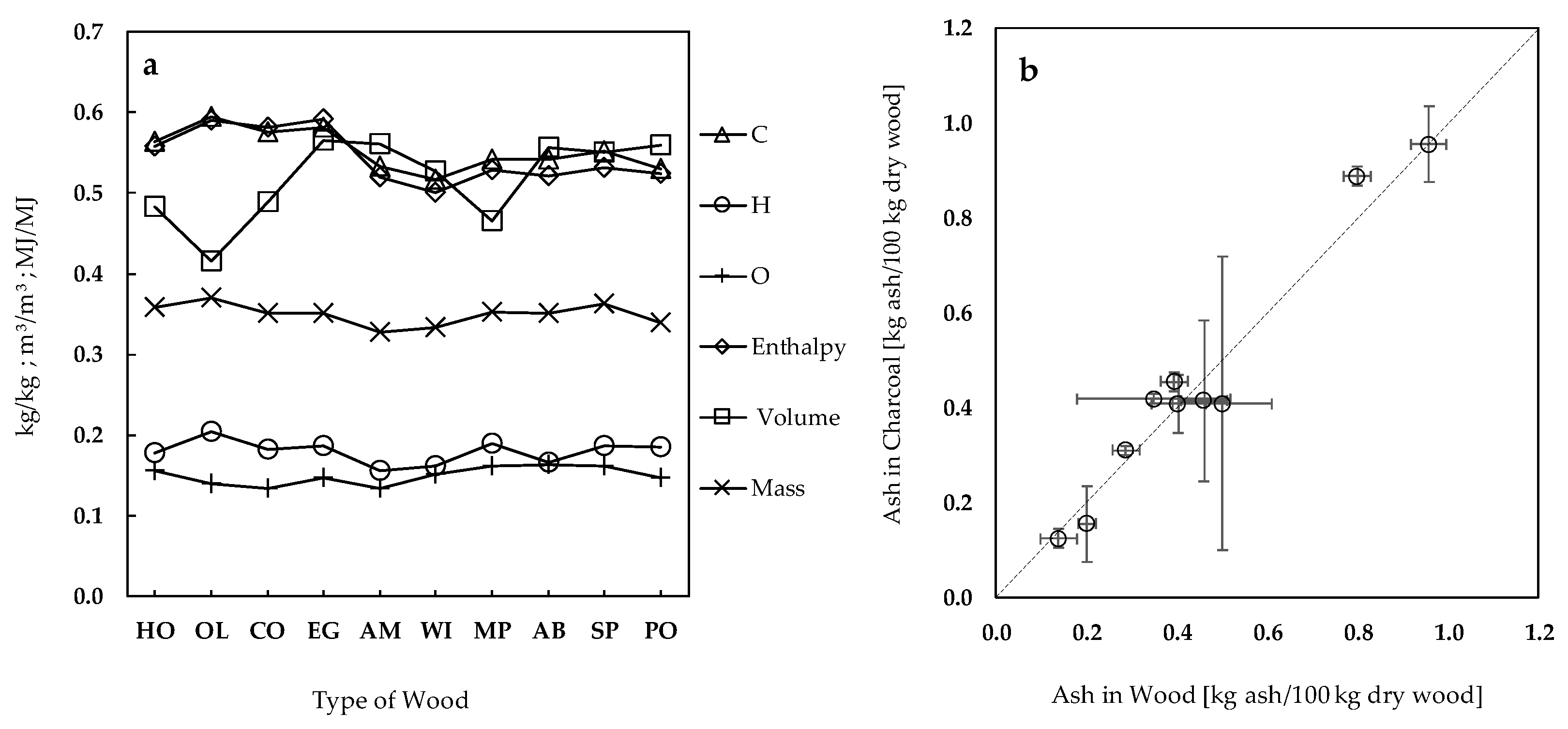
Figure 2 plots the relevant properties of the wood feedstocks tested in this work, which are expressed as ratios relative to those of holm-oak wood. It can be seen that apart from the apparent density, the physicochemical properties of wood vary little between species, being typically within 0.8 and 1.2 of those observed for holm oak wood. Differences in apparent density are larger and highlight holm oak as the densest wood, with around 900 kg/m
3, and poplar as the least dense wood, with just above 400 kg/m
3. In this regard, three groups of wood can be drawn: group I with up to around 500 kg/m
3 includes maritime pine, Australian blackwood stone pine, and poplar; group II within around 500 and 700 kg/m
3 includes eucalyptus globulus, acacia mimosa, and gray willow; and group III with above 700 kg/m
3 includes holm oak, olive tree, and cork oak. The lignin content also varies somewhat between woods, with the two pine species showing the highest values (>28 wt %), and eucalyptus and acacias showing the lowest (<20 wt %). As expected, the lignin content shows a slight negative correlation with the respective O/C mass ratios of wood (
Figure 2b); in the opposite direction goes the volatile matter content, which shows a positive correlation with the respective O/C ratios. The ash content falls outside the 0.8–1.2 range when plotted as ratios relative to that of holm oak, although the values obtained for the whole number of woods tested show a small variation if expressed by dry fuel mass: between 0.20 and 0.96 wt % (see
Table 1). Indeed, with the exceptions of holm oak and cork oak, the other wood types showed ash contents below ≈0.5 wt %.
3.3. Effect of Final Carbonization Temperature
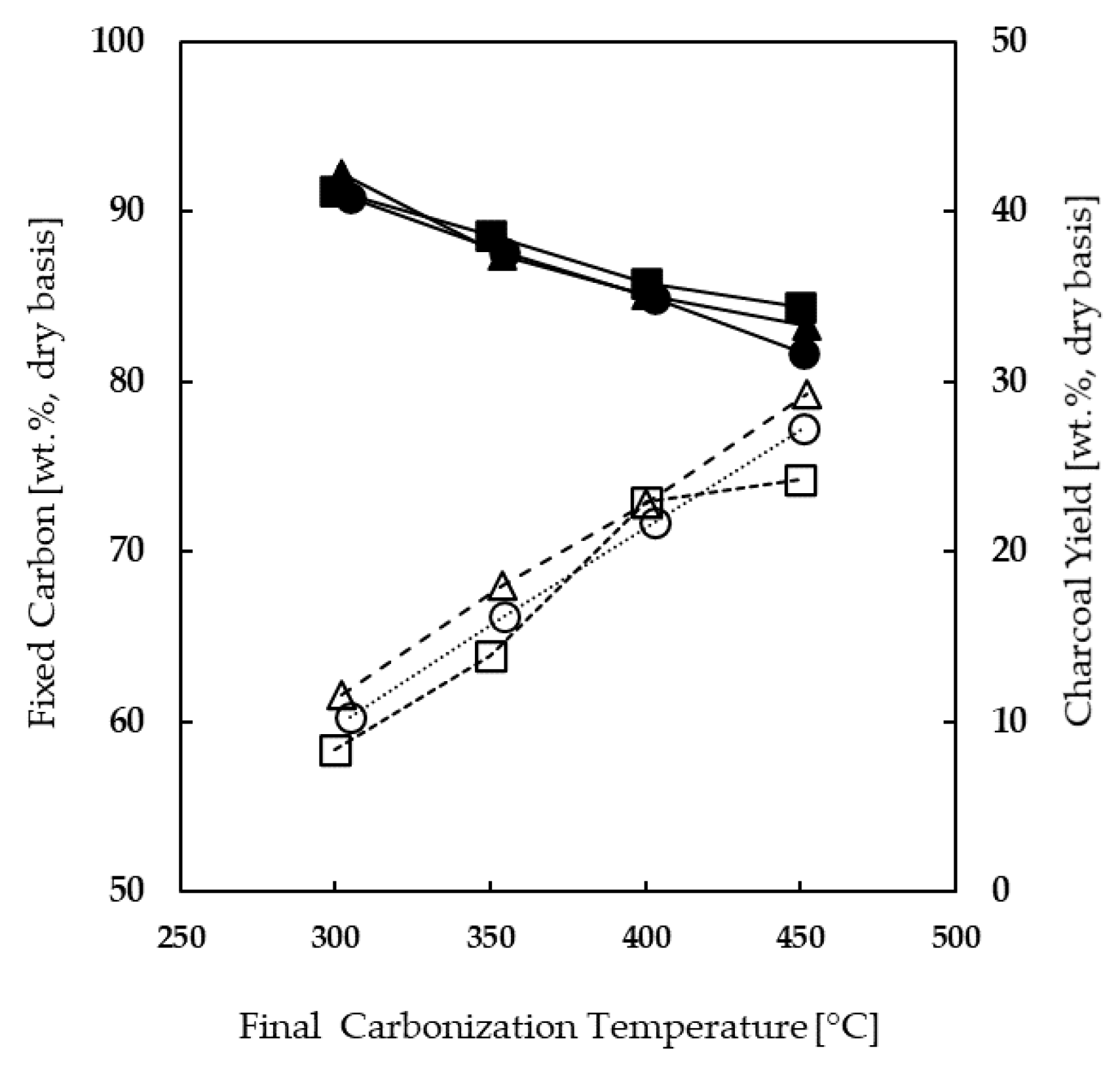
Figure 3 shows the charcoal’s fixed carbon content and yield as a function of final carbonization temperature, using a constant heating rate of 1 °C/min. The fact that similar trends were observed for holm oak, cork oak, and eucalyptus globulus, which exhibit quite different properties (
Table 1), suggests that this behavior will be valid for the remainder wood types tested in this work. The temperature causes different effects on charcoal’s fixed carbon content and yield. The fixed carbon content increases with temperature increase, while the respective yield decreases. The effect of temperature is more evident in the case of fixed carbon content. An increase in temperature from 300 to 450 °C leads to an increase in fixed carbon content from around 60 wt % to almost 80 wt %, while the respective yields decrease from around 40 wt % to just below 35 wt %.
These results show that a minimum final carbonization temperature of about 400 °C is required for the charcoal to reaches the threshold of 75 wt % (dry basis) of fixed carbon content, as established in the EN1860-2-2005:E standard for barbeque charcoal. Higher temperatures allow the production of charcoal with higher fixed carbon contents, albeit at the expense of lower mass yields. For this reason, and considering that some works dealing with practices of charcoal production report kiln temperatures typically around 400 °C [
27,
28], a final carbonization temperature of 400 °C was used during the tests aimed to evaluate the effect of wood type on the carbonization process.
3.4. Effect of Wood Type
3.4.1. Total Mass, Elemental, Ash, and Enthalpy Balances and Particle Shrinkage
Figure 4a provides the quantities of total mass, elements (CHO), volume, and enthalpy that remain in the charcoal particle after carbonization at 400 °C and 1 °C/min, which are expressed as ratios relative to the respective quantities initially present in the dry wood particles; actually, the mass and enthalpy ratios are the same as the usual operational parameters of mass and energy efficiencies, respectively. The most visible result is that the values obtained for the balances depend highly upon the quantity under focus and notably less on the type of wood. The values for charcoal’s yield (dry basis) varied from 0.33 kg/kg for acacia mimosa and 0.37 kg/kg for olive tree (holm oak, i.e., the reference wood, yielded 0.36 kg/kg). This range of charcoal’s yield is small considering the number of wood types tested and the ±2% uncertainty associated to this parameter. It also shows that the differences observed for e.g., lignin content or density of wood (
Figure 2) do not give rise to major differences in the charcoal’s yield. If this analysis is done in terms of specific elements, then it turns out that only a small fraction of the masses of hydrogen and oxygen initially present in the wood particles remain in the charcoal particles. Depending on the wood type, we observed a fraction ranging from 0.15 to 0.20 for H and from 0.13 to 0.16 for O. In the case of elemental carbon, this amount rises to more than half: between 0.52 and 0.6 of the mass of carbon in the dry wood remains in the charcoal. This underlines how the volatile products released during the carbonization of dry wood convey high amounts of oxygen and hydrogen, e.g., in the form of carbon oxides and water, as also noted with other operating conditions [
7]. Regarding particle shrinkage, the volume of the charcoal particles is within 0.42 to 0.55 (average of 0.51) of the volume of the dry wood particles. This corresponds to average shrinkages of ≈21% in diameter and ≈15% in length. Particle volume shrinkage is the parameter with major variations among the ten wood types tested, with a slight trend for the three densest woods (holm oak, cork oak, and olive tree) to afford larger volume contractions. In general, the charcoal particles kept the cylindrical shape of the original wood particles, with the exception being the eucalyptus charcoal with a barrel-like geometry. Almost all charcoal particles exhibited cracks—even if no cracks were observed in the original wood particles—while fragmentation was negligible. Concerning the enthalpy balances—i.e., based on the LHV of wood and charcoal—the results show that charcoal retains a fraction between 0.50 and 0.59 of the energy presents in the original wood particle (
Figure 4a). This places the carbonization process at a high level in terms of energy efficiency, with values comparable to those typical of biomass gasification and combustion (to electricity) processes [
29,
30], even if the energy released as volatile matter is discarded.
Concerning the ash balance—i.e., mass of ash in the charcoal particles per unit mass of ash in the dry wood particles—the values obtained are within 0.8–1.2. Values above 1 are unrealistic because the mass of ash in the charcoal particles should not be greater than the mass of ash initially present in the wood particles. However, the low ash contents of wood and charcoal can raise the uncertainties associated to the ash balances, and this should be considered when interpreting the results. To highlight uncertainties contributions,
Figure 4b details the ash balance separately. In general, the uncertainty values are within ±0.1 kg of ash per 100 kg of dry wood with the exceptions being the balances for poplar and the two acacias. There are a few cases with values above 1 even if uncertainties are taken into account. In these cases, it is possible that the wood samples subjected to analysis may have slightly different compositions than the wood samples placed in the reactor for carbonization, which leads to higher uncertainties. However, what can be stated from an overall analysis of
Figure 4b is that considering the uncertainties, it is not possible to rule out that the totality of the wood ash remains in the corresponding charcoal particles.
3.4.2. Charcoal Properties
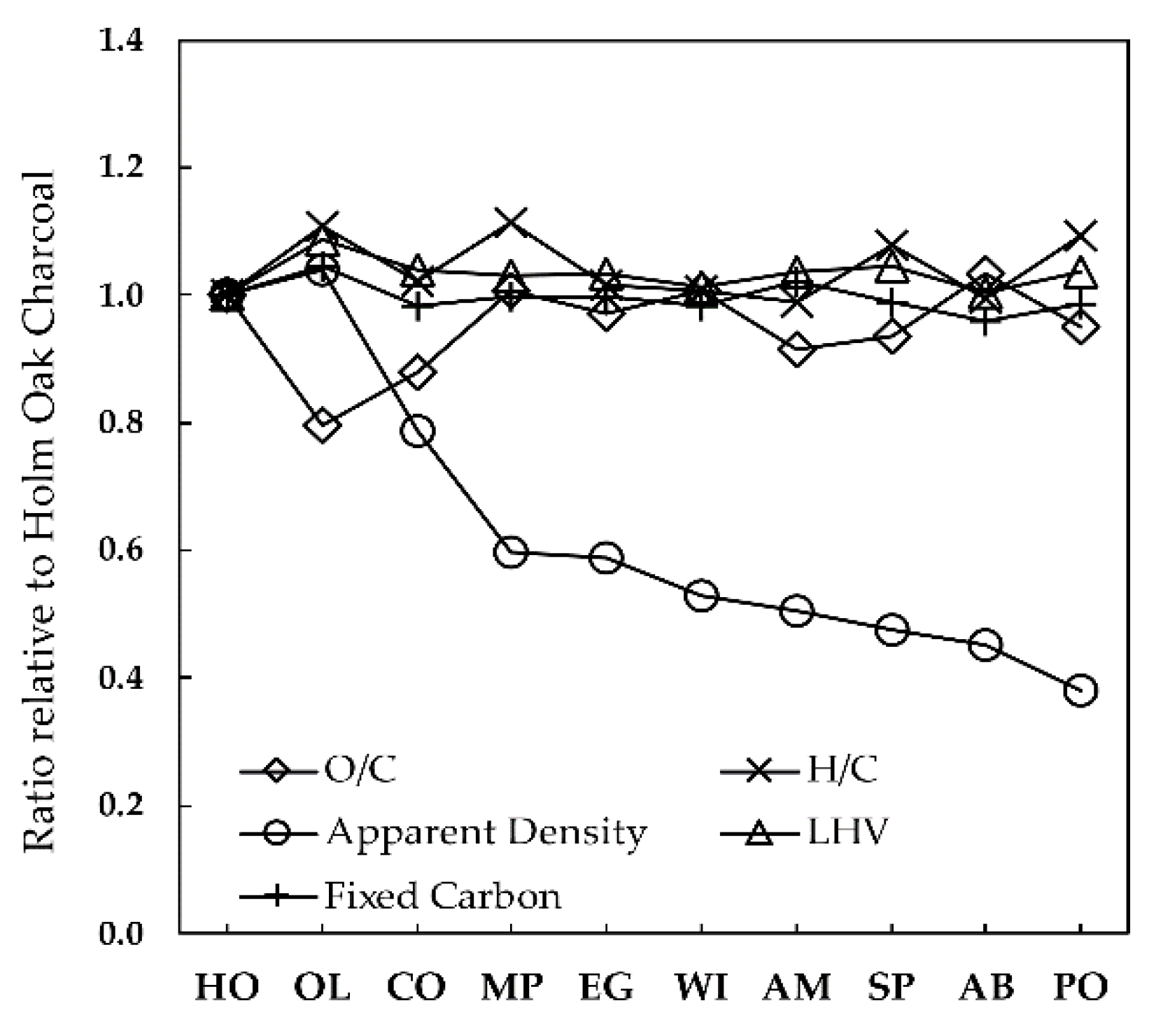
Following the analysis about the properties of the wood feedstocks in
Figure 2 (
Section 3.2),
Figure 5 shows the properties of the respective charcoals obtained at 400 °C and 1 °C/min, again given as ratios relative to those of holm oak charcoal—the base data are also given in
Table 2.
As discussed in
Section 3.4.1, the carbonization process leads to a massive release of elemental oxygen and hydrogen from the converting solid fuel, while the release of elemental carbon is much lower. The result is that the solid fraction arising from the process becomes highly enriched in both elemental carbon and fixed carbon, as compared to feedstock. For holm-oak—i.e., the reference fuel in this work—the results show an increase of elemental carbon content from 47.3 wt %, in dry wood, to 74.6 wt %, in dry charcoal. The corresponding increase for the fixed carbon content is from 16.3 wt % to 73.0 wt %. If this analysis is extended to the whole set of woods, it can be seen that the range of values for elemental carbon and fixed carbon enrichments is similar. For the elemental carbon content of charcoal, the values are between 0.99 and 1.05 of the values for holm-oak charcoal, or between 74.6 wt % and 78.6 wt % if expressed on a dry charcoal basis. This range is of the same order of magnitude of the measurement uncertainties associated with the elemental analysis (±3%), thus suggesting that the carbon content of charcoal has a small dependence on the type of wood for the samples studied. The same applies to the charcoal’s fixed carbon contents, with values within 0.95 to 1.05 of that obtained for holm-oak charcoal, or within 70.1–76.4 wt % on a dry charcoal basis. The slightly higher elemental carbon contents as compared to fixed carbon contents (e.g., 74.6 wt % C vs. 73.0 wt % FC for the case of holm-oak charcoal) are due to the small amount of volatile matter still present in the charcoal owing to the mild temperatures used in this work. These high carbon contents of charcoal make the respective O/C and H/C elemental mass ratios drop sharply as compared to those of wood: from 0.83–1.01 kgO/kgC and 0.14–0.15 kgH/kgC in the original woods, to 0.21–0.27 kgO/kgC and 0.04–0.05 kgH/kgC in the charcoals (see
Table 1 and
Table 2). Again, a similar range of O/C and H/C ratios were obtained among the 10 charcoal types analyzed. In its turn, the low O/C mass ratios enable the charcoal to show significantly higher lower heating values (LHV) as compared to wood: from 16.4–19.0 MJ/kg, for dry wood, to 26.7–29.0 MJ/kg, for dry charcoal, which represents an increment of about 50%. This also corresponds to about 80–90% of the LHV of graphite (32.8 MJ/kg [
31]), thus showing that the carbonization process generates high-quality charcoals without the need of harsh conditions. The results highlight that the ash content of charcoals is 2.7 to 3 times higher than those of woods, which is in line with the fact that the major part of ash in the feedstock remains in the charcoal. In contrast to the small differences found for the proximate and elemental compositions, and LHV of charcoals, the respective apparent density varies considerably, as also observed for the case of wood in
Figure 2. As a rule, there is a significant reduction of solid density when wood is converted into charcoal. Under the conditions of this study—carbonization of dry wood particles at 1 °C/min to 400 °C final temperature—the apparent density of charcoal is on average 68 ± 9% of that of the original wood particles, which is comparable with results from other studies [
32,
33]. Holm oak and olive tree are the wood types producing the densest charcoals (>650 kg/m
3 in
Table 2), while poplar produces the least dense charcoal (≈250 kg/m
3 or a ratio of 0.36 if expressed in relation to holm oak charcoal as in
Figure 5). Concerning other dominant tree species in Portugal (and Southern Europe), cork oak produces the third densest charcoal with just above 500 kg/m
3, while maritime pine, stone pine, and eucalyptus produce charcoals with less than 400 kg/m
3.
To get insight into the extent to which the apparent density of charcoals depends on the cellular macrostructure of the original wood particles, pore size measurements were performed for the charcoals obtained from holm oak, cork oak, eucalyptus globulus, maritime pine, and poplar. The results show small variations in the skeletal pore volume—between 0.0024 and 0.0034 cm
3/g—which corresponds to charcoal skeletal porosities of less than 1%. This reveals a non-porous skeletal structure with minimum spaces between graphite-like layers of flat aromatic carbon clusters. Nevertheless, the high porosities obtained following the Archimedes’ principle—up to ≈90% for poplar charcoal, which is equivalent to ≈250 kg/m
3 of apparent density—suggest a major presence of macro-porosity that is not associated with the skeletal. These results agree with other studies showing that charcoal macro-porosity accounts for more than 95% of total porosity [
34,
35]. Further SEM analysis of charcoal’s surface confirmed the presence of complex cellular structures typical of wood (see
Figure 6 for the cases of poplar and eucalyptus charcoals). This indicates that the cellular structure of wood remains intact after carbonization and defines charcoal’s apparent density.
3.5. Effect of the Heating Rate and Wood Moisture
Two additional variables that vary considerably during carbonization operations are the heating rate of the wood bed and wood moisture, due to the widely different carbonization systems under use [
16,
36] and because wood stacking is often carried out under atmospheric conditions. To get an initial impression of the effect of these variables, some additional carbonization tests were performed with holm oak wood, in which the heating rate was changed between 0.1, 1, and 5 °C/min and wood moisture between 0 and 30 wt % (while keeping the heating rate at 1 °C/min). To mimic the wetting process of the wood in real conditions, the original wood particles were moistened by immersion in distilled water for a couple of days to reach the desired moisture content.
Visually, the effect of increasing the heating rate is that the charcoal particles exhibit more cracks on the surface. This may be due to larger temperature gradients inside the converting wood particles that promote a faster release of volatiles and subsequent breakdown of wood structures during intra-particle transport. Comparing with the results at 1 °C/min, carbonization at 5 °C/min leads to a 15% decrease in charcoal yield, while the effect on charcoal properties is smaller (fixed carbon content decreases only ≈5%, and the ratio between the apparent densities of charcoal and wood particles decreases ≈10%). The corresponding variations due to decreasing the heating rate from 1 to 0.1 °C/min are even smaller and insignificant if the uncertainties of measurements are considered. In any case, the trend is that decreasing the heating rate to 0.1 °C/min improves both charcoal’s yield and properties (e.g., carbon content, LHV). If wet wood particles (i.e., 30 wt % moisture) are used, the result is that the cracking phenomenon of the charcoal particles is exacerbated. The explanation for this might be due to the formation and transport of larger amounts of steam inside the particles that can lead to the rupture of particle structures. Regarding its effect on charcoal’s yield and properties, carbonization of the wet wood particles results in slightly lower values of mass yield, fixed carbon content, and apparent density (decreases of less than 10%) as compared to the carbonization of dry wood particles.
3.6. Evaluation of Charcoal Properties in Relation to the European Standard and Barbeque Grill Market
The EN1860-2-2005:E standard (Appliances, solid fuels, and firelighters for barbecueing—Part 2: Barbecue charcoal and barbecue charcoal briquettes—Requirements and test methods) establishes a set of requirements for barbeque charcoal, including a minimum fixed carbon content of dry charcoal of 75 wt %, maximum ash content of dry charcoal of 8 wt %, maximum moisture content of as-received charcoal of 8 wt %, charcoal particle size distribution up to 150 mm, and minimum charcoal bulk density of 130 kg/m³. Among these parameters, charcoal moisture, particle size distribution, and bulk density were not tested in this work. Moisture was not tested because the charcoal particles recovered from the reactor were already dry. For the other two unmeasured parameters, the reason is that this work deals with the properties of single charcoals particles, while the standard addresses the properties of a mass of many particles.
In terms of fixed carbon content, most charcoals obtained at 400 °C and 1 °C/min approach the minimum 75 wt % threshold, with the exception being the olive charcoal with 76.3 wt %. The average value among the different charcoal types obtained in this work nearly coincides with the fixed carbon content of holm oak charcoal (73.0 wt %). However, the results in
Figure 3 show that the fixed carbon content of charcoal can be easily increased by adjusting the final carbonization temperature to above 400 °C. Therefore, it seems possible to prepare charcoals meeting the threshold of 75 wt % of fixed carbon content from all types of wood tested in this work. From the point of view of consumers, high fixed carbon contents are associated with high-quality charcoals for barbecueing. For instance, a study on marketing aspects of barbeque charcoal [
36] showed that volatile matter contents above 30 wt % are associated with embers that easily lead to flame and high-smoke emissions that might contain some quantity of harmful compounds. This can be particularly relevant during the refueling process of the barbeque with fresh charcoal particles. In this work, all charcoal types showed volatile matter contents under 30 wt % (maximum was 28.7 wt % for Australian blackwood charcoal). The ash content of charcoal is also a relevant parameter for consumers. High ash charcoals demand frequent cleaning and maintenance of barbequing systems, as well as it creates ticker ash layers over the embers that can be dragged to the food. However, all charcoal types obtained here exhibited ash contents well below the 8 wt % threshold given in the standard (actually below 3 wt %,
Table 2).
Another two charcoal properties not referred in the standard but that might be important for consumers are the LHV and apparent density. High LHV charcoals are associated with high-temperature embers, quick food preparation, and less charcoal feed during barbequing. The LHV of holm oak charcoal, ≈27 MJ/kg (dry basis), can be taken as a reference value, since this charcoal is perceived by consumers (and producers) as of high quality; again, minor differences in LHV were found between the 10 charcoal types obtained here (see
Table 2). Regarding density, Júnior [
37] referred that consumers often associate high-density charcoals with quality barbeques, having the notion that it means lower friability, fewer fines, and the possibility of preparing more food. In addition, there are other aspects linking density to charcoal quality perceptions. Charcoal is sometimes sold in bags with a given volume, which means that consumers have the notion that it is more economical to buy a bag with denser charcoal as it accommodates more mass of fuel. In addition, there is the perception that a bed of denser charcoal burns more slowly, which can be essential for some end-users. Indeed, the existence of two sub-markets for barbeque charcoal—i.e., professional users and residential users—can raise the interest for the production of low-density charcoals from alternative wood types. These low-density charcoals may be good enough for residential barbeques requiring small amounts of charcoal and where embers are needed during short periods (typically less than 30 min). In this sense, charcoals with smaller particle sizes may be also more suitable for these users, since burnout times are proportional to particle size [
38]. It has been shown that the carbonization of 50 mm diameter wood pieces (at 400 °C and 1 °C/min) originates charcoal particles of suitable diameters for the market (see
Section 3.4.1). On the contrary, professional barbecues such as restaurants and barbeque grill street food demand long operational times (several hours), which means that high-density charcoals such as those from holm oak and cork oak can be especially suited for them. Finally, from the point of view of producers and logistics operators, density is also relevant as it influences both costs and fines generation during screening, bagging, road transport, and storage.
4. Conclusions
A bench-scale fixed bed facility was used to investigate the carbonization behavior of 10 wood types common in Portugal and Southern Europe, under operating conditions that are relevant for charcoal production systems (heating rate within 0.1–5 °C/min, final temperature within 300–450 °C, large wood particle sizes, wood moisture up to 30 wt %). Apart from a detailed physical–chemical analysis of the original woods, the resulting charcoals were collected to evaluate the balances of the process and to obtain its most relevant properties.
Wood apparent density varies considerably more than other wood properties studied in this work (proximate and elemental analysis, lignin content, LHV), and its structural arrangement is preserved after withstanding temperatures typical of carbonization kilns. Then, the structure of charcoal is linked to that of the original wood, and its apparent density is positively correlated to the apparent density of the original wood. As a result, the apparent density of charcoal also varies widely compared to other charcoal properties studied. If the focus is on the top-five wood types in the Portuguese forestry sector (cork oak, holm oak, eucalyptus globulus, maritime pine, and stone pine), then it turns out that holm-oak wood produces significantly denser charcoals, which agrees with the fact that it is considered a reference product in the market. However, it is possible to produce charcoals complying with EN1860-2-2005:E standard for barbeque charcoal from all wood types tested in this work. The attainment of suitable fixed carbon contents—minimum 75 wt %, dry basis, according to the standard—is a critical issue that can be overcome by proper adjustment of the operating conditions. Raising the carbonization temperature is a straightforward way of improving charcoal quality, albeit at the expense of lower yields. On the other hand, reducing the heating rate from 1.0 to 0.1 °C/min improves charcoal quality and yield, although the gains are marginal and may not compensate for the much higher carbonization times.
Carbonization enables the conversion of various types of wood into charcoal—a valuable product for which both the production infrastructure and markets already exist. For the specific case of barbecue charcoal, the utilization of alternative wood types to the two types currently used on a large scale in Portugal—holm oak and cork oak—is only a market issue, including the aspects related to wood availability and charcoal price/quality relation. In this context, the existence of two main sub-markets for barbeque charcoal—professional and household users, the latter with lower quality requisites—can raise the interest for the carbonization of undervalued wood varieties or even wood feedstocks with smaller particle sizes, both of which can lead to charcoals of lower quality but yet suitable for household applications. This can contribute to diversifying the fuel supply chain associated with current charcoal activities in Portugal, with marketing and information actions aimed at both producers and consumers being needed to explore these opportunities within the barbeque market. Moreover, future work can also unveil specialized markets in e.g., industrial and agricultural sectors, which can raise the interest for the carbonization of specific wood types.