Two Excited State Collaboration of Heteroleptic Ir(III)-Coumarin Complexes for H2 Evolution Dye-Sensitized Photocatalysts †
Abstract
1. Introduction
2. Materials and Methods
2.1. Syntheses
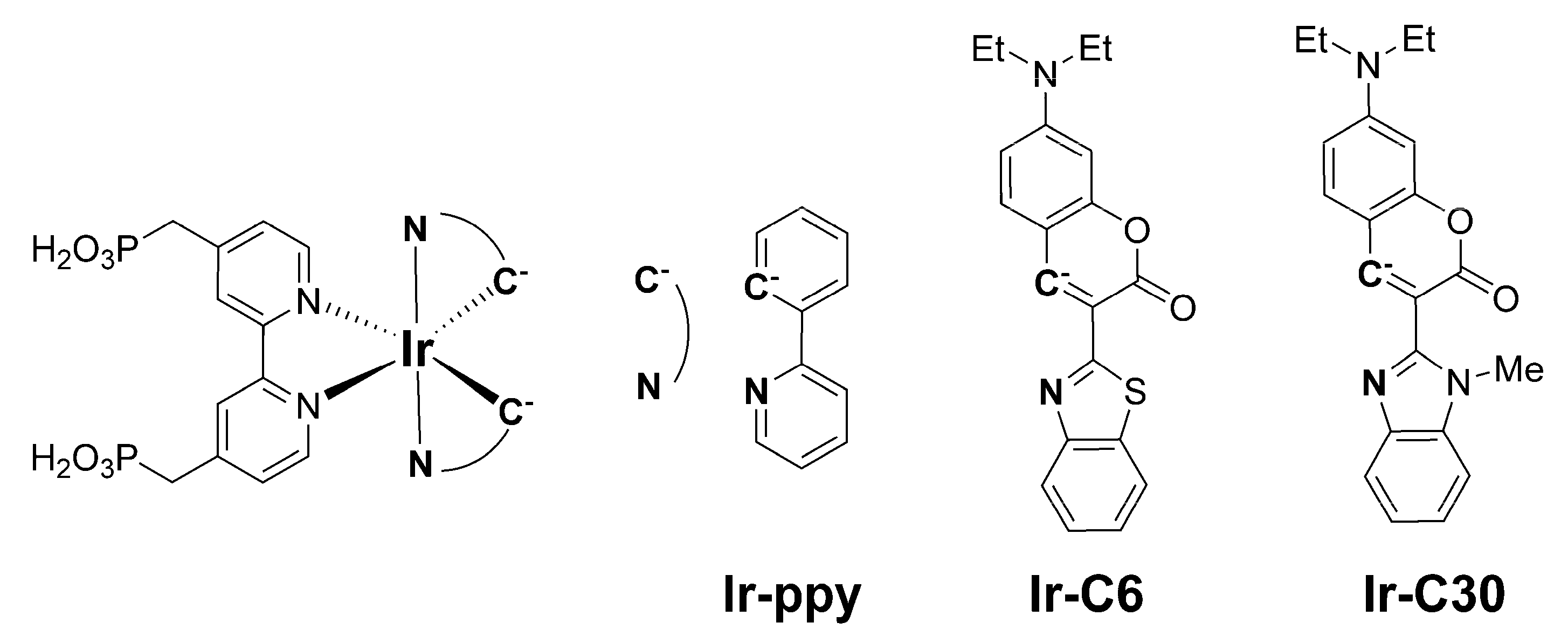
2.1.1. Synthesis of [Ir(C6)2(H4CPbpy)]Cl (Ir-C6)
2.1.2. Synthesis of [Ir(C30)2(H4CPbpy)]Cl (Ir-C30)
2.1.3. Preparation of the Nanoparticle Photocatalysts (Ir-CX@Pt-TiO2)
2.2. Measurements
2.2.1. Characterization
2.2.2. Photophysical and Electrochemical Measurements
2.3. Photocatalytic Hydrogen Evolution Reactions
2.4. Theoretical Calculations
3. Results and Discussion
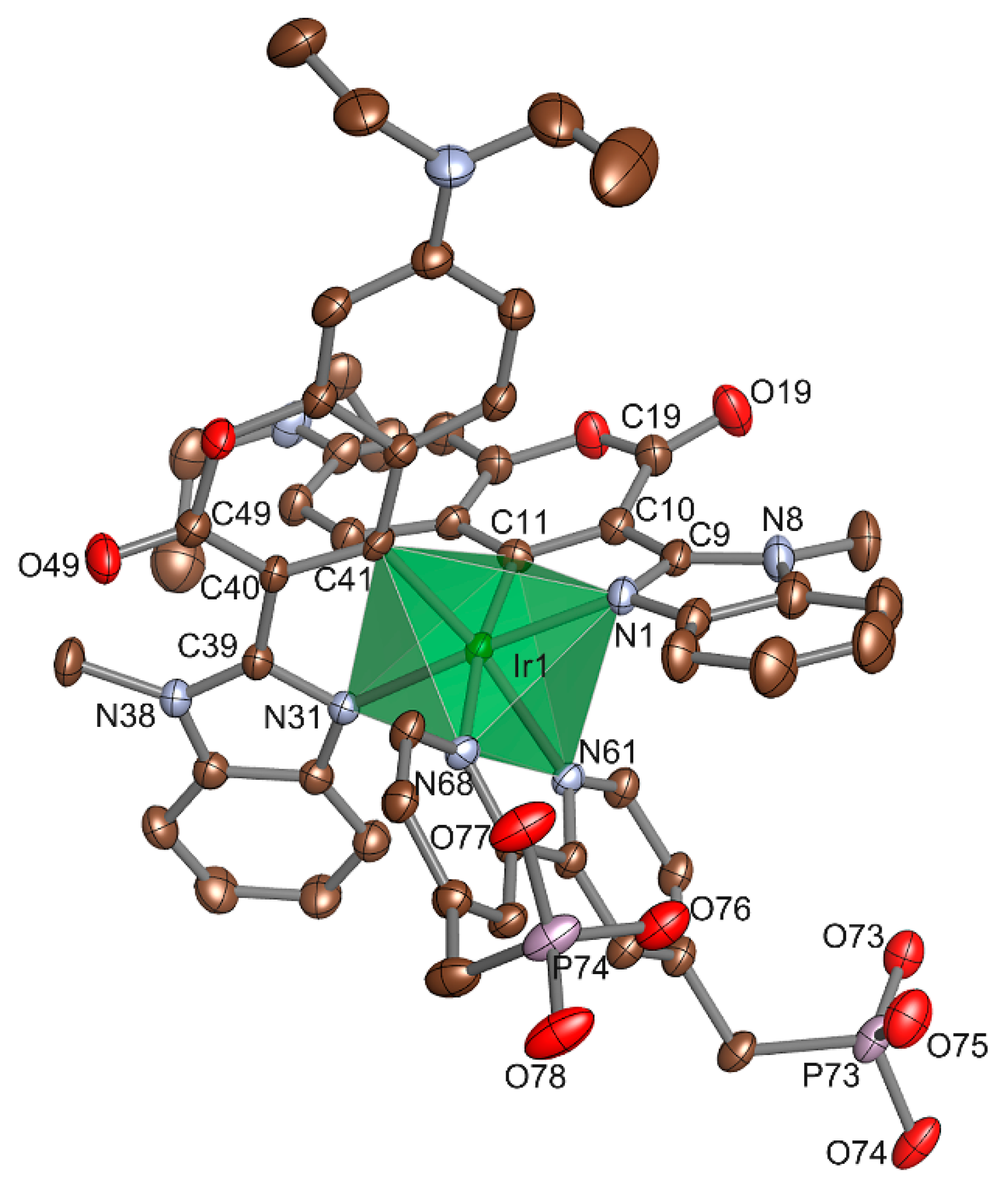
3.1. Molecular Structure of Ir-C30
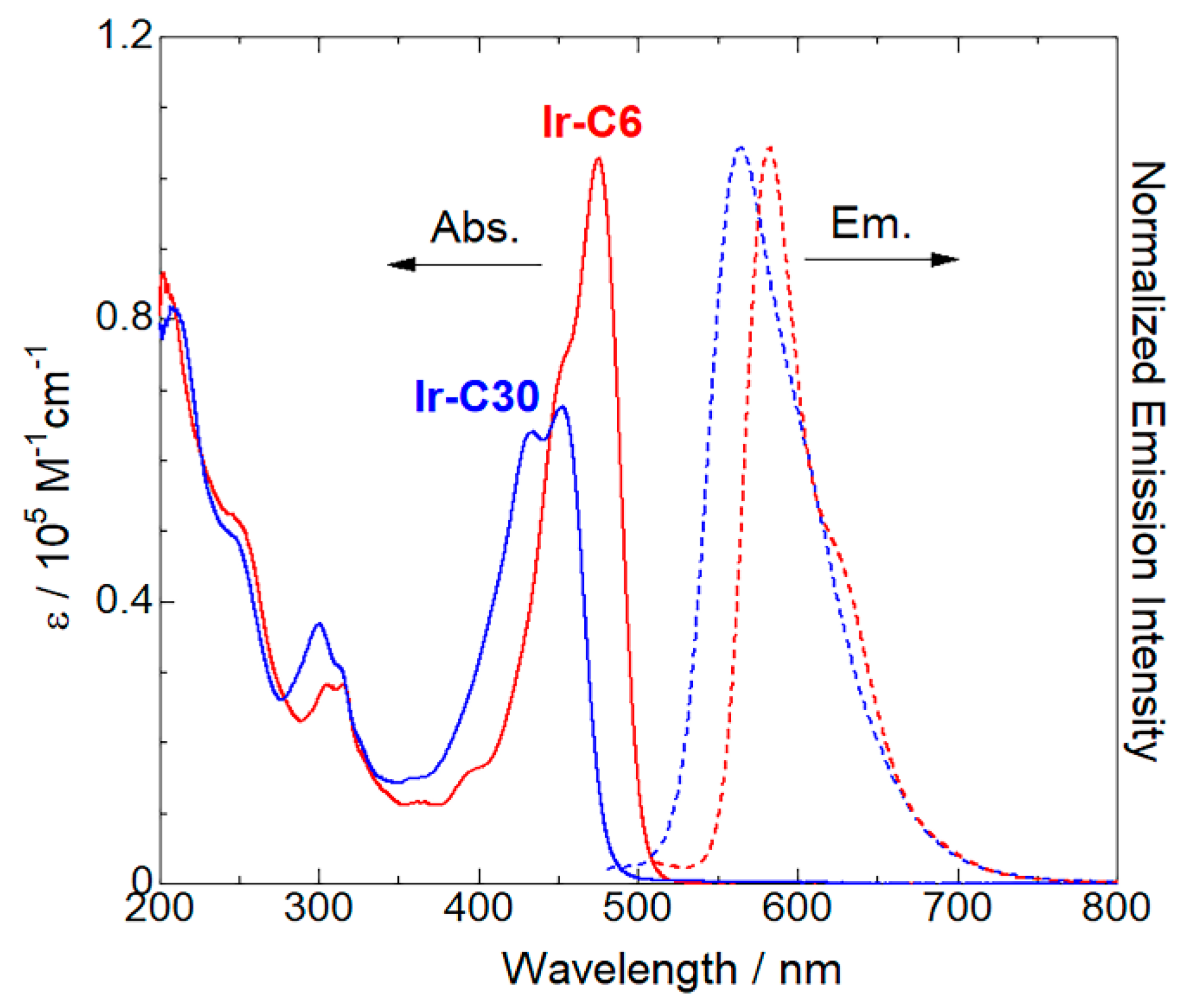
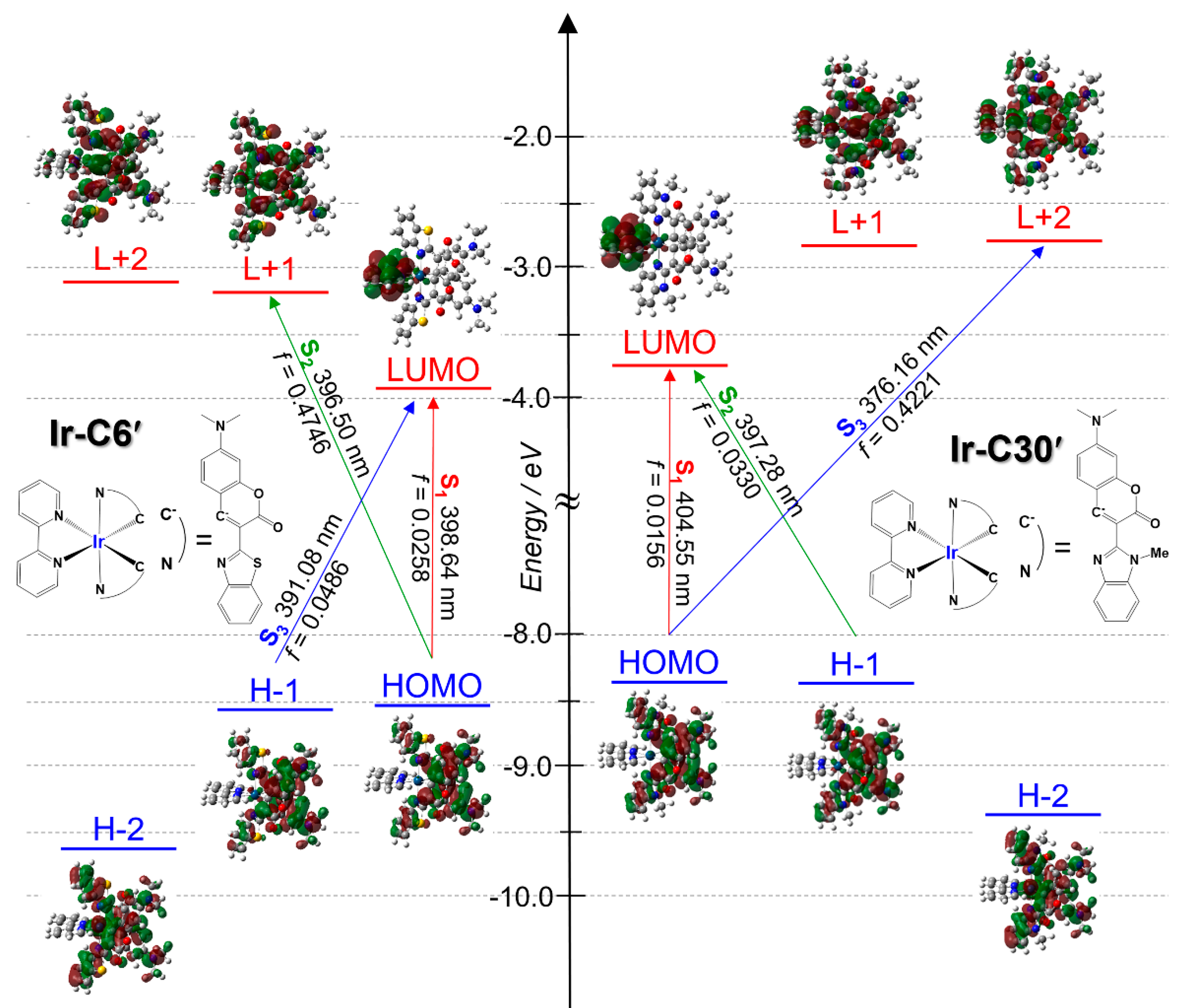
3.2. Photophysical and Electrochemical Properties
3.3. Synthesis and Characterization of the Ir-PS-Immobilized Pt-TiO2 Nanoparticles
3.4. Photocatalytic H2 Evolution Reactions
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fujishima, A.; Honda, K. Electrochemical Photolysis of Water at a Semiconductor Electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef]
- O’Regan, B.; Grätzel, M. A low-cost, high-efficiency solar cell based on dye-sensitized colloidal TiO2 films. Nature 1991, 353, 737–740. [Google Scholar] [CrossRef]
- Pelaez, M.; Nolan, N.T.; Pillai, S.C.; Seery, M.K.; Falaras, P.; Kontos, A.G.; Dunlop, P.S.M.; Hamilton, K.W.J.; Byrne, J.A.; O’Shea, K.; et al. A review on the visible light active titanium dioxide photocatalysts for environmental applications. Appl. Catal. B 2012, 125, 331–349. [Google Scholar] [CrossRef]
- Zhang, X.; Peng, T.; Song, S. Recent advances in dye-sensitized semiconductor systems for photocatalytic hydrogen produc-tion. J. Mater. Chem. A 2016, 4, 2365–2402. [Google Scholar] [CrossRef]
- Yuan, Y.-J.; Yu, Z.-T.; Chen, D.-Q.; Zou, Z.-G. Metal-complex chromophores for solar hydrogen generation. Chem. Soc. Rev. 2017, 46, 603–631. [Google Scholar] [CrossRef]
- You, Y.; Nam, W. Photofunctional triplet excited states of cyclometalated Ir(iii) complexes: Beyond electroluminescence. Chem. Soc. Rev. 2012, 41, 7061–7084. [Google Scholar] [CrossRef] [PubMed]
- Mills, I.N.; Porras, J.A.; Bernhard, S. Judicious Design of Cationic, Cyclometalated Ir(III) Complexes for Photochemical Energy Conversion and Optoelectronics. Acc. Chem. Res. 2018, 51, 352–364. [Google Scholar] [CrossRef] [PubMed]
- Goldsmith, J.I.; Hudson, W.R.; Lowry, M.S.; Anderson, T.H.; Bernhard, S. Discovery and High-Throughput Screening of Heteroleptic Iridium Complexes for Photoinduced Hydrogen Production. J. Am. Chem. Soc. 2005, 127, 7502–7510. [Google Scholar] [CrossRef]
- Lowry, M.S.; Goldsmith, J.I.; Slinker, J.D.; Rohl, R.; Pascal, R.A.; Malliaras, G.G.; Bernhard, S. Single-layer electroluminescent devices and photoinduced hydrogen production from an ionic iridium(III) complex. Chem. Mater. 2005, 17, 5712–5719. [Google Scholar] [CrossRef]
- Tinker, L.L.; McDaniel, N.D.; Curtin, P.N.; Smith, C.K.; Ireland, M.J.; Bernhard, S. Visible Light Induced Catalytic Water Reduction without an Electron Relay. Chem. Eur. J. 2007, 13, 8726–8732. [Google Scholar] [CrossRef]
- Metz, S.; Bernhard, S. Robust photocatalytic water reduction with cyclometalated Ir(iii) 4-vinyl-2,2′-bipyridine complexes. Chem. Commun. 2010, 46, 7551–7553. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.-H.; Ling, J.-W.; Lai, S.-H.; Huang, M.-J.; Cheng, C.H.; Chen, I.C. Dynamics of the Excited States of [Ir(ppy)2bpy]+with Triple Phosphorescence. J. Phys. Chem. A 2010, 114, 10339–10344. [Google Scholar] [CrossRef] [PubMed]
- Gärtner, F.; Cozzula, D.; Losse, S.; Boddien, A.; Anilkumar, G.; Junge, H.; Schulz, T.; Marquet, N.; Spannenberg, A.; Gladiali, S.; et al. Synthesis, Characterisation and Application of Iridium(III) Photosensitisers for Catalytic Water Reduction. Chem. Eur. J. 2011, 17, 6998–7006. [Google Scholar] [CrossRef]
- Yuan, Y.-J.; Yu, Z.-T.; Chen, X.-Y.; Zhang, J.-Y.; Zou, Z.-G. Visible-Light-Driven H2 Generation from Water and CO2 Conversion by Using a Zwitterionic Cyclometalated Iridium(III) Complex. Chem. Eur. J. 2011, 17, 12891–12895. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.-T.; Yuan, Y.-J.; Cai, J.-G.; Zou, Z.-G. Charge-neutral amidinate-containing iridium complexes capable of efficient pho-tocatalytic water reduction. Chem. Eur. J. 2013, 19, 1303–1310. [Google Scholar] [CrossRef] [PubMed]
- Neubauer, A.; Grell, G.; Friedrich, A.; Bokarev, S.I.; Schwarzbach, P.; Gärtner, F.; Surkus, A.-E.; Junge, H.; Beller, M.; Kühn, O.; et al. Electron- and Energy-Transfer Processes in a Photocatalytic System Based on an Ir(III)-Photosensitizer and an Iron Catalyst. J. Phys. Chem. Lett. 2014, 5, 1355–1360. [Google Scholar] [CrossRef]
- Fan, S.; Zong, X.; Shaw, P.E.; Wang, X.; Geng, Y.; Smith, A.R.G.; Burn, P.L.; Wang, L.; Lo, S.-C. Energetic requirements of iridium(iii) complex based photosensitisers in photocatalytic hydrogen generation. Phys. Chem. Chem. Phys. 2014, 16, 21577–21585. [Google Scholar] [CrossRef]
- Kawashima, M.; Mori, K.; Aoyama, J.; Yamashita, H. Synthesis and Characterization of Ir and Rh Complexes Supported on Layered K4Nb6O17 as a Heterogeneous Photocatalyst for Visible-Light-Induced Hydrogen Evolution. Bull. Chem. Soc. Jpn. 2014, 87, 874–881. [Google Scholar] [CrossRef]
- Cai, J.-G.; Yu, Z.-T.; Yuan, Y.-J.; Li, F.; Zou, Z.-G. Dinuclear iridium(III) complexes containing bibenzimidazole and their ap-plication to water photoreduction. ACS Catal. 2014, 4, 1953–1963. [Google Scholar] [CrossRef]
- Yuan, Y.-J.; Yu, Z.-T.; Liu, X.-J.; Cai, J.-G.; Guan, Z.-J.; Zou, Z.G. Hydrogen Photogeneration Promoted by Efficient Electron Transfer from Iridium Sensitizers to Colloidal MoS2 Catalysts. Sci. Rep. 2014, 4, 4045. [Google Scholar] [CrossRef]
- Paul, A.; Das, N.; Halpin, Y.; Vos, J.G.; Pryce, M.T. Carboxy derivatised Ir(III) complexes: Synthesis, electrochemistry, pho-tophysical properties and photocatalytic hydrogen generation. Dalton Trans. 2015, 44, 10423–10430. [Google Scholar] [CrossRef] [PubMed]
- Cheong, H.-Y.; Kim, S.-Y.; Cho, Y.-J.; Cho, D.W.; Kim, C.H.; Son, H.-J.; Pac, C.; Kang, S.O. Photosensitization Behavior of Ir(III) Complexes in Selective Reduction of CO2 by Re(I)-Complex-Anchored TiO2 Hybrid Catalyst. Inorg. Chem. 2017, 56, 12042–12053. [Google Scholar] [CrossRef]
- Schönweiz, S.; Heiland, M.; Anjass, M.; Jacob, T.; Rau, S.; Streb, C. Experimental and Theoretical Investigation of the Light-Driven Hydrogen Evolution by Polyoxometalate-Photosensitizer Dyads. Chem. Eur. J. 2017, 23, 15370–15376. [Google Scholar] [CrossRef]
- Torres, J.; Carrión, M.C.; Leal, J.; Jalón, F.A.; Cuevas, J.V.; Rodríguez, A.M.; Castañeda, G.; Manzano, B.R. Cationic bis(cyclometalated) Ir(III) complexes with pyridine−carbene ligands. Photophysical properties and photocatalytic hydrogen production from water. Inorg. Chem. 2018, 57, 970–984. [Google Scholar] [CrossRef]
- Kobayashi, A.; Watanabe, S.; Yoshida, M.; Kato, M. Importance of the Molecular Orientation of an Iridium(III)-Heteroleptic Photosensitizer Immobilized on TiO2 Nanoparticles. ACS Appl. Energy Mater. 2018, 1, 2882–2890. [Google Scholar] [CrossRef]
- Bevernaegie, R.; Wehlin, S.A.M.; Piechota, E.J.; Abraham, M.; Philouze, C.; Meyer, G.J.; Elias, B.; Troian-Gautier, L. Improved visible light absorption of potent iridium(III) photo-oxidants for excited-state electron transfer chemistry. J. Am. Chem. Soc. 2020, 142, 2732–2737. [Google Scholar] [CrossRef]
- Mayo, E.I.; Kilså, K.; Tirrell, T.; Djurovich, P.I.; Tamayo, A.; Thompson, M.E.; Lewis, N.S.; Gray, H.B. Cyclometalated iridium(iii)-sensitized titanium dioxide solar cells. Photochem. Photobiol. Sci. 2006, 5, 871–873. [Google Scholar] [CrossRef]
- Shinpuku, Y.; Inui, F.; Nakai, M.; Nakabayashi, Y. Synthesis and characterization of novel cyclometalated iridium(III) com-plexes for nanocrystalline TiO2-based dye-sensitized solar cells. J. Photochem. Photobiol. A 2011, 222, 203–209. [Google Scholar] [CrossRef]
- Gennari, M.; Légalité, F.; Zhang, L.; Pellegrin, Y.; Blart, E.; Fortage, J.; Brown, A.M.; Deronzier, A.; Collomb, M.-N.; Boujtita, M.; et al. Long-lived charge separated state in NiO-based p type dye sensitized solar cells with simple cyclometalated iridium complexes. J. Phys. Chem. Lett. 2014, 5, 2254–2258. [Google Scholar] [CrossRef] [PubMed]
- Tschierlei, S.; Neubauer, A.; Rockstroh, N.; Karnahl, M.; Schwarzbach, P.; Junge, H.; Bellerd, M.; Lochbrunner, S. Ultrafast excited state dynamics of iridium(III) complexes and their changes upon immobilization onto titanium dioxide layers. Phys. Chem. Chem. Phys. 2016, 18, 10682–10687. [Google Scholar] [CrossRef] [PubMed]
- Bobo, M.V.; Paul, A.; Robb, A.J.; Arcidiacono, A.M.; Smith, M.D.; Hanson, K.; Vannucci, A.K. Bis-Cyclometalated Iridium Complexes Containing 4,4′-Bis(phosphonomethyl)-2,2′-bipyridine Ligands: Photophysics, Electrochemistry, and High-Voltage Dye-Sensitized Solar Cells. Inorg. Chem. 2020, 59, 6351–6358. [Google Scholar] [CrossRef]
- Takizawa, S.; Pérez-Bolívar, C.; Anzenbacher, P., Jr.; Murata, S. Cationic iridium complexes coordinated with coumarin dyes—Sensitizers for visible-light-driven hydrogen generation. Eur. J. Inorg. Chem. 2012, 2012, 3975–3979. [Google Scholar] [CrossRef]
- Ma, L.; Guo, H.; Li, Q.; Guo, S.; Zhao, J. Visible light-harvesting cyclometalated Ir(iii) complexes as triplet photosensitizers for triplet–triplet annihilation based upconversion. Dalton Trans. 2012, 41, 10680–10689. [Google Scholar] [CrossRef]
- Yi, X.; Zhang, C.; Guo, S.; Ma, J.; Zhao, J. Strongly emissive long-lived3IL excited state of coumarins in cyclometalated Ir(iii) complexes used as triplet photosensitizers and application in triplet–triplet annihilation upconversion. Dalton Trans. 2014, 43, 1672–1683. [Google Scholar] [CrossRef]
- Happ, B.; Kübel, J.; Pfeffer, M.G.; Winter, A.; Hager, M.D.; Dietzek, B.; Rau, S.; Schubert, U.S. Towards Hydrogen Evolution Initiated by LED Light: 2-(1H -1,2,3-Triazol-4-yl)pyridine-Containing Polymers as Photocatalyst. Macromol. Rapid Commun. 2015, 36, 671–677. [Google Scholar] [CrossRef]
- Sinopoli, A.; Wood, C.J.; Gibson, E.A.; Elliott, P.I.P. Hybrid Cyclometalated Iridium Coumarin Complex as a Sensitiser of Both n- and p-Type DSSCs. Eur. J. Inorg. Chem. 2016, 2016, 2887–2890. [Google Scholar] [CrossRef]
- Takizawa, S.; Ikuta, N.; Zeng, F.; Komaru, S.; Sebata, S.; Murata, S. Impact of substituents on excited-state and photosensitizing properties in cationic iridium(III) complexes with ligands of coumarin 6. Inorg. Chem. 2016, 55, 8723–8735. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Conway-Kenny, R.; Wang, J.; Cui, X.; Zhao, J.; Draper, S.M. Exploiting coumarin-6 as ancillary ligands in 1,10-phenanthroline Ir(iii) complexes: Generating triplet photosensitisers with high upconversion capabilities. Dalton Trans. 2018, 47, 8585–8589. [Google Scholar] [CrossRef] [PubMed]
- Sebata, S.; Takizawa, S.; Ikuta, N.; Murata, S. Photofunctions of iridium(iii) complexes in vesicles: Long-lived excited states and visible-light sensitization for hydrogen evolution in aqueous solution. Dalton Trans. 2019, 48, 14914–14925. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Guo, S.; Wang, H.-J.; Chen, K.-K.; Zhang, N.; Zhang, Z.-M.; Lu, T.-B. A broadband and strong visible-light-absorbing photosensitizer boosts hydrogen evolution. Nat. Comm. 2019, 10, 3155. [Google Scholar] [CrossRef] [PubMed]
- Park, H.; Choi, W.; Hoffmann, M.R. Effects of the preparation method of the ternary CdS/TiO2/Pt hybrid photocatalysts on visible light-induced hydrogen production. J. Mater. Chem. 2008, 18, 2379–2385. [Google Scholar] [CrossRef]
- Borisov, S.M.; Klimant, I. Ultrabright Oxygen Optodes Based on Cyclometalated Iridium(III) Coumarin Complexes. Anal. Chem. 2007, 79, 7501–7509. [Google Scholar] [CrossRef]
- Norris, M.R.; Concepcion, J.J.; Glasson, C.R.K.; Fang, Z.; Lapides, A.M.; Ashford, D.L.; Templeton, J.L.; Meyer, T.J. Synthesis of phosphonic acid derivatized bipyridine ligands and their ruthenium complexes. Inorg. Chem. 2013, 52, 12492–12501. [Google Scholar] [CrossRef]
- Furugori, S.; Kobayashi, A.; Watanabe, A.; Yoshida, M.; Kato, M. Impact of photosensitizing multilayered structure on ruthe-nium(II)-dye-sensitized TiO2-nanoparticle photocatalysts. ACS Omega 2017, 2, 3901–3912. [Google Scholar] [CrossRef]
- CrysAlisPro, Version 1.171.39.45h; Rigaku Corporation: Oxford, UK, 2018.
- Sheldrick, G.M. A short history of SHELX. Acta Crystallogr. 2008, A64, 112–122. [Google Scholar] [CrossRef]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Pushmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Kiyota, J.; Yokoyama, J.; Yoshida, M.; Masaoka, S.; Sakai, K. Electrocatalytic O2 evolution from water at an ITO electrode modified with [Ru(terpy){4,4′-(CH2PO3H2)2-2,2′-bpy}(OH2)]2+: Evidence for a unimolecular pathway. Chem. Lett. 2010, 39, 1146–1148. [Google Scholar] [CrossRef]
- Yanai, T.; Tew, D.P.; Handy, N.C. A new hybrid exchange–correlation functional using the Coulomb-attenuating method (CAM-B3LYP). Chem. Phys. Lett. 2004, 393, 51–57. [Google Scholar] [CrossRef]
- Hehre, W.J.; Ditchfield, R.; Pople, J.A. Self—Consistent Molecular Orbital Methods. XII. Further Extensions of Gaussian—Type Basis Sets for Use in Molecular Orbital Studies of Organic Molecules. J. Chem. Phys. 1972, 56, 2257–2261. [Google Scholar] [CrossRef]
- Andrae, D.; Haussermann, U.; Dolg, M.; Stoll, H.; Preuss, H. Energy-adjusted ab initio pseudopotentials for the second and third row transition elements. Theor. Chim. Acta 1990, 77, 123–141. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09, Revision E.01; Gaussian, Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Lee, C.; Yang, W.; Parr, R.G. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B 1988, 37, 785–789. [Google Scholar] [CrossRef] [PubMed]
- Dennington, R.; Keith, T.; Millam, J. GaussView 5.0; Semichem Inc.: Shawnee Mission, KS, USA, 2009. [Google Scholar]
- DeRosa, M.C.; Hodgson, D.J.; Enright, G.D.; Dawson, B.; Evans, C.E.B.; Crutchley, R.J. Iridium Luminophore Complexes for Unimolecular Oxygen Sensors. J. Am. Chem. Soc. 2004, 126, 7619–7626. [Google Scholar] [CrossRef] [PubMed]
- Natali, M. Elucidating the Key Role of pH on Light-Driven Hydrogen Evolution by a Molecular Cobalt Catalyst. ACS Catal. 2017, 7, 1330–1339. [Google Scholar] [CrossRef]
- Rothenberger, G.; Fitzmaurice, D.; Grätzel, M. Spectroscopy of Conduction Band Electrons in Transparent Metal Oxide Semiconductor Films: Optical Determination of the Fiatband Potential of Colloidal Titanium Dioxide Films. J. Phys. Chem. 1992, 96, 5983–5988. [Google Scholar] [CrossRef]

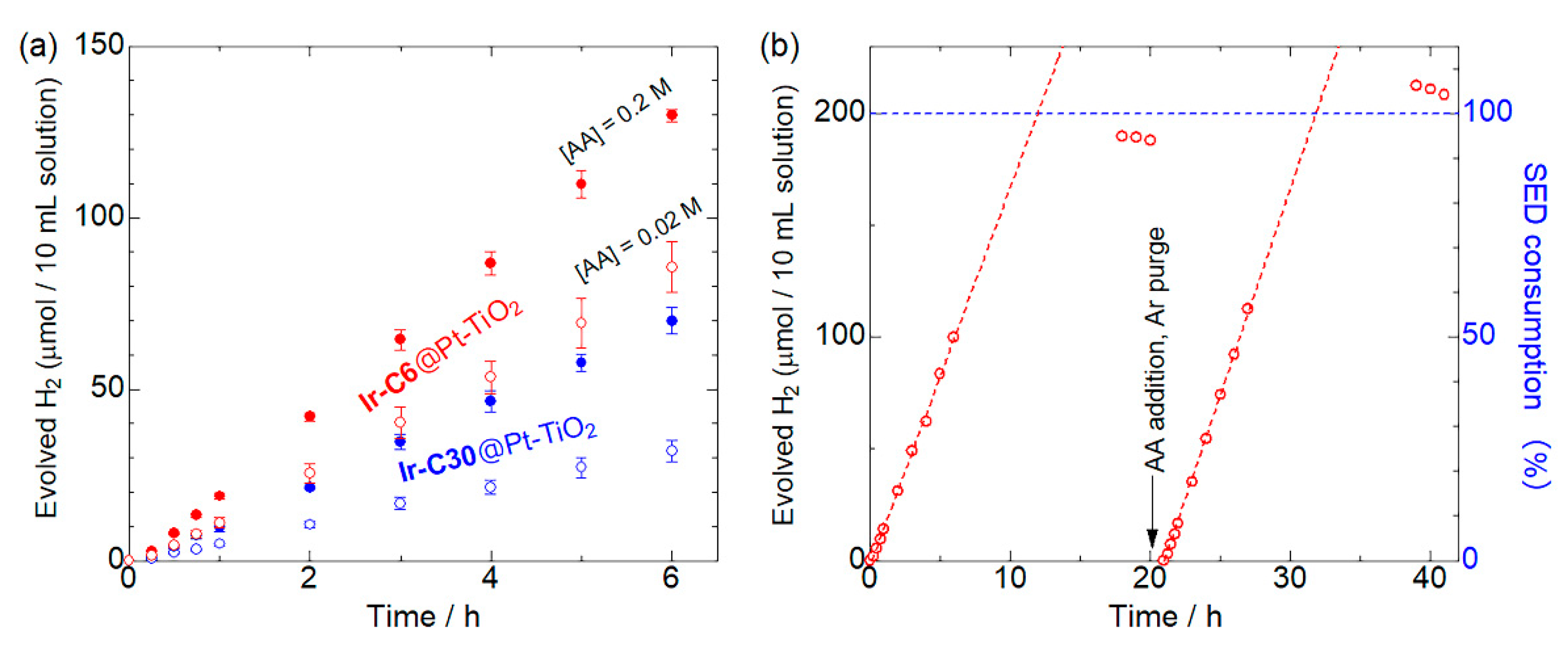
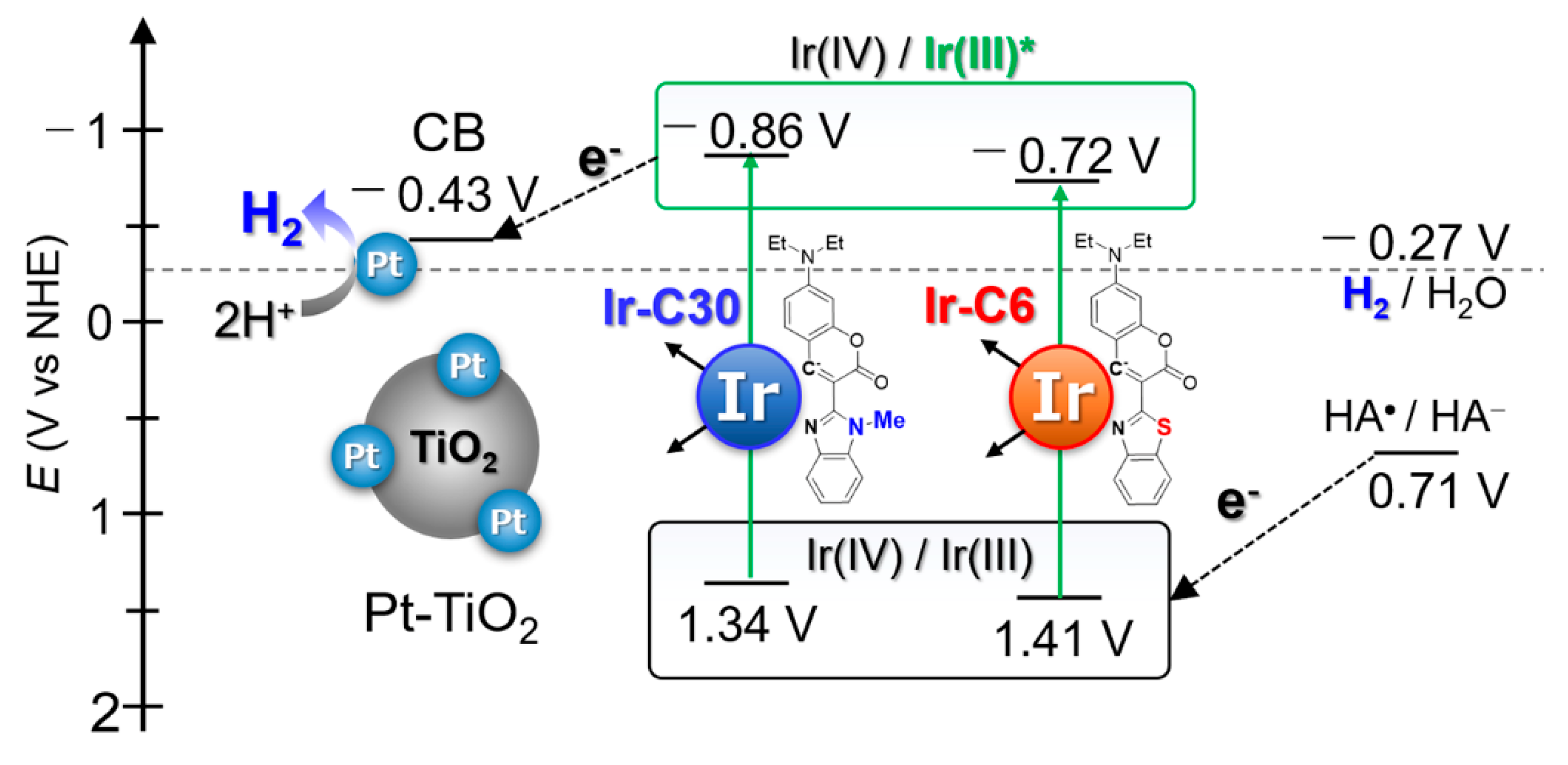
| Complex | λabs (ε) /nm | λem a /nm | τemb/μs | Φ c | krd /s−1 | knre/s−1 | Eoxf/V vs. NHE | E*oxg/V vs. NHE | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| Ir-C30 | 452 (6.76 × 104) | 564 | 7.03 | 0.10 | 1.5 × 104 | 1.3 × 105 | +1.34 | −0.86 | This work |
| Ir-C6 | 475 (10.3 × 104) | 582 | 19.2 | 0.39 | 2.1 × 104 | 3.2 × 104 | +1.41 | −0.72 | This work |
| Ir-ppy | 464 (6.1 × 102) | 587 | 0.29 | 0.10 | 3.5 × 105 | 3.1 × 106 | +1.67 | −0.63 | [25] |
| Photocatalyst | Amount of Immobilized Ir(III) Complex (nmol/1 mg TiO2) a | Surface Coverage (nmol/cm2) | Molecular Footprint of Ir(III) Complex (nm2) |
|---|---|---|---|
| Ir-C6@Pt-TiO2 | 130 | 7.61 × 10−2 | 2.17 |
| Ir-C30@Pt-TiO2 | 137 | 8.10 × 10−2 | 2.08 |
| Ir-ppy@Pt-TiO2 b | 175 | 10.5 × 10−2 | 1.62 |
| Photocatalyst | [Ir] (μM) | [AA] (M) | λirr (nm) | H2 (μmol) a | TON a,b | TOF b | Ref. |
|---|---|---|---|---|---|---|---|
| Ir-C6@Pt-TiO2 | 10 | 0.2 | 420 − 740 | 130 | 1299 | 217 | |
| Ir-C6@Pt-TiO2 | 10 | 0.02 | 420 − 740 | 85.6 | 856 | 143 | |
| Ir-C6@Pt-TiO2 | 40 | 0.2 | 450 ± 10 | 9.33 | 23.3 | 3.89 | |
| Ir-C30@Pt-TiO2 | 10 | 0.2 | 420 − 740 | 69.9 | 699 | 117 | |
| Ir-C30@Pt-TiO2 | 10 | 0.02 | 420 − 740 | 32.0 | 320 | 53.3 | |
| Ir-C30@Pt-TiO2 | 40 | 0.2 | 450 ± 10 | 8.66 | 21.7 | 3.61 | |
| Ir-ppy@Pt-TiO2 | 40 | 0.2 | 420 − 740 | 15.6 | 39 | 6.5 | [25] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kobayashi, A.; Muramatsu, E.; Yoshida, M.; Kato, M. Two Excited State Collaboration of Heteroleptic Ir(III)-Coumarin Complexes for H2 Evolution Dye-Sensitized Photocatalysts. Energies 2021, 14, 2425. https://doi.org/10.3390/en14092425
Kobayashi A, Muramatsu E, Yoshida M, Kato M. Two Excited State Collaboration of Heteroleptic Ir(III)-Coumarin Complexes for H2 Evolution Dye-Sensitized Photocatalysts. Energies. 2021; 14(9):2425. https://doi.org/10.3390/en14092425
Chicago/Turabian StyleKobayashi, Atsushi, Eiichirou Muramatsu, Masaki Yoshida, and Masako Kato. 2021. "Two Excited State Collaboration of Heteroleptic Ir(III)-Coumarin Complexes for H2 Evolution Dye-Sensitized Photocatalysts" Energies 14, no. 9: 2425. https://doi.org/10.3390/en14092425
APA StyleKobayashi, A., Muramatsu, E., Yoshida, M., & Kato, M. (2021). Two Excited State Collaboration of Heteroleptic Ir(III)-Coumarin Complexes for H2 Evolution Dye-Sensitized Photocatalysts. Energies, 14(9), 2425. https://doi.org/10.3390/en14092425







