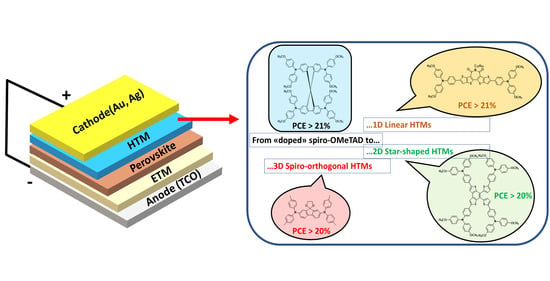
Dopant-Free All-Organic Small-Molecule HTMs for Perovskite Solar Cells: Concepts and Structure–Property Relationships
Abstract
1. Introduction
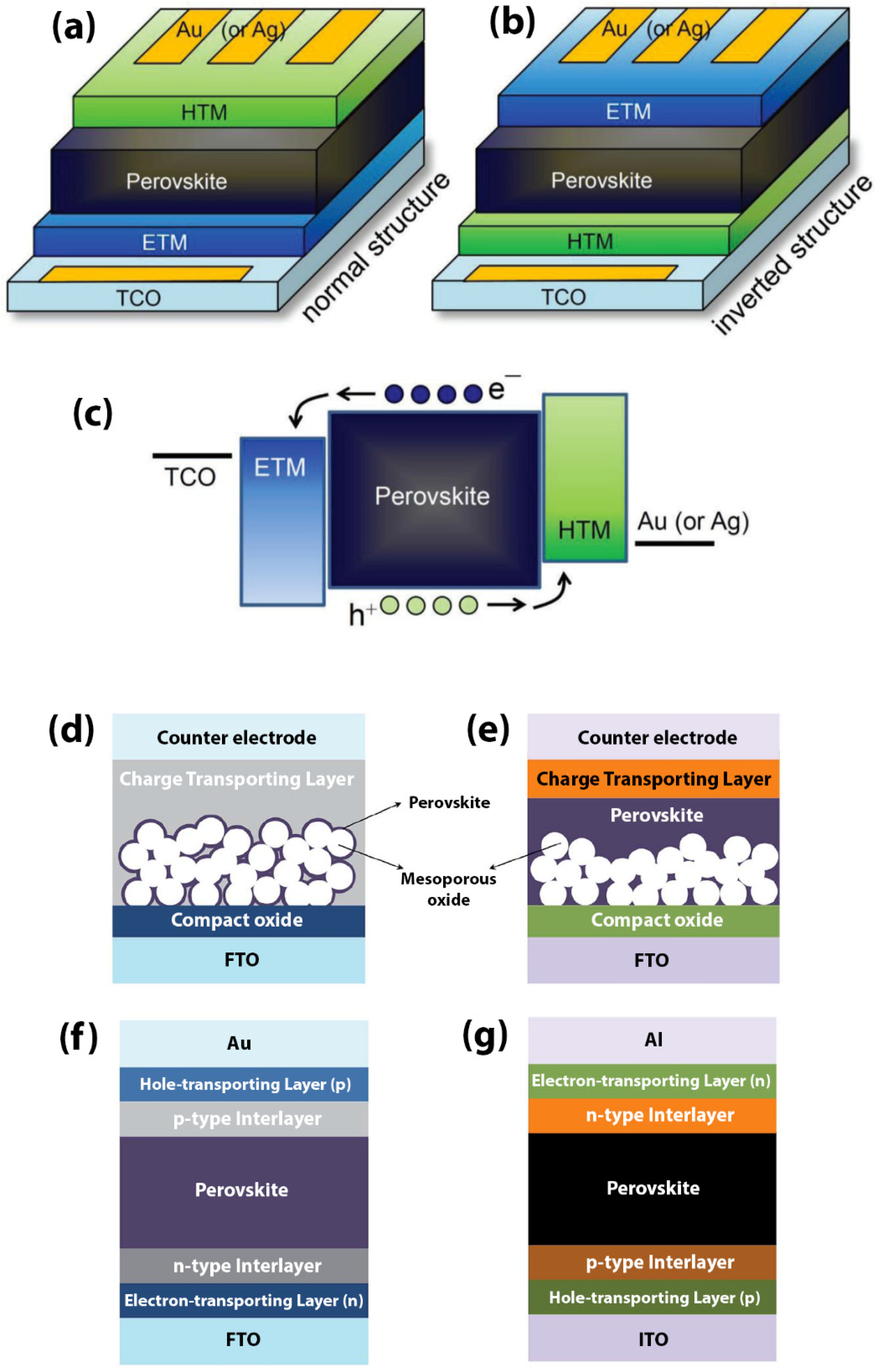
2. Perovskite Solar Cells
3. Figures of Merit of Dopant-Free HTMs
4. Organic Small-Molecule Dopant-Free HTMs: Structure–Activity Relationships
4.1. Linear 1D Structures
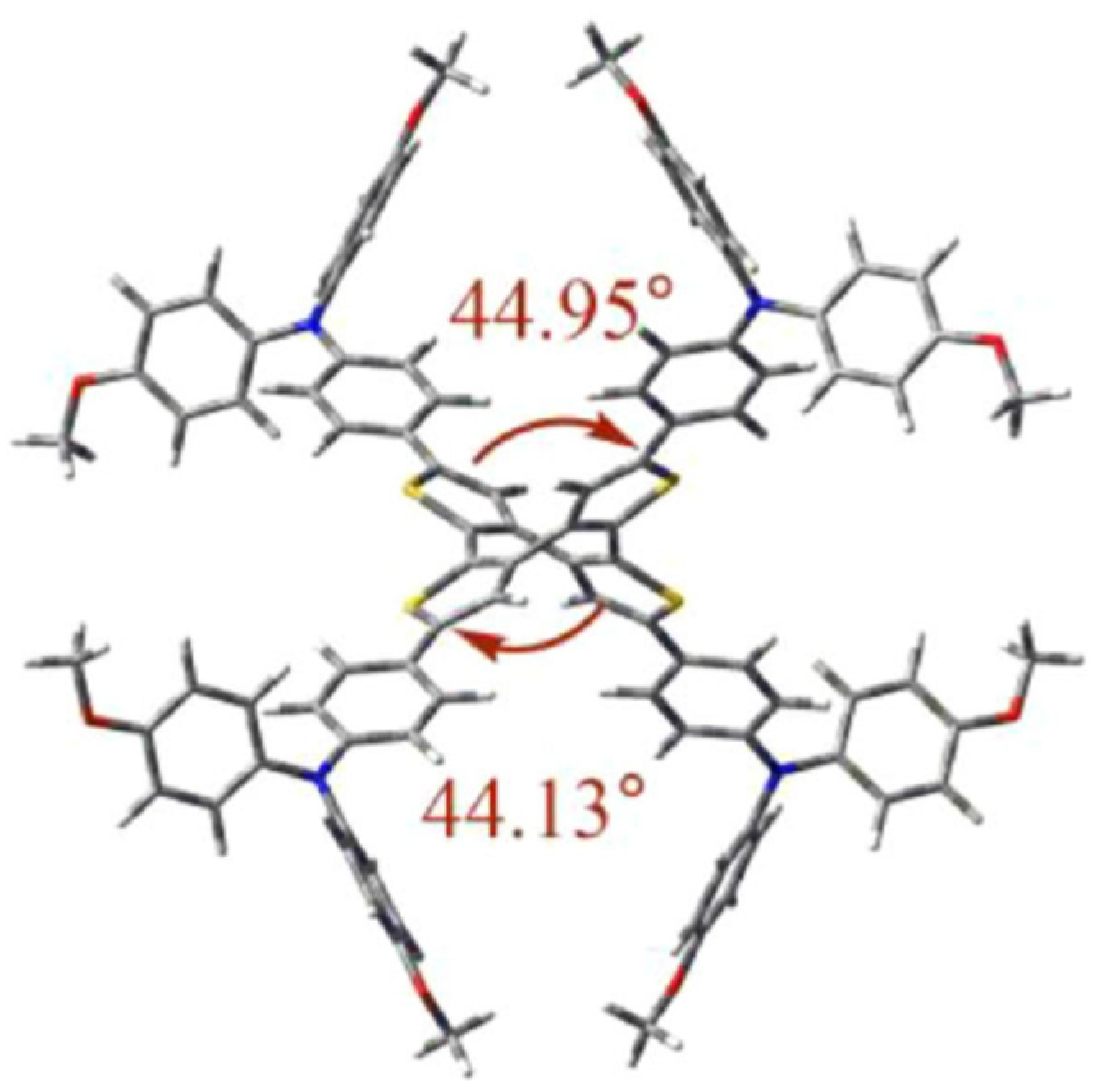
4.2. Two-Dimensional Star-Shaped Structures
4.3. Three-Dimensional Spiro–Orthogonal Structures
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Acronyms
| ACE | acenaphthene |
| A–D–A | Acceptor–Donor–Acceptor |
| ANT | anthanthrone |
| BCP | bathocuproine |
| BDT | benzodithiophene |
| BTZ | benzothiadiazole |
| CB | conduction band |
| CsPbI3 | cesium lead iodide perovskite |
| CsFAMA | Cesium Formamidinium/Methylammonium Perovskite |
| D–A | Donor–Acceptor |
| D–π–A | Donor–π-conjugated bridge-Acceptor |
| D–π–D | Donor–π-conjugated bridge-Donor |
| DPA | diphenylamine |
| DSSCs | Dye-Sensitized Solar Cells |
| EDOT | 3,4-ethylenedioxythiophene |
| ETL | Electron Transporting Layer |
| ETM | Electron Transporting Material |
| FAI | formamidinium lead iodide |
| FAPbI3 | formamidinium lead tri-iodide perovskite |
| FCTC | Flexible Core with Tunable Conformation |
| FF | fill factor |
| GIWAXS | Grazing-Incidence Wide-Angle X-Ray Scattering |
| GIXD | grazing incidence in-plane X-ray diffraction |
| HM | hole mobility |
| HOMO | highest occupied molecular orbital |
| HTL | Hole Transporting Layer |
| HTM | Hole Transporting Material |
| Imp | Current at Maximum Power |
| IDF | 6,6,12,12-tetrahexyl-6, 12-dihydroindeno[1,2-b] fluorene |
| IDT | indacenodithiophene |
| IDTT | indacenodithienothiophene |
| ITO | indium tin oxide |
| Jsc | short-circuit current density |
| LUMO | lowest unoccupied molecular orbital |
| MAPbBr3 | methylammonium Lead Bromide Perovskite |
| MAPbI3 | methylammonium Lead Iodide Perovskite |
| MAPbI3-xClx | methylammonium Lead Iodide/Chloride |
| m-TiO2 | Mesoporous titanium dioxide |
| NE | non-encapsulated devices |
| NMR | nuclear magnetic resonance |
| OTR | Oxygen Transmission Rate |
| Pin | incident Power at constant flux of photons |
| PC61BM | [6,6]-phenyl C61 butyric acid methyl ester |
| PCE | Power Conversion Efficiency |
| PCPM | phenyl-C61-butyric acid methyl ester |
| PEDOT | PSS: poly(3,4-ethylenedioxythiophene) polystyrene sulfonate |
| PEN | polyethylene naphthalate |
| PET | polyethylene terephthalate |
| PESA | Photoelectron Spectroscopy in Air |
| PSC | Perovskite Solar Cell |
| PSK | perovskite |
| PTAA | poly triarylamine |
| RH | relative humidity |
| SFX | spiro(fluorene-9,9′-Xanthene) |
| Spiro-OMeTAD | 2,2′,7,7′-Tetrakis[N,N-di(4-methoxyphenyl)amino]-9,9′-spirobifluorene |
| Tg | Glass Transition Temperature |
| TCO | Transparent Conductive Oxide |
| TPA | triphenylamine |
| TTE | tetrathienylethylene |
| UV–Vis | Ultraviolet–Visible |
| Vmp | potential at maximum power |
| VOC | open circuit voltage |
| VB | valence band |
| WVTR | Water Vapor Transmission Rate |
| XRD | X-Ray Diffraction |
References
- Chiang, C.K.; Fincher, C.R.; Park, Y.W.; Heeger, A.J.; Shirakawa, H.; Louis, E.J.; Gau, S.C.; MacDiarmid, A.G. Electrical Conductivity in Doped Polyacetylene. Phys. Rev. Lett. 1977, 39, 1098–1101. [Google Scholar] [CrossRef]
- Chandrasekhar, P. Conducting Polymers, Fundamentals and Applications, 2nd ed.; Springer: Cham, Switzerland, 2018; 850p. [Google Scholar]
- Foot, P.J.S.; Kaiser, A.B. Conducting polymers. In Kirk-Othmer Encyclopedia of Chemical Technology; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2004; pp. 513–550. [Google Scholar]
- Garnier, F. Functionalized conducting polymers—Towards intelligent materials. Angew. Chem. 1989, 101, 529–533. [Google Scholar] [CrossRef]
- Wessling, B. Conductive polymers as organic nanometals. In Handbook of Nanostructured Materials and Nanotechnology; Academic Press: San Diego, CA, USA, 2000; Volume 5, pp. 501–575. [Google Scholar]
- Chen, J.; Park, N.-G. Causes and Solutions of Recombination in Perovskite Solar Cells. Adv. Mater. 2019, 31, e1803019. [Google Scholar] [CrossRef] [PubMed]
- Swayamprabha, S.S.; Nagar, M.R.; Yadav, R.A.K.; Gull, S.; Dubey, D.K.; Jou, J.-H. Hole-transporting materials for organic light-emitting diodes: An overview. J. Mater. Chem. C 2019, 7, 7144–7158. [Google Scholar]
- Rakstys, K.; Paek, S.; Gao, P.; Gratia, P.; Marszalek, T.; Grancini, G.; Cho, K.T.; Genevicius, K.; Jankauskas, V.; Pisula, W.; et al. Molecular engineering of face-on oriented dopant-free hole transporting material for perovskite solar cells with 19% PCE. J. Mater. Chem. A 2017, 5, 7811–7815. [Google Scholar] [CrossRef]
- Nazeeruddin, M.K.; Snaith, H. Methylammonium lead triiodide perovskite solar cells: A new paradigm in photovoltaics. MRS Bull. 2015, 40, 641–645. [Google Scholar] [CrossRef]
- Yang, W.S.; Park, B.-W.; Jung, E.H.; Jeon, N.J.; Kim, Y.C.; Lee, D.U.; Shin, S.S.; Seo, J.; Kim, E.K.; Noh, J.H.; et al. Iodide management in formamidinium-lead-halide–based perovskite layers for efficient solar cells. Science 2017, 356, 1376–1379. [Google Scholar] [CrossRef]
- NREL. Available online: https://www.nrel.gov/pv/cell-efficiency.html (accessed on 29 April 2020).
- Li, M.-H.; Shen, P.-S.; Wang, K.-C.; Guo, T.-F.; Chen, P. Inorganic p-type contact materials for perovskite-based solar cells. J. Mater. Chem. A 2015, 3, 9011–9019. [Google Scholar] [CrossRef]
- Cetin, C.; Chen, P.; Hao, M.; He, D.; Bai, Y.; Lyu, M.; Yun, J.-H.; Wang, L. Inorganic p-Type Semiconductors as Hole Conductor Building Blocks for Robust Perovskite Solar Cells. Adv. Sustain. Syst. 2018, 2, 1800032. [Google Scholar] [CrossRef]
- Chen, J.; Park, N.-G. Inorganic Hole Transporting Materials for Stable and High Efficiency Perovskite Solar Cells. J. Phys. Chem. C 2018, 122, 14039–14063. [Google Scholar] [CrossRef]
- Urieta-Mora, J.; García-Benito, I.; Molina-Ontoria, A.; Martín, N. Hole transporting materials for perovskite solar cells: A chemical approach. Chem. Soc. Rev. 2018, 47, 8541–8571. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Wen, Z.; Gao, P. Less is More: Dopant-Free Hole Transporting Materials for High-Efficiency Perovskite Solar Cells. Adv. Energy Mater. 2018, 8, 1702512. [Google Scholar] [CrossRef]
- Ummadisingu, A.; Seo, J.-Y.; Stojanovic, M.; Zakeeruddin, S.M.; Grätzel, M.; Hagfeldt, A.; Vlachopoulos, N.; Saliba, M. Additives, Hole Transporting Materials and Spectroscopic Methods to Characterize the Properties of Perovskite Films. Chim. Int. J. Chem. 2017, 71, 754–761. [Google Scholar] [CrossRef]
- Calió, L.; Kazim, S.; Grätzel, M.; Ahmad, S. Hole-Transport Materials for Perovskite Solar Cells. Angew. Chem. Int. Ed. 2016, 55, 14522–14545. [Google Scholar] [CrossRef] [PubMed]
- MacDiarmid, A.G. “Synthetic Metals”: A Novel Role for Organic Polymers (Nobel Lecture). Angew. Chem. Int. Ed 2001, 40, 2581–2590. [Google Scholar] [CrossRef]
- Heeger, A.J. Semiconducting and metallic polymers: The fourth generation of polymeric materials. Synth. Met. 2001, 125, 23–42. [Google Scholar] [CrossRef]
- Jiang, Q.; Chu, Z.; Wang, P.; Pengyang, W.; Liu, H.; Wang, Y.; Yin, Z.; Wu, J.; Zhang, X.; You, J. Planar-Structure Perovskite Solar Cells with Efficiency beyond 21%. Adv. Mater. 2017, 29, 1703852. [Google Scholar] [CrossRef]
- Liu, D.; Yongsheng, L. Recent progress of dopant-free organic hole-transporting materials in perovskite solar cells. J. Semicond. 2017, 38, 011005. [Google Scholar] [CrossRef]
- Sun, X.; Zhao, D.; Li, Z. Recent advances in the design of dopant-free hole transporting materials for highly efficient perovskite solar cells. Chin. Chem. Lett. 2018, 29, 219–231. [Google Scholar] [CrossRef]
- Teh, C.H.; Daik, R.; Lim, E.L.; Yap, C.C.; Ibrahim, M.A.; Ludin, N.A.; Sopian, K.; Teridi, M.A.M. A review of organic small molecule-based hole-transporting materials for meso-structured organic–inorganic perovskite solar cells. J. Mater. Chem. A 2016, 4, 15788–15822. [Google Scholar] [CrossRef]
- Kim, H.-S.; Lee, C.-R.; Im, J.-H.; Lee, K.-B.; Moehl, T.; Marchioro, A.; Moon, S.-J.; Humphry-Baker, R.; Yum, J.-H.; Moser, J.; et al. Lead Iodide Perovskite Sensitized All-Solid-State Submicron Thin Film Mesoscopic Solar Cell with Efficiency Exceeding 9%. Sci. Rep. 2012, 2, 591. [Google Scholar] [CrossRef] [PubMed]
- Wali, Q.; Elumalai, N.K.; Iqbal, Y.; Uddin, A.; Jose, R. Tandem perovskite solar cells. Renew. Sustain. Energy Rev. 2018, 84, 89–110. [Google Scholar] [CrossRef]
- Grätzel, M. The light and shade of perovskite solar cells. Nat. Mater. 2014, 13, 838–842. [Google Scholar] [CrossRef] [PubMed]
- Djurišić, A.B.; Liu, F.Z.; Tam, H.W.; Wong, M.K.; Ng, A.; Surya, C.; Chen, W.; He, Z.B. Perovskite solar cells—An overview of critical issues. Prog. Quantum Electron. 2017, 53, 1–37. [Google Scholar] [CrossRef]
- Jung, H.S.; Han, G.S.; Park, N.-G.; Ko, M.J. Flexible Perovskite Solar Cells. Joule 2019, 3, 1850–1880. [Google Scholar] [CrossRef]
- De Rossi, F.; Renno, G.; Taheri, B.; Yaghoobi Nia, N.; Ilieva, V.; Fin, A.; Di Carlo, A.; Bonomo, M.; Barolo, C.; Brunetti, F. Modified P3HT materials as hole transport layers for flexible perovskite solar cells. J. Power Sources 2021, 494, 229735. [Google Scholar] [CrossRef]
- Boyd, C.C.; Cheacharoen, R.; Leijtens, T.; McGehee, M.D. Understanding Degradation Mechanisms and Improving Stability of Perovskite Photovoltaics. Chem. Rev. 2019, 119, 3418–3451. [Google Scholar] [CrossRef] [PubMed]
- De Quilettes, D.W.; Vorpahl, S.M.; Stranks, S.D.; Nagaoka, H.; Eperon, G.E.; Ziffer, M.E.; Snaith, H.J.; Ginger, D.S. Solar cells. Impact of microstructure on local carrier lifetime in perovskite solar cells. Science 2015, 348, 683–686. [Google Scholar] [CrossRef]
- Salim, T.; Sun, S.; Abe, Y.; Krishna, A.; Grimsdale, A.C.; Lam, Y.M. Perovskite-based solar cells: Impact of morphology and device architecture on device performance. J. Mater. Chem. A 2015, 3, 8943–8969. [Google Scholar] [CrossRef]
- Yin, X.; Zhou, J.; Song, Z.; Dong, Z.; Bao, Q.; Shrestha, N.; Bista, S.S.; Ellingson, R.J.; Yan, Y.; Tang, W. Dithieno[3,2-b:2′,3′-d]pyrrol-cored hole transport material enabling over 21% efficiency dopant-free perovskite solar cells. Adv. Funct. Mater. 2019, 29, 1904300. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, W.; Wang, L.; Tu, B.; Chen, T.; Liu, B.; Yang, K.; Koh, C.W.; Zhang, X.; Sun, H.; et al. Dopant-Free Small-Molecule Hole-Transporting Material for Inverted Perovskite Solar Cells with Efficiency Exceeding 21%. Adv. Mater. 2019, 31, e1902781. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Fu, W.; Li, C.-Z.; Zhang, Z.; Qiu, W.; Shi, M.; Heremans, P.; Jen, A.K.-Y.; Chen, H. Dopant-Free Hole-Transporting Material with a C3h Symmetrical Truxene Core for Highly Efficient Perovskite Solar Cells. J. Am. Chem. Soc. 2016, 138, 2528–2531. [Google Scholar] [CrossRef] [PubMed]
- Meroni, S.M.P.; Worsley, C.; Raptis, D.; Watson, T.M. Triple-Mesoscopic Carbon Perovskite Solar Cells: Materials, Processing and Applications. Energies 2021, 14, 386. [Google Scholar] [CrossRef]
- Berhe, T.A.; Su, W.-N.; Chen, C.-H.; Pan, C.-J.; Cheng, J.-H.; Chen, H.-M.; Tsai, M.-C.; Chen, L.-Y.; Dubale, A.A.; Hwang, B.-J. Organometal halide perovskite solar cells: Degradation and stability. Energy Environ. Sci. 2016, 9, 323–356. [Google Scholar] [CrossRef]
- Conings, B.; Drijkoningen, J.; Gauquelin, N.; Babayigit, A.; D’Haen, J.; D’Olieslaeger, L.; Ethirajan, A.; Verbeeck, J.; Manca, J.; Mosconi, E.; et al. Intrinsic Thermal Instability of Methylammonium Lead Trihalide Perovskite. Adv. Energy Mater. 2015, 5, 1500477. [Google Scholar] [CrossRef]
- Domanski, K.; Alharbi, E.A.; Hagfeldt, A.; Grätzel, M.; Tress, W. Systematic investigation of the impact of operation conditions on the degradation behaviour of perovskite solar cells. Nat. Energy 2018, 3, 61–67. [Google Scholar] [CrossRef]
- Holzhey, P.; Saliba, M. A full overview of international standards assessing the long-term stability of perovskite solar cells. J. Mater. Chem. A 2018, 6, 21794–21808. [Google Scholar] [CrossRef]
- Bonomo, M.; Taheri, B.; Bonandini, L.; Castro-Hermosa, S.; Brown, T.M.; Zanetti, M.; Menozzi, A.; Barolo, C.; Brunetti, F. Thermosetting Polyurethane Resins as Low-Cost, Easily Scalable, and Effective Oxygen and Moisture Barriers for Perovskite Solar Cells. ACS Appl. Mater. Interfaces 2020, 12, 54862–54875. [Google Scholar] [CrossRef]
- Niu, G.; Guo, X.; Wang, L. Review of recent progress in chemical stability of perovskite solar cells. J. Mater. Chem. A 2015, 3, 8970–8980. [Google Scholar] [CrossRef]
- Zhao, Z.; Gu, F.; Rao, H.; Ye, S.; Liu, Z.; Bian, Z.; Huang, C. Metal Halide Perovskite Materials for Solar Cells with Long-Term Stability. Adv. Energy Mater. 2019, 9, 1802671. [Google Scholar] [CrossRef]
- Castro-Hermosa, S.; Top, M.; Dagar, J.; Fahlteich, J.; Brown, T.M. Quantifying Performance of Permeation Barrier—Encapsulation Systems for Flexible and Glass-Based Electronics and Their Application to Perovskite Solar Cells. Adv. Electron. Mater. 2019, 5, 1800978. [Google Scholar] [CrossRef]
- Uddin, A.; Upama, M.B.; Yi, H.; Duan, L. Encapsulation of Organic and Perovskite Solar Cells: A Review. Coatings 2019, 9, 65. [Google Scholar] [CrossRef]
- Schloemer, T.H.; Christians, J.A.; Luther, J.M.; Sellinger, A. Doping strategies for small molecule organic hole-transport materials: Impacts on perovskite solar cell performance and stability. Chem. Sci. 2019, 10, 1904–1935. [Google Scholar] [CrossRef]
- Herz, L.M. Charge-Carrier Mobilities in Metal Halide Perovskites: Fundamental Mechanisms and Limits. ACS Energy Lett. 2017, 2, 1539–1548. [Google Scholar] [CrossRef]
- Liu, J.; Wu, Y.; Qin, C.; Yang, X.; Yasuda, T.; Islam, A.; Zhang, K.; Peng, W.; Chen, W.; Han, L. A dopant-free hole-transporting material for efficient and stable perovskite solar cells. Energy Environ. Sci. 2014, 7, 2963–2967. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, Q.; Duan, H.-S.; Zhou, H.; Yang, Y.; Chen, H.; Luo, S.; Song, T.-B.; Dou, L.; Hong, Z.; et al. A dopant-free organic hole transport material for efficient planar heterojunction perovskite solar cells. J. Mater. Chem. A 2015, 3, 11940–11947. [Google Scholar] [CrossRef]
- Kakiage, K.; Aoyama, Y.; Yano, T.; Oya, K.; Fujisawa, J.-I.; Hanaya, M. Highly-efficient dye-sensitized solar cells with collaborative sensitization by silyl-anchor and carboxy-anchor dyes. Chem. Commun. 2015, 51, 15894–15897. [Google Scholar] [CrossRef] [PubMed]
- Cheng, M.; Aitola, K.; Chen, C.; Zhang, F.; Liu, P.; Sveinbjörnsson, K.; Hua, Y.; Kloo, L.; Boschloo, G.; Sun, L. Acceptor–Donor–Acceptor type ionic molecule materials for efficient perovskite solar cells and organic solar cells. Nano Energy 2016, 30, 387–397. [Google Scholar] [CrossRef]
- Chen, C.; Cheng, M.; Liu, P.; Gao, J.; Kloo, L.; Sun, L. Application of benzodithiophene based A–D–A structured materials in efficient perovskite solar cells and organic solar cells. Nano Energy 2016, 23, 40–49. [Google Scholar] [CrossRef]
- Liu, Y.; Hong, Z.; Chen, Q.; Chen, H.; Chang, W.-H.; Yang, Y.; Song, T.-B. Perovskite Solar Cells Employing Dopant-Free Organic Hole Transport Materials with Tunable Energy Levels. Adv. Mater. 2016, 28, 440–446. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Xu, X.; Cai, N.; Qian, S.; Luo, R.; Huo, Y.; Tsang, S.-W. Rational Design of Dopant-Free Coplanar D-π-D Hole-Transporting Materials for High-Performance Perovskite Solar Cells with Fill Factor Exceeding 80%. Adv. Energy Mater. 2019, 9, 1901268. [Google Scholar] [CrossRef]
- Salunke, J.; Guo, X.; Lin, Z.; Vale, J.R.; Candeias, N.R.; Nyman, M.; Dahlström, S.; Österbacka, R.; Priimagi, A.; Chang, J.; et al. Phenothiazine-Based Hole-Transporting Materials toward Eco-friendly Perovskite Solar Cells. ACS Appl. Energy Mater. 2019, 2, 3021–3027. [Google Scholar] [CrossRef]
- Park, S.J.; Jeon, S.; Lee, I.K.; Zhang, J.; Jeong, H.; Park, J.-Y.; Bang, J.; Ahn, T.K.; Shin, H.-W.; Kim, B.-G.; et al. Inverted planar perovskite solar cells with dopant free hole transporting material: Lewis base-assisted passivation and reduced charge recombination. J. Mater. Chem. A 2017, 5, 13220–13227. [Google Scholar] [CrossRef]
- Ameen, S.; Nazim, M.; Akhtar, M.S.; Nazeeruddin, M.K.; Shin, H.-S. A novel perovskite solar cell design using aligned TiO2 nano-bundles grown on a sputtered Ti layer and a benzothiadiazole-based, dopant-free hole-transporting material. Nanoscale 2017, 9, 17544–17550. [Google Scholar] [CrossRef] [PubMed]
- Kwon, H.; Reddy, S.S.; Arivunithi, V.M.; Jin, H.; Park, H.-Y.; Cho, W.; Song, M.; Jin, S.-H. A linear D–π–A based hole transport material for high performance rigid and flexible planar organic–inorganic hybrid perovskite solar cells. J. Mater. Chem. C 2019, 7, 13440–13446. [Google Scholar] [CrossRef]
- Heo, J.H.; Park, S.; Im, S.H.; Son, H.J. Development of Dopant-Free Donor–Acceptor-type Hole Transporting Material for Highly Efficient and Stable Perovskite Solar Cells. ACS Appl. Mater. Interfaces 2017, 9, 39511–39518. [Google Scholar] [CrossRef] [PubMed]
- Lin, Q.; Armin, A.; Nagiri, R.C.R.; Burn, P.L.; Meredith, P. Electro-optics of perovskite solar cells. Nat. Photon. 2015, 9, 106–112. [Google Scholar] [CrossRef]
- Hwang, H.; Park, S.; Heo, J.H.; Kim, W.; Ahn, H.; Kim, T.-S.; Im, S.H.; Son, H.J. Enhancing performance and stability of perovskite solar cells using hole transport layer of small molecule and conjugated polymer blend. J. Power Sources 2019, 418, 167–175. [Google Scholar] [CrossRef]
- Zhang, J.; Xu, L.J.; Huang, P.; Zhou, Y.; Zhu, Y.Y.; Yuan, N.Y.; Ding, J.N.; Zhang, Z.G.; Li, Y.F. A simple and dopant-free hole-transporting material based on (2-ethylhexyl)-9H-carbazole for efficient planar perovskite solar cells. J. Mater. Chem. C 2017, 5, 12752–12757. [Google Scholar] [CrossRef]
- Qiu, J.; Liu, H.; Li, X.; Wang, S.; Zhang, F. Impact of 9-(4-methoxyphenyl) Carbazole and Benzodithiophene Cores on Performance and Stability for Perovskite Solar Cells Based on Dopant-Free Hole-Transporting Materials. Sol. RRL 2019, 3, 1900202. [Google Scholar] [CrossRef]
- Wang, S.-Y.; Chen, C.-P.; Chung, C.-L.; Hsu, C.-W.; Hsu, H.-L.; Wu, T.-H.; Zhuang, J.-Y.; Chang, C.-J.; Chen, H.M.; Chang, Y.J. Defect Passivation by Amide-Based Hole-Transporting Interfacial Layer Enhanced Perovskite Grain Growth for Efficient p–i–n Perovskite Solar Cells. ACS Appl. Mater. Interfaces 2019, 11, 40050–40061. [Google Scholar] [CrossRef] [PubMed]
- Cai, B.; Yang, X.; Jiang, X.; Yu, Z.; Hagfeldt, A.; Sun, L. Boosting the power conversion efficiency of perovskite solar cells to 17.7% with an indolo[3,2-b]carbazole dopant-free hole transporting material by improving its spatial configuration. J. Mater. Chem. A 2019, 7, 14835–14841. [Google Scholar] [CrossRef]
- Shang, R.; Zhou, Z.; Nishioka, H.; Halim, H.; Furukawa, S.; Takei, I.; Ninomiya, N.; Nakamura, E. Disodium Benzodipyrrole Sulfonate as Neutral Hole-Transporting Materials for Perovskite Solar Cells. J. Am. Chem. Soc. 2018, 140, 5018–5022. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Wu, F.; Ling, W.; Tu, Z.; Zhang, J.; Wei, Z.; Zhu, L.; Li, Q.; Li, Z. Facile-Effective Hole-Transporting Materials Based on Dibenzo[a,c]carbazole: The Key Role of Linkage Position to Photovoltaic Performance of Perovskite Solar Cells. ACS Energy Lett. 2019, 4, 2514–2521. [Google Scholar] [CrossRef]
- Azmi, R.; Nam, S.Y.; Sinaga, S.; Akbar, Z.A.; Lee, C.-L.; Yoon, S.C.; Jung, I.H.; Jang, S.-Y. High-performance dopant-free conjugated small molecule-based hole-transport materials for perovskite solar cells. Nano Energy 2018, 44, 191–198. [Google Scholar] [CrossRef]
- Bharath, D.; Sasikumar, M.; Chereddy, N.R.; Vaidya, J.R.; Pola, S. Synthesis of new 2-((5-(4-alkyl-4H-dithieno[3,2-b:2′,3′-d]pyrrol-2-yl)thiophen-2-yl)methylene)malononitrile: Dopant free hole transporting materials for perovskite solar cells with high power conversion efficiency. Sol. Energy 2018, 174, 130–138. [Google Scholar] [CrossRef]
- Zhou, J.; Yin, X.; Dong, Z.; Ali, A.; Song, Z.; Shrestha, N.; Bista, S.S.; Bao, Q.; Ellingson, R.J.; Yan, Y.; et al. Dithieno[3,2-b:2′,3′-d]pyrrole Cored p-Type Semiconductors Enabling 20% Efficiency Dopant-Free Perovskite Solar Cells. Angew. Chem. Int. Ed. 2019, 58, 13717–13721. [Google Scholar] [CrossRef]
- Tu, B.; Wang, Y.; Chen, W.; Liu, B.; Feng, X.; Zhu, Y.; Yang, K.; Zhang, Z.; Shi, Y.; Guo, X.; et al. Side-Chain Engineering of Donor–Acceptor Conjugated Small Molecules as Dopant-Free Hole-Transport Materials for Efficient Normal Planar Perovskite Solar Cells. ACS Appl. Mater. Interfaces 2019, 11, 48556–48563. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Scheel, K.R.; Clevenger, R.G.; Shou, W.; Pan, H.; Kilway, K.V.; Peng, Z. Highly Efficient and Stable Perovskite Solar Cells Using a Dopant-Free Inexpensive Small Molecule as the Hole-Transporting Material. Adv. Energy Mater. 2018, 8, 1801248. [Google Scholar] [CrossRef]
- Pham, H.D.; Do, T.T.; Kim, J.; Charbonneau, C.; Manzhos, S.; Feron, K.; Tsoi, W.C.; Durrant, J.R.; Jain, S.M.; Sonar, P. Molecular Engineering Using an Anthanthrone Dye for Low-Cost Hole Transport Materials: A Strategy for Dopant-Free, High-Efficiency, and Stable Perovskite Solar Cells. Adv. Energy Mater. 2018, 8, 1703007. [Google Scholar] [CrossRef]
- Sun, X.; Xue, Q.; Zhu, Z.; Xiao, Q.; Jiang, K.; Yip, H.-L.; Yan, H.; Li, Z. Fluoranthene-based dopant-free hole transporting materials for efficient perovskite solar cells. Chem. Sci. 2018, 9, 2698–2704. [Google Scholar] [CrossRef]
- Sun, X.; Wu, F.; Zhong, C.; Zhu, L.; Li, Z. A structure-property study of fluoranthene-cored hole-transporting materials enables 19.3% efficiency in dopant-free perovskite solar cells. Chem. Sci. 2019, 10, 6899–6907. [Google Scholar] [CrossRef]
- Zhang, D.; Xu, P.; Wu, T.; Ou, Y.; Yang, X.; Sun, A.; Cui, B.; Sun, H.; Hua, Y. Cyclopenta[hi]aceanthrylene-based dopant-free hole-transport material for organic–inorganic hybrid and all-inorganic perovskite solar cells. J. Mater. Chem. A 2019, 7, 5221–5226. [Google Scholar] [CrossRef]
- Qiu, J.; Liu, H.; Li, X.; Wang, S. Position effect of arylamine branches on pyrene-based dopant-free hole transport materials for efficient and stable perovskite solar cells. Chem. Eng. J. 2020, 387, 123965. [Google Scholar] [CrossRef]
- Pham, H.D.; Jain, S.M.; Li, M.; Manzhos, S.; Feron, K.; Pitchaimuthu, S.; Liu, Z.; Motta, N.; Wang, H.; Durrant, J.R.; et al. Dopant-free novel hole-transporting materials based on quinacridone dye for high-performance and humidity-stable mesoporous perovskite solar cells. J. Mater. Chem. A 2019, 7, 5315–5323. [Google Scholar] [CrossRef]
- Lin, Y.-S.; Abate, S.Y.; Lai, K.-W.; Chu, C.-W.; Lin, Y.-D.; Tao, Y.-T.; Sun, S.-S. New Helicene-Type Hole-Transporting Molecules for High-Performance and Durable Perovskite Solar Cells. ACS Appl. Mater. Interfaces 2018, 10, 41439–41449. [Google Scholar] [CrossRef] [PubMed]
- Qin, P.; Paek, S.; Dar, M.I.; Pellet, N.; Ko, J.; Grätzel, M.; Nazeeruddin, M.K. Perovskite Solar Cells with 12.8% Efficiency by Using Conjugated Quinolizino Acridine Based Hole Transporting Material. J. Am. Chem. Soc. 2014, 136, 8516–8519. [Google Scholar] [CrossRef]
- Zhao, X.; Zhang, F.; Yi, C.; Bi, D.; Bi, X.; Wei, P.; Luo, J.; Liu, X.; Wang, S.; Li, X.; et al. A novel one-step synthesized and dopant-free hole transport material for efficient and stable perovskite solar cells. J. Mater. Chem. A 2016, 4, 16330–16334. [Google Scholar] [CrossRef]
- Zhang, F.; Liu, X.; Yi, C.; Bi, D.; Luo, J.; Wang, S.; Li, X.; Xiao, Y.; Zakeeruddin, S.M.; Grätzel, M. Dopant-Free Donor (D)–π–D–π–D Conjugated Hole-Transport Materials for Efficient and Stable Perovskite Solar Cells. ChemSusChem 2016, 9, 2578–2585. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Yi, C.; Wei, P.; Bi, X.; Luo, J.; Jacopin, G.; Wang, S.; Li, X.; Xiao, Y.; Zakeeruddin, S.M.; et al. A Novel Dopant-Free Triphenylamine Based Molecular “Butterfly” Hole-Transport Material for Highly Efficient and Stable Perovskite Solar Cells. Adv. Energy Mater. 2016, 6, 1600401. [Google Scholar] [CrossRef]
- Zhang, F.; Zhao, X.; Yi, C.; Bi, D.; Bi, X.; Wei, P.; Liu, X.; Wang, S.; Li, X.; Zakeeruddin, S.M.; et al. Dopant-free star-shaped hole-transport materials for efficient and stable perovskite solar cells. Dye. Pigment. 2017, 136, 273–277. [Google Scholar] [CrossRef]
- Qi, P.; Zhang, F.; Zhao, X.; Liu, X.; Bi, X.; Wei, P.; Xiao, Y.; Li, X.; Wang, S. Efficient, Stable, Dopant-Free Hole-Transport Material with a Triphenylamine Core for CH3 NH3 PbI3 Perovskite Solar Cells. Energy Technol. 2017, 5, 1173–1178. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, F.; Liu, Z.; Xiao, Y.; Wang, S.; Li, X. Dopant-free and low-cost molecular “bee” hole-transporting materials for efficient and stable perovskite solar cells. J. Mater. Chem. C 2017, 5, 11429–11435. [Google Scholar] [CrossRef]
- Paek, S.; Qin, P.; Lee, Y.; Cho, K.T.; Gao, P.; Grancini, G.; Oveisi, E.; Gratia, P.; Rakstys, K.; Al-Muhtaseb, S.A.; et al. Dopant-Free Hole-Transporting Materials for Stable and Efficient Perovskite Solar Cells. Adv. Mater. 2017, 29, 1606555. [Google Scholar] [CrossRef]
- Goodby, J.W.; Collings, P.J.; Kato, T.; Tschierske, C.; Gleeson, H.; Raynes, P. Handbook of Liquid Crystals; Wiley-VCH: Weinheim, Germany, 1998. [Google Scholar]
- Cao, J.; Liu, Y.-M.; Jing, X.; Yin, J.; Li, J.; Xu, B.; Tan, Y.-Z.; Zheng, N. Well-Defined Thiolated Nanographene as Hole-Transporting Material for Efficient and Stable Perovskite Solar Cells. J. Am. Chem. Soc. 2015, 137, 10914–10917. [Google Scholar] [CrossRef]
- Sun, C.; Wu, Z.; Yip, H.-L.; Zhang, H.; Jiang, X.-F.; Xue, Q.; Hu, Z.; Hu, Z.; Shen, Y.; Wang, M.; et al. Amino-Functionalized Conjugated Polymer as an Efficient Electron Transport Layer for High-Performance Planar-Heterojunction Perovskite Solar Cells. Adv. Energy Mater. 2016, 6, 1501534. [Google Scholar] [CrossRef]
- Kim, Y.H.; Cho, H.; Heo, J.H.; Kim, T.S.; Myoung, N.; Lee, C.L.; Im, S.H.; Lee, T.W. Multicolored organic/inorganic hybrid perovskite light-emitting diodes. Adv. Mater. 2015, 27, 1248–1254. [Google Scholar] [CrossRef]
- Calió, L.; Momblona, C.; Gil-Escrig, L.; Kazim, S.; Sessolo, M.; Sastre-Santos, Á.; Bolink, H.J.; Ahmad, S. Vacuum deposited perovskite solar cells employing dopant-free triazatruxene as the hole transport material. Sol. Energy Mater. Sol. Cells 2017, 163, 237–241. [Google Scholar] [CrossRef]
- Wang, B.; Zeng, Q.; Sun, Z.; Xue, S.; Liang, M. Molecularly engineering of truxene-based dopant-free hole-transporting materials for efficient inverted planar perovskite solar cells. Dye. Pigment. 2019, 165, 81–89. [Google Scholar] [CrossRef]
- Molina-Ontoria, A.; Zimmermann, I.; Garcia-Benito, I.; Gratia, P.; Roldán-Carmona, C.; Aghazada, S.; Graetzel, M.; Nazeeruddin, M.K.; Martín, N. Benzotrithiophene-Based Hole-Transporting Materials for 18.2 % Perovskite Solar Cells. Angew. Chem. Int. Ed. 2016, 55, 6270–6274. [Google Scholar] [CrossRef]
- Peng, Y.-K.; Lee, K.-M.; Ting, C.-C.; Hsu, M.-W.; Liu, C.-Y. Making benzotrithiophene derivatives dopant-free for perovskite solar cells: Step-saving installation of π-spacers by a direct C–H arylation strategy. J. Mater. Chem. A 2019, 7, 24765–24770. [Google Scholar] [CrossRef]
- Chen, H.; Fu, W.; Huang, C.; Zhang, Z.; Li, S.; Ding, F.; Shi, M.; Li, C.-Z.; Jen, A.K.-Y.; Chen, H. Molecular Engineered Hole-Extraction Materials to Enable Dopant-Free, Efficient p-i-n Perovskite Solar Cells. Adv. Energy Mater. 2017, 7, 1700012. [Google Scholar] [CrossRef]
- Xue, Y.; Wu, Y.; Li, Y. Readily synthesized dopant-free hole transport materials with phenol core for stabilized mixed perovskite solar cells. J. Power Sources 2017, 344, 160–169. [Google Scholar] [CrossRef]
- Zhang, Y.; Heng, P.; Su, H.; Li, J.; Guo, J.; Ning, P.; Wu, W.; Ren, T.; Wang, L.; Zhang, J. Star-Shaped Molecules as Dopant-Free Hole Transporting Materials for Efficient Perovskite Solar Cells: Multiscale Simulation. Chem. Rec. 2018, 938–946. [Google Scholar] [CrossRef]
- Liu, N.; Zong, X.; Wang, Z.; Cui, T.; Liang, M.; Zhang, Y.; Xue, S. LiTFSI/TBP-free hole transport materials with nonlinear π-conjugation for efficient inverted perovskite solar cells. Electrochim. Acta 2019, 296, 283–293. [Google Scholar] [CrossRef]
- Duan, L.; Chen, Y.; Yuan, J.; Zong, X.; Sun, Z.; Wu, Q.; Xue, S. Dopant-free X-shaped D-A type hole-transporting materials for p-i-n perovskite solar cells. Dye. Pigment. 2020, 178, 108334. [Google Scholar] [CrossRef]
- Duan, L.; Chen, Y.; Jia, J.; Zong, X.; Sun, Z.; Wu, Q.; Xue, S. Dopant-Free Hole-Transport Materials Based on 2,4,6-Triarylpyridine for Inverted Planar Perovskite Solar Cells. ACS Appl. Energy Mater. 2020, 3, 1672–1683. [Google Scholar] [CrossRef]
- Bi, C.; Wang, Q.; Shao, Y.; Yuan, Y.; Xiao, Z.; Huang, J. Non-wetting surface-driven high-aspect-ratio crystalline grain growth for efficient hybrid perovskite solar cells. Nat. Commun. 2015, 6, 7747. [Google Scholar] [CrossRef] [PubMed]
- Cui, B.-B.; Han, Y.; Yang, N.; Yang, S.; Zhang, L.; Wang, Y.; Jia, Y.; Zhao, L.; Zhong, Y.-W.; Chen, Q. Propeller-Shaped, Triarylamine-Rich, and Dopant-Free Hole-Transporting Materials for Efficient n-i-p Perovskite Solar Cells. ACS Appl. Mater. Interfaces 2018, 10, 41592–41598. [Google Scholar] [CrossRef] [PubMed]
- Meng, F.; Jia, Y.; Wang, J.; Huang, X.; Gui, Z.; Huang, L.; Li, R.; Chen, R.; Xu, J.; Chen, W.; et al. Dopant-Free Hole Transporting Molecules for Highly Efficient Perovskite Photovoltaic with Strong Interfacial Interaction. Sol. RRL 2019, 3, 1900319. [Google Scholar] [CrossRef]
- Chen, Y.-C.; Li, Y.-H.; Chung, C.-L.; Hsu, H.-L.; Chen, C.-P. Triphenylamine dibenzofulvene-derived dopant-free hole transporting layer induces micrometer-sized perovskite grains for highly efficient near 20% for p-i-n perovskite solar cells. Prog. Photovolt. 2020, 28, 49–59. [Google Scholar] [CrossRef]
- Lai, X.; Du, M.; Meng, F.; Li, G.; Li, W.; Kyaw, A.K.K.; Wen, Y.; Liu, C.; Ma, H.; Zhang, R.; et al. High-Performance Inverted Planar Perovskite Solar Cells Enhanced by Thickness Tuning of New Dopant-Free Hole Transporting Layer. Small 2019, 15, e1904715. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.; Wu, Y.; Zhang, H.; Li, E.; Zhang, W.; Xu, X.; Wu, W.; Tian, H.; Zhu, W.-H. Semi-Locked Tetrathienylethene as a Building Block for Hole-Transporting Materials: Toward Efficient and Stable Perovskite Solar Cells. Angew. Chem. Int. Ed. 2019, 58, 3784–3789. [Google Scholar] [CrossRef] [PubMed]
- Dong, Z.; Yin, X.; Ali, A.; Zhou, J.; Bista, S.S.; Chen, C.; Yan, Y.; Tang, W. A dithieno[3,2-b:2′,3′-d]pyrrole-cored four-arm hole transporting material for over 19% efficiency dopant-free perovskite solar cells. J. Mater. Chem. C 2019, 7, 9455–9459. [Google Scholar] [CrossRef]
- Li, Z.; Zhu, Z.; Chueh, C.-C.; Jo, S.B.; Luo, J.; Jang, S.-H.; Jen, A.K.-Y. Rational Design of Dipolar Chromophore as an Efficient Dopant-Free Hole-Transporting Material for Perovskite Solar Cells. J. Am. Chem. Soc. 2016, 138, 11833–11839. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zheng, X.; Wang, Y.; Chen, Z.; Yao, F.; Zhang, Q.; Fang, G.; Chen, Z.-K.; Huang, W.; Xu, Z.-X. Dopant-Free Hole-Transport Materials Based on Methoxytriphenylamine-Substituted Indacenodithienothiophene for Solution-Processed Perovskite Solar Cells. ChemSusChem 2017, 10, 2833–2838. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Xu, B.; Yang, L.; Mingorance, A.; Ruan, C.; Hua, Y.; Wang, L.; Vlachopoulos, N.; Lira-Cantú, M.; Boschloo, G.; et al. Incorporation of Counter Ions in Organic Molecules: New Strategy in Developing Dopant-Free Hole Transport Materials for Efficient Mixed-Ion Perovskite Solar Cells. Adv. Energy Mater. 2017, 7, 1602736. [Google Scholar] [CrossRef]
- Zhang, J.; Sun, Q.; Chen, Q.; Wang, Y.; Zhou, Y.; Song, B.; Yuan, N.; Ding, J.; Li, Y. High efficiency planar p-i-n perovskite solar cells using low-cost fluorene-based hole transporting material. Adv. Funct. Mater. 2019, 29, 1900484. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, J.; Liu, P.; Xu, B.; Zhang, B.; Chen, H.; Inge, A.K.; Li, Y.; Wang, H.; Cheng, Y.-B.; et al. Design and synthesis of dopant-free organic hole-transport materials for perovskite solar cells. Chem. Commun. 2018, 54, 9571–9574. [Google Scholar] [CrossRef]
- Guo, K.; Wu, M.; Yang, S.; Wang, Z.; Li, J.; Liang, X.; Zhang, F.; Liu, Z.; Wang, Z. Introduction of fluorine into spiro[fluorene-9,9′-xanthene]-based hole transport material to obtain sensitive-dopant-free, high efficient and stable perovskite solar cells. Sol. RRL 2019, 3, 1800352. [Google Scholar] [CrossRef]
- Liu, F.; Wu, F.; Tu, Z.; Liao, Q.; Gong, Y.; Zhu, L.; Li, Q.; Li, Z. Hole Transport Materials Based on 6,12-Dihydroindeno[1,2-b]fluorine with Different Periphery Groups: A New Strategy for Dopant-Free Perovskite Solar Cells. Adv. Funct. Mater. 2019, 29, 1901296. [Google Scholar] [CrossRef]
- Cao, Y.; Li, Y.; Morrissey, T.; Lam, B.; Patrick, B.O.; Dvorak, D.J.; Xia, Z.; Kelly, T.L.; Berlinguette, C.P. Dopant-free molecular hole transport material that mediates a 20% power conversion efficiency in a perovskite solar cell. Energy Environ. Sci. 2019, 12, 3502–3507. [Google Scholar] [CrossRef]
- Yin, C.; Lu, J.; Xu, Y.; Yun, Y.; Wang, K.; Li, J.; Jiang, L.; Sun, J.; Scully, A.D.; Huang, F.; et al. Low-Cost N,N′-Bicarbazole-Based Dopant-Free Hole-Transporting Materials for Large-Area Perovskite Solar Cells. Adv. Energy Mater. 2018, 8, 1800538. [Google Scholar] [CrossRef]
- Petrus, M.L.; Bein, T.; Dingemans, T.J.; Docampo, P. A low cost azomethine-based hole transporting material for perovskite photovoltaics. J. Mater. Chem. A 2015, 3, 12159–12162. [Google Scholar] [CrossRef]
- Pham, H.D.; Gil-Escrig, L.; Feron, K.; Manzhos, S.; Albrecht, S.; Bolink, H.J.; Sonar, P. Boosting inverted perovskite solar cell performance by using 9,9-bis(4-diphenylaminophenyl)fluorene functionalized with triphenylamine as a dopant-free hole transporting material. J. Mater. Chem. A 2019, 7, 12507–12517. [Google Scholar] [CrossRef]
- Yang, X.; Xi, J.; Sun, Y.; Zhang, Y.; Zhou, G.; Wong, W.-Y. A dopant-free twisted organic small-molecule hole transport material for inverted planar perovskite solar cells with enhanced efficiency and operational stability. Nano Energy 2019, 64, 103946. [Google Scholar] [CrossRef]
- Lai, X.; Meng, F.; Zhang, Q.-Q.; Wang, K.; Li, G.; Wen, Y.; Ma, H.; Li, W.; Li, X.; Kyaw, A.K.K.; et al. A Bifunctional Saddle-Shaped Small Molecule as a Dopant-Free Hole Transporting Material and Interfacial Layer for Efficient and Stable Perovskite Solar Cells. Sol. RRL 2019, 3, 1900011. [Google Scholar] [CrossRef]
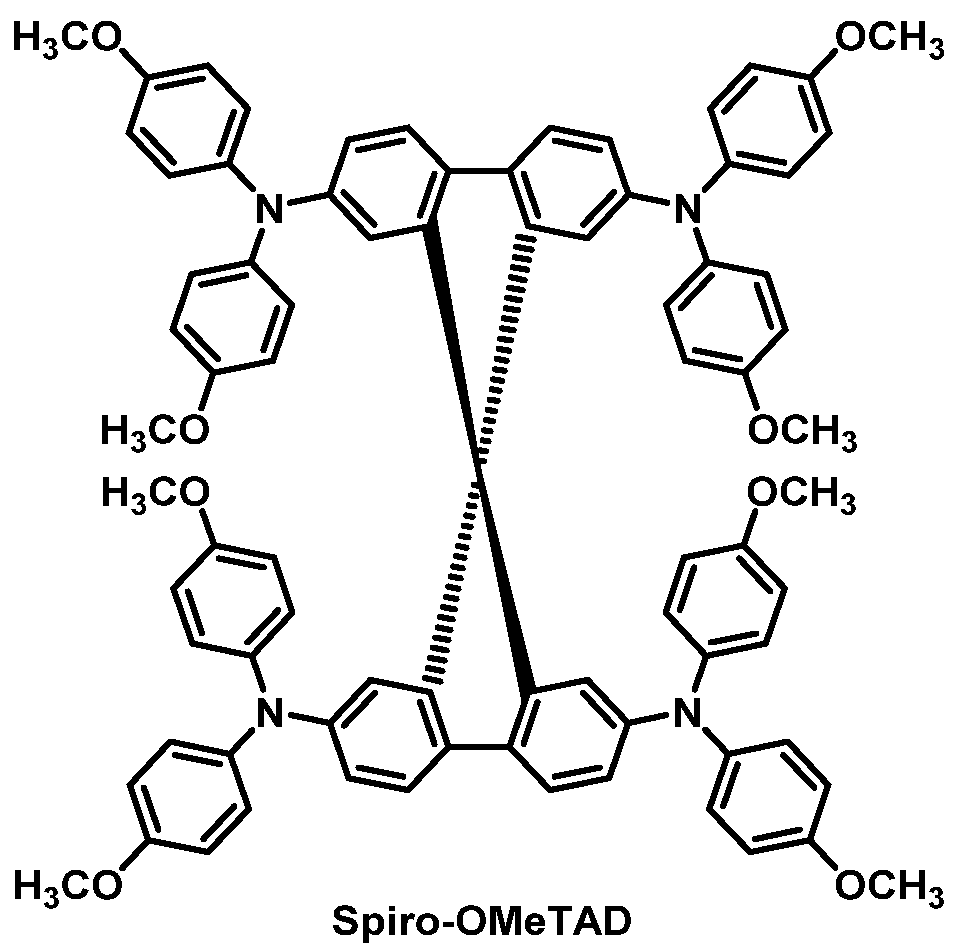
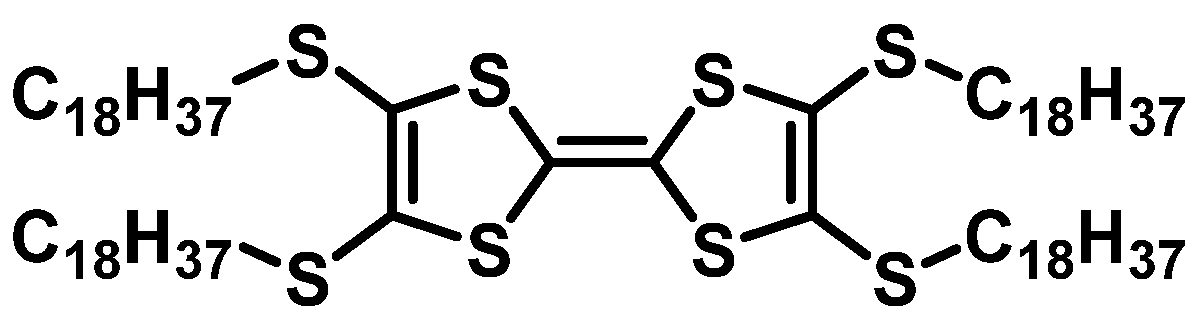
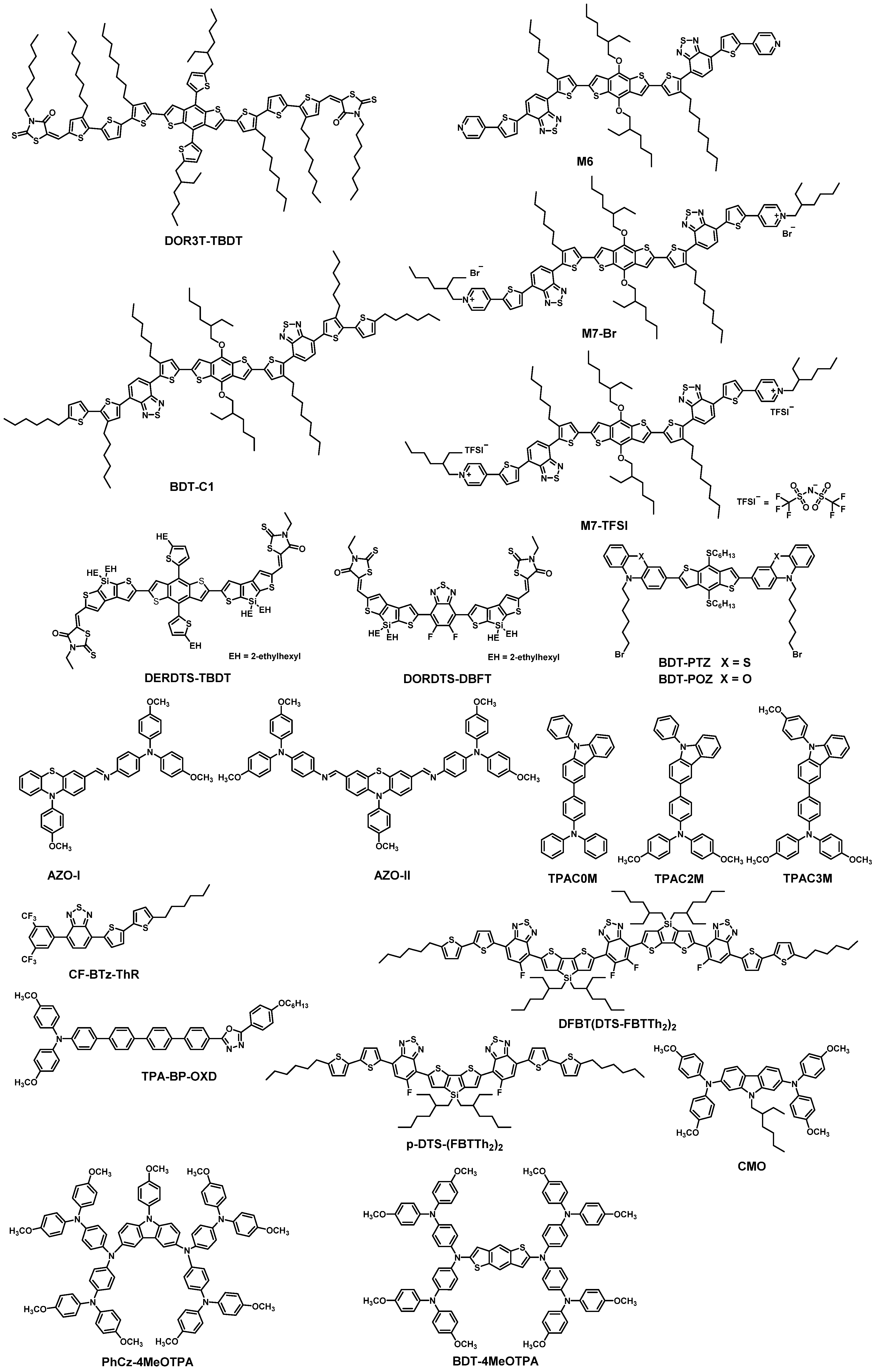
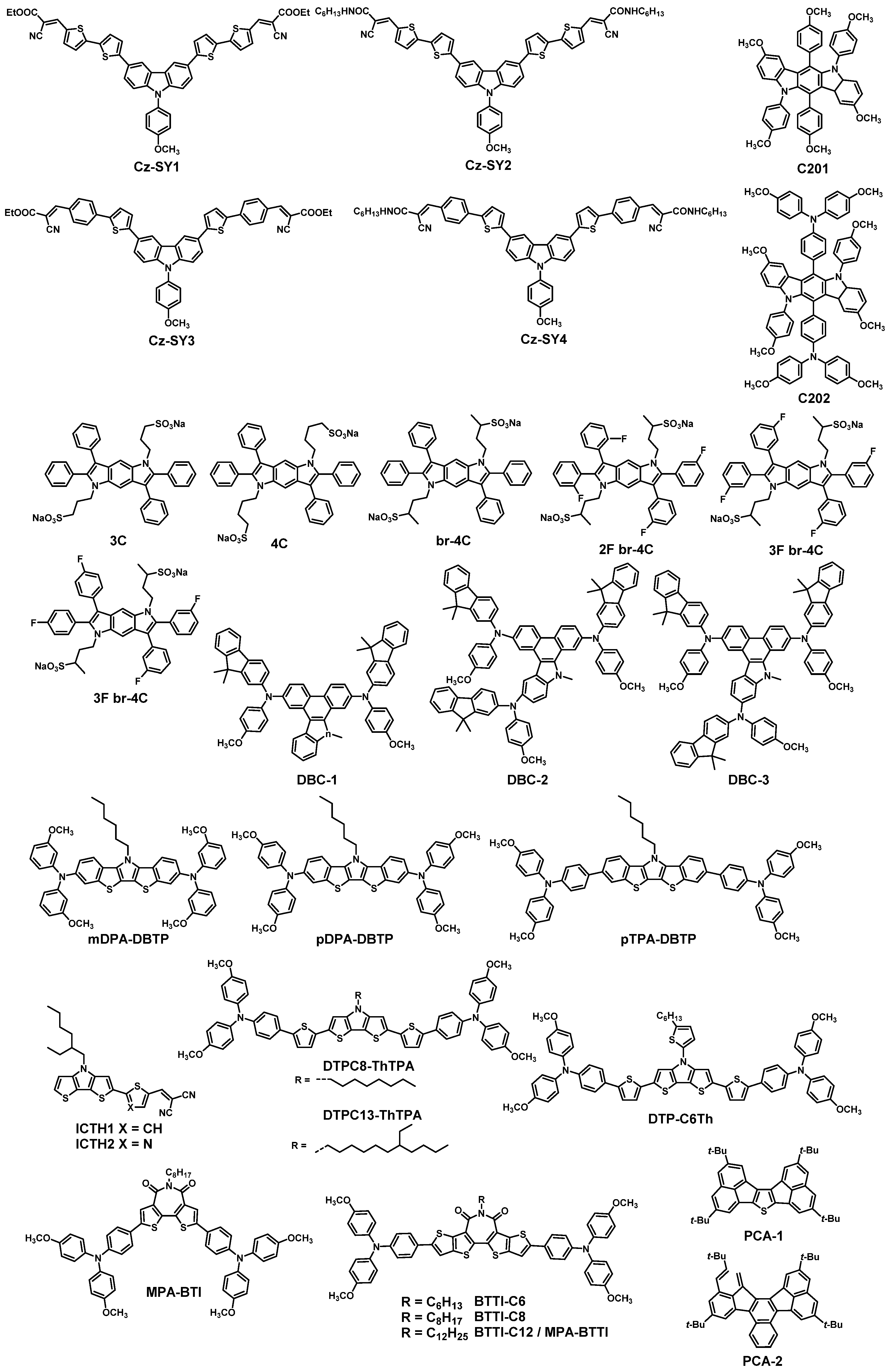
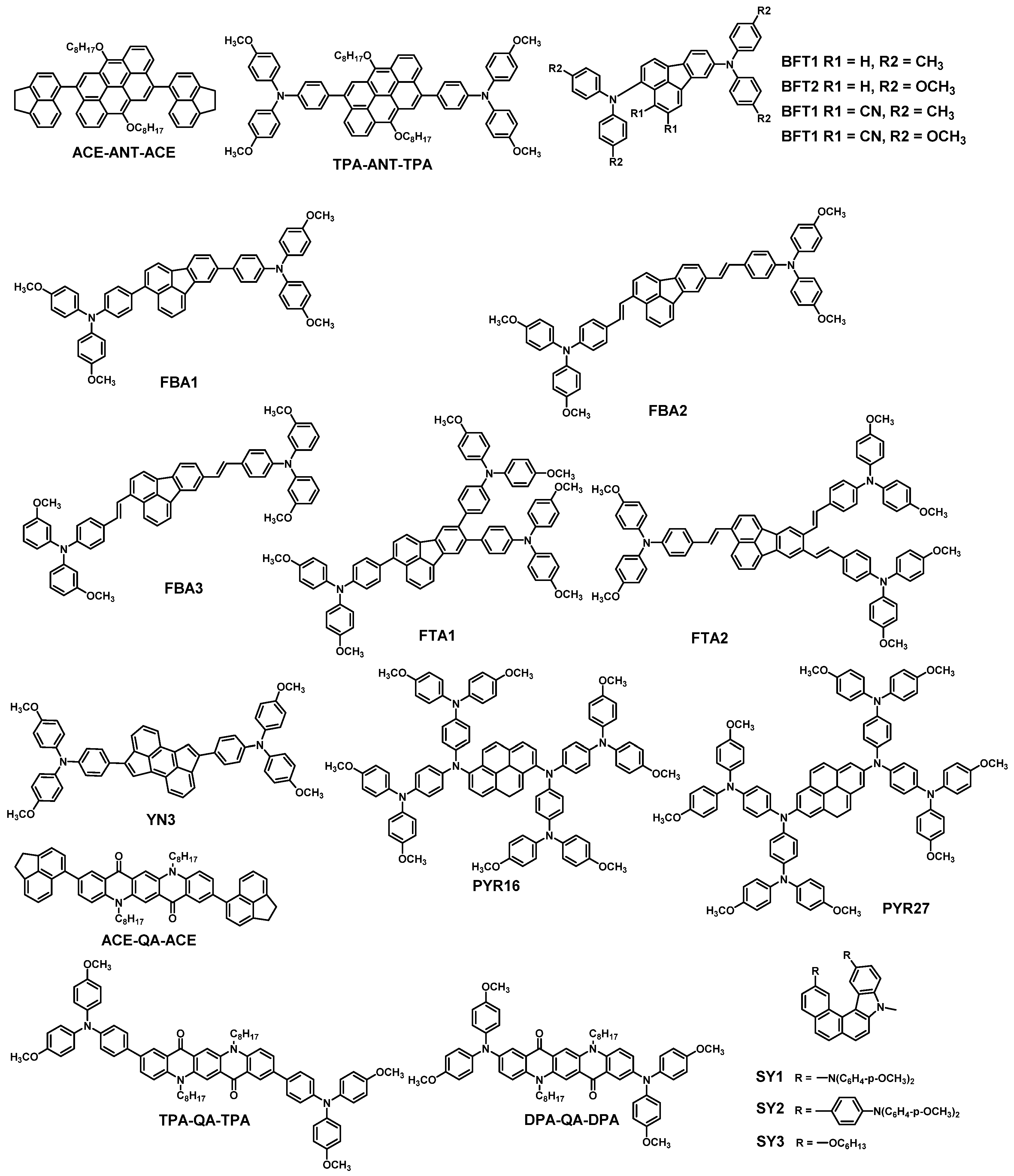
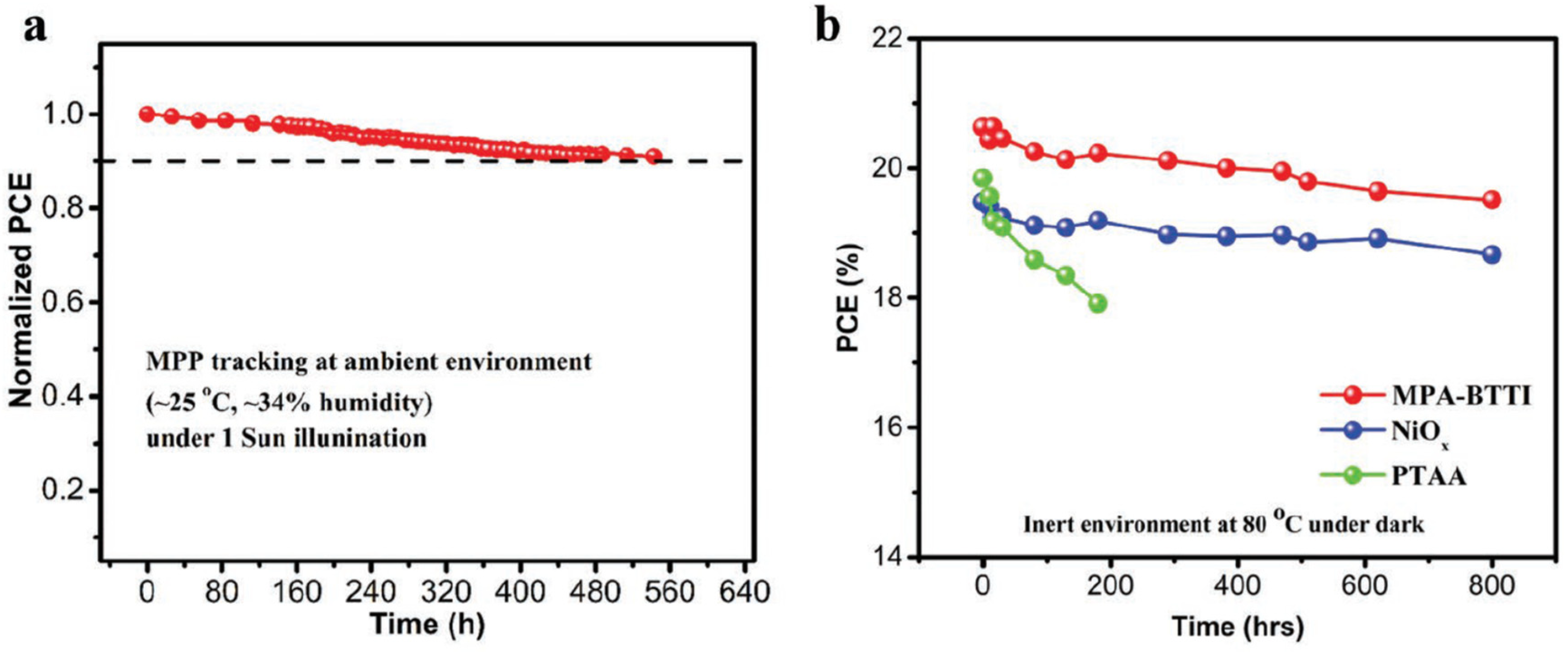
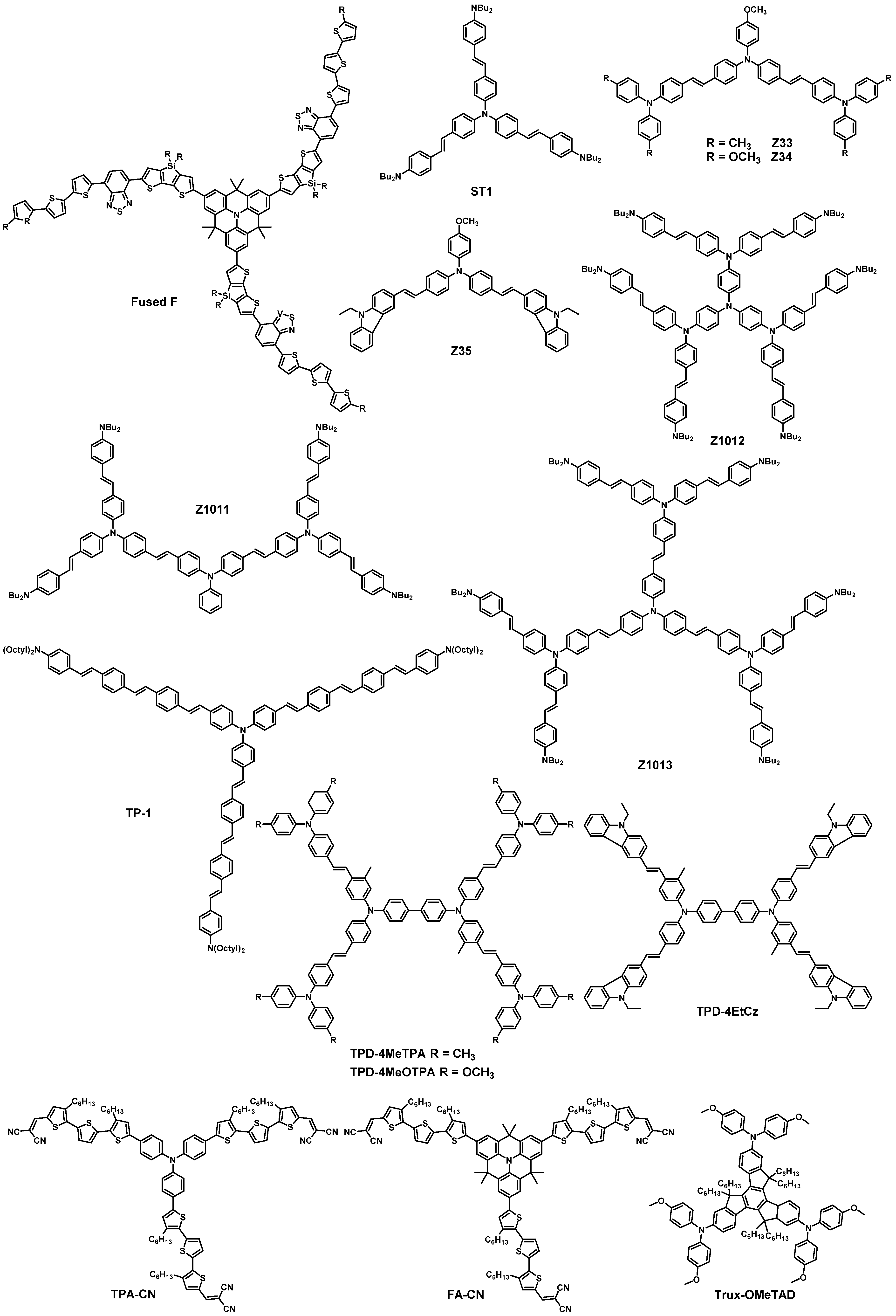
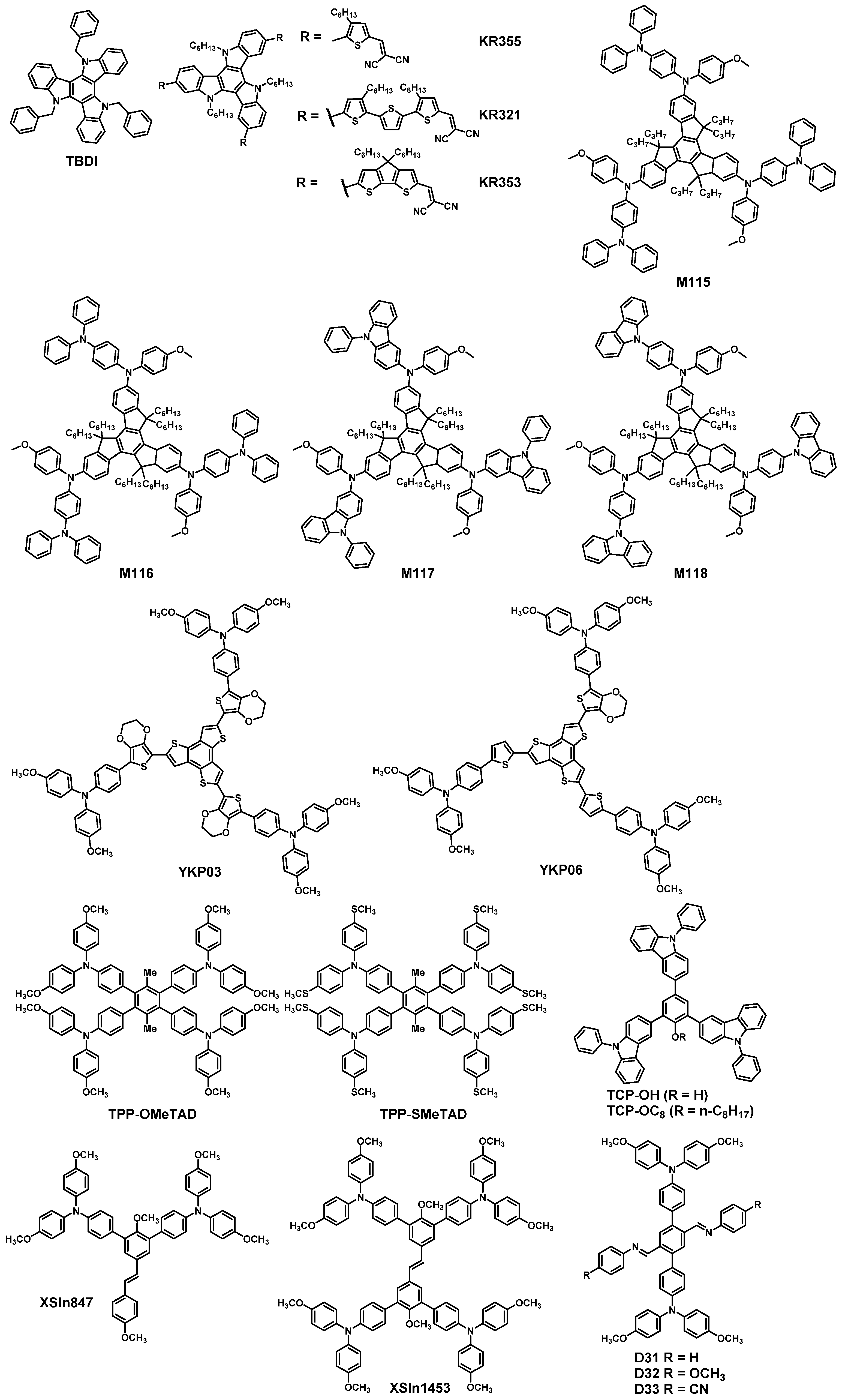
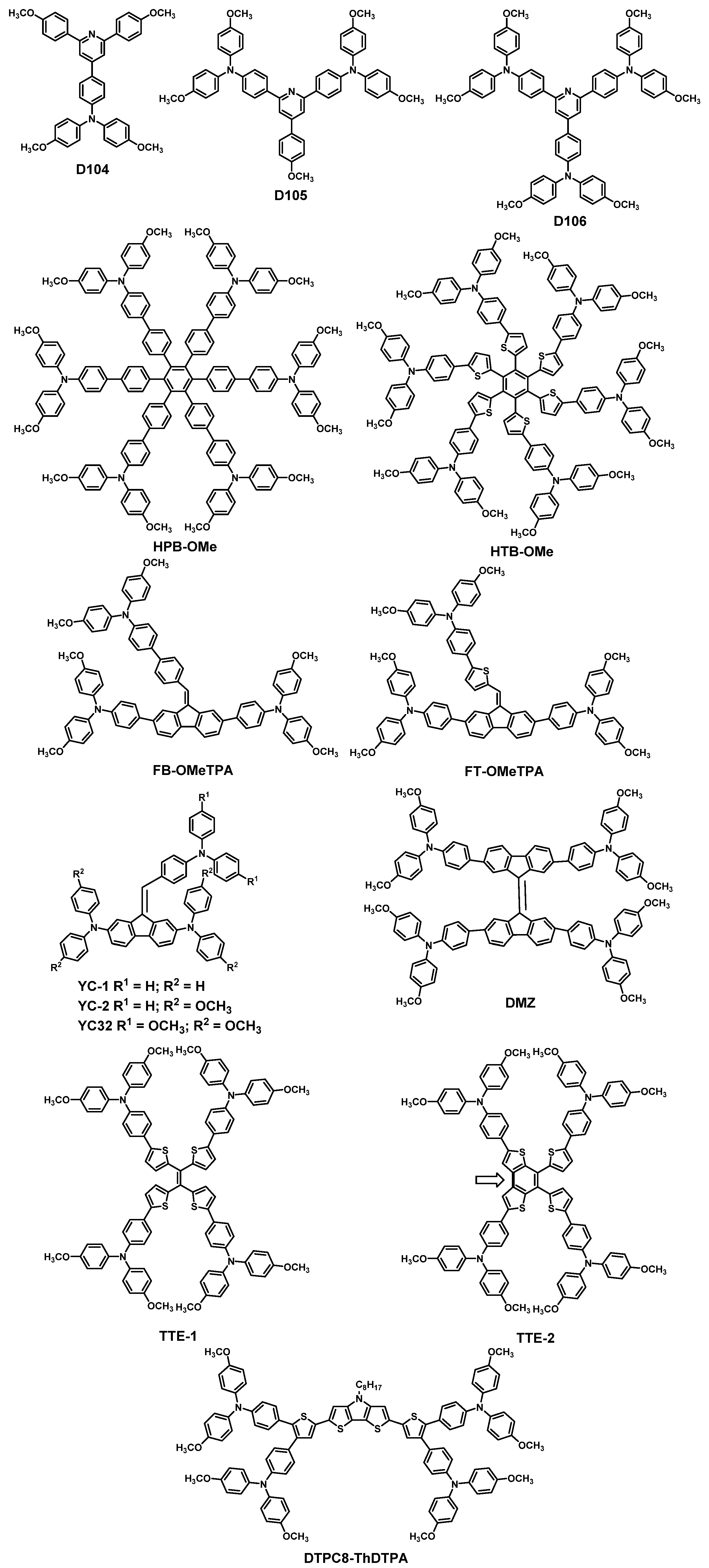
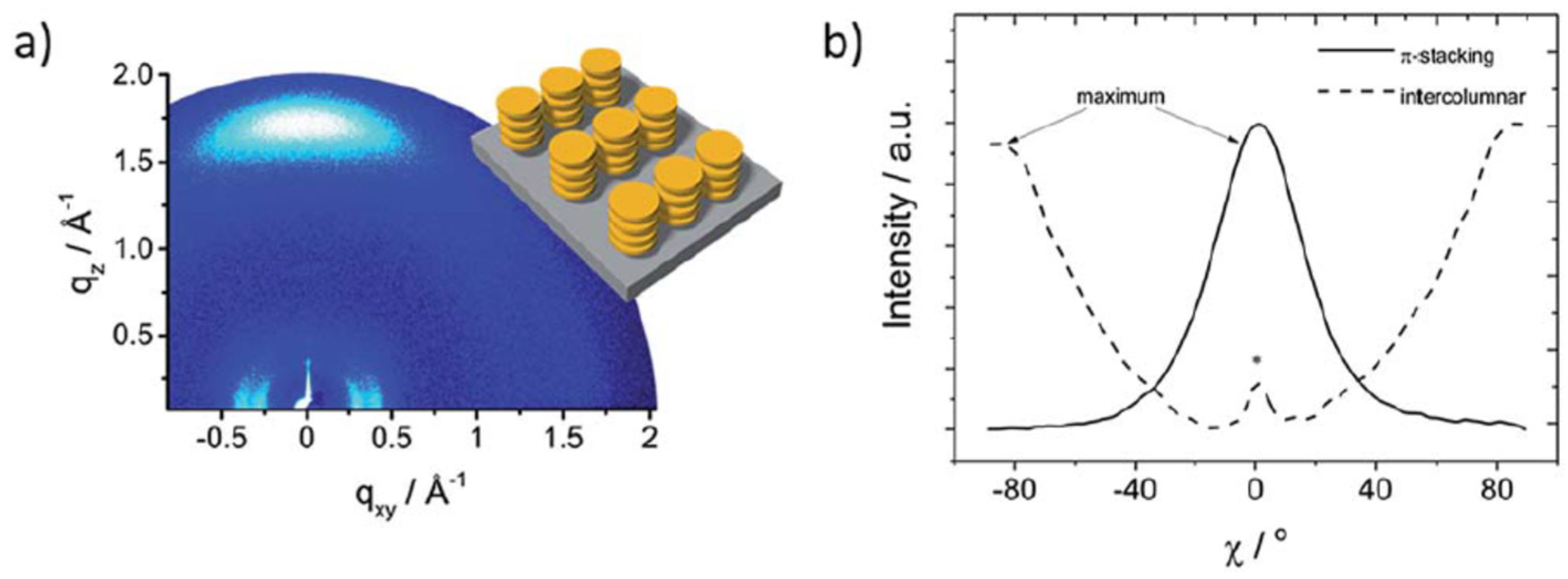
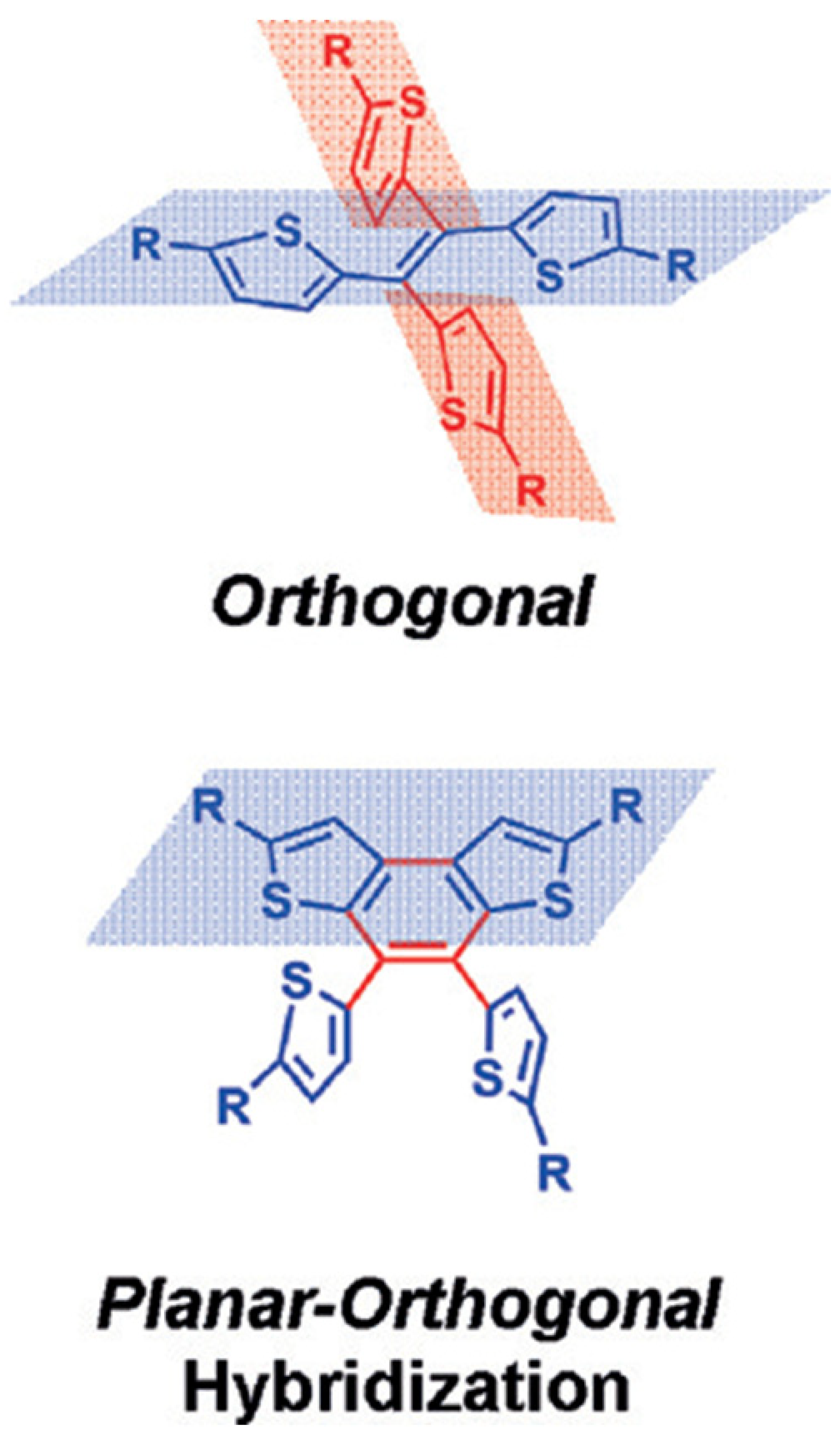
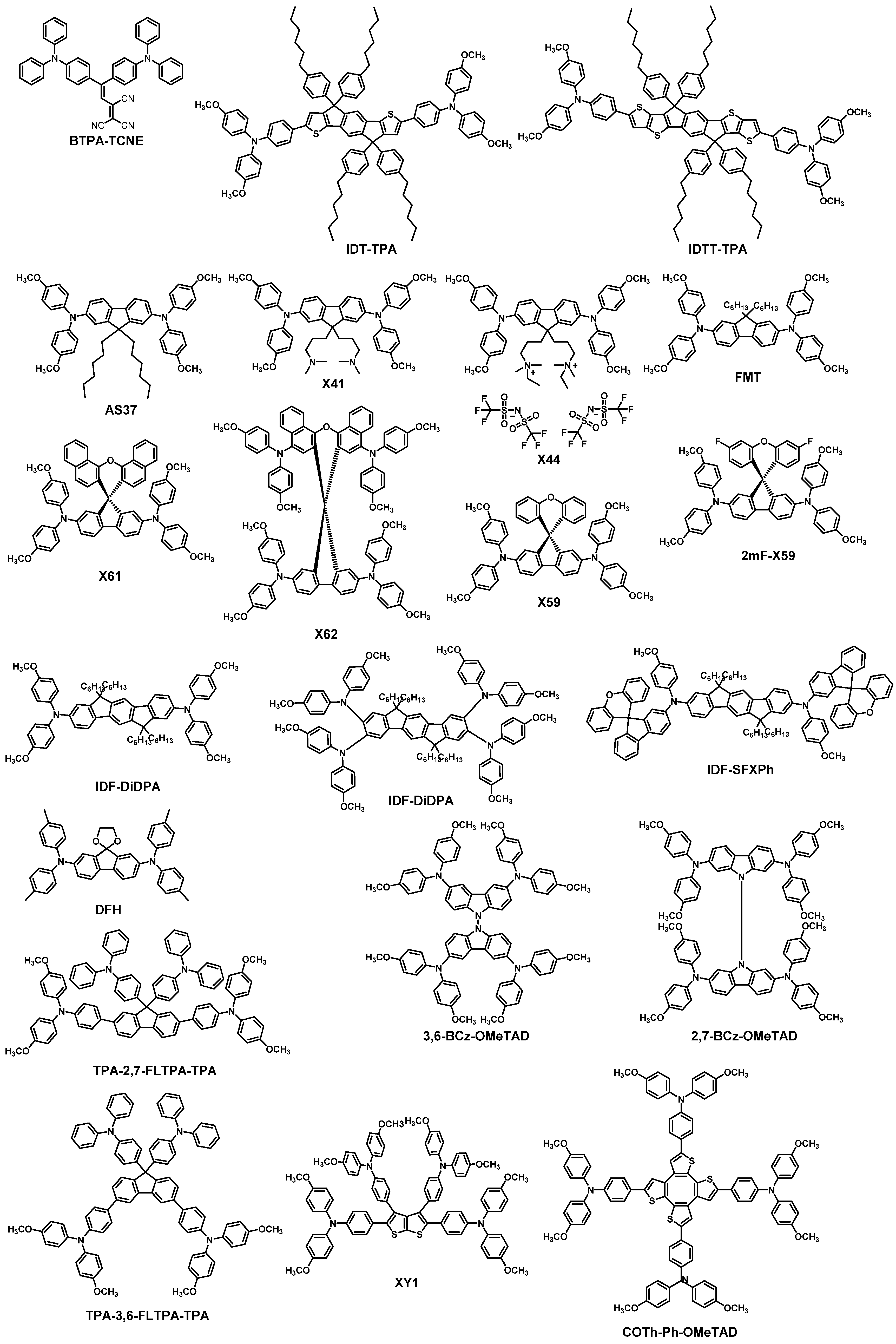
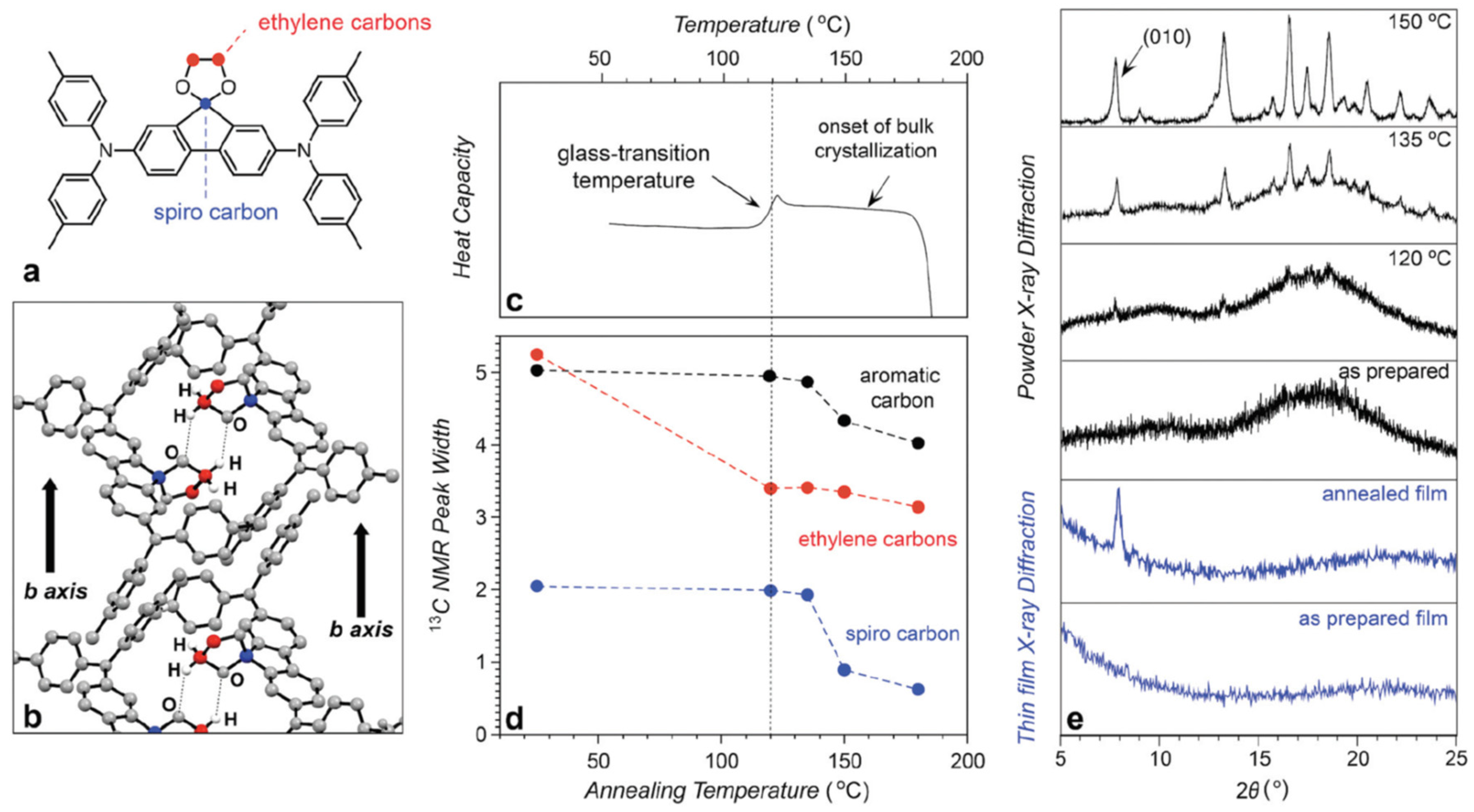
| HTM | PSK | Cell a | PCE (%) | Standard PCE (%) | Voc (mV) | Jsc (mA cm−2) | FF (%) | HOMO (eV) | Bg (eV) | HM f (cm2 V−1 s−1) | Conductivity (S cm−1) | Stability g (h) | Reference |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TTF-1 | MAPbI3 | M | 11.03 | 6.18 b/11.4 | 860 | 19.9 | 64.4 | −5.05 | 3.07 | 0.1 | - | 550h (40% RH) | [49] |
| DOR3T-TBDT | MAPbIxCl-1x | M | 14.9 | 3.5 b/14.0 | 0.97 | 20.7 | 74 | −5.5 | 1.77 | 0.26 | 4.0 × 10−4 | - | [50] |
| M7-Br | (FAPbI3)0.85(MABr3)0.15 | M | 15.5 | 17.9b | 1045 | 21.94 | 67.6 | −5.28 | 1.7 | 3.04 × 10−4 | 1.58 × 10−4 | 76%, 30 d (30% RH, rt) | [52] |
| M7-TFSI | (FAPbI3)0.85(MABr3)0.15 | M | 17.7 | 17.9b | 1093 | 22.84 | 70.6 | −5.28 | 1.7 | 3.24 × 10−4 | 2.01 × 10−4 | 64%, 30 d (30% RH, rt) | [52] |
| DERDTS-TBDT | MAPbIxCl-1x | P | 16.2 | 5.4 b | 1050 | 21.2 | 72.8 | −5.09 | 1.83 | 1.0 × 10−4 | - | - | [54] |
| BDT-PTZ | MAPbI3 | I | 18.26 (best) 16.91 (average) | 17.85d (best) 16.82 (average) | 1.02 | 22.43 | 79.8 | −5.42 | 2.62 | 9.8 × 10−5 | - | 80%, 400 h, NE (60% RH, rt) | [55] |
| BDT-POZ | MAPbI3 | I | 19.16 (best) 18.10 (average) | 17.85d (best) 16.82 (average) | 1.04 | 22.56 | 81.6 | −5.35 | 2.57 | 2.1 × 10−4 | - | 80%, 400 h, NE (60% RH, rt) | [55] |
| AZO-II | Cs0.05MA1−yFAyPbI3−xClx | P | 15.6 (best) 14.0 (average) | 19.3b | 0.95 | 21.6 | 71 | −4.94 | 2.2 | 2.0 × 10−5 | - | 91%, 60 d, NE (ambient air, rt) | [56] |
| TPAC2M | MAPbI3 p-in | P | 15.77 (best) 15.20 (average) | 12.60 c (best) 11.44 (average) | 990 | 22.58 | 71 | −4.98 | 3.25 | 1.0 × 10−5 | - | - | [57] |
| TPAC3M | MAPbI3 p-in | P | 17.54 (best) 16.58 (average) | 12.60 c | 1000 | 22.79 | 78 | −4.96 | 3.25 | 1.1 × 10−5 | - | - | [57] |
| CF-BTz-ThR | MAPbI3 | M | 15.4 | 10.4 b | 1020 | 22.42 | 67 | −5.42 | 2.03 | - | 8.4 × 10−4 | - | [58] |
| TPA-PB-OXD | MAPBI3 | I | 15.46 (rig) 15.29 (flex) | 14.04 c (rig) 10.35 (flex) | 1.03 | 21.23 | 70.56 | −5.1 | 2.67 | 3.12 × 10−5 | - | 80%, 720 h, NE (35% RH, rt) | [59] |
| DFBT(DTS-FBTTh2)2 | MAPbI3 | P | 17.3 | 17.4b | 1100 | 20.7 | 76 | −5.27 | 1.41 | 1.78 × 10−4 | - | 80%, 500 h, NE (60% RH, light, rt) | [60] |
| p-DTS(FBTTh2)2/PCDTBT | MAPbI3 | P | 18.0 (best) 15.90 (average) | - | 1.1 | 20.6 | 79.4 | −5.15 | n.a | 1.07 × 10−4 | - | 78%, 100 min, NE (85% RH, 85°C) | [62] |
| CMO | MAPbI3 | P | 15.92 | 16.70b | 930 | 25.19 | 67.9 | −4.78 | 2.96 | 1.4 × 10−5 | - | - | [63] |
| PhCz-4OMeTPA | Cs0.05(MA0.17FA0.83)0.95Pb(I0.83Br0.17)3 | M | 16.04 | 19.20b | 1.08 | 21.52 | 69 | −5.06 | 3.12 | 1.13 × 10−4 | - | 92%, 1200h, NE (ambient air, dark, 80°C) | [64] |
| Cz-SY1 | MAPbI3 | I | 18.18 (best) 16.73 (average) | 12.33 c 16.92 e | 1.099 | 21.57 | 76.7 | −5.29 | 2.22 | 8.19 × 10−6 | - | 85%, 216h, NE (40% RH, light, rt) | [65] |
| Cz-SY2 | MAPbI3 | I | 18.96 (best) 17.67 (average) | 12.33 c 16.92 e | 1.102 | 21.76 | 79.1 | −5.26 | 2.27 | 9.41 × 10−5 | - | 85%, 216h, NE (40% RH, light, rt) | [65] |
| Cz-SY3 | MAPbI3 | I | 15.86 (best) 14.39 (average) | 12.33 c 16.92 e | 1.06 | 20.44 | 73.2 | −5.28 | 2.39 | 6.79 × 10−6 | - | 88%, 216h, NE (49% RH, light, rt) | [65] |
| Cz-SY4 | MAPbI3 | I | 18.44 (best) 17.42 (average) | 12.33 c 16.92 e | 1.102 | 21.71 | 77.1 | −5.21 | 2.45 | 1.81 × 10−5 | - | 84%, 216 h, NE (40% RH, light, rt) | [65] |
| C202 | (FAPbI3)0.85(MAPbBr3)0.15 | M | 17.7 (best) 16.9 (average) | 19.8b | 1.05 | 23 | 73.1 | −5.23 | 2.74 | 1.5 × 10−4 | - | 72%, 288 h NE (40% RH, 22°C) | [66] |
| DBC-1 | CH3NH3PbI3−xClx | P | 18.81 | 18.18b | 1.05 | 22.14 | 80.91 | −5.26 | 2.77 | 7.09 × 10−4 | - | - | [68] |
| DBC-2 | CH3NH3PbI3−xClx | P | 20.02 | 18.18b | 1.11 | 22.69 | 78.78 | −5.22 | 2.73 | 9.85 × 10−4 | - | 83%, 1 month, NE (N2, rt) | [68] |
| DBC-3 | CH3NH3PbI3−xClx | P | 16.77 | 18.18b | 1.06 | 21.44 | 73.79 | −5.14 | 2.76 | 2.23 × 10−4 | - | - | [68] |
| Br-4C | MAPbI3 p-i-n | P | 15.6 | 12.5 c | 1000 | 19.5 | 79 | −5.22 | 2.97 | - | - | [67] | |
| 3-F-br-4C | MAPbI3 p-i-n | P | 16.9 | 12.5 c | 1040 | 19.8 | 80 | −5.37 | 2.99 | 4.0 × 10−5 | - | 80%, 50 d, NE (50% RH, rt) | [67] |
| mDPA-DBTP | MAPbI3 | P | 18.09 | 6.85 b/17.82 | 1120 | 21.13 | 76 | −5.31 | 2.87 | 6.34 × 10−4 | - | 81%, 33 d, NE. (ambient air, light, rt) | [69] |
| pTPA-DBTP | MAPbI3 | P | 15.63 | 6.85 b/17.82 | 1090 | 20.1 | 72 | −5.2 | 2.73 | 3.52 × 10−4 | - | - | [69] |
| ICTH1 | MAPbI3 | M | 17.91 | 14.74b | 1010 | 24.56 | 72.2 | −5.3 | 2.12 | 3.2 × 10−4 | - | 74%, 0 d, NE. (ambient air, rt) | [70] |
| ICTH2 | MAPbI3 | M | 18.75 | 14.74b | 1030 | 24.78 | 73.5 | −5.41 | 2.23 | 3.1 × 10−4 | - | 86%, 40 d, NE (ambient air, rt) | [70] |
| DTPC8-ThTPA | MA0.7FA0.3PbI2.85Br0.15 | P | 18.37 (best) 17.74 (average) | 19.85b | 1.094 | 22.66 | 74.1 | −4.82 | 2.21 | 2.14 × 10−4 | - | - | [71] |
| DTPC13-ThTPA | MA0.7FA0.3PbI2.85Br0.15 | P | 20.38 (best) 19.30 (average) | 19.85b | 1.135 | 22.82 | 78.7 | −4.82 | 2.21 | 3.48 × 10−5 | - | - | [71] |
| DTP-C6Th | MA0.7FA0.3PbI3 | P | 21.04 (best) 20.36 (average) | 20.61b | 1.157 | 22.76 | 79.9 | −4.87 | 2.22 | 4.18 × 10−4 | - | 85%, 60 d, NE (35% RH, rt) | [34] |
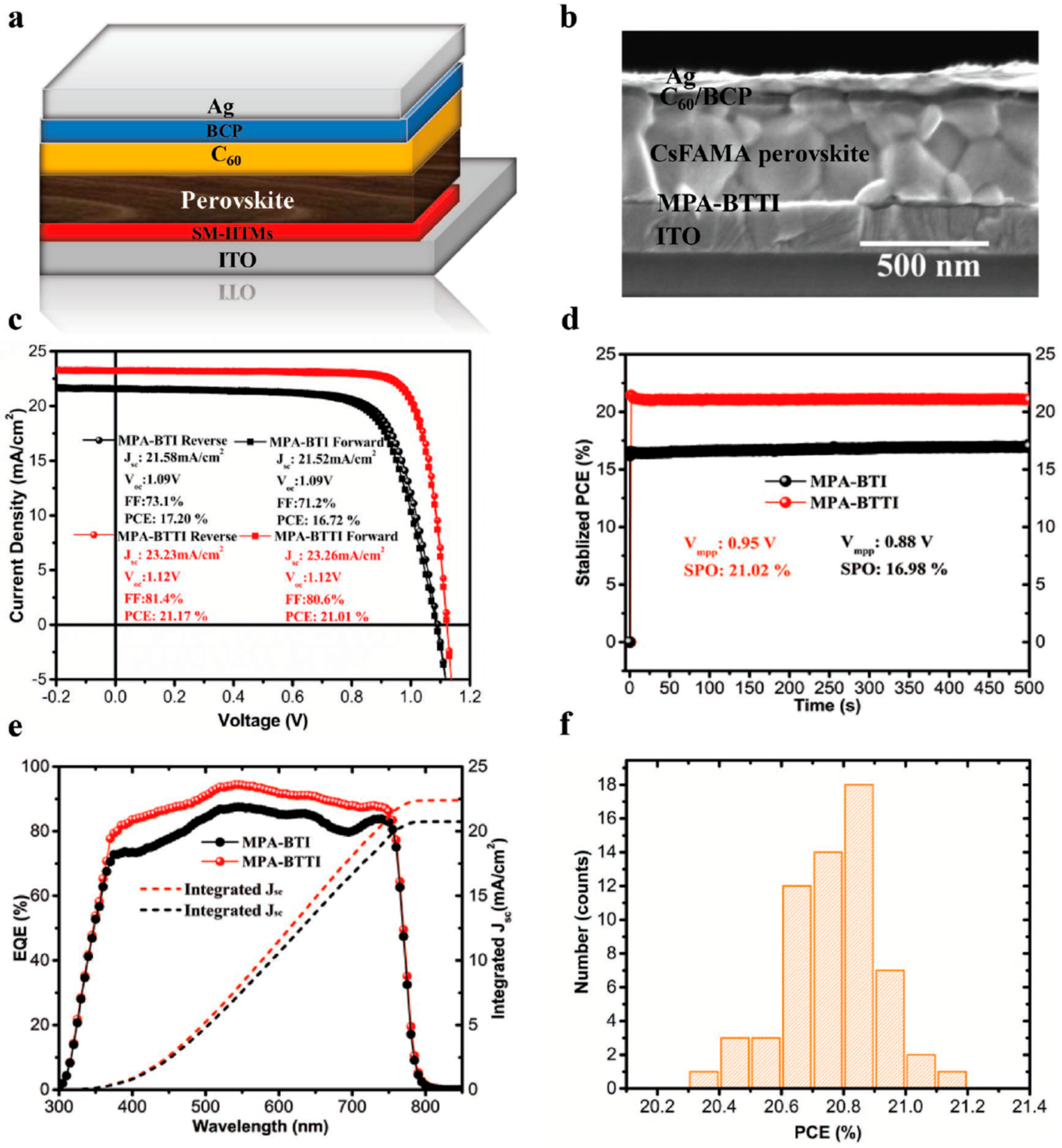
| MPA-BTI | Cs-FAMA | I | 17.2 | 19.85 b | 1.09 | 21.58 | 73.1 | −5.28 | 2.12 | 3.99 × 10−5 | 6.90 × 10−6 | - | [35] |
| MPA-BTTI (same as BTTI-C12) | Cs-FAMA | I | 21.17 (best) 20.7 (average) | 19.85 b | 1.12 | 23.23 | 81.4 | −5.24 | 1.92 | 2.02 × 10−4 | 1.35 × 10−5 | 90%, 500 h, NE (24% RH, light, rt) | [35] |
| BTTI-C6 | Cs-FAMA | P | 19.69 8best) 19.06 (average) | 19.90b | 1.1 | 24 | 74.6 | −5.25 | 1.92 | 2.19 × 10−4 | 3.02 × 10−5 | 85%, 100 d, NE (24% RH, rt) | [72] |
| BTTI-C8 | Cs-FAMA | P | 18.89 (best) 18.04 (average) | 19.90b | 1.09 | 24.1 | 71.36 | −5.25 | 1.92 | 2.08 × 10−4 | 2.94 × 10−5 | - | [72] |
| BTTI-C112 (same as: MPA-BTTI) | Cs-FAMA | P | 17.49 (best) 16.83 (average) | 19.90b | 1.03 | 24.26 | 69.91 | −5.24 | 1.92 | 2.02 × 10−4 | 2.79 × 10−5 | - | [72] |
| PCA-1 | MAPbI3 | P | 15.59 (best) 14.88 (average) | 16.5b | 1024 | 20.81 | 73.2 | −5.34 | 2.66 | 8.3 × 10−5 not annealed | - | 85.2%, 400 h, NE. (ambient air, rt) | [73] |
| 8.31 × 10−2 annealed | |||||||||||||
| PCA-1 | MAPbI3 | M | 18.17 (best) 17.23 (average) | 18.30b | 1062 | 22.3 | 76.7 | −5.34 | 2.66 | 8.3 × 10−5 not annealed | - | 85%, 400 h, NE (ambient air, rt) | [73] |
| 8.31 × 10−2 annealed | |||||||||||||
| TPA-ANT-TPA | MAPbI3 | M | 17.5 (best) 16.0 (average) | 16.8b (best) 13.8 (average) | 1030 | 21.07 | 79.6 | −5.41 | 2.48 | 2.6 × 10−4 | - | 84%, 50 h, NE (58% RH, 22 °C) | [74] |
| BTF3 | (FAPbI3)0.85(MAPbBr3)0.15 | P | 16.34 | 9.33 b/18.8 16.42 c | 1080 | 20.4 | 74.3 | −5.19 | 1.7 | 6.36 × 10−5 | - | - | [75] |
| BTF4 | (FAPbI3)0.85(MAPbBr3)0.15 | P | 18.03 | 9.33 b/18.8 16.42 c | 1060 | 22.5 | 75.6 | −5.02 | 1.59 | 1.17 × 10−4 | - | - | [75] |
| FBA1 | MAPbIxCl3-x | P | 16.80 (best) 16.24 (average) | 17.57b | 1.05 | 21.57 | 74.2 | −5 | 2.48 | 8.91 × 10−4 | - | 80% 180 h, NE (30% RH, rt) | [76] |
| FBA2 | MAPbIxCl3−x | P | 18.70 (best) 17.97 (average) | 17.57b | 1.06 | 22.32 | 79 | −4.98 | 2.24 | 1.36 × 10−4 | - | 80% 180 h, NE (30% RH, rt) | [76] |
| FBA3 | MAPbIxCl3−x | P | 19.27 (best) 18.46 (average) | 17.57b | 1.09 | 22.12 | 79.9 | −5.07 | 2.29 | 2.12 × 10−4 | - | 80% 180 h, NE (30% RH, rt) | [76] |
| FTA1 | MAPbIxCl3−x | P | 15.15 (best) 14.52 (average) | 17.57b | 1.01 | 20.76 | 72.3 | −5 | 2.49 | 4.83 × 10−5 | - | 80% 180 h, NE (30% RH, rt) | [76] |
| FTA2 | MAPbIxCl3−x | P | 17.73 (best) 17.12 (average) | 17.57b | 1.03 | 22.04 | 78.1 | −4.99 | 2.21 | 1.07 × 10−4 | - | 80% 180 h, NE (30% RH, rt) | [76] |
| YN3 | (FAPbI3)0.85(MAPbBr3)0.15 | M | 18.84 | 18.41b | 1.12 | 22.43 | 75 | −5.31 | 1.54 | 2.25 × 10−4 | 1.98 × 10−4 | 92%, 300 h, NE (45% RH, rt) | [77] |
| PYR16 | [Cs0.05(FA0.83MA0.17)0.95]Pb(I0.83Br0.17)3 | M | 17 | 19.74b | 1.11 | 21.56 | 71 | −5.14 | 2.36 | 1.19 × 10−4 | - | 98%, 1080 h, NE (ambient air, dark, 80 °C) | [78] |
| ACE-QA-ACE | MAPbI3 | M | 18.2 | 15.2b | 1.06 | 22.41 | 77 | −5.59 | 2.12 | 2.3 × 10−4 | - | 81%, 30 d, NE (75% RH, dark, rt) | [79] |
| TPA-QA-TPA | MAPbI3 | M | 16.6 | 15.2b | 0.99 | 22.4 | 75.1 | −5.41 | 1.98 | 1.6 × 10−4 | - | 78%, 30 d, NE (75% RH, dark, rt) | [79] |
| DPA-QA-DPA | MAPbI3 | M | 15.5 | 15.2b | 0.95 | 22.38 | 73.2 | −5.28 | 1.87 | 1.2 × 10−4 | - | 78%, 30 d, NE (75% RH, dark, rt) | [79] |
| SY1 | MAPbI3 | M | 17.34 (best) 16.20 (average) | 18.14b (best) 16.57 (average) | 1010 | 23.68 | 72.1 | 0.94140625 | 2.74 | 2.55 × 10−5 | - | 504 h, NE (30% RH, 35 °C) | [80] |
| SY2 | MAPbI3 | M | 16.10 (best) 14.46 (average) | 18.14b (best) 16.57 (average) | 1000 | 21.87 | 73.2 | 0.942857143 | 3.01 | 3.46 × 10−5 | - | 504 h, not enc. (30% RH, 35 °C) | [80] |
| HTM | PSK | Cell a | PCE (%) | Standard PCE (%) b | Voc (mV) | Jsc (mA cm−2) | FF (%) | HOMO (eV) | Bg (eV) | HM (cm2 V−1 s−1) | Conductivity (S cm−1) | Stability (h) | Reference |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ST1 | MAPbI3 | M | 15.4 | 16.3b | 1059 | 21.07 | 66 | −5.24 | 2.61 | 4.57 × 10−4 | - | 80%, 30 d, NE (30% RH, rt) | [82] |
| Z33 | MAPbI3 | M | 15.4 (best) 15.3 (average) | 3.9 b/16.6 | 1087 | 20.46 | 66 | −5.34 | 2.72 | 4.67 × 10−4 | - | >100%, (incr.), 1000 h, NE (30% RH, rt) | [83] |
| Z34 | MAPbI3 | M | 16.1 (best) 15.9 (average) | 3.9 b/16.6 | 1053 | 21.27 | 69 | −5.14 | 2.71 | 7.46 × 10−4 | - | >100%, (incr.), 1000 h, NE (30% RH, rt) | [83] |
| Z1011 | MAPbI3 | M | 16.3 | 9.6 b/16.5 | 1096 | 20.52 | 70 | −5.21 | 2.64 | 8.49 × 10−4 | - | >100% (incr.), 1000 h, NE (dry air, rt) | [84] |
| Z1013 | MAPbI3 | M | 15.4 | 16.7b | 1027 | 21.33 | 70.2 | −5.14 | 2.6 | 6.67 × 10−4 | - | >100%, (incr.), 1000 h, NE (30% RH, 80 °C) | [85] |
| TPD-4MeOTPA | (FAPbI3)0.85(MAPbBr3)0.15 | M | 15.28 | 17.26b | 1099 | 20.84 | 66.7 | −5.28 | 2.59 | 4.92 × 10−4 | - | 92%, 600 h, NE (RH 30%, rt) | [87] |
| TPA-CN | (FAPbI3)0.85(MAPbBr3)0.15 | M | 17.5 (best) 16.4 (average) | 19.2b (best) 18.06 (average) | 1090 | 20.85 | 77 | −5.38 | 2.03 | 1.1 × 10−4 | 25%, 1300 h, NE (Ar, light, rt) | [88] | |
| FA-CN | (FAPbI3)0.85(MAPbBr3)0.15 | M | 18.9 (best) 17.2 (average) | 19.2b (best) 18.06 (average) | 1130 | 21.71 | 77 | −5.3 | 1.99 | 1.2 × 10−4 | 65%, 1300 h, NE (Ar, light, rt) | [88] | |
| Trux-OMeTAD | MAPbI3 p-i-n | M | 18.6 | 16.3b16.2c 16.1 e | 1020 | 23.2 | 79 | −5.28 | 2.98 | 3.6 × 10−3 | - | - | [36] |
| TBDI | MAPbI3 p-i-n | P | 14.85 | 15.30 d | 1.09 | 18.73 | 72.8 | −5.25 | 3.27 | 5.95 × 10−3 | - | - | [93] |
| KR321 | (FAPbI3)0.85(MAPbBr3)0.15 | M | 19.03 | 19.01b | 1130 | 21.7 | 78 | −5.24 | 2.05 | 2.6 × 10−4 | - | - | [8] |
| M116 | MAPbI3 | P | 15.5 | 14.4 c | 1.03 | 21.1 | 72 | −5.05 | 2.95 | 1.42 × 10−3 | - | - | [94] |
| M117 | MAPbI3 | P | 15.7 | 14.4 c | 1.03 | 21.7 | 70 | −5.08 | 2.88 | 2.41 × 10−3 | - | - | [94] |
| M118 | MAPbI3 | P | 17.1 | 14.4 c | 1.06 | 22.4 | 72 | −5.27 | 3.1 | 1.75 × 10−3 | - | - | [94] |
| YKP03 | MAPbI3 | M | 16.15 | 17.59b | 1.03 | 23.07 | 68 | −5.16 | 2.6 | 5.80 × 10−4 | - | 80%, 800 h, NE 5% RH, glove-box, rt) | [96] |
| TPP-OMeTAD | MAPbI3 p-i-n | P | 14.6 | - | 1000 | 18.56 | 79 | −5.08 | 3.2 | - | - | - | [97] |
| TPP-SMeTAD | MAPbI3 p-i-n | P | 16.6 | - | 1007 | 20.15 | 77 | −5.18 | 3.25 | - | - | - | [97] |
| TCP-OC8 | (FAI)0.81(PbI2)0.85(MAPbBr3)0.15) | M | 15.28 | 13.26 b/18.85 | 1090 | 22.38 | 61.4 | −5.56 | 3.45 | 2.56 × 10−7 | - | 87%, 720 h, E (ambient air, 45 °C) | [98] |
| TCP-OH | (FAI)0.81(PbI2)0.85(MAPbBr3)0.15) | M | 16.97 | 13.26 b/18.85 | 1070 | 23.15 | 66.7 | −5.47 | 3.45 | 5.85 × 10−6 | - | approx. 85%, 720h, E (ambient air, 45 °C) | [98] |
| XSIn847 | MAPbI3 p-i-n | P | 15.02 | 11.95 c | 1090 | 21.58 | 65.7 | −5.26 | 3.16 | 7.75 × 10−5 | - | - | [100] |
| D32 | MAPbI3 | I | 15.83 (best) 14.32 (average) | 13.16 c 16.19 e | 1 | 20.86 | 75.9 | −5.36 | 2.62 | 1.39 × 10−4 | - | 48%, 400 h, NE (35% RH, rt) | [101] |
| D33 | MAPbI3 | I | 17.85 (best) 16.38 (average) | 13.16 c 16.19 e | 1.02 | 22.19 | 78.8 | −5.38 | 2.32 | 2.41 × 10−4 | - | 70%,400 h, NE (35% RH, rt) | [101] |
| D104 | MAPbI3 | I | 16.28 | 14.37 c | 1.05 | 22.22 | 73.4 | −5.36 | 2.88 | 7.1 × 10−5 | - | 33%, 275 h, NE (30% RH, 20 °C) | [102] |
| D105 | MAPbI3 | I | 17.4 | 14.37 c | 1.04 | 21.96 | 76.1 | −5.3 | 2.94 | 2.41 × 10−4 | - | 70%, 275 h, NE (30% RH, 20 °C) | [102] |
| D106 | MAPbI3 | I | 18.24 | 14.37 c | 1.05 | 22.32 | 77.8 | −5.29 | 2.9 | 1.65 × 10−4 | - | 75%, 275h, NE (30% RH, 20°C) | [102] |
| HTB-OMe | MAPbI3 | P | 17.29 | - | 1030 | 22.79 | 73.7 | −5.33 | 2.78 | 5.48 × 10−4 | - | - | [104] |
| FT-OMeTPA | (CsPbI3)0.05[(FAPbI3)0.83(MAPbBr3)0.17]0.95 | I | 17.57 (best) 16.65 (average) | 18.67 e | 1.09 | 20.5 | 78.43 | −5.11 | 2.03 | 1.42 × 10−5 | - | - | [105] |
| YC-1 | MAPbI3 | I | 16.53 (best) 15.78 (average) | 14.38 c | 1.022 | 20.98 | 72.9 | −5.28 | 2.57 | - | - | 93%, 1000 h, NE (30% RH, rt) | [106] |
| YC-1/NiOx | MAPbI3 | I | 19.37 (best) 18. 18 (average) | 1.069 | 22.14 | 79.5 | −5.28 | 2.57 | - | - | 96%, 1000 h, NE (30% RH, rt) | [106] | |
| DMZ | MAPBI3 | I | 18.61 (best) 17.62 (average) | 12.03 c | 1.02 | 22.62 | 81.05 | −5.15 | 2.27 | 3.71 × 10−5 | - | 90%, 556 h, NE (50% RH, rt) | [107] |
| TTE-2 | (FAPbI3)0.95(MAPbBr3)0.05 | P | 20.04 | - | 1.11 | 23.26 | 77.53 | −5.3 | 2.62 | 6.18 × 10−4 | 85%, 1000 h, NE (35% RH, rt) | [108] | |
| DTPC8-ThDTPA | MA0.7FA0.3PbI2.85Br0.15 | P | 19.42 (best) 18.37 (average) | 12.83 b/19.59 | 1.14 | 23.02 | 74.1 | −4.85 | 2.26 | 6.50 × 10−5 | - | - | [109] |
| HTM | PSK | Cell a | PCE (%) | Standard PCE b (%) | Voc (mV) | Jsc (mA cm−2) | FF (%) | HOMO (eV) | Bg (eV) | HM e (cm2 V−1 s−1) | Conductivity (S cm−1) | Stability f (h) | Reference |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BTPA-TCNE | MAPbI3 n-i-p | P | 17.0 | 4.96 b/15.70 | 1040 | 20.84 | 78 | −5.35 | 1.62 | 3.14 × 10−5 | - | - | [110] |
| IDTT-TPA | SnO2/PCBM/ MAPbI3 | P | 15.7 | 7.8 b/17.0 | 1050 | 21.25 | 70.5 | −5.00 | 2.50 | 6.46 × 10−4 | - | 96%, 168 h, NE (air, rt) | [111] |
| X44 | (FAPbI3)0.85(MAPbBr3)0.15 | M | 15.2 | 7.5 b | 1080 | 21.04 | 67.0 | −5.06 | 2.94 | 9.03 × 10−4 | - | 106% (>6%), 15 d, NE. (20% RH, rt) | [112] |
| FMT | MAPbI3 | I | 19.06 | 13.9 c | 1.07 | 22.52 | 79.27 | −4.89 | 2.95 | 2.28 × 10−6 | - | 86%, 270 h, NE (N2,rt) | [113] |
| X62 | (FAPbI3)0.85(MAPbBr3)0.15 | M | 15.9 | 10.8 b | 1010 | 22.4 | 70.4 | −5.14 | 2.92 | 7.95 × 10−5 | 5.14x10-6 | 80%, 10 d, NE (50% RH, rt) | [114] |
| 2mF-X59 | MAPbI3 | P | 15.45 | 18.22b | 0.97 | 24.00 | 66.33 | −5.14 | 2.82 | 7.14 × 10−4 | - | 90%, 500 h, NE (30% RH, rt) | [115] |
| IDF-SFXPh | CH3NH3PbI3−xClx | P | 17.6 | 17.6b | 1.05 | 21.5 | 77.3 | −5.22 | 2.79 | 1.41 × 10−4 | - | 92% 500 h, NE (N2, rt) | [116] |
| DFH | MA0.9FA0.1PbI3-xClx | I | 20.6 (best)/19.3 (average) | 19.2d | 1.08 | 22.0 | 0.81 | −5.27 | 2.98 | 1.5 × 10−3 | - | - | [117] |
| 3,6-BCz-OMeTAD | Cs0.05FA0.79MA0.16PbI2.49Br0.51 | M | 17.0 | 18.5b | 1121 | 21.34 | 71.4 | −5.11 | 2.81 | 1.13 × 10−4 | - | 74%, 85 d, NE (30% RH, rt) | [118] |
| 2,7-BCz-OMeTAD | Cs0.05FA0.79MA0.16PbI2.49Br0.51 | M | 17.6 | 18.5b | 1089 | 22.38 | 72.5 | −5.15 | 2.91 | 9.5 × 10−5 | - | 90%, 85 d, NE (RH 30%, rt) | [118] |
| TPA-2,7-FLTPA-TPA | MAPbI3 | I | 17.1 | 15.2b | 1.05 | 19.85 | 78 | −5.45 | 2.95 | - | - | - | [120] |
| XY1 | (CsPbI3)0.05[(FAPbI3)0.83(MAPbBr3)0.17]0.95 | P | 18.78 | 16.31 c | 1.11 | 22.21 | 76.18 | −5.44 | 3.02 | 3.76 × 10−4 | - | 86%, 480 h, NE (glove-box, light, rt) | [121] |
| COTh-Ph-OMeTAD | CsFAMA | M | 17.22 | 16.83b | 1.05 | 21.51 | 76.37 | −5.30 | 2.39 | 2.88 × 10−4 | - | 86%, 800 h, NE (dry air, rt) | [122] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Desoky, M.M.H.; Bonomo, M.; Buscaino, R.; Fin, A.; Viscardi, G.; Barolo, C.; Quagliotto, P. Dopant-Free All-Organic Small-Molecule HTMs for Perovskite Solar Cells: Concepts and Structure–Property Relationships. Energies 2021, 14, 2279. https://doi.org/10.3390/en14082279
Desoky MMH, Bonomo M, Buscaino R, Fin A, Viscardi G, Barolo C, Quagliotto P. Dopant-Free All-Organic Small-Molecule HTMs for Perovskite Solar Cells: Concepts and Structure–Property Relationships. Energies. 2021; 14(8):2279. https://doi.org/10.3390/en14082279
Chicago/Turabian StyleDesoky, Mohamed M. H., Matteo Bonomo, Roberto Buscaino, Andrea Fin, Guido Viscardi, Claudia Barolo, and Pierluigi Quagliotto. 2021. "Dopant-Free All-Organic Small-Molecule HTMs for Perovskite Solar Cells: Concepts and Structure–Property Relationships" Energies 14, no. 8: 2279. https://doi.org/10.3390/en14082279
APA StyleDesoky, M. M. H., Bonomo, M., Buscaino, R., Fin, A., Viscardi, G., Barolo, C., & Quagliotto, P. (2021). Dopant-Free All-Organic Small-Molecule HTMs for Perovskite Solar Cells: Concepts and Structure–Property Relationships. Energies, 14(8), 2279. https://doi.org/10.3390/en14082279