Evaporated MAPbI3 Perovskite Planar Solar Cells with Different Annealing Temperature
Abstract
1. Introduction
2. Materials and Methods
2.1. Fabrication of Mesoporous TiO2/Compact TiO2/FTO Structure (TiO2/FTO)
2.2. Fabrication of PCBM/TiO2/FTO (ETL)
2.3. Fabrication of MAPbI3/PCBM/TiO2/FTO (Perovskite)
2.4. Fabrication of Spiro-OMeTAD/MAPbI3/PCBM/TiO2/FTO (HTL)
2.5. Fabrication of Ag/Spiro-OMeTAD/MAPbI3/PCBM/TiO2/FTO (PSC)
2.6. Performance Measurement
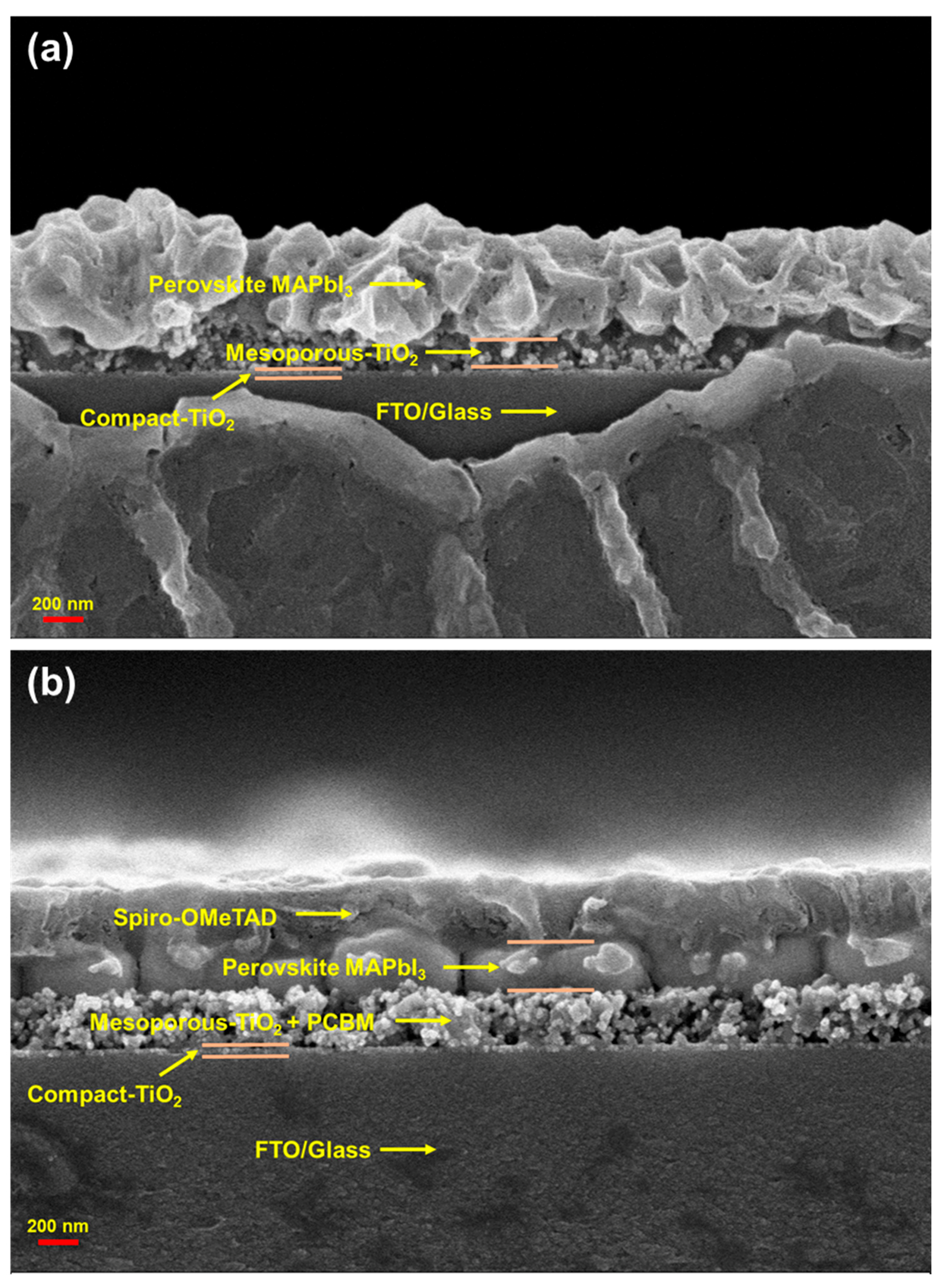
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- National Renewable Energy Laboratory. Best Research-Cell Efficiencies. Available online: https://www.nrel.gov/pv/assets/pdfs/best-research-cell-efficiencies.20200104.pdf (accessed on 4 January 2021).
- Lim, S.-H.; Seok, H.-J.; Kwak, M.-J.; Choi, D.-H.; Kim, S.-K.; Kim, D.-H.; Kim, H.-K. Semi-transparent perovskite solar cells with bidirectional transparent electrodes. Nano Energy 2021, 82, 105703. [Google Scholar] [CrossRef]
- Wei, Z.; Smith, B.; De Rossi, F.; Searle, J.R.; Worsley, D.A.; Watson, T.M. Efficient and semi-transparent perovskite solar cells using a room-temperature processed MoOx/ITO/Ag/ITO electrode. J. Mater. Chem. C 2019, 7, 10981–10987. [Google Scholar] [CrossRef]
- Islam, M.B.; Yanagida, M.; Shirai, Y.; Nabetani, Y.; Miyano, K. Highly stable semi-transparent MAPbI3 perovskite solar cells with operational output for 4000 h. Sol. Energy Mater. Sol. Cells 2019, 195, 323–329. [Google Scholar] [CrossRef]
- Ahmad, T.; Wilk, B.; Radicchi, E.; Pineda, R.F.; Spinelli, P.; Herterich, J.; Castriotta, L.A.; Dasgupta, S.; Mosconi, E.; De Angelis, F.; et al. New fullerene derivative as an n-type material for highly efficient, flexible perovskite solar cells of a p-i-n configuration. Adv. Funct. Mater. 2020, 30, 2004357. [Google Scholar] [CrossRef]
- Yang, H.; Kwon, H.-C.; Ma, S.; Kim, K.M.; Yun, S.-C.; Jang, G.; Park, J.; Lee, H.; Goh, S.; Moon, J. Energy level-graded al-doped ZnO protection layers for copper nanowire-based window electrodes for efficient flexible perovskite solar cells. ACS Appl. Mater. Interfaces 2020, 12, 13824–13835. [Google Scholar] [CrossRef] [PubMed]
- Tavakoli, M.M.; Yadav, P.; Prochowicz, D.; Tavakoli, R. Efficient, hysteresis-free, and flexible inverted perovskite solar cells using all-vacuum processing. Sol. RRL 2021, 5. [Google Scholar] [CrossRef]
- Yang, D.; Yang, R.; Priya, S.; Liu, S. Recent advances in flexible perovskite solar cells: Fabrication and applications. Angew. Chem. Int. Ed. 2019, 58, 4466–4483. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Jia, C.; Fan, Z.; Lin, Z.; Lee, S.-J.; Atallah, T.L.; Caram, J.R.; Huang, Y.; Duan, X. Large-area synthesis and patterning of all-inorganic lead halide perovskite thin films and heterostructures. Nano Lett. 2021, 21, 1454–1460. [Google Scholar] [CrossRef]
- Zhao, Y.; Deng, Q.; Guo, R.; Wu, Z.; Li, Y.; Duan, Y.; Shen, Y.; Zhang, W.; Shao, G. Sputtered Ga-doped SnOx electron transport layer for large-area all-inorganic perovskite solar cells. ACS Appl. Mater. Interfaces 2020, 12, 54904–54915. [Google Scholar] [CrossRef]
- Lee, D.-K.; Jeong, D.-N.; Ahn, T.K.; Park, N.-G. Precursor engineering for a large-area perovskite solar cell with >19% efficiency. ACS Energy Lett. 2019, 4, 2393–2401. [Google Scholar] [CrossRef]
- Lai, X.; Meng, F.; Zhang, Q.; Wang, K.; Li, G.; Wen, Y.; Ma, H.; Li, W.; Li, X.; Kyaw, A.K.K.; et al. A bifunctional saddle-shaped small molecule as a dopant-free hole transporting material and interfacial layer for efficient and stable perovskite solar cells. Sol. RRL 2019, 3, 5. [Google Scholar] [CrossRef]
- Liu, S.; Li, S.; Wu, J.; Wang, Q.; Ming, Y.; Zhang, D.; Sheng, Y.; Hu, Y.; Rong, Y.; Mei, A.; et al. Amide additives induced a fermi level shift to improve the performance of hole-conductor-free, printable mesoscopic perovskite solar cells. J. Phys. Chem. Lett. 2019, 10, 6865–6872. [Google Scholar] [CrossRef] [PubMed]
- Yoo, J.J.; Seo, G.; Chua, M.R.; Park, T.G.; Lu, Y.; Rotermund, F.; Kim, Y.-K.; Moon, C.S.; Jeon, N.J.; Correa-Baena, J.-P.; et al. Efficient perovskite solar cells via improved carrier management. Nature 2021, 590, 587–593. [Google Scholar] [CrossRef]
- Bowman, A.R.; Lang, F.; Chiang, Y.-H.; Jiménez-Solano, A.; Frohna, K.; Eperon, G.E.; Ruggeri, E.; Abdi-Jalebi, M.; Anaya, M.; Lotsch, B.V.; et al. Relaxed current matching requirements in highly luminescent perovskite tandem solar cells and their fundamental efficiency limits. ACS Energy Lett. 2021, 6, 612–620. [Google Scholar] [CrossRef] [PubMed]
- Al-Ashouri, A.; Köhnen, E.; Li, B.; Magomedov, A.; Hempel, H.; Caprioglio, P.; Márquez, J.A.; Vilches, A.B.M.; Kasparavicius, E.; Smith, J.A.; et al. Monolithic perovskite/silicon tandem solar cell with >29% efficiency by enhanced hole extraction. Science 2020, 370, 1300–1309. [Google Scholar] [CrossRef] [PubMed]
- Schulze, P.S.C.; Bett, A.J.; Bivour, M.; Caprioglio, P.; Gerspacher, F.M.; Kabaklı, Ö.Ş.; Richter, A.; Stolterfoht, M.; Zhang, Q.; Neher, D.; et al. 25.1% high-efficiency monolithic perovskite silicon tandem solar cell with a high bandgap perovskite absorber. Sol. RRL 2020, 4, 2000152. [Google Scholar] [CrossRef]
- Chen, B.; Yu, Z.J.; Manzoor, S.; Wang, S.; Weigand, W.; Yu, Z.; Yang, G.; Ni, Z.; Dai, X.; Holman, Z.C.; et al. Blade-coated perovskites on textured silicon for 26%-efficient monolithic perovskite/silicon tandem solar cells. Joule 2020, 4, 850–864. [Google Scholar] [CrossRef]
- Si, F.; Tang, F.; Xue, H.; Qi, R. Effects of defect states on the performance of perovskite solar cells. J. Semicond. 2016, 37, 072003. [Google Scholar] [CrossRef]
- Cao, K.; Li, H.; Liu, S.; Cui, J.; Shen, Y.; Wang, M. MAPbI3−xBrxmixed halide perovskites for fully printable mesoscopic solar cells with enhanced efficiency and less hysteresis. Nanoscale 2016, 8, 8839–8846. [Google Scholar] [CrossRef]
- Wang, F.; Yang, M.; Zhang, Y.; Du, J.; Yang, S.; Yang, L.; Fan, L.; Sui, Y.; Sun, Y.; Yang, J. Full-scale chemical and field-effect passivation: 21.52% efficiency of stable MAPbI3 solar cells via benzenamine modification. Nano Res. 2021, 1–7. [Google Scholar] [CrossRef]
- Jung, M.-C.; Qi, Y. Dopant interdiffusion effects in n-i-p structured spiro-OMeTAD hole transport layer of organometal halide perovskite solar cells. Org. Electron. 2016, 31, 71–76. [Google Scholar] [CrossRef]
- Jena, A.K.; Ikegami, M.; Miyasaka, T. Severe morphological deformation of spiro-OMeTAD in (CH3NH3)PbI3 solar cells at high temperature. ACS Energy Lett. 2017, 2, 1760–1761. [Google Scholar] [CrossRef]
- Li, J.; Wang, H.; Chin, X.Y.; Dewi, H.A.; Vergeer, K.; Goh, T.W.; Lim, J.W.M.; Lew, J.H.; Loh, K.P.; Soci, C.; et al. Highly efficient thermally co-evaporated perovskite solar cells and mini-modules. Joule 2020, 4, 1035–1053. [Google Scholar] [CrossRef]
- Roß, M.; Gil-Escrig, L.; Al-Ashouri, A.; Tockhorn, P.; Jošt, M.; Rech, B.; Albrecht, S. Co-evaporated p-i-n perovskite solar cells beyond 20% efficiency: Impact of substrate temperature and hole-transport layer. ACS Appl. Mater. Interfaces 2020, 12, 39261–39272. [Google Scholar] [CrossRef] [PubMed]
- Kottokkaran, R.; Gaonkar, H.A.; Abbas, H.A.; Noack, M.; Dalal, V. Performance and stability of co-evaporated vapor deposited perovskite solar cells. J. Mater. Sci. Mater. Electron. 2019, 30, 5487–5494. [Google Scholar] [CrossRef]
- Yang, W.S.; Noh, J.H.; Jeon, N.J.; Kim, Y.C.; Ryu, S.; Seo, J.; Seok, S.I. High-performance photovoltaic perovskite layers fabricated through intramolecular exchange. Science 2015, 348, 1234–1237. [Google Scholar] [CrossRef] [PubMed]
- Liang, G.-X.; Fan, P.; Gu, D.; Zheng, Z.-H.; Zhang, D.-P.; Luo, J.-T.; Zhang, X.-H.; Ma, H.-L. Enhanced crystallinity and performance of CH3NH3PbI3 thin film prepared by controlling hot CH3NH3I solution onto evaporated PbI2 nanocrystal. IEEE J. Photovoltaics 2016, 6, 1537–1541. [Google Scholar] [CrossRef]
- Zhu, N.; Qi, X.; Zhang, Y.; Liu, G.; Wu, C.; Wang, D.; Guo, X.; Luo, W.; Li, X.; Hu, H.; et al. High efficiency (18.53%) of flexible perovskite solar cells via the insertion of potassium chloride between SnO2 and CH3NH3PbI3 layers. ACS Appl. Energy Mater. 2019, 2, 3676–3682. [Google Scholar] [CrossRef]
- Carrillo, J.; Guerrero, A.; Rahimnejad, S.; Almora, O.; Zarazua, I.; Mas-Marza, E.; Bisquert, J.; Garcia-Belmonte, G. Ionic reactivity at contacts and aging of methylammonium lead triiodide perovskite solar cells. Adv. Energy Mater. 2016, 6, 1502246. [Google Scholar] [CrossRef]
- Xia, J.; Luo, J.; Yang, H.; Wan, Z.; Malik, H.A.; Shi, Y.; Yao, X.; Jia, C. Interface induced in-situ vertical phase separation from MAPbI3:Spiro-OMeTAD precursors for perovskite solar cells. Sol. Energy Mater. Sol. Cells 2020, 216, 110689. [Google Scholar] [CrossRef]
- Ponseca, C.S.; Hutter, E.M.; Piatkowski, P.; Cohen, B.; Pascher, T.; Douhal, A.; Yartsev, A.; Sundström, V.; Savenije, T.J. Mechanism of charge transfer and recombination dynamics in organo metal halide perovskites and organic electrodes, PCBM, and spiro-OMeTAD: Role of dark carriers. J. Am. Chem. Soc. 2015, 137, 16043–16048. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Tu, L.; Li, H.; Li, S.; Yang, S.-E.; Chen, Y. A feasible and effective post-treatment method for high-quality CH3NH3PbI3 films and high-efficiency perovskite solar cells. Crystals 2018, 8, 44. [Google Scholar] [CrossRef]
- Wu, S.; Zheng, Y.; Liu, Q.; Li, R.; Peng, T. Low cost and solution-processable zinc phthalocyanine as alternative hole transport material for perovskite solar cells. RSC Adv. 2016, 6, 107723–107731. [Google Scholar] [CrossRef]
- Wang, Y.; Mahmoudi, T.; Yang, H.-Y.; Bhat, K.S.; Yoo, J.-Y.; Hahn, Y.-B. Fully-ambient-processed mesoscopic semitransparent perovskite solar cells by islands-structure-MAPbI3-xClx-NiO composite and Al2O3/NiO interface engineering. Nano Energy 2018, 49, 59–66. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, T.; Xu, F.; Wang, Y.; Li, G.; Yang, Y.; Zhao, Y. CH3NH3Cl assisted solvent engineering for highly crystallized and large grain size mixed-composition (FAPbI3)0.85(MAPbBr3)0.15 perovskites. Crystals 2017, 7, 272. [Google Scholar] [CrossRef]
- Shan, D.; Tong, G.; Cao, Y.; Tang, M.; Xu, J.; Yu, L.; Chen, K. The effect of decomposed PbI2 on microscopic mechanisms of scattering in CH3NH3PbI3 films. Nanoscale Res. Lett. 2019, 14, 208. [Google Scholar] [CrossRef]
- Jiang, Y.; Pan, L.; Wei, D.; Li, W.; Li, S.; Yang, S.-E.; Shi, Z.; Guo, H.; Xia, T.; Zang, J.; et al. The modified multi-step thermal annealing process for highly efficient MAPbI3-based perovskite solar cells. Sol. Energy 2018, 174, 218–224. [Google Scholar] [CrossRef]
- Chen, L.-C.; Lee, K.-L.; Wu, W.-T.; Hsu, C.-F.; Tseng, Z.-L.; Sun, X.H.; Kao, Y.-T. Effect of different CH3NH3PbI3 morphologies on photovoltaic properties of perovskite solar cells. Nanoscale Res. Lett. 2018, 13, 140. [Google Scholar] [CrossRef]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, Y.-T.; Tien, C.-H.; Lee, K.-Y.; Tung, Y.-S.; Chen, L.-C. Evaporated MAPbI3 Perovskite Planar Solar Cells with Different Annealing Temperature. Energies 2021, 14, 2145. https://doi.org/10.3390/en14082145
Chang Y-T, Tien C-H, Lee K-Y, Tung Y-S, Chen L-C. Evaporated MAPbI3 Perovskite Planar Solar Cells with Different Annealing Temperature. Energies. 2021; 14(8):2145. https://doi.org/10.3390/en14082145
Chicago/Turabian StyleChang, Yi-Tsung, Ching-Ho Tien, Kun-Yi Lee, Yu-Shen Tung, and Lung-Chien Chen. 2021. "Evaporated MAPbI3 Perovskite Planar Solar Cells with Different Annealing Temperature" Energies 14, no. 8: 2145. https://doi.org/10.3390/en14082145
APA StyleChang, Y.-T., Tien, C.-H., Lee, K.-Y., Tung, Y.-S., & Chen, L.-C. (2021). Evaporated MAPbI3 Perovskite Planar Solar Cells with Different Annealing Temperature. Energies, 14(8), 2145. https://doi.org/10.3390/en14082145







