Low-Rank Coal Supported Ni Catalysts for CO2 Methanation
Abstract
1. Introduction
2. Experimental
2.1. Preparation of Catalysts
2.2. Evaluation of CO2 Methanation Activity
2.3. Instrumental Analysis
3. Results and Discussion
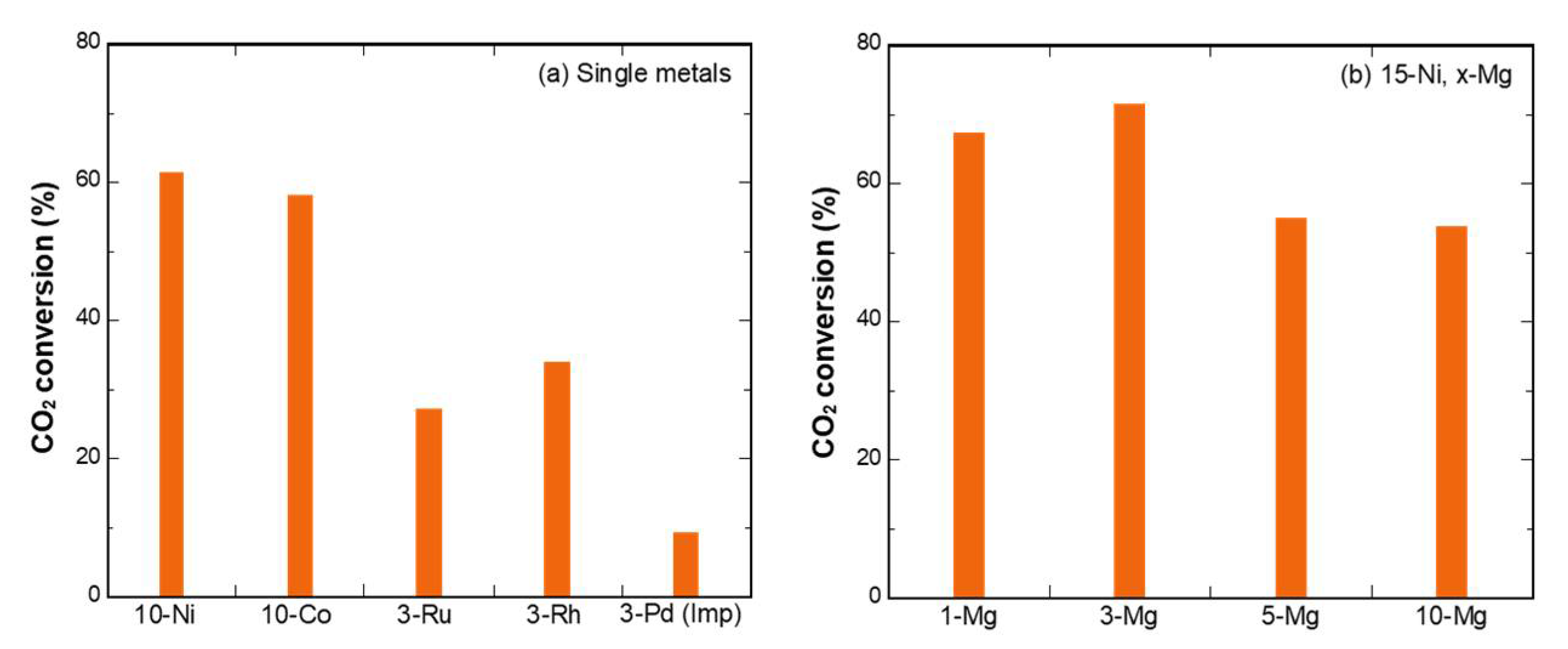
3.1. Metal Screening for CO2 Methanation
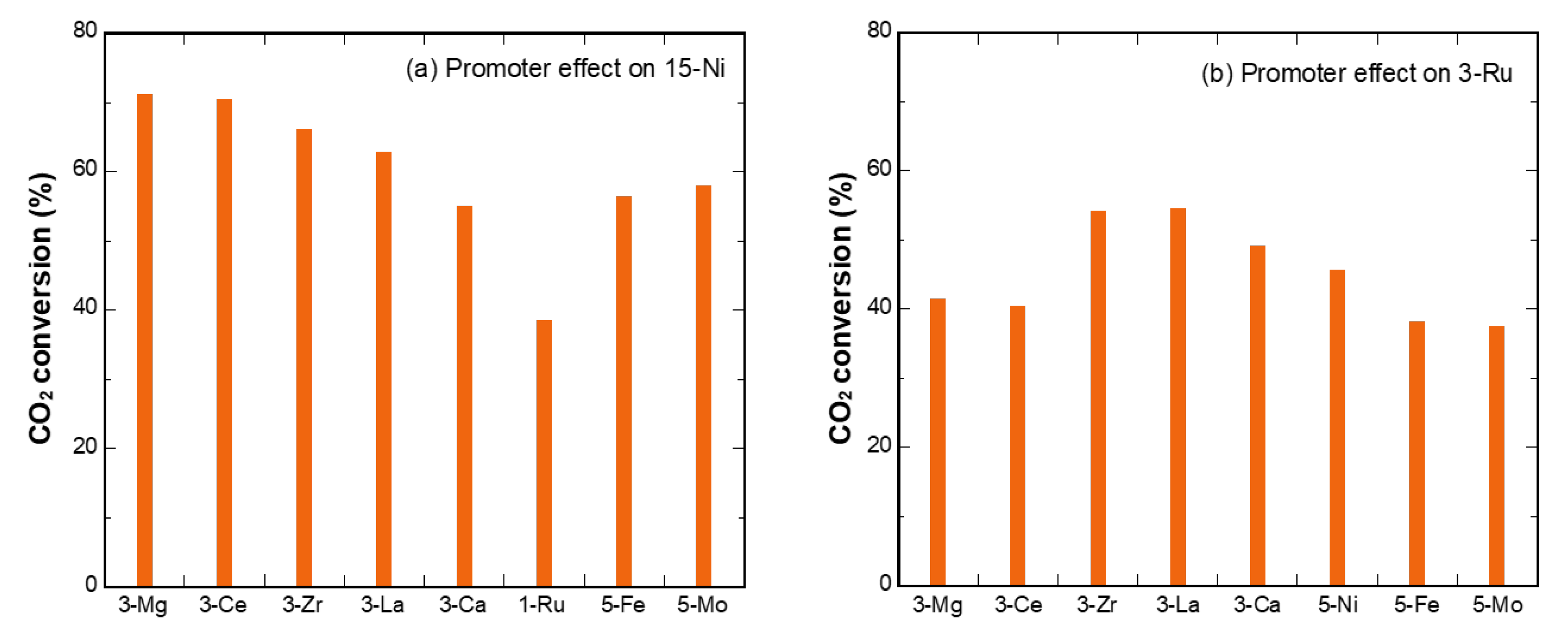
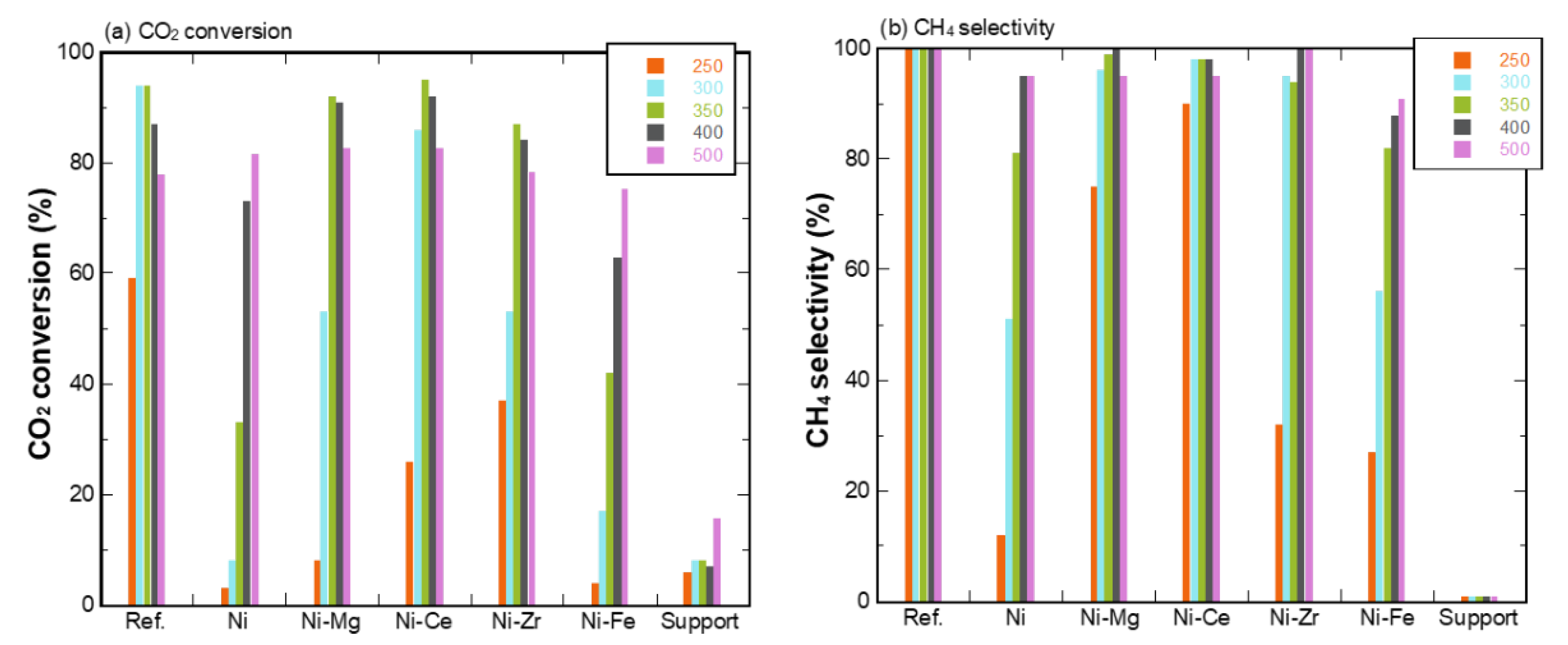
3.2. Temperature Dependence of Promoter-Doped Ni/Eco
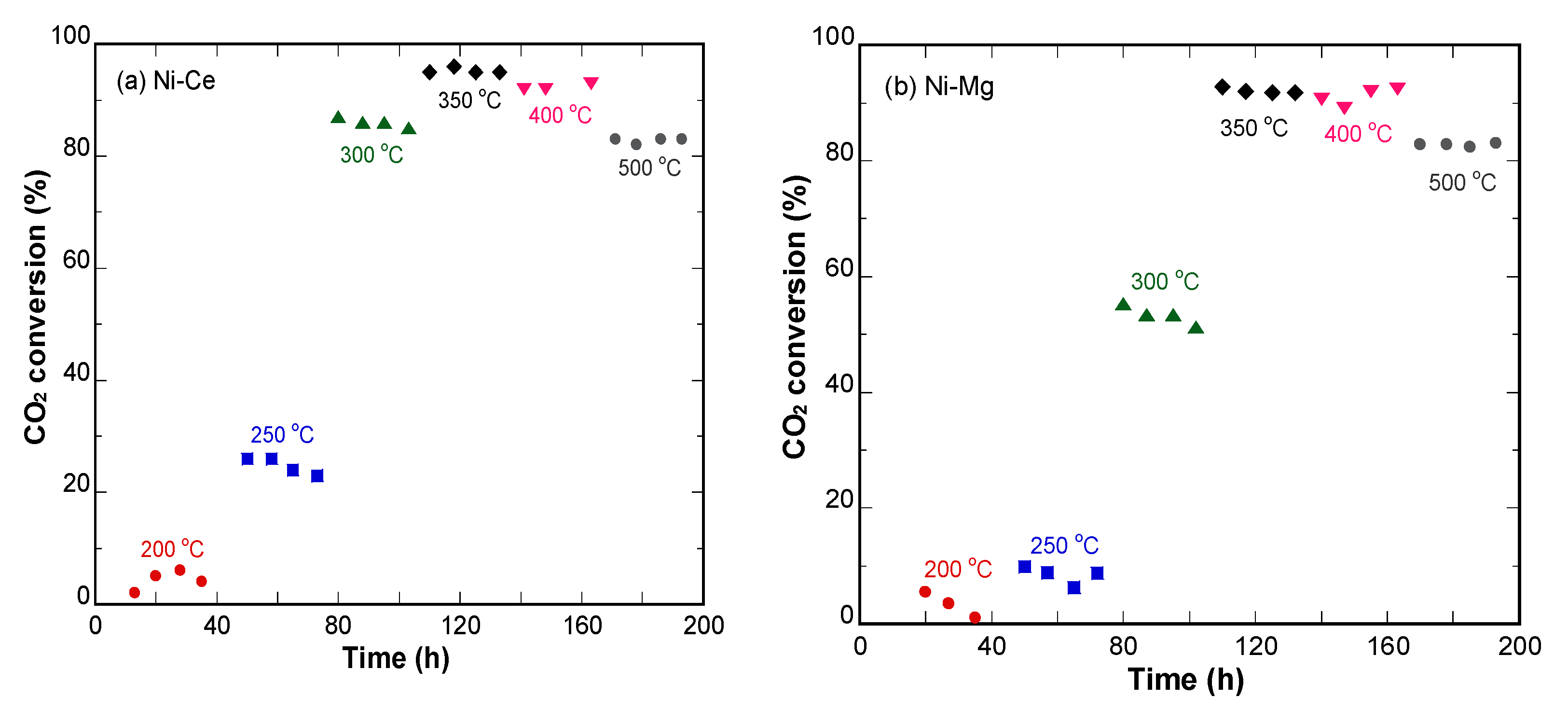
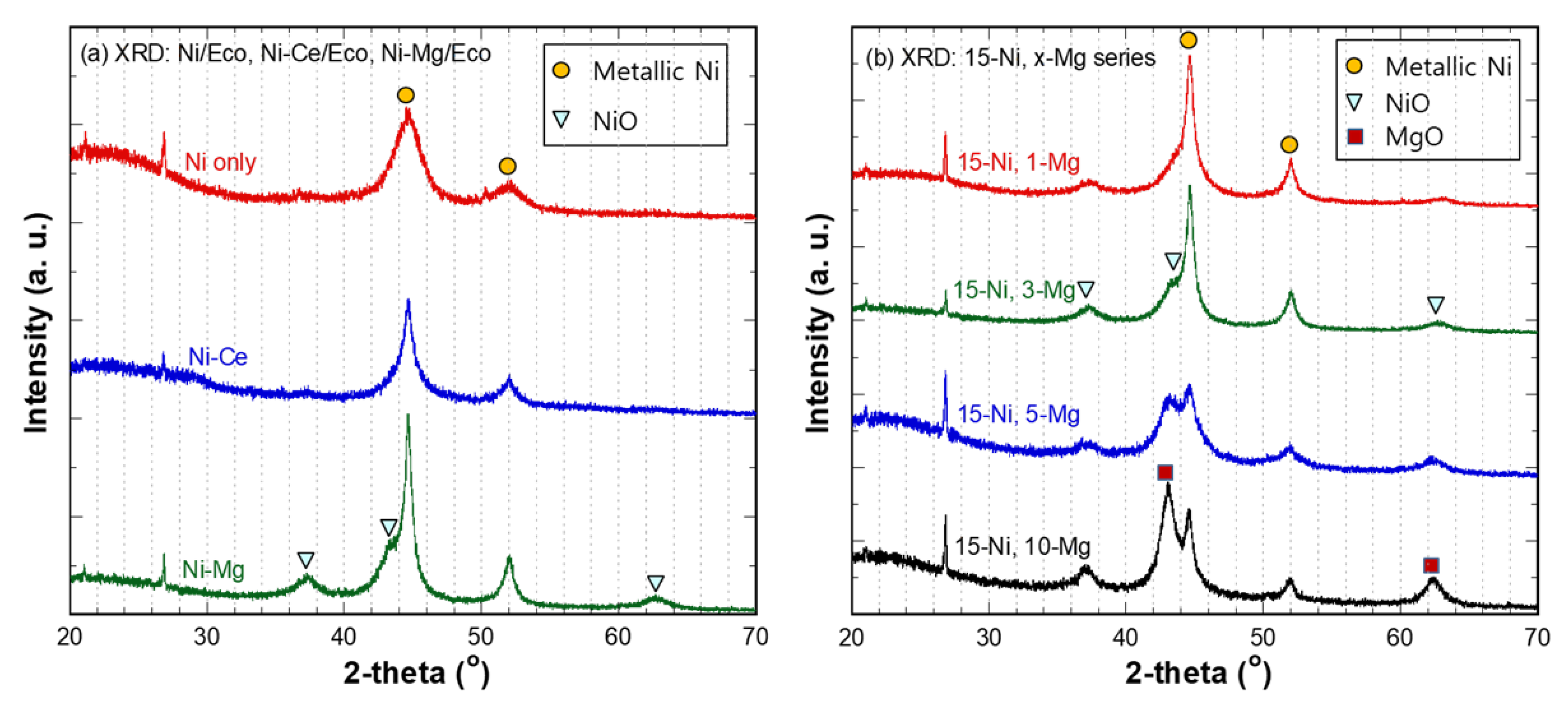
3.3. Characterization of Catalysts
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zhou, S.; Sun, K.; Wu, Z.; Gu, W.; Wu, G.; Li, Z.; Li, J. Optimized operation method of small and medium-sized integrated energy system for P2G equipment under strong uncertainty. Energy 2020, 199, 117269. [Google Scholar] [CrossRef]
- Götz, M.; Lefebvre, J.; Mörs, F.; Koch, A.M.; Graf, F.; Bajohr, S.; Reimert, R.; Kolb, T. Renewable Power-to-Gas: A technological and economic review. Renew. Energy 2016, 85, 1371–1390. [Google Scholar] [CrossRef]
- Guandalini, G.; Campanari, S.; Romano, M.C. Power-to-gas plants and gas turbines for improved wind energy dispatchability: Energy and economic assessment. Appl. Energy 2015, 147, 117–130. [Google Scholar] [CrossRef]
- Baysal, Z.; Kureti, S. CO2 methanation on Mg-promoted Fe catalysts. Appl. Catal. B Environ. 2020, 262, 118300. [Google Scholar] [CrossRef]
- Beuls, A.; Swalus, C.; Jacquemin, M.; Heyen, G.; Karelovic, A.; Ruiz, P. Methanation of CO2: Further insight into the mechanism over Rh/γ-Al2O3 catalyst. Appl. Catal. B Environ. 2012, 113, 2–10. [Google Scholar] [CrossRef]
- Bassano, C.; Deiana, P.; Lietti, L.; Visconti, C.G. P2G movable modular plant operation on synthetic methane production from CO2 and hydrogen from renewables sources. Fuel 2019, 253, 1071–1079. [Google Scholar] [CrossRef]
- Mebrahtu, C.; Krebs, F.; Abate, S.; Perathoner, S.; Centi, G.; Palkovits, R. CO2 Methanation: Principles and Challenges. Rapid Acting Antidepressants 2019, 178, 85–103. [Google Scholar] [CrossRef]
- Aziz, M.A.A.; Jalil, A.A.; Triwahyono, S.; Ahmad, A. CO2 methanation over heterogeneous catalysts: Recent progress and future prospects. Green Chem. 2015, 17, 2647–2663. [Google Scholar] [CrossRef]
- Schlereth, D.; Hinrichsen, O. A fixed-bed reactor modeling study on the methanation of CO2. Chem. Eng. Res. Des. 2014, 92, 702–712. [Google Scholar] [CrossRef]
- Ocampo, F.; Louis, B.; Kiwi-Minsker, L.; Roger, A.-C. Effect of Ce/Zr composition and noble metal promotion on nickel based CexZr1−xO2 catalysts for carbon dioxide methanation. Appl. Catal. A Gen. 2011, 392, 36–44. [Google Scholar] [CrossRef]
- Hidalgo, D.; Martín-Marroquín, J. Power-to-methane, coupling CO2 capture with fuel production: An overview. Renew. Sustain. Energy Rev. 2020, 132, 110057. [Google Scholar] [CrossRef]
- Esa, Y.A.M.; Sapawe, N. A short review on carbon dioxide (CO2) methanation process. Mater. Today Proc. 2020, 31, 394–397. [Google Scholar] [CrossRef]
- Renda, S.; Ricca, A.; Palma, V. Precursor salts influence in Ruthenium catalysts for CO2 hydrogenation to methane. Appl. Energy 2020, 279, 115767. [Google Scholar] [CrossRef]
- Nam, H.; Kim, J.H.; Kim, H.; Kim, M.J.; Jeon, S.-G.; Jin, G.-T.; Won, Y.; Hwang, B.W.; Lee, S.-Y.; Baek, J.-I.; et al. CO2 methanation in a bench-scale bubbling fluidized bed reactor using Ni-based catalyst and its exothermic heat transfer analysis. Energy 2021, 214, 118895. [Google Scholar] [CrossRef]
- Song, H.; Yang, J.; Zhao, J.; Chou, L. Methanation of Carbon Dioxide over a Highly Dispersed Ni/La2O3 Catalyst. Chin. J. Catal. 2010, 31, 21–23. [Google Scholar] [CrossRef]
- Ye, R.-P.; Gong, W.; Sun, Z.; Sheng, Q.; Shi, X.; Wang, T.; Yao, Y.; Razink, J.J.; Lin, L.; Zhou, Z.; et al. Enhanced stability of Ni/SiO2 catalyst for CO2 methanation: Derived from nickel phyllosilicate with strong metal-support interactions. Energy 2019, 188, 116059–116068. [Google Scholar] [CrossRef]
- Liu, Q.; Tian, Y. One-pot synthesis of NiO/SBA-15 monolith catalyst with a three-dimensional framework for CO2 methanation. Int. J. Hydrogen Energy 2017, 42, 12295–12300. [Google Scholar] [CrossRef]
- Aziz, M.; Jalil, A.; Triwahyono, S.; Mukti, R.; Taufiq-Yap, Y.; Sazegar, M. Highly active Ni-promoted mesostructured silica nanoparticles for CO2 methanation. Appl. Catal. B Environ. 2014, 147, 359–368. [Google Scholar] [CrossRef]
- Yin, S.; Zhu, L.; Liu, Y.; Wang, X.; Liu, Y.; Wang, S. Effect of Ni Precipitation Method on CO Methanation over Ni/TiO2 Catalysts. Chem. Res. Chin. Univ. 2018, 34, 296–301. [Google Scholar] [CrossRef]
- Lippi, R.; Howard, S.C.; Barron, H.; Easton, C.D.; Madsen, I.C.; Waddington, L.J.; Vogt, C.; Hill, M.R.; Sumby, C.J.; Doonan, C.J.; et al. Highly active catalyst for CO2 methanation derived from a metal organic framework template. J. Mater. Chem. A 2017, 5, 12990–12997. [Google Scholar] [CrossRef]
- Ye, R.-P.; Liao, L.; Reina, T.R.; Liu, J.; Chevella, D.; Jin, Y.; Fan, M.; Liu, J. Engineering Ni/SiO2 catalysts for enhanced CO2 methanation. Fuel 2021, 285, 119151. [Google Scholar] [CrossRef]
- Rui, N.; Zhang, X.; Zhang, F.; Liu, Z.; Cao, X.; Xie, Z.; Zou, R.; Senanayake, S.D.; Yang, Y.; Rodriguez, J.A.; et al. Highly active Ni/CeO2 catalyst for CO2 methanation: Preparation and characterization. Appl. Catal. B Environ. 2021, 282, 119581. [Google Scholar] [CrossRef]
- Lu, H.; Yang, X.; Gao, G.; Wang, J.; Han, C.; Liang, X.; Li, C.; Li, Y.; Zhang, W.; Chen, X. Metal (Fe, Co, Ce or La) doped nickel catalyst supported on ZrO2 modified mesoporous clays for CO and CO2 methanation. Fuel 2016, 183, 335–344. [Google Scholar] [CrossRef]
- Taherian, Z.; Khataee, A.; Orooji, Y. Promoted nickel-based catalysts on modified mesoporous silica support: The role of yttria and magnesia on CO2 methanation. Microporous Mesoporous Mater. 2020, 306, 110455. [Google Scholar] [CrossRef]
- Lippi, R.; D’Angelo, A.M.; Li, C.; Howard, S.C.; Madsen, I.C.; Wilson, K.; Lee, A.F.; Sumby, C.J.; Doonan, C.J.; Patel, J.; et al. Unveiling the structural transitions during activation of a CO2 methanation catalyst Ru0/ZrO2 synthesised from a MOF precursor. Catal. Today 2020. [Google Scholar] [CrossRef]
- Swalus, C.; Jacquemin, M.; Poleunis, C.; Bertrand, P.; Ruiz, P. CO2 methanation on Rh/γ-Al2O3 catalyst at low temperature: “In situ” supply of hydrogen by Ni/activated carbon catalyst. Appl. Catal. B Environ. 2012, 125, 41–50. [Google Scholar] [CrossRef]
- Wang, X.; Liu, Y.; Zhu, L.; Li, Y.; Wang, K.; Qiu, K.; Tippayawong, N.; Aggarangsi, P.; Reubroycharoen, P.; Wang, S. Biomass derived N-doped biochar as efficient catalyst supports for CO2 methanation. J. CO2 Util. 2019, 34, 733–741. [Google Scholar] [CrossRef]
- Wang, X.; Yang, M.; Zhu, X.; Zhu, L.; Wang, S. Experimental study and life cycle assessment of CO2 methanation over biochar supported catalysts. Appl. Energy 2020, 280, 115919. [Google Scholar] [CrossRef]
- Romero-Sáez, M.; Dongil, A.; Benito, N.; Espinoza-González, R.; Escalona, N.; Gracia, F. CO2 methanation over nickel-ZrO2 catalyst supported on carbon nanotubes: A comparison between two impregnation strategies. Appl. Catal. B Environ. 2018, 237, 817–825. [Google Scholar] [CrossRef]
- Wang, J.; You, Z.; Zhang, Q.; Deng, W.; Wang, Y. Synthesis of lower olefins by hydrogenation of carbon dioxide over supported iron catalysts. Catal. Today 2013, 215, 186–193. [Google Scholar] [CrossRef]
- Kwak, J.H.; Kovarik, L.; Szanyi, J. Heterogeneous Catalysis on Atomically Dispersed Supported Metals: CO2 Reduction on Multifunctional Pd Catalysts. ACS Catal. 2013, 3, 2094–2100. [Google Scholar] [CrossRef]
- Kim, S.; Prajitno, H.; Yoo, J.; Kim, S.; Chun, D.; Lim, J.; Choi, H.; Lee, S.; Im, H. Dispersion behavior of various single metals on carbonaceous coal supports and their reactivity in methanol steam reforming. J. Ind. Eng. Chem. 2021, 94, 317–325. [Google Scholar] [CrossRef]
- Kim, S.; Chun, D.; Rhim, Y.; Lim, J.; Kim, S.; Choi, H.; Lee, S.; Yoo, J. Catalytic reforming of toluene using a nickel ion-exchanged coal catalyst. Int. J. Hydrogen Energy 2015, 40, 11855–11862. [Google Scholar] [CrossRef]
- Ruhswurmova, N.; Kim, S.; Yoo, J.; Chun, D.; Rhim, Y.; Lim, J.; Kim, S.; Choi, H.; Lee, S. Nickel supported on low-rank coal for steam reforming of ethyl acetate. Int. J. Hydrogen Energy 2018, 43, 15880–15890. [Google Scholar] [CrossRef]
- Frontera, P.; Macario, A.; Ferraro, M.; Antonucci, P. Supported Catalysts for CO2 Methanation: A Review. Catalysts 2017, 7, 59. [Google Scholar] [CrossRef]
- Karelovic, A.; Ruiz, P. CO2 hydrogenation at low temperature over Rh/γ-Al2O3 catalysts: Effect of the metal particle size on catalytic performances and reaction mechanism. Appl. Catal. B Environ. 2012, 113, 237–249. [Google Scholar] [CrossRef]
- Veith, G.M.; Lupini, A.R.; Rashkeev, S.; Pennycook, S.J.; Mullins, D.R.; Schwartz, V.; Bridges, C.A.; Dudney, N.J. Thermal stability and catalytic activity of gold nanoparticles supported on silica. J. Catal. 2009, 262, 92–101. [Google Scholar] [CrossRef]
- Rodríguez-Reinoso, F. The role of carbon materials in heterogeneous catalysis. Carbon 1998, 36, 159–175. [Google Scholar] [CrossRef]
- Takano, H.; Shinomiya, H.; Izumiya, K.; Kumagai, N.; Habazaki, H.; Hashimoto, K. CO2 methanation of Ni catalysts supported on tetragonal ZrO2 doped with Ca2+ and Ni2+ ions. Int. J. Hydrogen Energy 2015, 40, 8347–8355. [Google Scholar] [CrossRef]
- Tada, S.; Shimizu, T.; Kameyama, H.; Haneda, T.; Kikuchi, R. Ni/CeO2 catalysts with high CO2 methanation activity and high CH4 selectivity at low temperatures. Int. J. Hydrogen Energy 2012, 37, 5527–5531. [Google Scholar] [CrossRef]
- Wang, X.; Zhen, T.; Yu, C. Application of Ni–Al-hydrotalcite-derived catalyst modified with Fe or Mg in CO2 methanation. Appl. Petrochem. Res. 2016, 6, 217–223. [Google Scholar] [CrossRef]
- Pan, Z.; Dong, M.; Meng, X.; Zhang, X.; Mu, X.; Zong, B. Integration of magnetically stabilized bed and amorphous nickel alloy catalyst for CO methanation. Chem. Eng. Sci. 2007, 62, 2712–2717. [Google Scholar] [CrossRef]
- Ashok, J.; Ang, M.; Kawi, S. Enhanced activity of CO2 methanation over Ni/CeO2-ZrO2 catalysts: Influence of preparation methods. Catal. Today 2017, 281, 304–311. [Google Scholar] [CrossRef]
- Westermann, A.; Azambre, B.; Bacariza, M.; Graça, I.; Ribeiro, M.; Lopes, J.; Henriques, C. Insight into CO2 methanation mechanism over NiUSY zeolites: An operando IR study. Appl. Catal. B Environ. 2015, 174–175, 120–125. [Google Scholar] [CrossRef]
- Rahmani, S.; Rezaei, M.; Meshkani, F. Preparation of promoted nickel catalysts supported on mesoporous nanocrystalline gamma alumina for carbon dioxide methanation reaction. J. Ind. Eng. Chem. 2014, 20, 4176–4182. [Google Scholar] [CrossRef]
- Auer, E.; Freund, A.; Pietsch, J.; Tacke, T. Carbons as supports for industrial precious metal catalysts. Appl. Catal. A Gen. 1998, 173, 259–271. [Google Scholar] [CrossRef]
- Bacariza, M.C.; Graça, I.; Bebiano, S.S.; Lopes, J.M.; Henriques, C. Magnesium as Promoter of CO2 Methanation on Ni-Based USY Zeolites. Energy Fuels 2017, 31, 9776–9789. [Google Scholar] [CrossRef]
- Karelovic, A.; Ruiz, P. Mechanistic study of low temperature CO2 methanation over Rh/TiO2 catalysts. J. Catal. 2013, 301, 141–153. [Google Scholar] [CrossRef]
- Marwood, M.; Doepper, R.; Renken, A. In-situ surface and gas phase analysis for kinetic studies under transient conditions the catalytic hydrogenation of CO2. Appl. Catal. A Gen. 1997, 151, 223–246. [Google Scholar] [CrossRef]
- Cárdenas-Arenas, A.; Cortés, H.S.; Bailón-García, E.; Davó-Quiñonero, A.; Lozano-Castelló, D.; Bueno-López, A. Active, selective and stable NiO-CeO2 nanoparticles for CO2 methanation. Fuel Process. Technol. 2021, 212, 106637. [Google Scholar] [CrossRef]
- Cai, M.; Wen, J.; Chu, W.; Cheng, X.; Li, Z. Methanation of carbon dioxide on Ni/ZrO2-Al2O3 catalysts: Effects of ZrO2 promoter and preparation method of novel ZrO2-Al2O3 carrier. J. Nat. Gas Chem. 2011, 20, 318–324. [Google Scholar] [CrossRef]
- Fu, T.; Li, Z. Review of recent development in Co-based catalysts supported on carbon materials for Fischer–Tropsch synthesis. Chem. Eng. Sci. 2015, 135, 3–20. [Google Scholar] [CrossRef]
- Storck, S.; Bretinger, H.; Maier, W.F. Characterization of micro- and mesoporous solids by physisorption methods and pore-size analysis. Appl. Catal. A Gen. 1998, 174, 137–146. [Google Scholar] [CrossRef]
- Sotomayor, F.; Cychosz, K.A.; Thommes, M. Characterization of Micro/Mesoporous Materials by physisorption: Concepts and Case Studies. Acc. Mater. Surf. Res. 2018, 3, 24–50. [Google Scholar]
- Wang, S.; Lu, G.M. Role of CeO2 in Ni/CeO2–Al2O3 catalysts for carbon dioxide reforming of methane. Appl. Catal. B Environ. 1998, 19, 267–277. [Google Scholar] [CrossRef]
- Wang, C.-Y.; Li, S.-Y.; Yang, X.-R.; Ren, J.; Chen, Y.-G. CH4CO2 reforming anti-carbon deposition catalyst. Energy Convers. Manag. 1996, 37, 1357–1361. [Google Scholar] [CrossRef]
- Zangouei, M.; Moghaddam, A.Z.; Arasteh, M. The influence of nickel loading on reducibility of NiO/Al2O3 catalysts synthesized by sol-gel method. Chem. Eng. Res. Bull. 2010, 14, 97–102. [Google Scholar] [CrossRef]
- Clause, O. Effect of the preparation method on the thermal stability of silica-supported nickel oxide as studied by EXAFS and TPR techniques. J. Catal. 1992, 138, 195–205. [Google Scholar] [CrossRef]
- Marconi, E.; Tuti, S.; Luisetto, I. Structure-Sensitivity of CO2 Methanation over Nanostructured Ni Supported on CeO2 Nanorods. Catalyst 2019, 9, 375. [Google Scholar] [CrossRef]
- Diskin, A.M.; Cunningham, R.H.; Ormerod, R. The oxidative chemistry of methane over supported nickel catalysts. Catal. Today 1998, 46, 147–154. [Google Scholar] [CrossRef]
- Kumar, M.; Aberuagba, F.; Gupta, J.; Rawat, K.; Sharma, L.; Dhar, G.M. Temperature-programmed reduction and acidic properties of molybdenum supported on MgO–Al2O3 and their correlation with catalytic activity. J. Mol. Catal. A Chem. 2004, 213, 217–223. [Google Scholar] [CrossRef]
- Laguna, O.; Centeno, M.A.; Sarria, F.R.; Odriozola, J.A. Oxidation of CO over gold supported on Zn-modified ceria catalysts. Catal. Today 2011, 172, 118–123. [Google Scholar] [CrossRef]
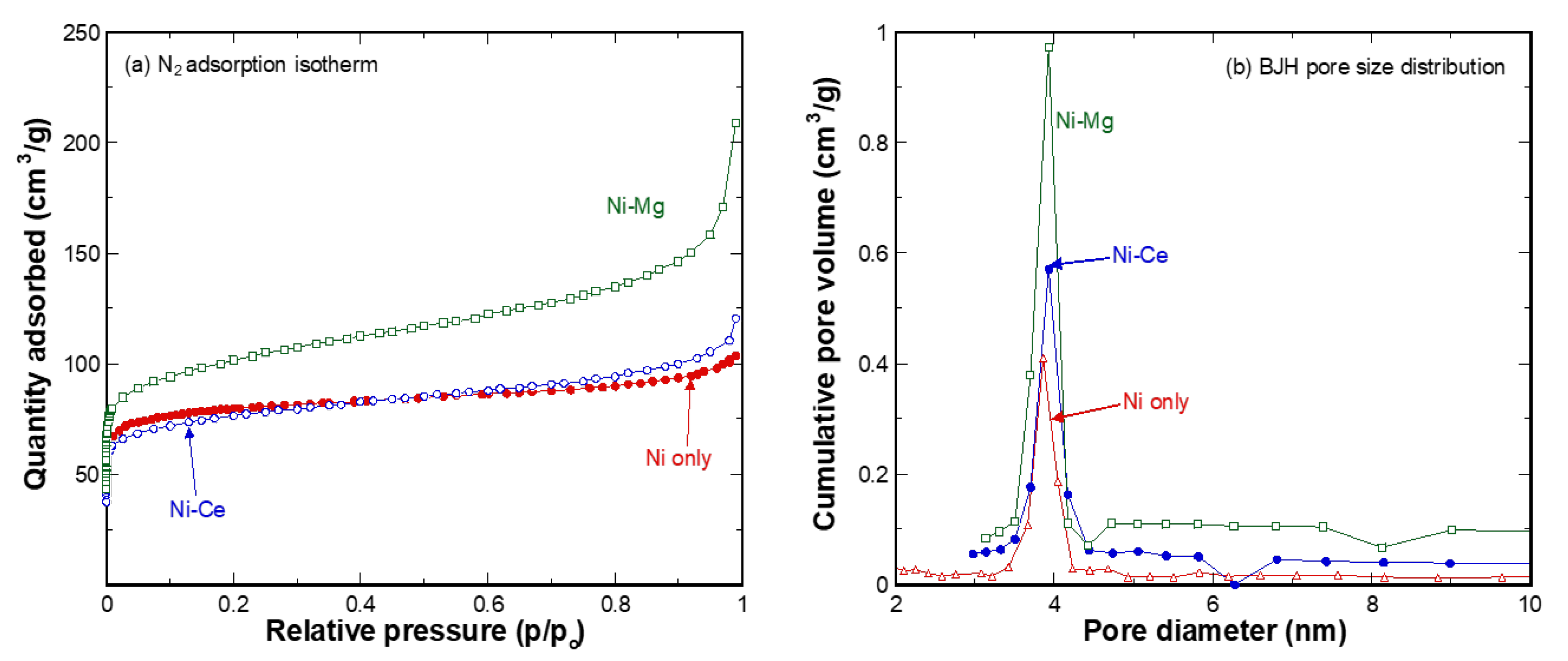


| Sample (wt.%) | Moisture | Volatile Matter * | Fixed Carbon * | Ash * | C ** | H ** | N ** | O ** | S ** |
|---|---|---|---|---|---|---|---|---|---|
| Eco | 11.1 | 53.4 | 42.4 | 4.2 | 70.3 | 5.2 | 0.9 | 23.4 | 0.1 |
| Catalyst | SBET (m2/g) | Vp (cm3/g) | Vmicro (cm3/g) | Vmicro Ratio (%) | Pore Size (nm) |
|---|---|---|---|---|---|
| Ni only | 308 | 0.163 | 0.120 | 73.6 | 2.1 |
| Ni-Ce | 286 | 0.163 | 0.090 | 55.2 | 2.6 |
| Ni-Mg | 373 | 0.187 | 0.110 | 58.8 | 3.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, S.; Yang, Y.; Lippi, R.; Choi, H.; Kim, S.; Chun, D.; Im, H.; Lee, S.; Yoo, J. Low-Rank Coal Supported Ni Catalysts for CO2 Methanation. Energies 2021, 14, 2040. https://doi.org/10.3390/en14082040
Kim S, Yang Y, Lippi R, Choi H, Kim S, Chun D, Im H, Lee S, Yoo J. Low-Rank Coal Supported Ni Catalysts for CO2 Methanation. Energies. 2021; 14(8):2040. https://doi.org/10.3390/en14082040
Chicago/Turabian StyleKim, Soohyun, Yunxia Yang, Renata Lippi, Hokyung Choi, Sangdo Kim, Donghyuk Chun, Hyuk Im, Sihyun Lee, and Jiho Yoo. 2021. "Low-Rank Coal Supported Ni Catalysts for CO2 Methanation" Energies 14, no. 8: 2040. https://doi.org/10.3390/en14082040
APA StyleKim, S., Yang, Y., Lippi, R., Choi, H., Kim, S., Chun, D., Im, H., Lee, S., & Yoo, J. (2021). Low-Rank Coal Supported Ni Catalysts for CO2 Methanation. Energies, 14(8), 2040. https://doi.org/10.3390/en14082040








