Batch Anaerobic Co-Digestion and Biochemical Methane Potential Analysis of Goat Manure and Food Waste
Abstract
1. Introduction
1.1. Anaerobic Digestion
1.2. Goat Manure Mismanagement in Concentrated Animal Feeding Operations
1.3. Environmental Impact of Food Waste
2. Materials and Methods
2.1. Substrates and Inoculum
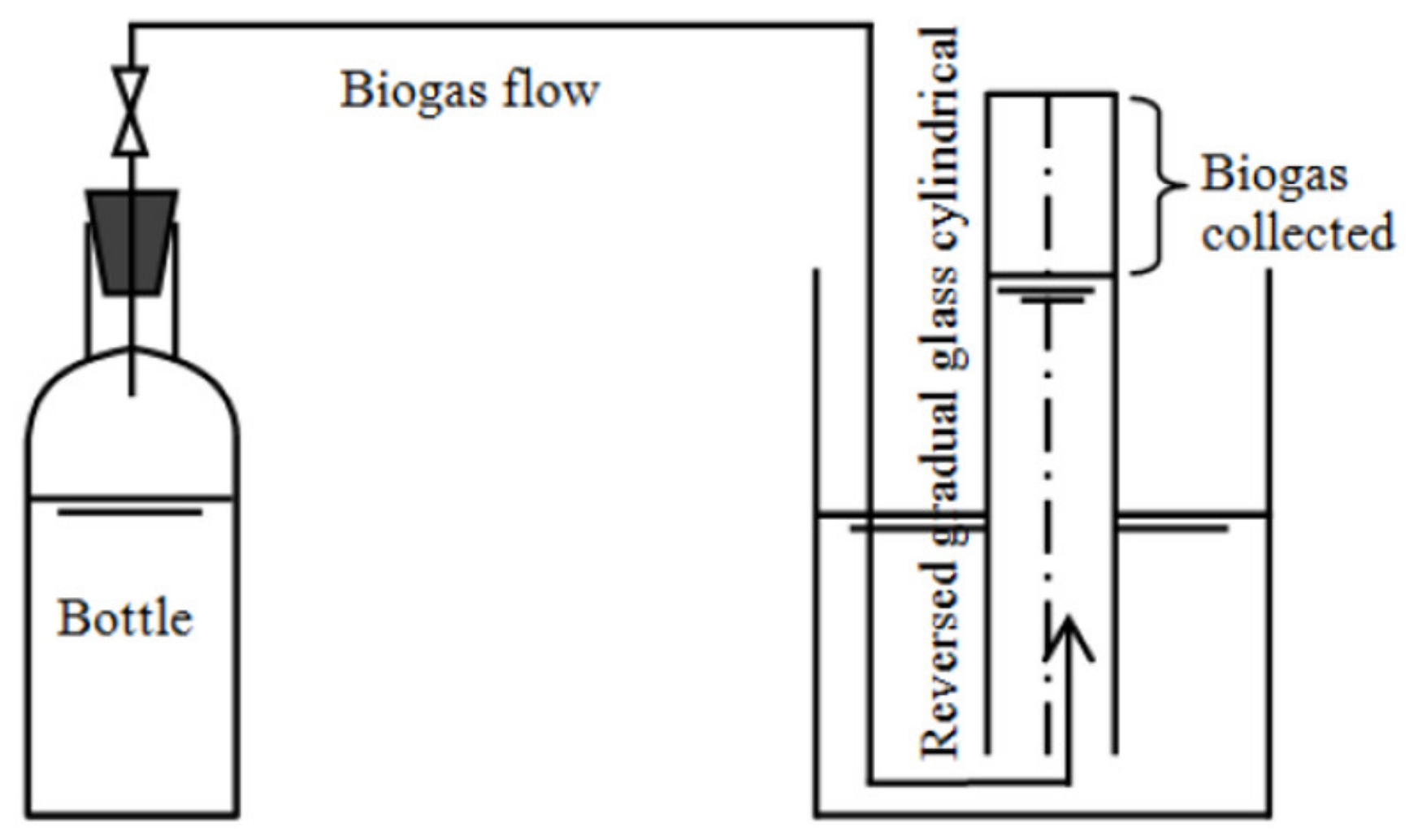
2.2. Experimental Design
2.3. Data Analysis
2.4. Theoretical Maximum Methane Yield and Biodegradability
2.5. Reaction Kinetics
2.5.1. First-order Kinetic Model
2.5.2. Modified Gompertz Model
2.5.3. Statistical Analysis
3. Results and Discussion
3.1. Substrate Characterization
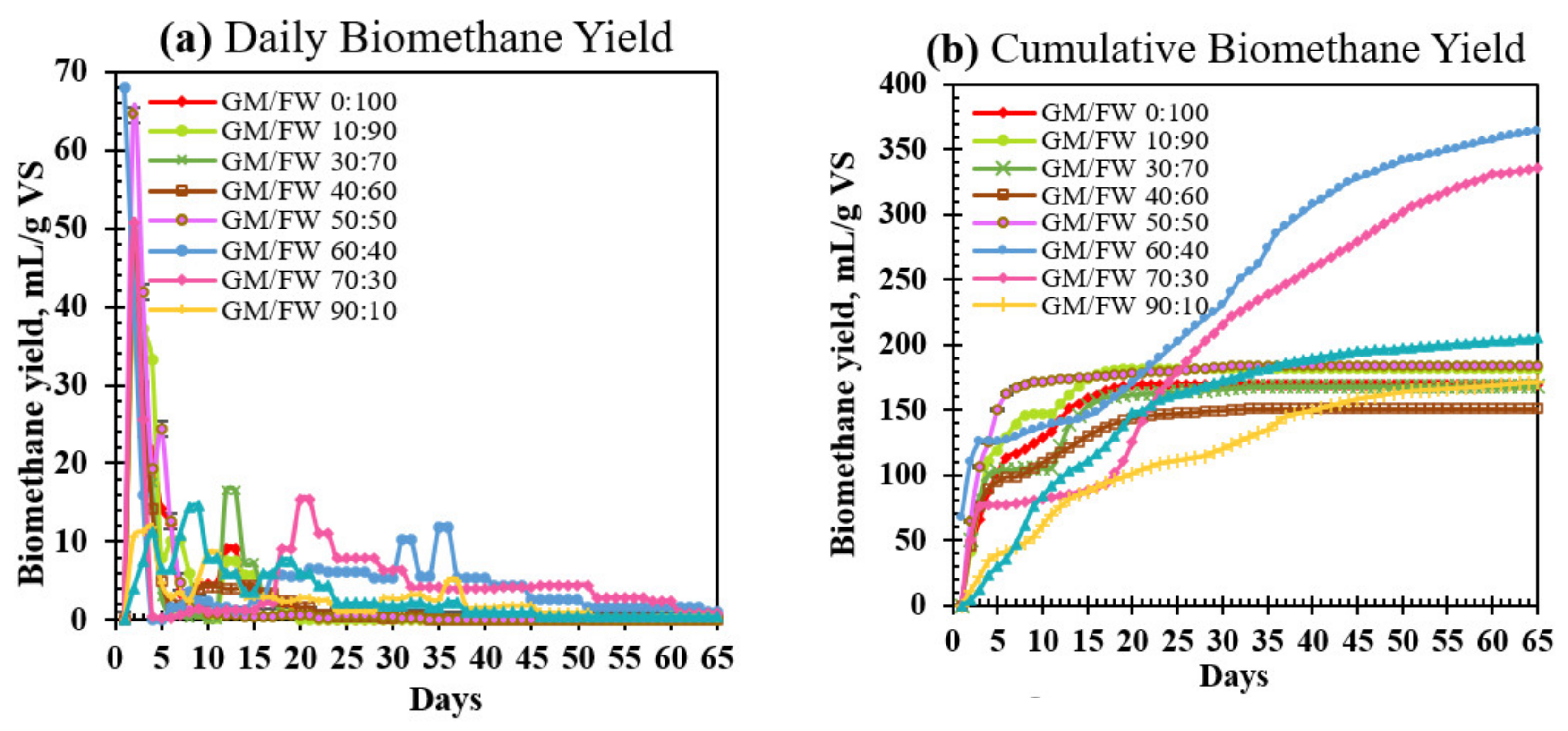
3.2. Daily Biomethane Yield
3.3. Cumulative Biomethane Yield
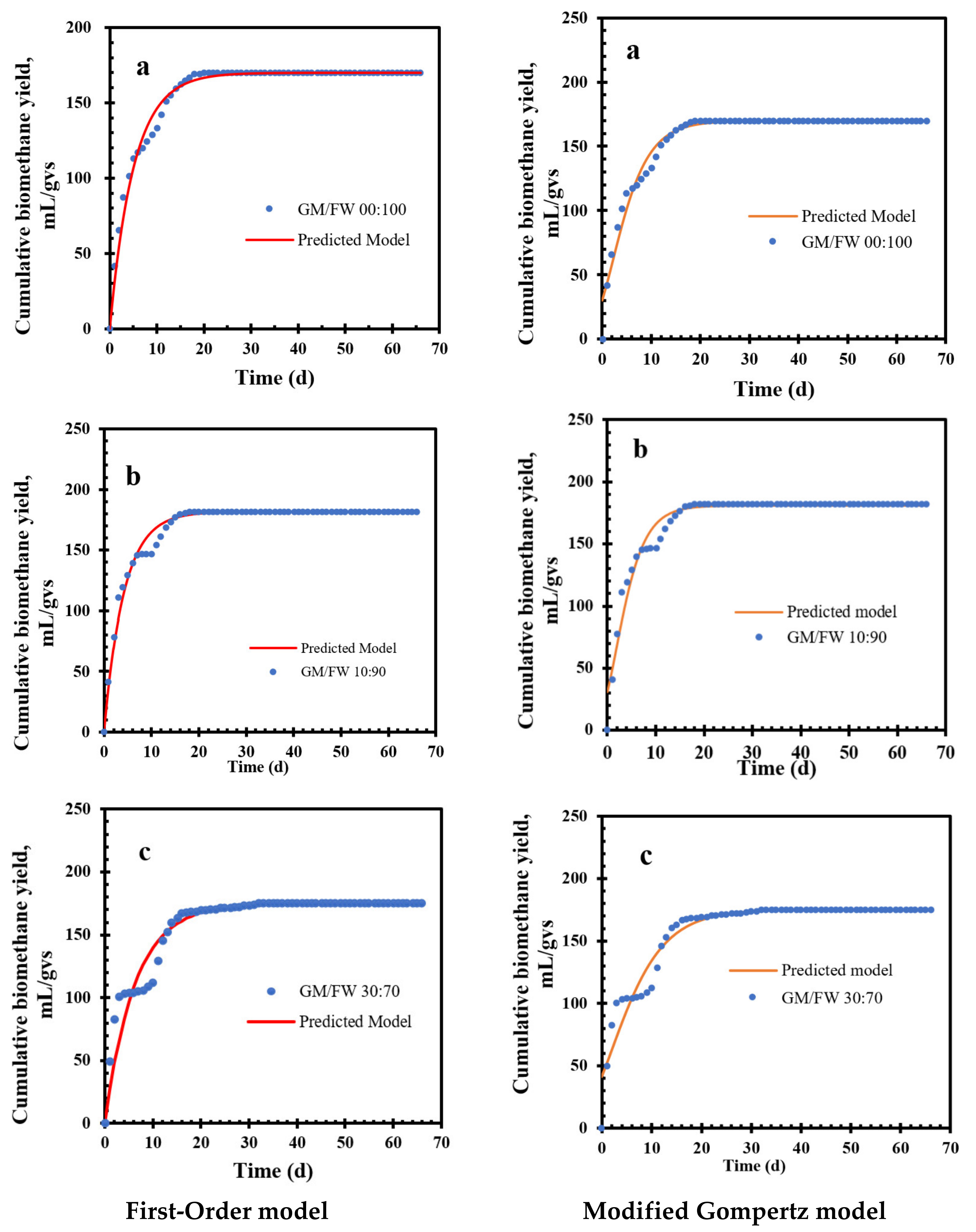
3.4. Kinetic Model Analysis
3.5. First-Order Kinetic Model
3.6. Modified Gompertz Kinetic Model
3.7. Comparison of First-Order and Modified Gompertz Model
3.8. Biodegradability
3.9. Process Stability
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Giusti, L. A review of waste management practices and their impact on human health. Waste Manag. 2009, 29, 2227–2239. [Google Scholar] [CrossRef] [PubMed]
- Scarlat, N.; Dallemand, J.-F.; Fahl, F. Biogas: Developments and perspectives in Europe. Renew. Energy 2018, 129, 457–472. [Google Scholar] [CrossRef]
- Nasir, I.M.; Ghazi, T.I.M.; Omar, R. Production of biogas from solid organic wastes through anaerobic digestion: A review. Appl. Microbiol. Biotechnol. 2012, 95, 321–329. [Google Scholar] [CrossRef] [PubMed]
- Afazeli, H.; Jafari, A.; Rafiee, S.; Nosrati, M. An investigation of biogas production potential from livestock and slaughterhouse wastes. Renew. Sustain. Energy Rev. 2014, 34, 380–386. [Google Scholar] [CrossRef]
- Braber, K. Anaerobic digestion of municipal solid waste: A modern waste disposal option on the verge of breakthrough. Biomass Bioenergy 1995, 9, 365–376. [Google Scholar] [CrossRef]
- Rolewicz-Kalińska, A.; Oniszk-Popławska, A.; Wesołowska, J.; Ryńska, E.D. Conditions for the development of anaerobic digestion technologies using the organic fraction of municipal solid waste: Perspectives for Poland. Environ. Dev. Sustain. 2016, 18, 1279–1296. [Google Scholar] [CrossRef]
- Dehkordi, S.M.M.N.; Jahromi, A.R.T.; Ferdowsi, A.; Shumal, M.; Dehnavi, A. Investigation of biogas production potential from mechanical separated municipal solid waste as an approach for developing countries (case study: Isfahan-Iran). Renew. Sustain. Energy Rev. 2020, 119, 109586. [Google Scholar] [CrossRef]
- Bedoić, R.; Špehar, A.; Puljko, J.; Čuček, L.; Ćosić, B.; Pukšec, T.; Duić, N. Opportunities and challenges: Experimental and kinetic analysis of anaerobic co-digestion of food waste and rendering industry streams for biogas production. Renew. Sustain. Energy Rev. 2020, 130, 109951. [Google Scholar] [CrossRef]
- Ma, G.; Ndegwa, P.; Harrison, J.H.; Chen, Y. Methane yields during anaerobic co-digestion of animal manure with other feedstocks: A meta-analysis. Sci. Total Environ. 2020, 728, 138224. [Google Scholar] [CrossRef]
- Panigrahi, S.; Sharma, H.B.; Dubey, B.K. Anaerobic co-digestion of food waste with pretreated yard waste: A comparative study of methane production, kinetic modeling and energy balance. J. Clean. Prod. 2020, 243, 118480. [Google Scholar] [CrossRef]
- Şenol, H.; Açıkel, Ü.; Demir, S.; Oda, V. Anaerobic digestion of cattle manure, corn silage and sugar beet pulp mixtures after thermal pretreatment and kinetic modeling study. Fuel 2020, 263, 116651. [Google Scholar] [CrossRef]
- Hartmann, H.; Ahring, B.K. Anaerobic digestion of the organic fraction of municipal solid waste: Influence of co-digestion with manure. Water Res. 2005, 39, 1543–1552. [Google Scholar] [CrossRef] [PubMed]
- Banks, C.J.; Zhang, Y.; Jiang, Y.; Heaven, S. Trace element requirements for stable food waste digestion at elevated ammonia concentrations. Bioresour. Technol. 2012, 104, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Nagao, N.; Tajima, N.; Kawai, M.; Niwa, C.; Kurosawa, N.; Matsuyama, T.; Yusoff, F.M.; Toda, T. Maximum organic loading rate for the single-stage wet anaerobic digestion of food waste. Bioresour. Technol. 2012, 118, 210–218. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Liu, L.; Song, Z.; Ren, G.; Feng, Y.; Han, X.; Yang, G. Biogas production by co-digestion of goat manure with three crop residues. PLoS ONE 2013, 8, e66845. [Google Scholar] [CrossRef]
- Gu, Y.; Chen, X.; Liu, Z.; Zhou, X.; Zhang, Y. Effect of inoculum sources on the anaerobic digestion of rice straw. Bioresour. Technol. 2014, 158, 149–155. [Google Scholar] [CrossRef]
- Xu, F.; Li, Y.; Ge, X.; Yang, L.; Li, Y. Anaerobic digestion of food waste—Challenges and opportunities. Bioresour. Technol. 2018, 247, 1047–1058. [Google Scholar] [CrossRef]
- Miller, B.A.; Lu, C.D. Current status of global dairy goat production: An overview. Asian Australas. J. Anim. Sci. 2019, 32, 1219–1232. [Google Scholar] [CrossRef]
- USDA; NASS. United States Summary and State Data, 2017. In Census of Agriculture; United States Department of Agriculture: Washington, DC, USA, 2019; Volume 1. Available online: https://www.nass.usda.gov/Publications/AgCensus/2017/ (accessed on 11 April 2019).
- Hribar, C. Understanding Concentrated Animal Feeding Operations and Their Impact on Communities; National Association of Local Boards of Health: Bowling Green, OH, USA, 2010; pp. 1–22. [Google Scholar]
- Burkholder, J.; Libra, B.; Weyer, P.; Heathcote, S.; Kolpin, D.; Thorne, P.S.; Wichman, M. Impacts of waste from concentrated animal feeding operations on water quality. Environ. Health Perspect. 2007, 115, 308–312. [Google Scholar] [CrossRef]
- Aneja, V.P.; Nelson, D.R.; Roelle, P.A.; Walker, J.T.; Battye, W. Agricultural ammonia emissions and ammonium concentrations associated with aerosols and precipitation in the southeast United States. J. Geophys. Res. 2003, 108, 4152. [Google Scholar] [CrossRef]
- Howard, A.; Botlaguduru, V.S.V.; Du, H.; Kommalapati, R.R.; Huque, Z. Measurements and comparative air quality analysis of a goat farm operation. Trans. ASABE 2019, 62, 1723–1733. [Google Scholar] [CrossRef]
- Hu, Y.; Cheng, H.; Tao, S. Environmental and human health challenges of industrial livestock and poultry farming in China and their mitigation. Environ. Int. 2017, 107, 111–130. [Google Scholar] [CrossRef]
- United States Government Accountability Office. EPA-Concentrated Animal Feeding Farm Operations; GAO: Washington, DC, USA, 2008. Available online: https://www.gao.gov/products/gao-08-944 (accessed on 24 September 2008).
- Zhang, R.; El-Mashad, H.M.; Hartman, K.; Wang, F.; Liu, G.; Choate, C.; Gamble, P. Characterization of food waste as feedstock for anaerobic digestion. Bioresour. Technol. 2007, 98, 929–935. [Google Scholar] [CrossRef]
- Li, R.; Chen, S.; Li, X. Biogas production from anaerobic co-digestion of food waste with dairy manure in a two-phase digestion system. Appl. Biochem. Biotechnol. 2010, 160, 643–654. [Google Scholar] [CrossRef]
- Han, S.-K.; Shin, H.-S. Biohydrogen production by anaerobic fermentation of food waste. Int. J. Hydrogen Energy 2004, 29, 569–577. [Google Scholar] [CrossRef]
- Kelley, T.R.; Walker, P.M. Bacterial concentration reduction in swine waste amended livestock feed using a single-screw dry-extrusion process. Bioresour. Technol. 2000, 75, 189–195. [Google Scholar] [CrossRef]
- Ohemeng-Ntiamoah, J.; Datta, T. Perspectives on variabilities in biomethane potential test parameters and outcomes: A review of studies published between 2007 and 2018. Sci. Total Environ. 2019, 664, 1052–1062. [Google Scholar] [CrossRef] [PubMed]
- Filer, J.; Ding, H.H.; Chang, S. Biochemical Methane Potential (BMP) assay method for anaerobic digestion research. Water 2019, 11, 921. [Google Scholar] [CrossRef]
- Raposo, F.; Fernández-Cegrí, V.; De La Rubia, M.A.; Borja, R.; Béline, F.; Cavinato, C.; Demirer, G.N.; Fernández, B.; Fernández-Polanco, M.; Frigon, J.C.; et al. Biochemical methane potential (BMP) of solid organic substrates: Evaluation of anaerobic biodegradability using data from an international interlaboratory study. J. Chem. Technol. Biotechnol. 2011, 86, 1088–1098. [Google Scholar] [CrossRef]
- Labatut, R.A.; Angenent, L.T.; Scott, N.R. Biochemical methane potential and biodegradability of complex organic substrates. Bioresour. Technol. 2011, 102, 2255–2264. [Google Scholar] [CrossRef]
- Rabii, A.; Aldin, S.; Dahman, Y.; Elbeshbishy, E. A review on anaerobic co-digestion with a focus on the microbial populations and the effect of multi-stage digester configuration. Energies 2019, 12, 1106. [Google Scholar] [CrossRef]
- Bong, C.P.C.; Lim, L.Y.; Lee, C.T.; Klemeš, J.J.; Ho, C.S.; Ho, W.S. The characterisation and treatment of food waste for improvement of biogas production during anaerobic digestion—A review. J. Clean. Prod. 2018, 172, 1545–1558. [Google Scholar] [CrossRef]
- Angelidaki, I.; Alves, M.M.; Bolzonella, D.; Borzacconi, L.; Campos, J.L.; Guwy, A.J.; Kalyuzhnyi, S.; Jenicek, P.; Van Lier, J.B. Defining the biomethane potential (BMP) of solid organic wastes and energy crops: A proposed protocol for batch assays. Water Sci. Technol. 2009, 59, 927–934. [Google Scholar] [CrossRef] [PubMed]
- Moody, L.; Burns, R.; Wu-haan, W.; Spajic, R. Use of biochemical methane potential (BMP) assays for predicting and enhancing anaerobic digester performance. In Proceedings of the 44th Croatian and the 4th International Symposium on Agriculture, Opatija, Croatia, 16–20 February 2009. [Google Scholar]
- Braguglia, C.; Mininni, G.; Tomei, M.; Rolle, E. Effect of feed/inoculum ratio on anaerobic digestion of sonicated sludge. Water Sci. Technol. 2006, 54, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Richards, B.K.; Cummings, R.J.; White, T.E.; Jewell, W.J. Methods for kinetic analysis of methane fermentation in high solids biomass digesters. Biomass Bioenergy 1991, 1, 65–73. [Google Scholar] [CrossRef]
- Boyle, W. Energy Recovery from Sanitary Landfills—A Review; Pergamon Press: Oxford, UK, 1977; pp. 119–138. [Google Scholar]
- Buswell, A.M.; Mueller, H.F. Mechanism of methane fermentation. Ind. Eng. Chem. 1952, 44, 550–552. [Google Scholar] [CrossRef]
- Lay, J.-J.; Li, Y.-Y.; Noike, T. Effect of moisture content and chemical nature on methane fermentation characteristics of municipal solid wastes. Doboku Gakkai Ronbunshu 1996, 101–108. [Google Scholar] [CrossRef]
- Lay, J.-J.; Li, Y.-Y.; Tatsuya, N. Influences of pH and moisture content on the methane production in high-solids sludge digestion. Water Res. 1997, 31, 1518–1524. [Google Scholar] [CrossRef]
- Bhattarai, S.; Oh, J.-H.; Euh, S.-H.; Kafle, G.K.; Kim, D.H. Simulation and model validation of sheet and tube type photovoltaic thermal solar system and conventional solar collecting system in transient states. Sol. Energy Mater. Sol. Cells 2012, 103, 184–193. [Google Scholar] [CrossRef]
- Kafle, G.K.; Kim, S.H.; Sung, K.I. Ensiling of fish industry waste for biogas production: A lab scale evaluation of biochemical methane potential (BMP) and kinetics. Bioresour. Technol. 2013, 127, 326–336. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Yang, G.; Feng, Y.; Ren, G.; Han, X. Optimizing feeding composition and carbon–nitrogen ratios for improved methane yield during anaerobic co-digestion of dairy, chicken manure and wheat straw. Bioresour. Technol. 2012, 120, 78–83. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Rodríguez, M.J.F.; Rincón, B.; Fermoso, F.G.; Jiménez, A.; Borja, R. Assessment of two-phase olive mill solid waste and microalgae co-digestion to improve methane production and process kinetics. Bioresour. Technol. 2014, 157, 263–269. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.-Z.; Shi, X.-S.; Yuan, C.-X.; Wang, Y.-P.; Qiu, Y.-L.; Guo, R.-B.; Wang, L.-S. Modeling anaerobic digestion of blue algae: Stoichiometric coefficients of amino acids acidogenesis and thermodynamics analysis. Water Res. 2014, 49, 113–123. [Google Scholar] [CrossRef] [PubMed]
- Mata-Alvarez, J. Biomethanization of the Organic Fraction of Municipal Solid Wastes; IWA Publishing: London, UK, 2003; ISBN 1900222140. [Google Scholar]
- Díaz, J.P.; Reyes, I.P.; Lundin, M.; Horváth, I.S. Co-digestion of different waste mixtures from agro-industrial activities: Kinetic evaluation and synergetic effects. Bioresour. Technol. 2011, 102, 10834–10840. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, C.; Astals, S.; Peces, M.; Campos, J.; Guerrero, L. Biochemical methane potential (BMP) tests: Reducing test time by early parameter estimation. Waste Manag. 2018, 71, 19–24. [Google Scholar] [CrossRef]
- Xie, S.; Hai, F.I.; Zhan, X.; Guo, W.; Ngo, H.H.; Price, W.E.; Nghiem, L.D. Anaerobic co-digestion: A critical review of mathematical modelling for performance optimization. Bioresour. Technol. 2016, 222, 498–512. [Google Scholar] [CrossRef] [PubMed]
- Raposo, F.; Borja, R.; Martín, A.; De La Rubia, M.Á.; Rincón, B. Influence of inoculum–substrate ratio on the anaerobic digestion of sunflower oil cake in batch mode: Process stability and kinetic evaluation. Chem. Eng. J. 2009, 149, 70–77. [Google Scholar] [CrossRef]
- Kafle, G.K.; Chen, L. Comparison on batch anaerobic digestion of five different livestock manures and prediction of biochemical methane potential (BMP) using different statistical models. Waste Manag. 2016, 48, 492–502. [Google Scholar] [CrossRef]
- Eronen, P.; Österberg, M.; Heikkinen, S.; Tenkanen, M.; Laine, J. Interactions of structurally different hemicelluloses with nanofibrillar cellulose. Carbohydr. Polym. 2011, 86, 1281–1290. [Google Scholar] [CrossRef]
- Ju, X.; Engelhard, M.; Zhang, X. An advanced understanding of the specific effects of xylan and surface lignin contents on enzymatic hydrolysis of lignocellulosic biomass. Bioresour. Technol. 2013, 132, 137–145. [Google Scholar] [CrossRef]
- Anderson, G.K.; Yang, G. Determination of bicarbonate and total volatile acid concentration in anaerobic digesters using a simple titration. Water Environ. Res. 1992, 64, 53–59. [Google Scholar] [CrossRef]
- Browne, J.D.; Murphy, J.D. Assessment of the resource associated with biomethane from food waste. Appl. Energy 2013, 104, 170–177. [Google Scholar] [CrossRef]
- Lahav, O.; Morgan, B. Titration methodologies for monitoring of anaerobic digestion in developing countries? A review. J. Chem. Technol. Biotechnol. 2004, 79, 1331–1341. [Google Scholar] [CrossRef]


| Parameters | Units | GM | FW | Inoculum |
|---|---|---|---|---|
| Total Solid (TS) | % | 64.9 ± 1.9 | 85.2 ± 0.6 | 3.9 ± 0.4 |
| Volatile Solid (VS) | % | 54.4 ± 1.3 | 44.3 ± 2.8 | 1.94 ± 0.2 |
| Moisture Content (MC) | % | 35.1 ± 1.7 | 14.8 ± 0.6 | 96 ± 0.1 |
| Ash | % | 10.5 ± 0.4 | 40.8 ± 2.5 | 1.9 ± 0.2 |
| COD | g/L | 1.9 ± 0.2 | 165.5 ± 3.4 | 3.0 ± 0.2 |
| pH | - | 7.3 ± 0.0 | 5.1 ± 0.1 | 7.1 ± 0.0 |
| C | % | 35.5 ± 0.4 | 47.0 ± 2.4 | - |
| H | % | 6.0 ± 0.1 | 7.3 ± 0.4 | - |
| N | % | 1.8 ± 0.0 | 2.7 ± 0.4 | - |
| O | % | 56.2 ± 0.3 | 42.9 ± 3.3 | - |
| S | % | 0.5 ± 0.0 | 0.2 ± 0.0 | - |
| C/N | % | 20:1 | 18:1 | - |
| VS/TS | % | 84.2 ± 0.2 | 52.1 ± 3.1 | 50.2 ± 0.6 |
| Chemical formula | - | C23.51H47.59O27.93N | C20.36H37.49O13.94N | - |
| First-Order | Kinetic | Model | ||||
|---|---|---|---|---|---|---|
| GM/FW Treatment | (mL/gVS) | CMY (mL/gVS) | K(d−1) | R2 | RMSE | % Difference |
| 0:100 | 169.8 | 169.7 | 0.1966 | 0.983 | 0.050 | 0.06 |
| 10:90 | 181.4 | 182.0 | 0.2379 | 0.981 | 0.037 | 0.29 |
| 30:70 | 174.6 | 174.8 | 0.1607 | 0.927 | 0.106 | 0.15 |
| 40:60 | 149.4 | 150.9 | 0.1869 | 0.933 | 0.091 | 1.01 |
| 50:50 | 181.9 | 183.8 | 0.4141 | 0.989 | 0.018 | 1.05 |
| 60:40 | 432.8 | 380.5 | 0.0293 | 0.921 | 0.221 | 1.16 |
| 70:30 | 497.6 | 337.3 | 0.0185 | 0.970 | 0.213 | 4.00 |
| 90:10 | 183.9 | 171.9 | 0.0419 | 0.989 | 0.089 | 0.34 |
| 100:0 | 212.2 | 206.0 | 0.0561 | 0.994 | 0.992 | 0.49 |
| GM: FW | (mL/gVS) | CMY (mL/gVS) | (mL/gVS) | λ (d) | R2 | RMSE | % Differnce |
|---|---|---|---|---|---|---|---|
| 0:100 | 169.6 | 167.7 | 15.1 | −1.853 | 0.972 | 0.038 | 0.06 |
| 10:90 | 184.1 | 182.0 | 20.2 | −1.407 | 0.959 | 0.049 | 0.49 |
| 30:70 | 175.3 | 174.8 | 10.9 | −3.749 | 0.997 | 0.066 | 0.26 |
| 40:60 | 150.5 | 150.9 | 9.7 | −4.145 | 0.998 | 0.050 | 0.27 |
| 50:50 | 181.3 | 183.8 | 42.3 | −0.300 | 0.999 | 0.003 | 1.35 |
| 60:40 | 449.3 | 380.5 | 5.9 | −11.472 | 0.996 | 0.086 | 3.69 |
| 70:30 | 386.9 | 337.3 | 6. | −2.655 | 0.997 | 0.097 | 2.13 |
| 90:10 | 176.1 | 171.9 | 4.1 | −4.916 | 0.998 | 0.203 | 0.49 |
| 100:0 | 200.0 | 206.0 | 8.0 | −0.1011 | 0.999 | 0.448 | 3.19 |
| GM:FW | TMY (mL/gVS) | EMY (mL/gVS) | BD (%) |
|---|---|---|---|
| 0:100 | 506.6 | 167.7 | 33.5 |
| 10:90 | 487.3 | 182.0 | 37.3 |
| 30:70 | 448.7 | 174.8 | 39.0 |
| 40:60 | 429.3 | 150.9 | 35.2 |
| 50:50 | 418.2 | 183.8 | 43.9 |
| 60:40 | 390.7 | 380.5 | 97.4 |
| 70:30 | 374.2 | 337.3 | 90.1 |
| 90:10 | 332.8 | 171.9 | 51.7 |
| 100:0 | 313.4 | 206.0 | 65.7 |
| GM/FW | pH | ||
|---|---|---|---|
| Initial | Adjusted | Final | |
| 0:100 | 5.1 ± 0.1 | 6.9± 0.1 | 5.4 ± 0.0 |
| 10:90 | 6.0 ± 0.1 | 6.9 ± 0.1 | 5.4 ± 0.0 |
| 30:70 | 6.4 ± 0.1 | 7.1 ± 0.0 | 5.6 ± 0.0 |
| 40:60 | 7.2 ± 0.2 | 7.2 ± 0.2 | 5.6 ± 0.1 |
| 50:50 | 7.0 ± 0.0 | 7.0 ± 0.0 | 5.2 ± 0.0 |
| 60:40 | 7.1 ± 0.1 | 7.1 ± 0.1 | 7.5 ± 0.0 |
| 70:30 | 7.1 ± 0.1 | 7.1 ± 0.1 | 7.2 ± 0.1 |
| 90:10 | 7.1 ± 0.0 | 7.1 ± 0.0 | 7.2 ± 0.0 |
| 100:0 | 7.3 ± 0.0 | 7.3 ± 0.0 | 7.1 ± 0.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Orangun, A.; Kaur, H.; Kommalapati, R.R. Batch Anaerobic Co-Digestion and Biochemical Methane Potential Analysis of Goat Manure and Food Waste. Energies 2021, 14, 1952. https://doi.org/10.3390/en14071952
Orangun A, Kaur H, Kommalapati RR. Batch Anaerobic Co-Digestion and Biochemical Methane Potential Analysis of Goat Manure and Food Waste. Energies. 2021; 14(7):1952. https://doi.org/10.3390/en14071952
Chicago/Turabian StyleOrangun, Ayobami, Harjinder Kaur, and Raghava R. Kommalapati. 2021. "Batch Anaerobic Co-Digestion and Biochemical Methane Potential Analysis of Goat Manure and Food Waste" Energies 14, no. 7: 1952. https://doi.org/10.3390/en14071952
APA StyleOrangun, A., Kaur, H., & Kommalapati, R. R. (2021). Batch Anaerobic Co-Digestion and Biochemical Methane Potential Analysis of Goat Manure and Food Waste. Energies, 14(7), 1952. https://doi.org/10.3390/en14071952