Life Cycle Assessment for Supporting Eco-Design: The Case Study of Sodium–Nickel Chloride Cells
Abstract
1. Introduction
- -
- Few studies are based on detailed data for the foreground processes. Thus, even if the topic of LCA of batteries has already been discussed in the literature, further research is needed on specific parts/components and processes related to batteries in order to gain more knowledge in this field;
- -
- The comparison of studies is often a difficult task, due to different methodological assumptions (e.g., functional units, system boundaries, cut-off rules, uncertainty of secondary data, variation in primary data, etc.) and battery features (e.g., cathode and anode composition, battery application, etc.).
- -
- Sodium–nickel chloride batteries are innovative storage systems used both in mobile and stationary applications [20,21]. They are characterized by a broad range of operating temperatures (−20 °C–+60 °C) without the need for complex thermal management systems, which makes them suitable for extreme cold or hot environments. In addition, these batteries do not require maintenance and are fully recyclable [22].
- -
2. LCA of Sodium–Nickel Chloride Cells
2.1. Goal and Scope Definition
2.2. Primary and Secondary Data
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- European Environment Agency. Circular by Design—Products in the Circular Economy, EEA Report No 6/2017; European Environment Agency: Copenhagen, Denmark, 2017; ISBN 1977-8449. [Google Scholar]
- Ardente, F.; Beccali, G.; Cellura, M. Eco-sustainable energy and environmental strategies in design for recycling: The software “ENDLESS”. Ecol. Model. 2003, 163, 101–118. [Google Scholar] [CrossRef]
- European Commission. Communication from the Commission to the European Parliament, the Council, the European Economic and Social Committee and the Committee of the Regions “A new Circular Economy Action Plan for a Cleaner and More Competitive Europe” COM(2020) 98 Final; European Commission: Brussles, Belgium, 2020. [Google Scholar]
- European Union. Directive 2006/66/EC of the European Parliament and of the Council of on batteries and accumulators and waste batteries and accumulators and repealing Directive 91/157/EEC. Off. J. Eur. Union 2006, 266, 1–14. [Google Scholar]
- European Battery Alliance. Priority Actions. Available online: www.eba250.com (accessed on 26 January 2021).
- European Commission. Report from the Commission to the European Parliament, the Council, the European Economic and Social Committee, the Committee of the Regions and the European Investment Bank on the Implementation of the Strategic Action Plan on Batteries: Building a Straegic Battery Value Chain in Europe; European Commission: Brussels, Belgium, 2019. [Google Scholar]
- Guarino, F.; Cassarà, P.; Longo, S.; Cellura, M.; Ferro, E. Load match optimisation of a residential building case study: A cross-entropy based electricity storage sizing algorithm. Appl. Energy 2015, 154, 380–391. [Google Scholar] [CrossRef]
- European Union. Directive 2009/125/EC of the European Parliament and of the Council of establishing a framework for the setting of ecodesign requirements for energy-related products (recast). Off. J. Eur. Union 2009, 285, 10. [Google Scholar]
- Cusenza, M.A.; Guarino, F.; Longo, S.; Ferraro, M.; Cellura, M. Energy and environmental benefits of circular economy strategies: The case study of reusing used batteries from electric vehicles. J. Energy Storage 2019, 25, 100845. [Google Scholar] [CrossRef]
- UNI EN ISO 14040. ‘Environmental Management—Life Cycle Assessment—Principles and Framework’. July 2006. Available online: https://www.iso.org/standard/37456.html (accessed on 26 January 2021).
- UNI EN ISO 14044. ‘Environmental Management—Life Cycle Assessment—Requirements and Guidelines’. July 2006. Available online: https://www.iso.org/standard/38498.html (accessed on 26 January 2021).
- Cusenza, M.A.; Bobba, S.; Ardente, F.; Cellura, M.; Di Persio, F. Energy and environmental assessment of a traction lithiumion battery pack for plug-in hybrid electric vehicles. J. Clean. Prod. 2019, 215, 634–649. [Google Scholar] [CrossRef]
- Longo, S.; Antonucci, V.; Cellura, M.; Ferraro, M. Life cycle assessment of storage systems: The case study of a sodium/nickel chloride battery. J. Clean. Prod. 2014, 85, 337–346. [Google Scholar] [CrossRef]
- Sullivan, J.L.; Gaines, L. A Review of Battery Life-cycle Analysis: State of Knowledge and Critical Needs. ANL/ESD/10-7. Argonne National Laboratory e Energy Systems Division. 2010. Available online: http://www.osti.gov (accessed on 19 November 2020).
- McManus, M. Environmental consequences of the use of batteries in low carbon systems: The impact of battery production. Appl. Energy 2012, 93, 288–295. [Google Scholar] [CrossRef]
- Schexnayder, S.M.; Das, S.; Dhingra, R.; Overly, J.G.; Tonn, B.E.; Peretz, J.H.; Waidley, G.; Davis, G.A. Environmental Evaluation of New Generation Vehicles and Vehicle Components. ORNL/TM-2001-266. Available online: http://wwwcta.ornl.gov (accessed on 19 November 2020).
- Notter, D.A.; Gauch, M.; Widmer, R.; Wager, P.; Stamp, A.; Zah, R.; Althaus, H.J. Contribution of Li-ion batteries to the environmental impact of electric vehicles, Environ. Sci. Technol. 2010, 44, 6550–6556. [Google Scholar] [CrossRef] [PubMed]
- Ellingsen, L.A.-W.; Hung, C.R.; Strømman, A.H. Identifying key assumptions and differences in life cycle assessment studies of lithium-ion traction batteries with focus on greenhouse gas emissions. Transp. Res. Part D Transp. Environ. 2017, 55, 82–90. [Google Scholar] [CrossRef]
- Majeau-Bettez, G.; Hawkins, T.R.; Strømman, A.H. Life Cycle Environmental Assessment of Lithium-Ion and Nickel Metal Hydride Batteries for Plug-In Hybrid and Battery Electric Vehicles. Environ. Sci. Technol. 2011, 45, 4548–4554. [Google Scholar] [CrossRef] [PubMed]
- Manzoni, R.; Metzger, M.; Crugnola, G. ZEBRA Electric Energy Storage System: From R&D to Market; HTE hi.tech.expo: Milan, Italy, 2008; pp. 25–58. [Google Scholar]
- Sudworth, J. The sodium/nickel chloride (ZEBRA) battery. J. Power Sources 2001, 100, 149–163. [Google Scholar] [CrossRef]
- Parkhided, B. Project: Storage Technologies for Hybrid Electric Buses, Subject: ZEBRA Battery, 2nd ed.; Electrochemical Energy Conversion and Storage Systems Group, RWTH Aachen University: Aachen, Germany, 2006; Available online: http://www.euromatic.no/ZEBRA_Aug17.pdf (accessed on 19 November 2020).
- Matheys, J.; Van Autenboer, W.; Van Mierlo, J. SUBAT: Sustainable Batteries—Work Package 5: Overall Assessment. Final Public Report; Vrije Universiteit Brussel and ETEC: Brussel, Belgium, 2004. [Google Scholar]
- Matheys, J.; Timmermans, J.M.; Van Autenboer, W.; Van Mierlo, J.; Maggetto, G.; Meyer, S.; De Groof, A.; Hecq, W.; Van den Bossche, P. Comparison of the Environmental impact of 5 Electric Vehicle Battery technologies using LCA. In Proceedings of the LCE 2006, Leuven, Belgium, 31 May–2 June 2006. [Google Scholar]
- Li, G.; Lu, X.; Kim, J.Y.; Meinhardt, K.D.; Chang, H.J.; Canfield, N.L.; Sprenkle, V.L. Advanced intermediate temperature sodium–nickel chloride batteries with ultra-high energy density. Nat. Commun. 2016, 7, 10683. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Coffey, G.; Meinhardt, K.; Sprenkle, V.; Yang, Z.; Lemmon, J.P. Lemmon, High Power Planar Sodium-Nickel Chloride Battery. ECS Trans. 2010, 28, 7–13. [Google Scholar] [CrossRef]
- Lu, X.; Li, G.; Kim, J.Y.; Lemmon, J.P.; Sprenkle, V.L.; Yang, Z. The effects of temperature on the electrochemical performance of sodium–nickel chloride batteries. J. Power Sources 2012, 215, 288–295. [Google Scholar] [CrossRef]
- Kim, S.-M.; Lee, S.-M.; Jung, K.; Park, Y.-C.; Cho, N.-U.; Kim, H.-S. Feasibility Study of a Planar-type Sodium-Nickel Chloride Battery. Bull. Korean Chem. Soc. 2016, 37, 695–699. [Google Scholar] [CrossRef]
- Kirchev, A. Battery management and battery diagnostics. In Electrochemical Energy Storage for Renewable Sources and Grid Balancing; Moseley, P.T., Garche, J., Eds.; Elsevier: Amsterdam, The Netherlands, 2015; pp. 411–435. [Google Scholar]
- Pilla, P.; Mehta, H.D. Basic Electrical Engineering; S. Chand Publishing: New Delhi, India, 2012. [Google Scholar]
- Li, G.; Lu, X.; Kim, J.Y.; Lemmon, J.P.; Sprenkle, V.L. Cell degradation of a Na–NiCl2 (ZEBRA) battery. J. Mater. Chem. A 2013, 1, 14935. [Google Scholar] [CrossRef]
- Sessa, S.D.; Crugnola, G.; Todeschini, M.; Zin, S.; Benato, R. Sodium nickel chloride battery steady-state regime model for stationary electrical energy storage, J. Energy Storage 2016, 6, 105–115. [Google Scholar] [CrossRef]
- Frischknecht, R.; Jungbluth, N.; Althaus, H.-J.; Bauer, C.; Doka, G.; Dones, R.; Hischier, R.; Hellweg, S.; Humbert, S.; Köllner, T. Implementation of Life Cycle Impact Assessment Methods. 2007. Available online: https://www.ecoinvent.org/ (accessed on 19 November 2020).
- European Commission-Joint Research Centre. Characterisation Factors of the ILCD Recommended Life Cycle Impact Assessment Methods: Database and Supporting Information; European Commission: Rome, Italy, 2012. [Google Scholar]
- Wernet, G.; Bauer, C.; Steubing, B.; Reinhard, J.; Moreno-Ruiz, E.; Weidema, B.P. The ecoinvent database version 3 (part I): Overview and methodology. Int. J. Life Cycle Assess. 2016, 21, 1218–1230. [Google Scholar] [CrossRef]
- Rabah, M.; Farghaly, F.; Motaleb, M.A.-E. Recovery of nickel, cobalt and some salts from spent Ni-MH batteries. Waste Manag. 2008, 28, 1159–1167. [Google Scholar] [CrossRef] [PubMed]
- European Commission. Commission Staff Working Document Report on Raw Materials for Battery Applications, SWD (2018) 245/2 Final; European Commission: Brussels, Belgium, 2018. [Google Scholar]
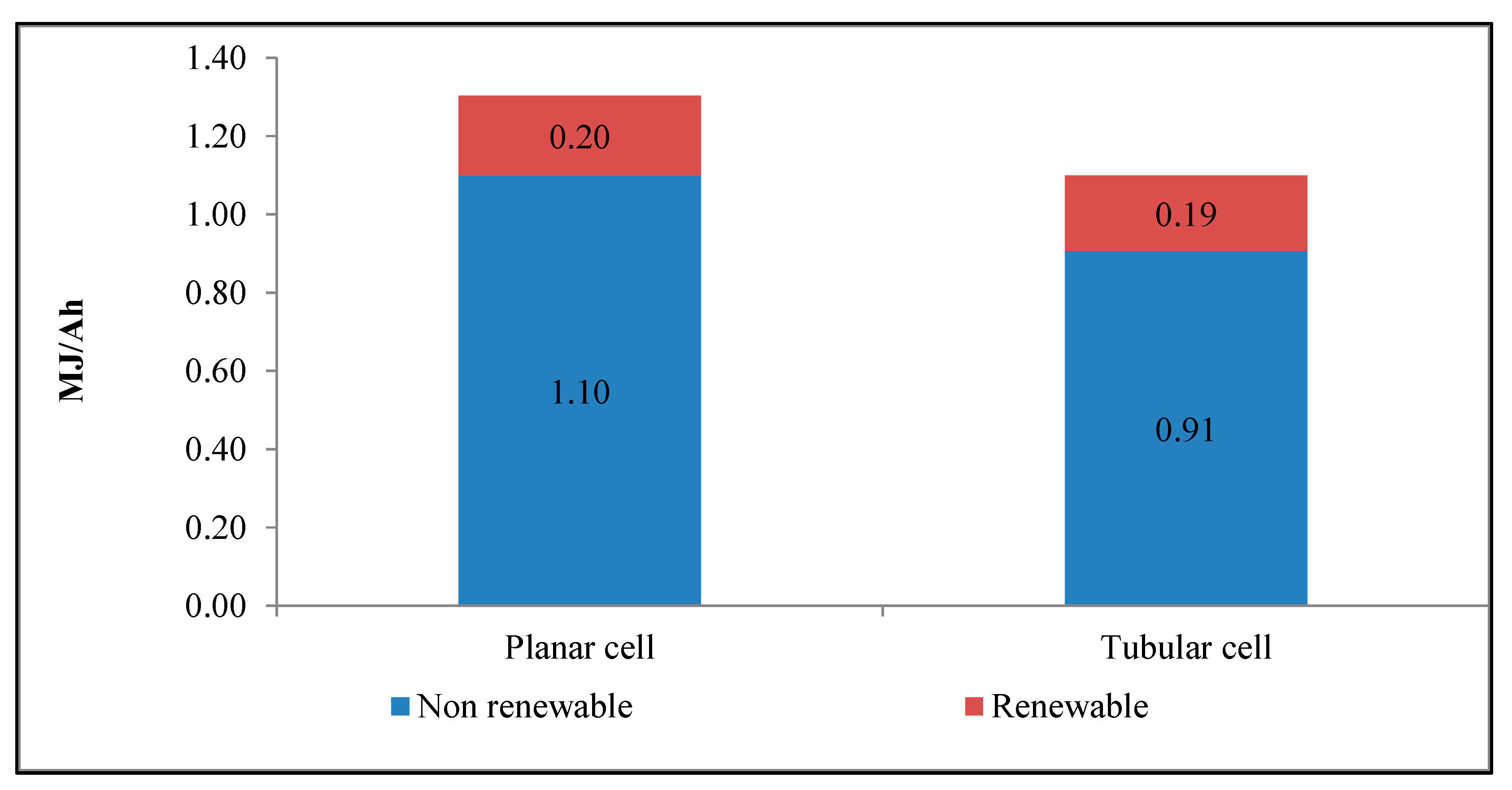
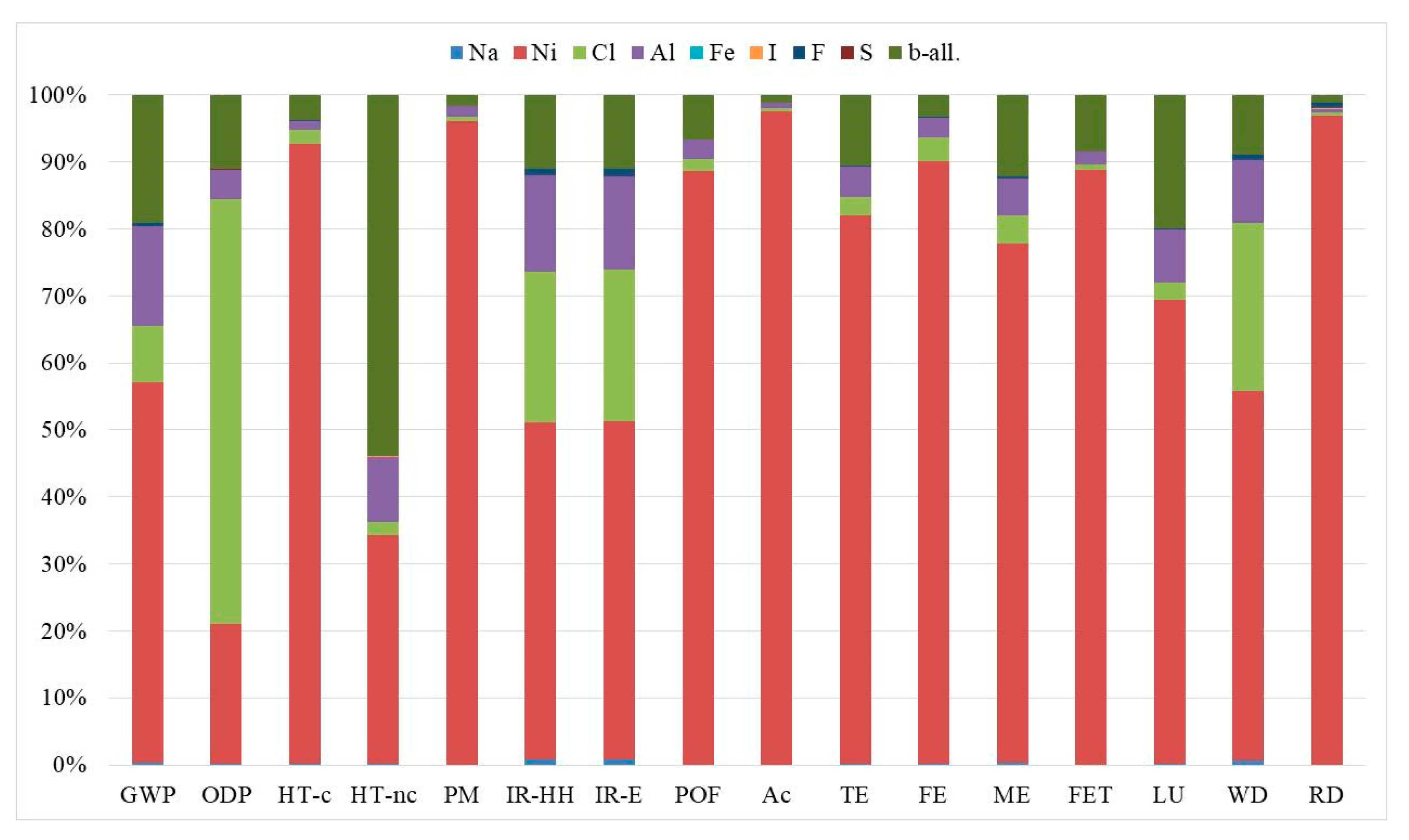
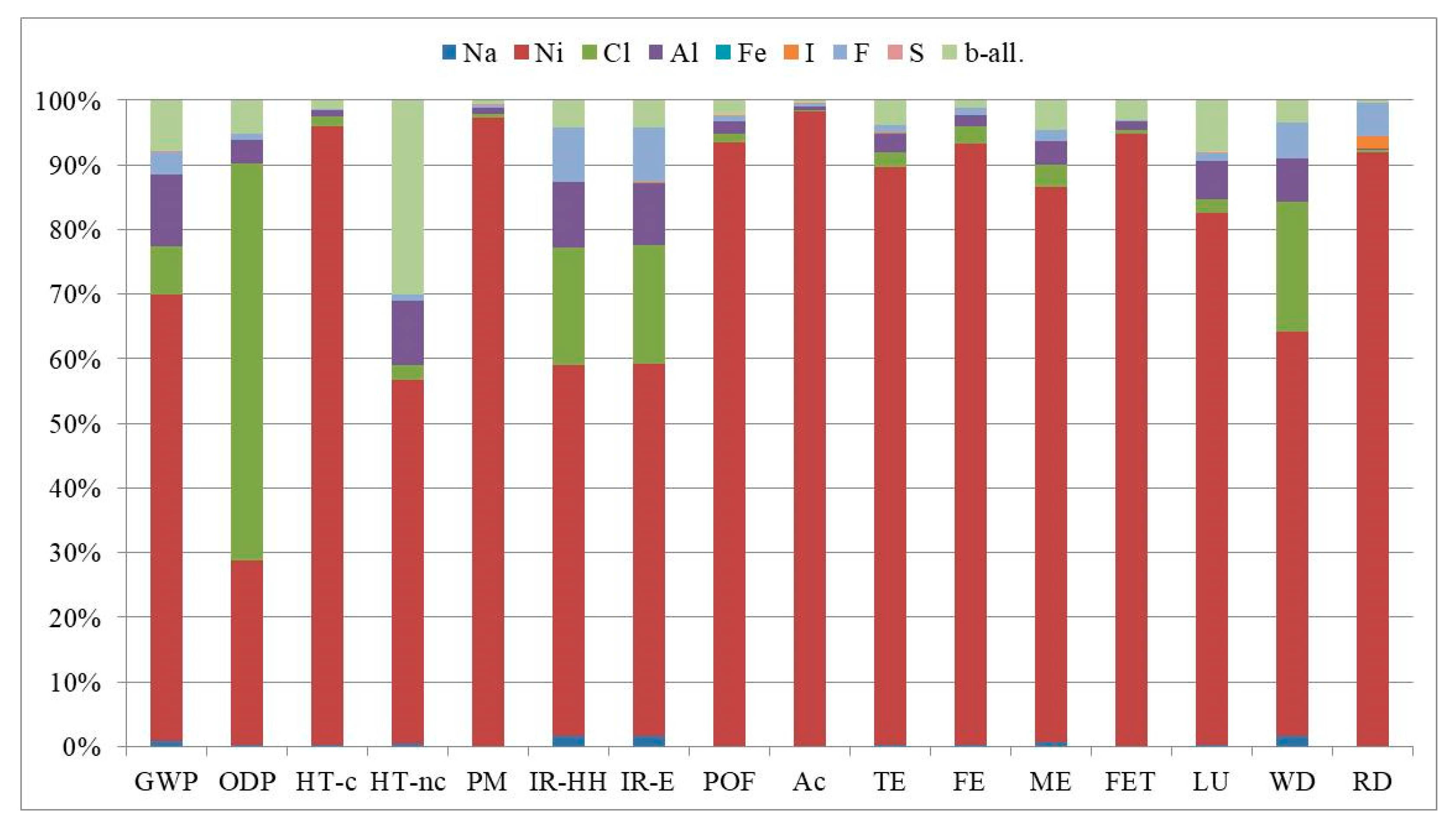
| Impact Category | Acronym | Unit of Measure |
|---|---|---|
| Global Energy Requirement | GER | MJ |
| Global Energy Requirement—non-renewable | GER-nr | MJ |
| Global Energy Requirement—renewable | GER-r | MJ |
| Global Warming Potential | GWP | kg CO2eq |
| Ozone Depletion Potential | ODP | kgCFC-11eq |
| Human Toxicity—Cancer effects | HT-c | CTUh |
| Human Toxicity—Non-cancer effects | HT-nc | CTUh |
| Particulate Matter | PM | kg PM2.5eq |
| Ionizing Radiations HH | IR-HH | kBq U235eq |
| Ionizing Radiations E | IR-E | CTUe |
| Photochemical Ozone Formation | POF | kg NMVOCeq |
| Acidification | Ac | molc H+eq |
| Terrestrial Eutrophication | TE | molc Neq |
| Freshwater Eutrophication | FE | kg Peq |
| Marine Eutrophication | ME | kg Neq |
| Freshwater Ecotoxicity | FET | CTUe |
| Land Use | LU | kg C deficit |
| Water Depletion | WD | m3 watereq |
| Resource Depletion—mineral, fossil, renewable | RD | kg Sbeq |
| Material | Planar Cell (kg/Ah) | Tubular Cell (kg/Ah) |
|---|---|---|
| Sodium (Na) | 1.65 × 10−3 | 3.22 × 10−3 |
| Nickel (Ni) | 3.96 × 10−3 | 4.03 × 10−3 |
| Chlorine (Cl) | 6.05 × 10−3 | 4.38 × 10−3 |
| Aluminum (Al) | 9.30 × 10−4 | 5.81 × 10−4 |
| Iron (Fe) | 5.10 × 10−5 | 2.50 × 10−4 |
| Iodine (I) | 2.70 × 10−5 | 1.97 × 10−4 |
| Fluorine (F) | 2.61 × 10−5 | 1.90 × 10−4 |
| Sulfur (S) | 5.10 × 10−5 | 3.71 × 10−4 |
| β-alumina | 1.18 × 10−2 | 4.06 × 10−3 |
| Total | 2.46 × 10−2 | 1.73 × 10−2 |
| Input | Process Name |
|---|---|
| Na | Adapted from sodium chloride, powder, at plant |
| Ni | Nickel, 99.5%, at plant |
| Cl | Chlorine, liquid, production mix, at plant |
| Al | Aluminum, primary, at plant |
| Fe | Iron ore, 46% Fe, at mine |
| I | Adapted from sodium chlorine, brine solution, at plant |
| F | Fluorine, liquid, at plant |
| S | Secondary sulfur, at refinery |
| β-alumina | Aluminum oxide, at plant |
| Impact Category | Unit | Planar Cell | Tubular Cell |
|---|---|---|---|
| GWP | kg CO2eq | 7.60 × 10−2 | 6.34 × 10−2 |
| ODP | kg CFC-11eq | 1.63 × 10−8 | 1.22 × 10−8 |
| HT-c | CTUh | 2.66 × 10−7 | 2.62 × 10−7 |
| HT-nc | CTUh | 3.22 × 10−8 | 1.99 × 10−8 |
| PM | kg PM2.5eq | 3.99 × 10−4 | 4.01 × 10−4 |
| IR-HH | kBq U235eq | 2.05 × 10−2 | 1.83 × 10−2 |
| IR-E | CTUe | 6.31 × 10−8 | 5.64 × 10−8 |
| POF | kg NMVOCeq | 8.49 × 10−4 | 8.20 × 10−4 |
| Ac | molc H+eq | 7.66 × 10−3 | 7.74 × 10−3 |
| TE | molc Neq | 1.85 × 10−3 | 1.72 × 10−3 |
| FE | kg Peq | 1.75 × 10−4 | 1.73 × 10−4 |
| ME | kg Neq | 1.47 × 10−4 | 1.35 × 10−4 |
| FET | CTUe | 7.26 | 6.93 |
| LU | kg C deficit | 1.43 × 10−1 | 1.22 × 10−1 |
| WD | m3 watereq | 4.48 × 10−4 | 4.02 × 10−4 |
| RD | kg Sbeq | 2.19 × 10−5 | 2.35 × 10−5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Longo, S.; Cellura, M.; Cusenza, M.A.; Guarino, F.; Mistretta, M.; Panno, D.; D’Urso, C.; Leonardi, S.G.; Briguglio, N.; Tumminia, G.; et al. Life Cycle Assessment for Supporting Eco-Design: The Case Study of Sodium–Nickel Chloride Cells. Energies 2021, 14, 1897. https://doi.org/10.3390/en14071897
Longo S, Cellura M, Cusenza MA, Guarino F, Mistretta M, Panno D, D’Urso C, Leonardi SG, Briguglio N, Tumminia G, et al. Life Cycle Assessment for Supporting Eco-Design: The Case Study of Sodium–Nickel Chloride Cells. Energies. 2021; 14(7):1897. https://doi.org/10.3390/en14071897
Chicago/Turabian StyleLongo, Sonia, Maurizio Cellura, Maria Anna Cusenza, Francesco Guarino, Marina Mistretta, Domenico Panno, Claudia D’Urso, Salvatore Gianluca Leonardi, Nicola Briguglio, Giovanni Tumminia, and et al. 2021. "Life Cycle Assessment for Supporting Eco-Design: The Case Study of Sodium–Nickel Chloride Cells" Energies 14, no. 7: 1897. https://doi.org/10.3390/en14071897
APA StyleLongo, S., Cellura, M., Cusenza, M. A., Guarino, F., Mistretta, M., Panno, D., D’Urso, C., Leonardi, S. G., Briguglio, N., Tumminia, G., Antonucci, V., & Ferraro, M. (2021). Life Cycle Assessment for Supporting Eco-Design: The Case Study of Sodium–Nickel Chloride Cells. Energies, 14(7), 1897. https://doi.org/10.3390/en14071897














