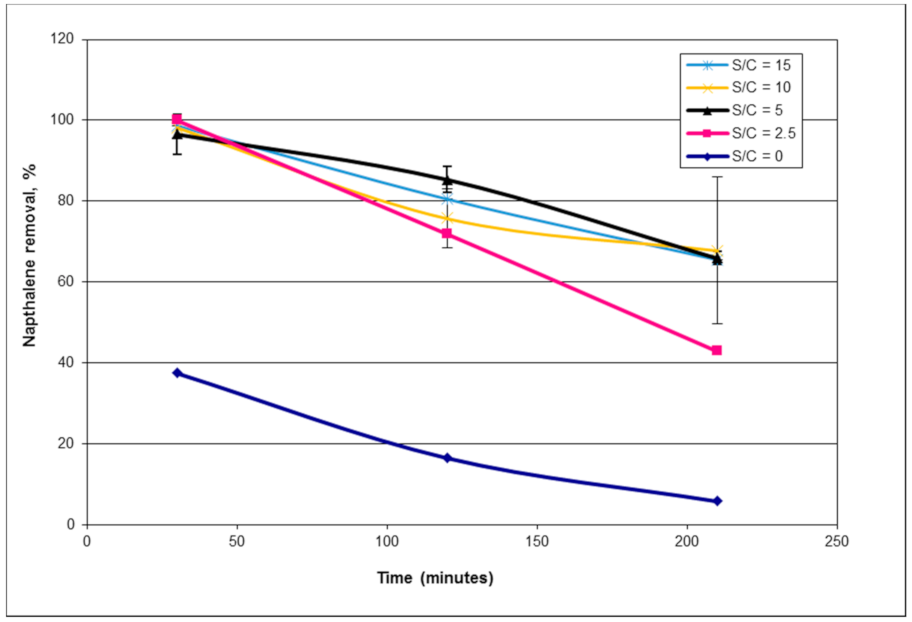
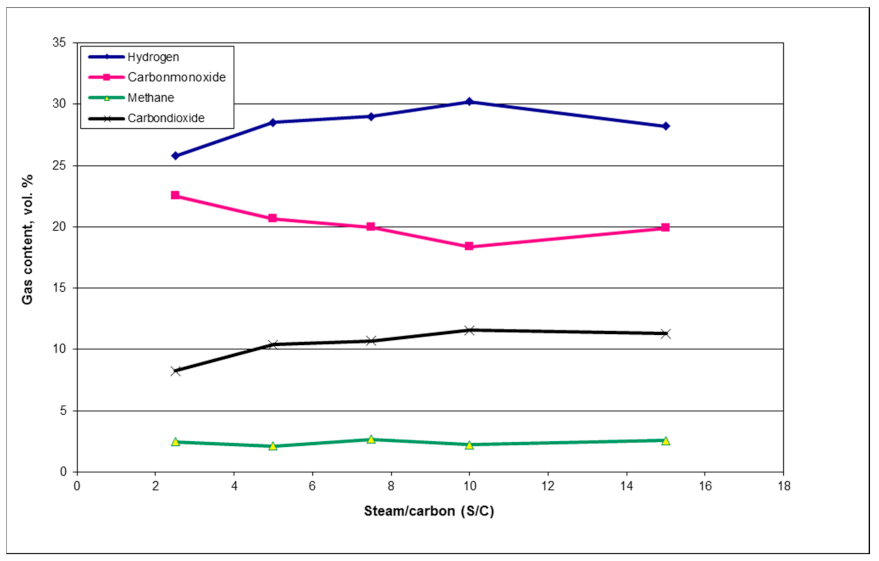
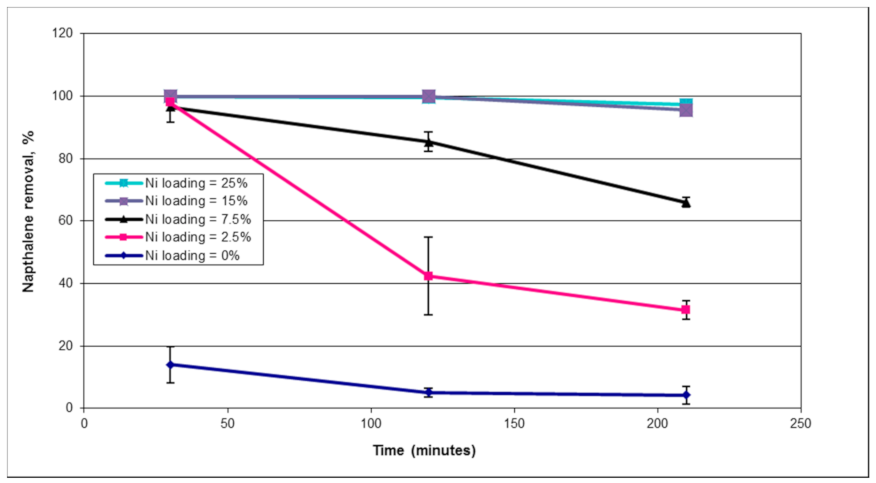
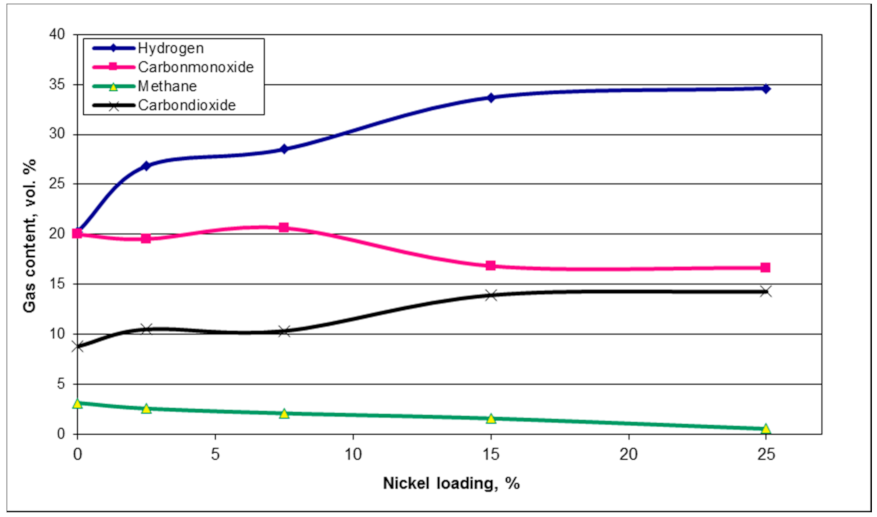
1. Introduction
With an increasing global population comes an increase in energy demand. The global energy demand has been increasing by an annual average of 1.8% over the past 10 years [
1]. This demand is expected to increase by 15% from 2015 to 2030 and then by an additional 11% by 2040 [
2]. Another concern is related to fossil fuels in their possible depletion as well as the effect the industry has towards climate change. Globally, more than 50% of the atmospheric CO
2 increase, about 50% of the rise in the mean surface temperature, and the sea level rise (26–32%) between 1980 to 2010 have been linked to 90 carbon producers. Fossil fuel aerosols have been linked to an increase in surface temperature of 0.97 °C during the same period [
3]. Owing to the increasing energy demand and overwhelming evidence of the negative impact of fossil fuels with regard to climate change, there has been a push for alternative and renewable energy sources. Lignocellulosic biomass is one of the renewable energy sources and is considered to be a potential feedstock for sustainable energy generation [
4] amongst others such as wind, solar, geothermal energy, etc. [
1,
2]. In 2015, 4% of electricity came from renewable energy (RE) sources; however, the investment in RE technology continues to increase with a 14.1% increase in 2016 alone [
1]. Moreover, biofuel energy sources are expected to increase to 25% by 2040 [
2]. Gasification is a thermochemical process of converting biomass/carbonaceous waste to syngas (producer gas or synthesis gas). Amongst many applications, syngas produced from the process can be used in electricity generation, as a feedstock in transportation fuels and specialty chemical production, or can be further processed and separated for the production of hydrogen fuel [
5]. From 2005 to 2015, the global biofuels production increased by 14.1% and by an additional 2.6% in 2016 alone [
1]. In addition to tars, many impurities are present in fuel gases, including ammonia, hydrogen sulfide, hydrogen chloride, alkali and non-alkali metals, and particulate matter [
6]. Tar removal has been proven to be a cost-intensive step in the purification of the resulting fuel gases. Tars are defined as a mixture of polycyclic hydrocarbons having molecular weights of greater than 72, ranging in size from one ring compound such as toluene to six-ring compounds such as coronene [
7]. Catalyst deactivation, plugging, corrosion, and blockages that result from tar buildup cause many operational and maintenance difficulties. These problems lead to efficiency losses in the overall gasification process in terms of energy and material. Further converting tars to fuel gases [
8] would improve efficiency as well as reduce the environmental and health impact that the carcinogenic tars pose [
9]. Owing to the abovementioned reasons, cleanup of tars from biomass syngas is essential and will allow for economic and effective use of syngas in various applications.
Typically, tar removal techniques have been categorized into (a) physical, (b) thermal, and (c) catalytic [
5]. The catalytic technique is of particular interest since instead of simply removing tars, they can be reformed into syngas, which will result in an increase in the gasification process efficiency. Catalysts for cleanup of tars have been categorized into the following groups: fluid catalytic cracking catalysts, calcined rocks, olivine, clay minerals, ferrous metal oxides, char, alkali metals, activated alumina, and transition metal catalysts [
10]. Most of the research on catalytic tar removal has been focused on using dolomites, olivine, alkali metals, and nickel. Several researchers [
4,
11,
12,
13,
14,
15,
16,
17,
18] have investigated dolomites owing to their availability, lower cost, and decent catalytic performance. However, issues with dolomites include deactivation due to coke formation leading to a gradual loss in the mechanical strength over time [
14,
16] and their relatively lower removal efficiencies [
19]. Alkali metals is another group of catalysts that has been extensively investigated. Alkali metals are typically introduced by mixing with biomass or through wet impregnation. These catalysts exhibit good tar removal activity; however, there are some disadvantages as well. At high temperatures, these catalysts exhibit loss of activity due to particle agglomeration and volatility [
10]. Recovery of these catalyst materials can be challenging owing to their mixing with biomass feed. Furthermore, separation of the char and ash containing alkalis can lead to additional expenses thus making the overall commercialization more difficult [
8].
One of the most widely evaluated catalysts for syngas cleanup (tar removal) are nickel-based. Owing to the high steam and dry reforming activity, nickel-based catalysts allow for complete tar removal. While the cost of these catalysts is relatively higher compared to dolomites, their activity is 8–10 times higher under similar operating conditions [
18,
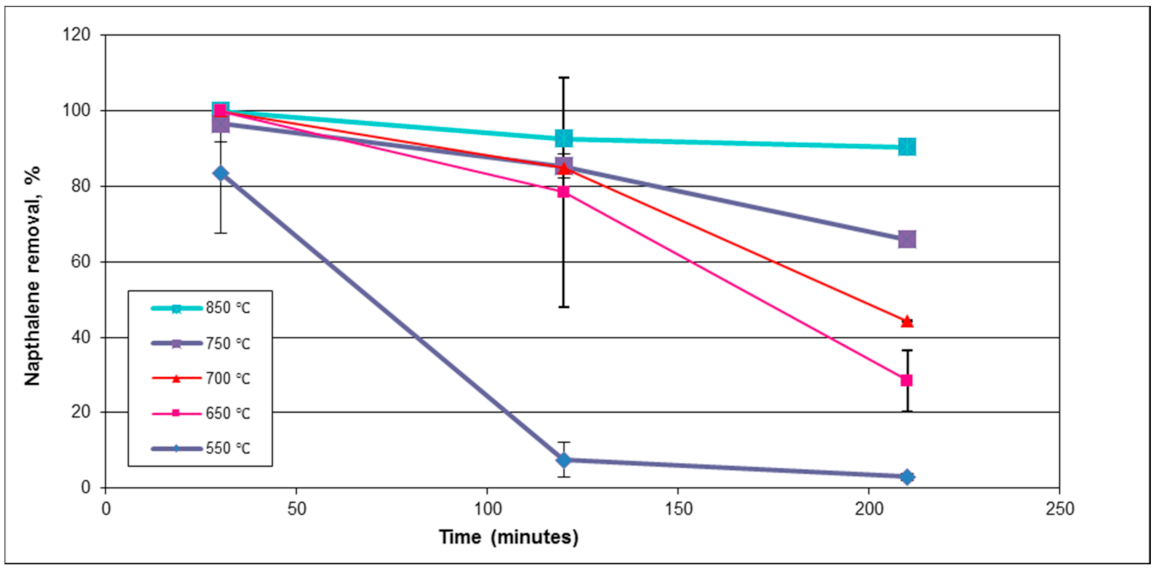
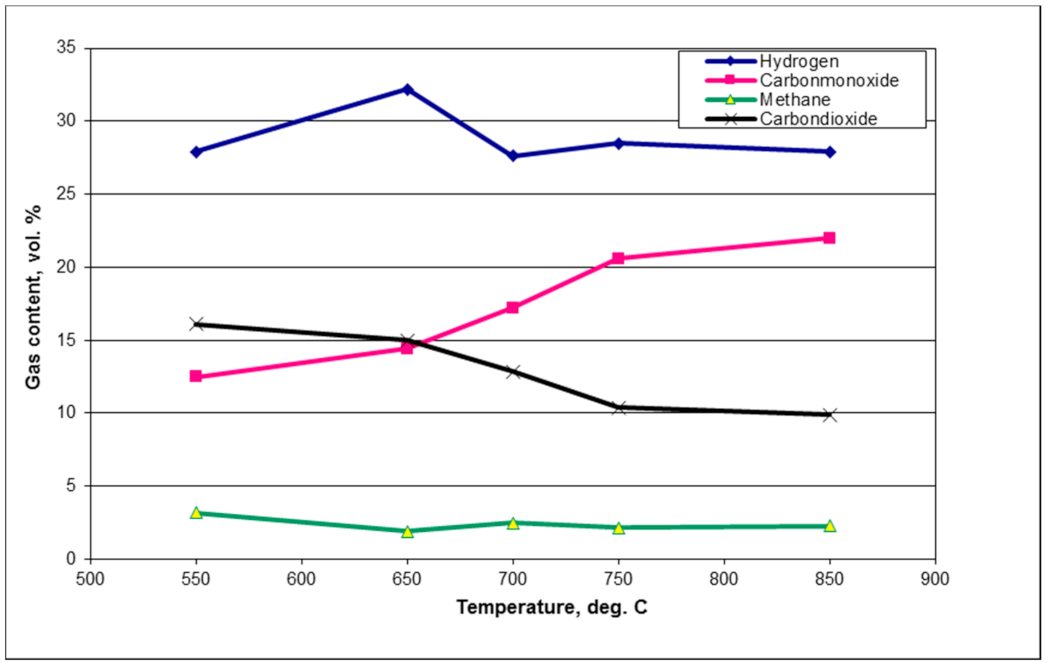
20]. The ideal catalytic steam reforming conditions were found to be between 650 °C and 800 °C for supported nickel catalysts; however, the optimum temperature might depend on the support media [
21,
22,
23,
24]. Operating in the upper end of the temperature range (near 800 °C) promotes greater activity and a longer lifetime for the nickel catalyst and operating at the lower end (around 650 °C) can lead to producing syngas rich in methane [
24]. It was also observed that the commercial nickel-based catalysts synthesized for heavy hydrocarbon steam reforming are more suitable and active for tar reforming compared to those tailored for light hydrocarbons [
25,
26]. Shortcomings of nickel include the gradual loss in its catalytic activity over time. This comes as a result of carbon deposition (coking), sintering, and fouling. Nickel sintering causes irreversible loss of activity due to material changes; however, coke deposition on the surface of the catalyst can be eliminated through regeneration and its activity can thereby be restored [
8].
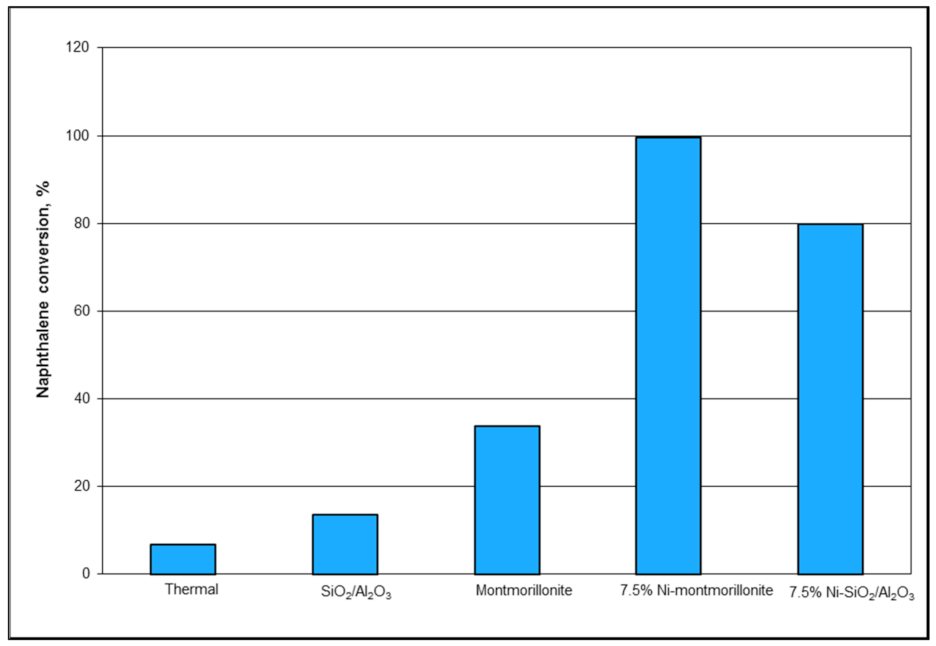
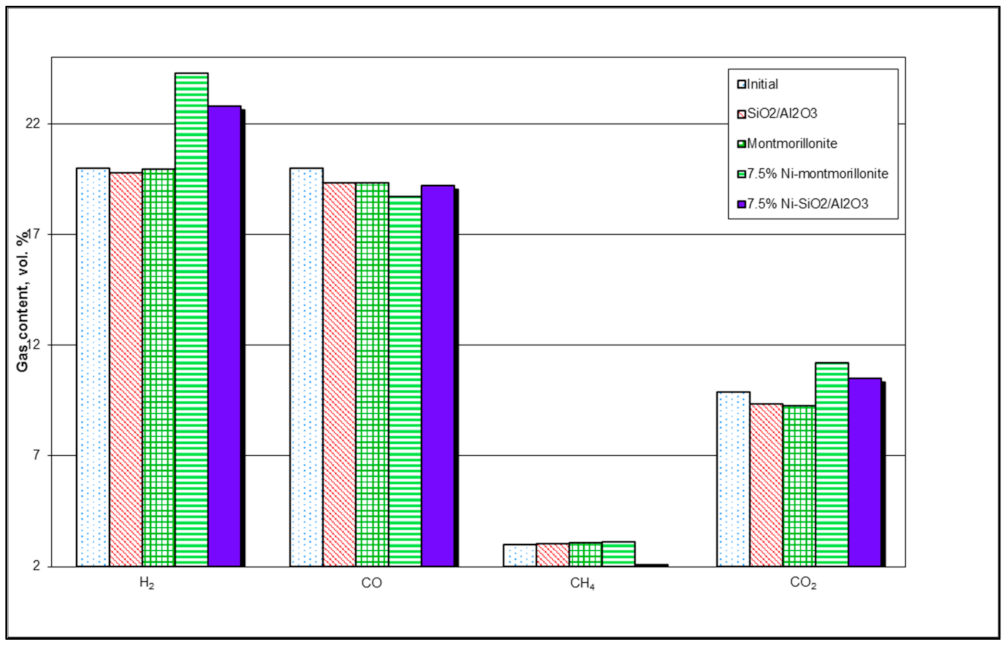
Dolomites and olivine catalysts exhibit decent activity towards the removal of tars, but their inability to reduce tars to desired low levels has prompted several researchers to explore nickel-based dolomite and olivine catalysts [
19,
27,
28,
29]. Tar removal efficiencies of 97% using dolomite-supported Ni were reported by Wang et al. [
19] while operating at 750 °C and maintaining a space velocity of 12,000 h
−1. At a space velocity of 6000 h
−1, Quitete et al. [
30] observed tar (toluene) conversions of 68%, 76%, and 79% using Ni/LaAl
11O
18, Ni/La
0.8Ce
0.2 Al
11O
19, and Ni/CaAl
12O
19, respectively, at 700 °C. Swierczynski et al. [
27] investigated the use of an olivine-supported Ni catalyst. They reported complete removal of toluene (which was used as a model compound for tar) with negligible coke deposition at temperatures above 650 °C [
27]. Excellent catalytic activity was comparable to commercial nickel-based steam reforming catalysts [
31]. At lower temperatures when there is risk of carbon deposition, it can be kept to a minimum by optimizing the loading of nickel on the support material. It was concluded that when using an olivine support, the ideal loading is 2.8 wt% Ni [
32]. These results suggest that catalysts consisting of Ni supported on olivine or dolomite have demonstrable high activity for tar removal. Further, these catalysts are relatively less expensive in comparison to commercial steam reforming catalysts.
The other group of materials that have the tar removal potential includes clay minerals [
10]. Clay minerals that are commonly available are montmorillonite, bentonite, kaolinite, and palygorskite. The catalytic activity of clay minerals for tar removal has been related to the effective pore diameter, high surface area, and acidity [
31,
33]. These minerals have several advantages owing to abundant availability and their being inexpensive. The main disadvantage of this material is its lower activity compared with dolomites and nickel catalysts. Liu et al. [
34] obtained 99.5% tar conversion using the highly active Fe–Ni on palygorskite support at 700 °C [
34].
Montmorillonite is a group of clay minerals composed of hydrous alluminosilicates. The surface area of montmorillonite (239 m
2/g) is relatively high compared with dolomites (12 m
2/g) and olivine (4 m
2/g). The pore size of montmorillonite reported by Wang et al. is 5.2 nm [
35]. High surface area results in an increase in active metal surface due to improved dispersion of metals on its surface. Wen et al. [
31] reported that the catalytic activity of clay minerals increases with pore diameters greater than 0.7 nm, increase in the surface area, and acid strength. This pore size allows for the diffusion of the high-abundance molecules within tars, naphthalene and acenaphthene, with measured sizes of 0.499–0.681 nm and 0.65–0.681 nm, respectively [
36]. The conclusion of increased activity correlating with acid strength agrees with Buchireddy et al. [
33] whereby nickel on zeolite supports of various SiO
2/Al
2O
3 were tested for tar (naphthalene) reforming.
Transition metal (group VIII) catalysts have been proven effective for steam and dry reforming applications. Owing to the relatively lower cost and high activity in comparison to cobalt, platinum, ruthenium, and rhodium, nickel has been widely used in such applications [
8,
37]. Thus, taking the advantages of clay mineral properties and nickel, this study evaluated montmorillonite-supported nickel for tar removal application. Owing to its stability and relatively high concentrations in tars, naphthalene was chosen as a tar model compound. Coll et al. [
38] concluded that naphthalene is the least reactive compound among several tar model compounds tested, including benzene, toluene, naphthalene, antharacine, and pyrene. Furthermore, naphthalene is one of the most abundant polycyclic hydrocarbons produced during biomass gasification [
10]. Moreover, several researchers [
33,
34,
35,
36] used naphthalene as a model compound in their studies and using the same model compound will allow comparison of kinetic data from this study. In this study, Ni-montmorillonite was synthesized, characterized, and tested for its efficiency in reforming naphthalene. Tests were also conducted using silica alumina (SiO
2/Al
2O
3), and SiO
2/Al
2O
3-supported nickel for comparison. Furthermore, the efficacy of the montmorillonite-supported nickel catalyst was tested as a function of nickel content, reaction temperature, naphthalene loading, steam-to-carbon ratio (S/C), and over extended time periods. Varying these operating conditions is common in experimental catalytic steam reforming using a variety of fuel sources [
39].