Increasing Parameters of Diesel Engines by Their Transformation for Methanol Conversion Products
Abstract
1. Introduction
- monogas fuel is significantly cheaper than diesel fuel, and diesel fuel is not supplied at all;
- resource of cylinder-piston group of convertible engines, due to smoother increase in combustion pressures, increases by 1.3–1.5 times;
- frequency of replacement of motor oils and oil filters of monogas engines is approximately doubled, due to the reduction of carbon formation, the absence of process of washing the oil film, and reducing vacuum of engine oil;
2. Materials and Methods
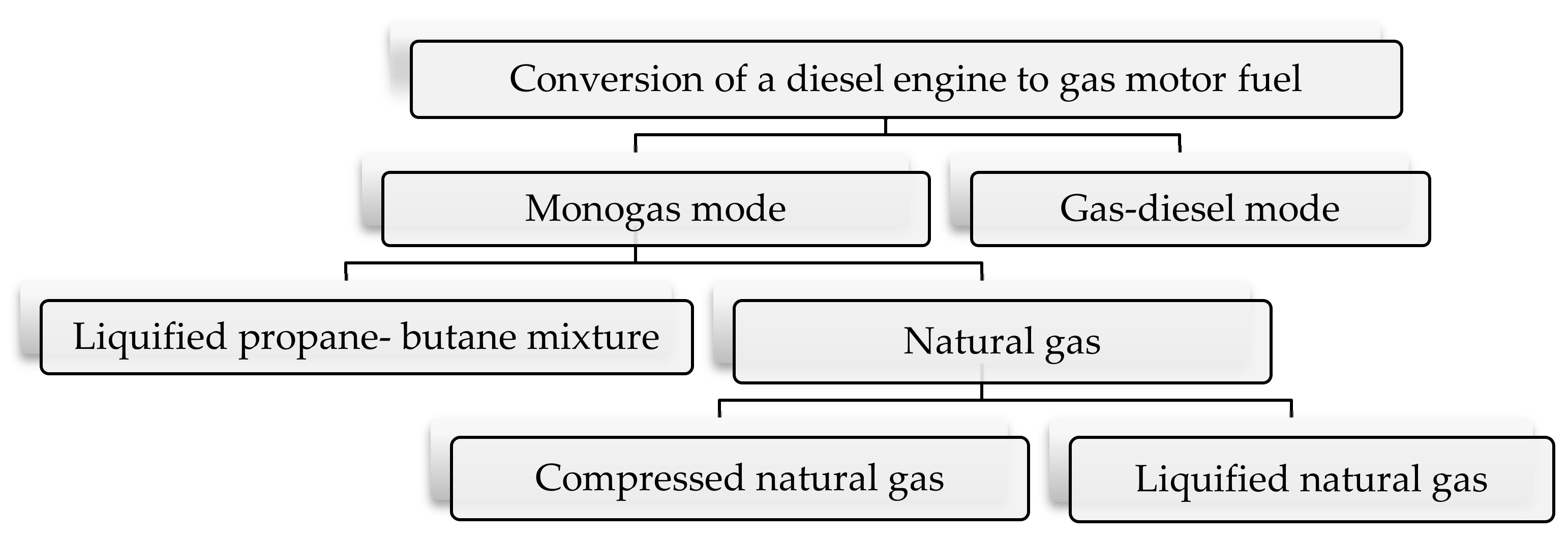
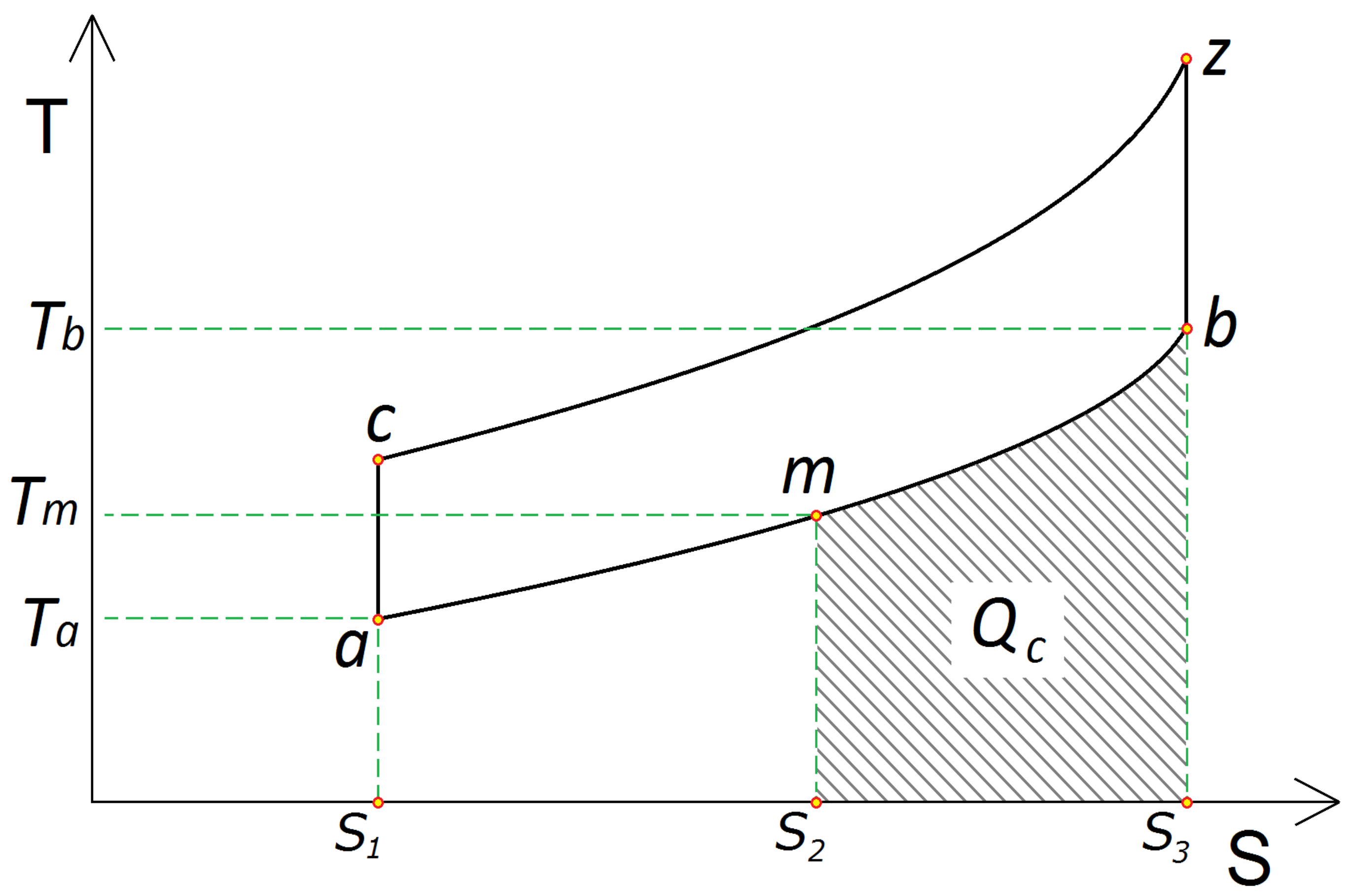
2.1. Fundamentals
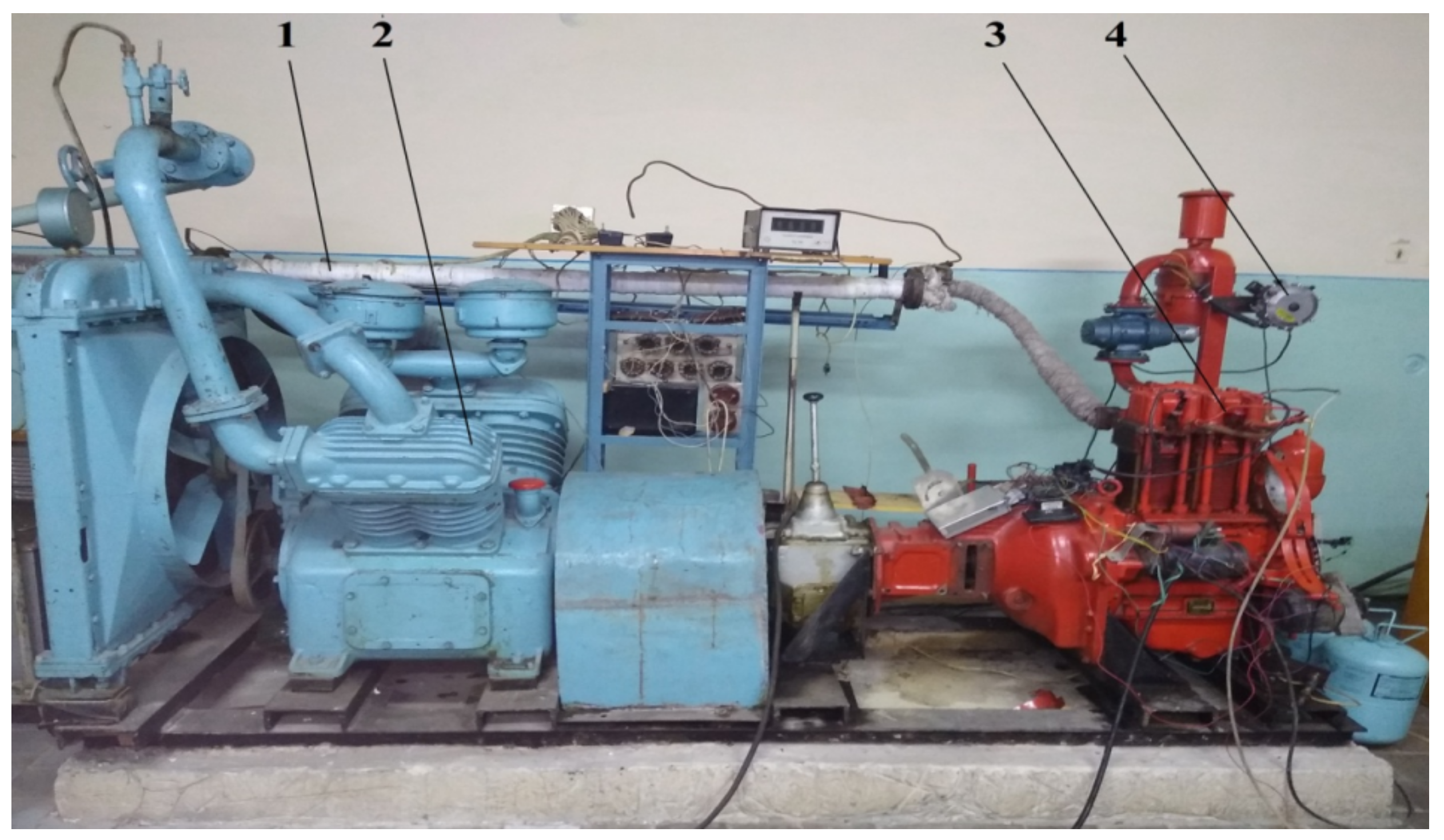
2.2. Equipment Used
2.3. Testing Methodology
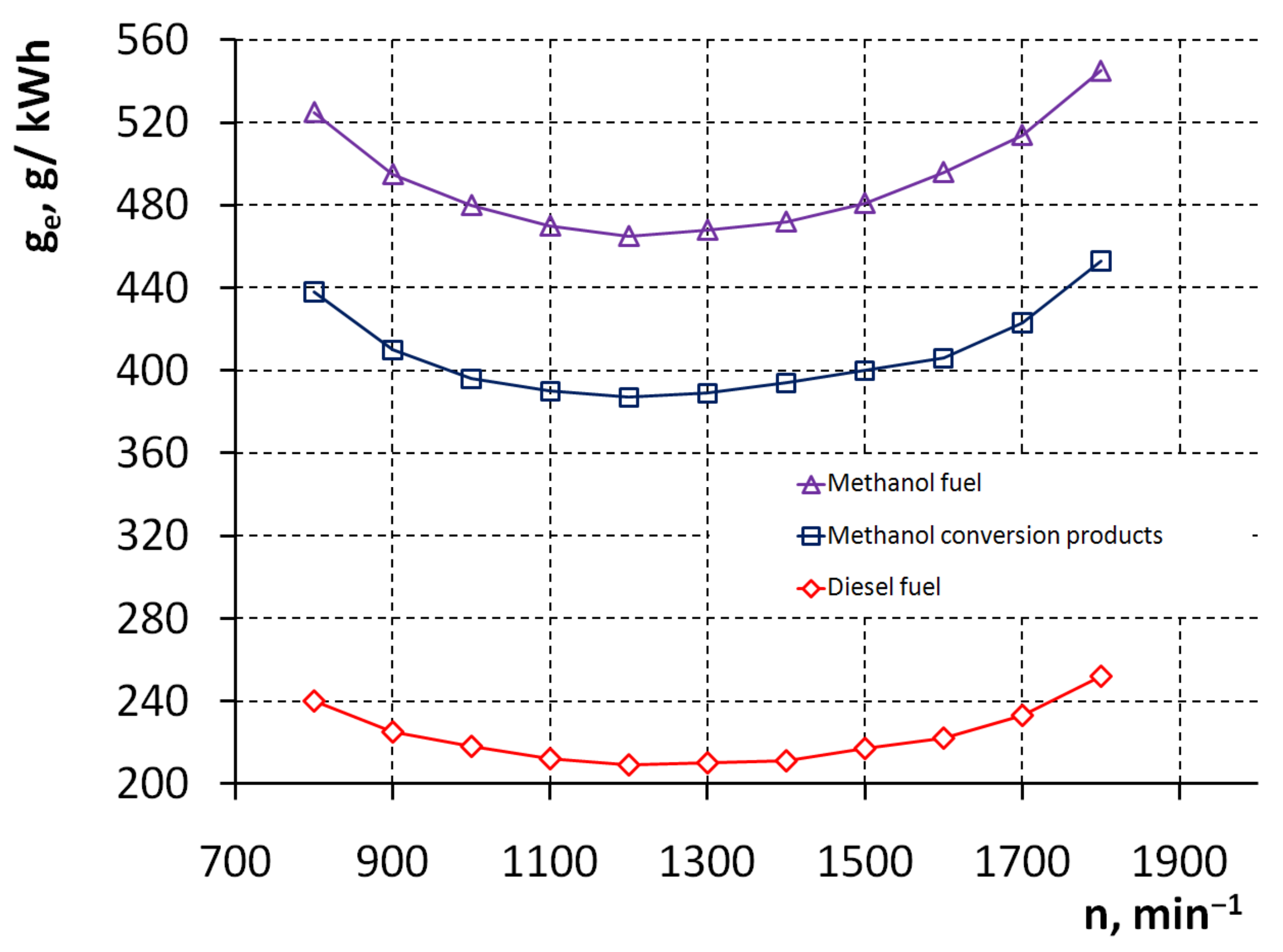
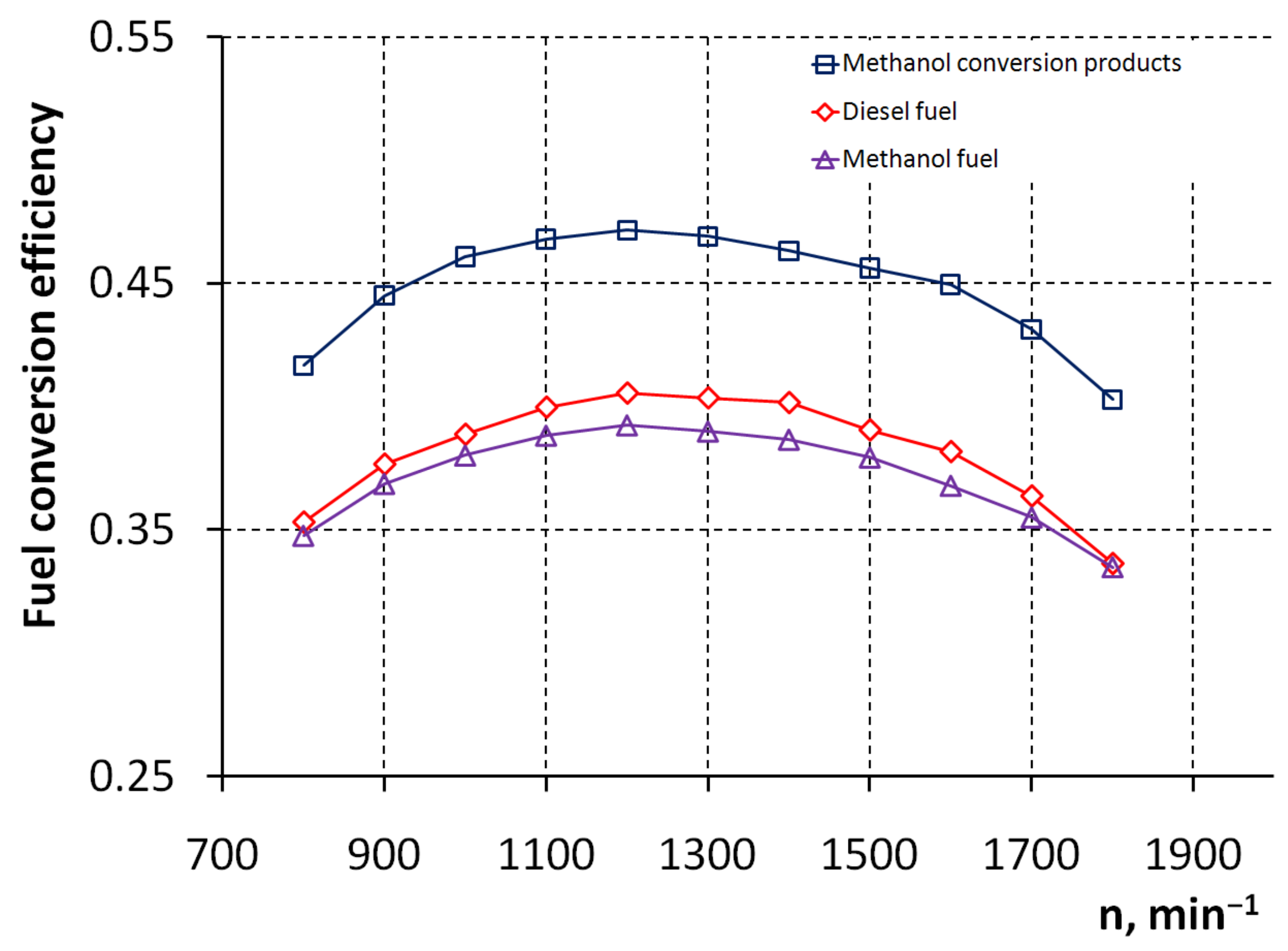
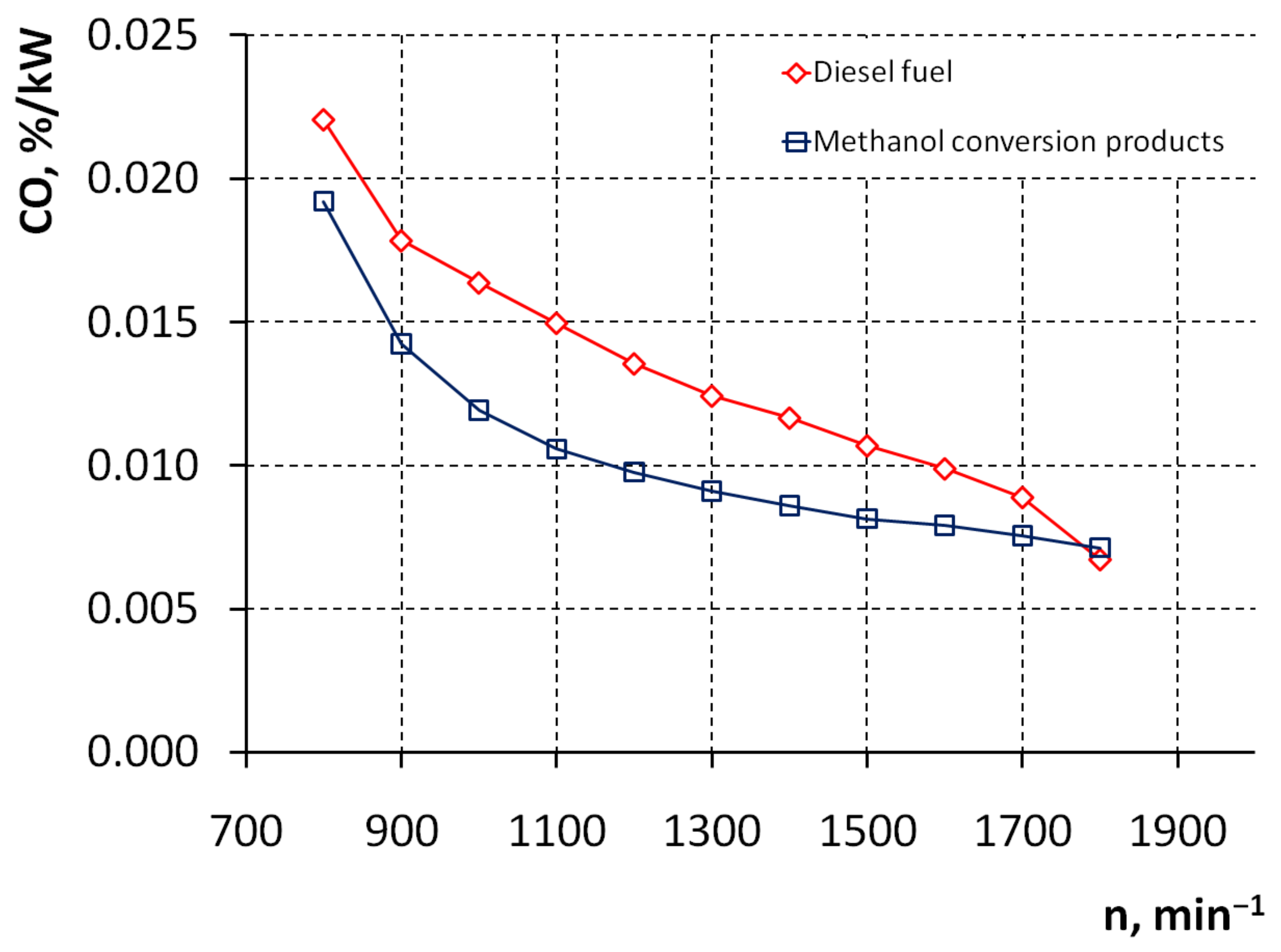
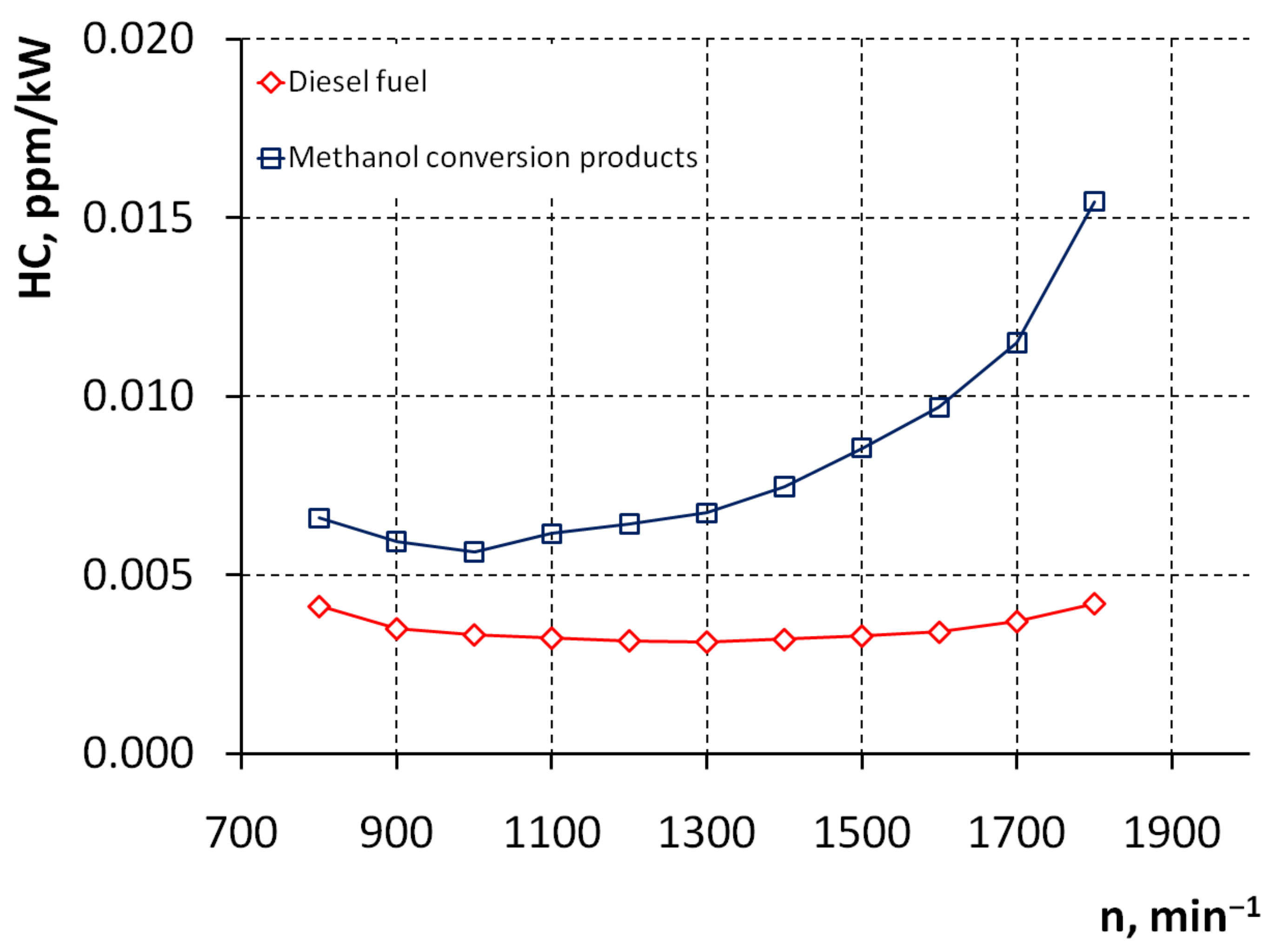
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Nomenclature
| Cμν | Average molar heat capacity of combustion products at constant volume |
| CO | Carbon monoxide |
| HU.C | Combustion heat |
| HU.D | Combustion heat of diesel fuel |
| HU.P.M | Combustion heat of gaseous products of methanol conversion |
| HU.M | Combustion heat of methanol |
| HC | Hydrocarbons |
| L | Work performed |
| Mpr.c | Number of combustion products at a constant volume |
| N | Power |
| NOx | Nitrogen oxides |
| S | Entropy |
| T | Temperature |
| Q | Heat |
| QC | Endothermic heat conversion quantity |
| QM | Exothermic thermal effect from the combustion of methanol-air mixture |
| QW | Waste heat |
| n | Crankshaft speed |
| ppm | parts per million |
| η | Efficiency |
References
- Panchuk, M.; Kryshtopa, S.; Sladkowski, A.; Panchuk, A.; Mandryk, I. Efficiency of production of motor biofuels for water and land transport. Nase More 2019, 66, 6–12. [Google Scholar] [CrossRef]
- Kovalov, S. Designing the shape of the combustion chambers for gas engines converted on the basis of the diesel engines. East. Eur. J. Enterp. Technol. 2020, 2, 23–31. [Google Scholar] [CrossRef]
- Panchuk, M.; Kryshtopa, S.; Sładkowski, A.; Panchuk, A. Environmental Aspects of the Production and Use of Biofuels in Transport. Lect. Notes Netw. Syst. 2020, 124, 115–168. [Google Scholar]
- Vershina, G.; Bystrenkov, O. Influence of Diesel Fuel Ignition Portion Value on Working Process Parameters of Gas-Diesel Engine. Sci. Tech. 2019, 18, 395–400. [Google Scholar] [CrossRef]
- Boretti, A. Advantages and Disadvantages of Diesel Single and Dual-Fuel Engines. Front. Mech. Eng. 2019, 5, 64. [Google Scholar] [CrossRef]
- Jurkovič, M.; Kalina, T.; Jancosek, L.; Kadnar, R.; Gorzelanczyk, P.; Jerabek, K. Proposal of Conversion the Tugboat Engines to Diesel-LNG Operation. Adv. Sci. Technol. Res. J. 2019, 13, 129–142. [Google Scholar] [CrossRef]
- Jovanović, S.; Knežević, M. Theoretical analysis of the cumulative costs of different diesel bus alternatives for a public transport in the city of Belgrade. Therm. Sci. 2017, 21, 669–681. [Google Scholar] [CrossRef][Green Version]
- Mateichyk, V.; Saga, M.; Smieszek, M.; Tsiuman, M.; Goridko, N.; Gritsuk, I.; Symonenko, R. Information and analytical system to monitor operating processes and environmental performance of vehicle propulsion systems. Iop Conf. Ser. Mater. Sci. Eng. 2020, 776, 012064. [Google Scholar] [CrossRef]
- Zhanga, K.; Xin, Q.; Mu, Z.; Niu, Z.; Wanga, Z. Numerical simulation of diesel combustion based on n-heptane and toluene. Propuls. Power Res. 2019, 8, 121–127. [Google Scholar] [CrossRef]
- Firmansyaha, A.; Aziz, A. Investigation of Auto-ignition of Several Single Fuels. MATEC Web of Conferences. 4th International Conference on Production. Energy Reliab. 2014, 13, 02013. [Google Scholar]
- Kryshtopa, S.; Melnyk, V.; Dolishnii, B.; Zakhara, I.; Voitsekhivska, T. Improvement of the model of forecasting heavy metals of exhaust gases of motor vehicles in the soil. East. Eur. J. Enterp. Technol. 2019, 4, 1–8. [Google Scholar] [CrossRef]
- Kryshtopa, S.; Kryshtopa, L.; Melnyk, V.; Prunko, I.; Demianchuk, Y. Experimental research on diesel engine working on a mixture of diesel fuel and fusel oils. Transp. Probl. 2017, 12, 53–63. [Google Scholar] [CrossRef]
- Abbasi, S.; Bahrami, H.; Ghobadian, B.; Kiani, M.; Kiani, D. Energy Analysis of a Diesel Engine Using Diesel and Biodiesel from Waste Cooking Oil. J. Agric. Mach. 2018, 8, 149–157. [Google Scholar]
- Afanas’ev, A.; Tret’yakov, A. Simulation of diesel engine energy conversion processes. J. Min. Inst. 2016, 222, 839–852. [Google Scholar]
- Panchuk, M.; Kryshtopa, S.; Panchuk, A.; Mandryk, I.; Sladkowski, A. Perspectives for developing and using the torrefaction technology in Ukraine. Int. J. Energy A Clean Environ. 2019, 20, 113–134. [Google Scholar] [CrossRef]
- Abbondanza, M.; Cavina, N.; Corti, E.; Moro, D.; Ponti, F.; Ravaglioli, V. Development of a Combustion Delay Model in the Control of Innovative Combustions. E3S WEB Conf. 2020, 197, 6013. [Google Scholar] [CrossRef]
- Cherednichenko, O. Efficiency Analysis of Methanol Usage for Marine Turbine Power Plant Operation Based on Waste Heat Chemical Regeneration. Probl. Energeticii Reg. 2019, 1, 102–111. [Google Scholar]
- Tselischev, O.; Kudryavtsev, S.; Loriya, M.; Boychenko, S.; Lanetsky, V.; Matveeva, I.; Leonenko, S.; Tselishcheva, M. Modification of motor gasoline with bioethanol in the cavitation field. Vopr. Khimii I Khimicheskoi Tekhnologii 2020, 6, 171–178. [Google Scholar]
- Bureika, G.; Matijošius, J.; Rimkus, A. Alternative Carbonless Fuels for Internal Combustion Engines of Vehicles. Lect. Notes Netw. Syst. 2020, 124, 1–49. [Google Scholar]
- Korpaniuk, T.; Ishchenko, Y.; Koval, N. Backgrounds for improving resource management of agricultural enterprises based on economic diagnostics of biofuel consumption. J. Soc. Sci. Res. 2019, 5, 367–380. [Google Scholar] [CrossRef]
- Bildirici, M.; Gökmenoğlu, S. Environmental pollution, hydropower energy consumption and economic growth: Evidence from G7 countries. Renew. Sustain. Energy Rev. 2016, 75, 68–85. [Google Scholar] [CrossRef]
- Bahman, N.; Sina, F.; Shahaboddin, S.; Kwok-wing, C.; Timon, R. Application of ANNs, ANFIS and RSM to estimating and optimizing the parameters that affect the yield and cost of biodiesel production. Eng. Appl. Comput. Fluid Mech. 2018, 12, 611–624. [Google Scholar]
- Havrysh, V.; Kalinichenko, A.; Minkova, O.; Lyashenko, S. Agricultural feedstock for solid and liquid biofuel production in Ukraine: Cluster analysis. Procedia Environ. Sci. Eng. Manag. 2019, 6, 649–658. [Google Scholar]
- Plashikhin, S.; Zelenin, E.; Semenyuk, N.; Safyants, A. Purification of a flue gas from solid particles and acidic impurities. Int. J. Energy Clean Environ. 2019, 20, 247–259. [Google Scholar] [CrossRef]
- Zhang, Z. Experimental Investigation on Regulated and Unregulated Emissions of a Diesel/ Methanol Compound Combustion Engine with and without Diesel Oxidation Catalyst. Sci. Total Environ. 2010, 408, 865–872. [Google Scholar] [CrossRef]
- Li, Y. Numerical Study on the Combustion and Emission Characteristics of a Methanol/Diesel Reactivity Controlled Compression Ignition (RCCI) Engine. Appl. Energy 2013, 106, 184–197. [Google Scholar] [CrossRef]
- Liu, Z. Economic Analysis of Methanol Production from Coal/Biomass Upgrading. Energy Sources Part B-Econ. Plan. Policy 2018, 13, 66–71. [Google Scholar] [CrossRef]
- He, L.; Fu, Y.; Lidstrom, M. Quantifying Methane and Methanol Metabolism of “Methylotuvimicrobium buryatense” 5GB1C under Substrate Limitation. Msystems 2019, 4, 748-19. [Google Scholar] [CrossRef]
- Liu, H.; Ma, J.; Tong, L.; Zheng, Z.; Yao, M. Investigation on the potential of high efficiency for internal combustion engines. Energies 2018, 11, 513. [Google Scholar]
- Zhennan, H.; Sulong, G.; Xi, Z.; Shipei, X.; Ping, A.; Jiguang, C.; Jun, Y.; Feng, L.; Suyi, Z.; Miao, L.; et al. Reaction decoupling in thermochemical fuel conversion and technical progress based on decoupling using fluidized bed. Carbon Resour. Convers. 2018, 1, 109–125. [Google Scholar]
- Mäyrä, O.; Leiviskä, K. Modeling in methanol synthesis. In Methanol; Elsevier: Amsterdam, The Netherlands, 2018; pp. 475–492. [Google Scholar]
- Yakovlieva, A.; Boichenko, S. Energy Efficient Renewable Feedstock for Alternative Motor Fuels Production: Solutions for Ukraine. Stud. Syst. Decis. Control 2020, 298, 247–259. [Google Scholar]
- Wargula, Ł.; Waluś Konrad, J.; Krawiec, P. The problems of measuring the temperature of the small engines (SI) on the example of a drive for non-road mobile machines. In Proceedings of the MATEC Web of Conferences, Rydzyna, Poland, 4–7 September 2018; Volume 254, pp. 4–7. [Google Scholar]
- Marty, P.; Hétet, J.; Chalet, D.; Corrignan, P. Exergy Analysis of Complex Ship Energy Systems. Entropy 2016, 18, 127. [Google Scholar] [CrossRef]
- Fernández-Yáñez, P.; Armas, O.; Gómez, A.; Gi, A. Developing Computational Fluid Dynamics (CFD) Models to Evaluate Available Energy in Exhaust Systems of Diesel Light-Duty Vehicles. Appl. Sci. 2017, 7, 590. [Google Scholar] [CrossRef]
- Alarifi, A.; Alsobhi, S.; Elkamel, A.; Croiset, E. Multiobjective optimization of methanol synthesis loop from synthesis gas via a multibed adiabatic reactor with additional interstage CO2 quenching. Energy Fuels 2015, 29, 530–537. [Google Scholar] [CrossRef]
- Tang, G.; Wang, S.; Zhang, L.; Shang, H. Diagnosis and Improvement of Combustion Characteristics of Methanol Miniature Reciprocating Piston Internal Combustion Engine. Micromachines 2020, 11, 96. [Google Scholar] [CrossRef] [PubMed]
- Tartakovsky, L.; Baibikov, V.; Veinblat, M. Modeling Methanol Steam Reforming for Internal Combustion Engine. Energy Power 2014, 4, 50–56. [Google Scholar]
- Korohodskyi, V.; Kryshtopa, S.; Migal, V.; Vasylenko, O.; Osetrov, O. Determining the characteristics for the rational adjusting of an fuel-air mixture composition in a two-stroke engine with internal mixture formation. East. Eur. J. Enterp. Technol. 2020, 2, 39–52. [Google Scholar]
- Syed, K.; Gaddale, A.P.R. Conversion of diesel engine into spark ignition engine to work with cng and lpg fuels for meeting new emission norms. Therm. Sci. 2010, 14, 913–922. [Google Scholar]
- Andersson, J.; Lundgren, J.; Marklund, M. Methanol production via pressurized entrained flow biomass gasification—Techno-economic comparison of integrated vs. stand-alone production. Biomass Bioenergy 2014, 64, 256–268. [Google Scholar] [CrossRef]
- Shamsul, N.S.; Kamarudin, S.K.; Rahman, N.A.; Kofli, N.T. An overview on the production of bio-methanol as potential renewable energy. Renew. Sustain. Energy Rev. 2014, 33, 578–588. [Google Scholar] [CrossRef]
- Dalena, F.; Senatore, A.; Marino, A.; Gordano, A.; Basile, M.; Basile, A. Methanol production and applications: An overview. In Methanol; Elsevier: Amsterdam, The Netherlands, 2018; pp. 3–28. [Google Scholar]






| Type of Fuel | Methane | Octane | Propane | Ethanol | Methanol |
|---|---|---|---|---|---|
| Cost (Euro/kg) | 0.05–0.1 | 0.55–0.8 | 0.4–0.75 | 0.2–0.3 | 0.15–0.25 |
| Conversion temperature (K) | 1000 | 1000 | 700 | 600 | 570 |
| Parameter | Diesel Fuel HU.D | Methanol HU.M | Converted Methanol HU.P.M |
|---|---|---|---|
| Absolute combustion heat (kJ/kg) | 42,500 | 19,700 | 23,870 |
| Relative combustion heat (%) | 100 | 46.35 | 56.17 |
| The Name of the Engine Parameters | Unit | Meaning |
|---|---|---|
| Type of diesel engine | - | 2-cylinder, 4-stroke, air-cooled |
| Diesel engine displacement | L | 2.08 |
| Diesel engine weight | kg | 280 |
| The method of mixing | - | Direct injection of diesel fuel |
| Rated engine power | kW | 18.4 |
| Efficient specific fuel consumption | g/kWh | 248 |
| Crankshaft speed at rated power | rpm | 1800 |
| Crankshaft speed at idling speed | rpm | 800 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kryshtopa, S.; Górski, K.; Longwic, R.; Smigins, R.; Kryshtopa, L. Increasing Parameters of Diesel Engines by Their Transformation for Methanol Conversion Products. Energies 2021, 14, 1710. https://doi.org/10.3390/en14061710
Kryshtopa S, Górski K, Longwic R, Smigins R, Kryshtopa L. Increasing Parameters of Diesel Engines by Their Transformation for Methanol Conversion Products. Energies. 2021; 14(6):1710. https://doi.org/10.3390/en14061710
Chicago/Turabian StyleKryshtopa, Sviatoslav, Krzysztof Górski, Rafał Longwic, Ruslans Smigins, and Liudmyla Kryshtopa. 2021. "Increasing Parameters of Diesel Engines by Their Transformation for Methanol Conversion Products" Energies 14, no. 6: 1710. https://doi.org/10.3390/en14061710
APA StyleKryshtopa, S., Górski, K., Longwic, R., Smigins, R., & Kryshtopa, L. (2021). Increasing Parameters of Diesel Engines by Their Transformation for Methanol Conversion Products. Energies, 14(6), 1710. https://doi.org/10.3390/en14061710







