Abstract
Hydrothermal pretreatment of biomass can improve fuel characteristics based on the decomposition properties of subcritical water. Thus, this study used a hydrothermal treatment to improve the fuel characteristics of empty fruit bunches (EFBs), which are generated as waste after palm oil extraction. The experimental reaction temperature was increased from 180 °C to 250 °C at an interval of 10 °C and the mass ratios between the dry sample and water content were set to 1:8 and 1:16 so that the sample was sufficiently immersed. Additionally, the material properties of EFB under hydrothermal treatment conditions were investigated using mass and energy yields, elemental analysis, proximate analysis, thermogravimetric analysis, derivative thermogravimetry, and Fourier transform infrared spectroscopy analysis of the reaction products. As the reaction temperature increased, the fixed carbon content and heating value increased because volatile matter, including oxygen, was removed first, which is similar characteristics to coal. All analyses revealed that the water content exhibited little influence on EFB material properties since the samples were sufficiently immersed in water. Thus, it is not necessary to add more water that required for sample immersion for the hydrothermal treatment of EFB.
1. Introduction
Fossil fuel energy sources have represented a large proportion of global power production over recent decades but have contributed to climate change through increased greenhouse gas emissions. To address this problem, coal can be replaced with renewable energy sources such as solar heat, wind power, and hydropower, as well as fuels obtained from waste and biomass or a mixture of the two [1,2,3].
As of 2015, the area of global palm cultivation amounted to 17.32 million ha, which leads to a large number of empty fruit bunches (EFBs) discharged as waste biomass after palm oil production, predominantly in Indonesia, Malaysia, and Thailand [4]. Most EFBs are currently buried or incinerated, with some left unattended, causing severe environmental problems [5]. Therefore, researchers have studied the possibility of using EFB as fuel for power generation [6]; however, this is complicated by its low heating value compared to coal and difficult pulverization due to large quantities of fiber components [4]. Therefore, torrefaction studies have been actively conducted to improve the fuel characteristics of EFB [7]. The carbon content and heating value of biomass increase during torrefaction due to the decomposition and removal of oxygen; its storage characteristics also improve as the fuel characteristics change from hydrophilicity to hydrophobicity [8].
Recently, hydrothermal treatment has attracted attention as a method of improving the fuel characteristics of biomass along with torrefaction technology [9], and many researchers have studied to investigate the effectiveness of hydrothermal treatment in improving the fuel properties of EFB as well as the possibility of utilizing the liquid residue for fertilizer [10,11]. Moriyasu et al. [12] performed hydrothermal treatment to upgrade low-rank coal and woody biomass mixture and Peitao et al. [13] studied to produce clean solid biofuel from high moisture content waste biomass by mild hydrothermal conversion processes. Subcritical water, in particular, has stronger dissolution characteristics than typical solvents due to the increased ionization of H+ and OH− [14]. The reaction in which the physical properties of subcritical water depolymerize organic polymers and refine particles by breaking the bonds of elements other than carbon is referred to as a hydrothermal reaction [15]. The hydrothermal reaction is generally performed in a high-pressure reactor to maintain high-pressure conditions [16,17]; however, it may exhibit higher carbonization efficiency at lower temperatures than typical torrefaction [18]. Additionally, various reaction mechanisms are formed depending on the experimental conditions, such as the sample type, reaction temperature, and water content [9]. The reaction temperature is an important parameter that determines various reaction mechanisms, including hydrolysis. The hydrothermal reaction occurs from 180 to 250 °C, which is the subcritical temperature range of water’s physical and chemical characteristics and the region in which hydrolysis begins to occur [9,19].
The water content is known to affect the occurrence of reactions such as hydrolysis, depolymerization, and condensation/polymerization during the hydrothermal reaction. However, no accurate quantitative value has been reported [20,21,22]. Nevertheless, previous studies have reported that it is desirable to add water so that biomass samples are sufficiently immersed; otherwise, the hydrothermal reaction will not occur for some types of biomass [9,23]. Therefore, the water content must be carefully determined considering the formation of an efficient reaction atmosphere, the load on the reactor, and the investment cost [24,25]. This study analyzes the influence of hydrothermal treatment on the solid fuel characteristics of EFB under various reaction temperatures and water content conditions. Furthermore, changes in the material properties of EFB are investigated through proximate analysis, elemental analysis, heating value analysis, thermogravimetric analysis (TGA), derivative thermogravimetry (DTG), and Fourier transform infrared spectroscopy (FT-IR) analysis of the reaction products.
2. Materials and Methods
2.1. Materials and Experimental Apparatus
Figure 1 shows a schematic diagram of the reactor used in this study. The reactor was a high-pressure reactor (Parr Instrument Co., Moline, IL, USA) with a capacity of 500 mL, a maximum temperature of 350 °C, a maximum pressure of 20.34 MPa, and a maximum stirring speed of 1700 rpm. The reaction temperature was controlled using a proportional–integral–derivative controller (Parr Instrument Co., USA). A cooling water circulation device was installed to prevent overheating of the motor during the stirring process. EFB generated after palm oil production was used after drying and the distilled water was also used in the hydrothermal treatment.
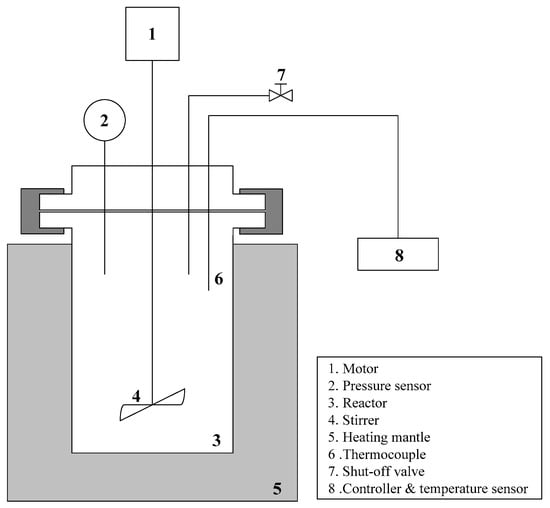
Figure 1.
Schematic diagram of the experimental apparatus used in this study.
2.2. Experimental Procedure and Analysis
For hydrothermal treatment, the material properties vary depending on the experimental conditions, such as the sample type, temperature, pressure, and water content. Therefore, in this study, experiments on the hydrothermal treatment of EFB were performed by setting the reaction temperature and water content as experimental parameters; the pressure inside the high-pressure reactor was also measured according to the reaction temperature. The reaction temperature was increased in 10-°C intervals from 180 °C to 250 °C. Furthermore, in this study, the minimum ratio of solid to water that the biomass samples were sufficiently immersed in was 1:8. Therefore, the mass ratios between the dry sample and water content were set to 1:8 and 1:16 to investigate the effect of the amount of water for the hydrothermal reaction. In this instance, the amount of dry EFB sample was 5 g, and the mass ratios between the sample and the water content were adjusted by adding water. The retention time after reaching each reaction temperature was set to 30 min to ensure sufficient reaction time [9], and the stirring speed was set to 180 rpm so that the water and EFB could be sufficiently mixed. To ensure the reliability of the experimental results, each experiment was performed three times. Upon completion of each experiment, the pressure was released through the shut-off valve and cooling was performed for 10 min using a cold water bath to prevent further reaction. The reaction product inside the reactor was collected and dried for 24 h at 105 °C. The mass and energy yields of the reaction product after hydrothermal treatment were calculated using the following equations:
Elemental analysis, proximate analysis, heating value analysis, FT-IR, TGA, and DTG analyses were conducted to investigate changes in the material properties through hydrothermal reaction. A bomb calorimeter (6100 Compensated Jacket Calorimeter, Parr Instrument Co., USA) was used for obtaining the heating value analysis, and the proximate analysis was conducted in accordance with the standards ASTM D 3175-89 and ASTM 3174-89 [26]. The elemental analysis of C, H, O, N, and S was conducted using an elemental analyzer (Flash 2000, Thermo Fisher Scientific K.K., Waltham, MA, USA) to mainly investigate changes in carbon and oxygen contents, which are the main indicators of hydrothermal treatment. To investigate changes in the chemical structure of the reaction product under different experimental conditions, an experiment was conducted at 2-cm−1 intervals in the 500–4000-cm−1 range at 25 °C using FT-IR (Spectrum 100, PerkinElmer, Buckinghamshire, UK). Finally, TGA and DTG analyses were conducted using a thermogravimetric analyzer (Pyris 1 TGA, PerkinElmer, UK) where the temperature was increased at a rate of 5 °C/min from room temperature to 700 °C under a nitrogen flow of 20 mL/min to investigate the thermal characteristics of the reaction product.
3. Results and Discussion
3.1. Mass and Energy Yields
Table 1 shows the proximate analysis, elemental analysis, and heating value analysis results for the dry EFB sample used in this study. Carbon and oxygen exhibited the highest amounts of 42.3% and 36.4%, respectively, and others were calculated by subtracting carbon, hydrogen, nitrogen, and oxygen from the total content. Moreover, it was seen that most of them are composed of inorganic components such as aluminum, silicon, calcium, and potassium [27]. This may be because the main component of EFB is lignin [28]. In addition, the higher heating value (HHV) was 16.4 MJ/kg.

Table 1.
Characteristics of dry empty fruit bunches (EFB) used in this study.
Figure 2 shows the HHV, mass yield and energy yields of the hydrothermally treated EFB according to the reaction temperature and water content. Figure 2a shows that the mass yield decreased due to the decomposition of some volatile organic matter while the heating value increased because of an increase in fixed carbon (FC) content as the reaction temperature increased. However, the water content had little influence on the mass yield and heating value. This is likely because all water content conditions in this study allowed the EFB to be sufficiently immersed in water. Therefore, it was concluded that no further addition of water is necessary if the water content allows the sample to be immersed for the hydrothermal treatment of EFB. Figure 2b shows the energy yield according to the reaction temperature and water content, which was calculated using Equation (2) based on the mass yield and heating value. During the experiment, the energy yield decreased as the reaction temperature increased, which is likely because the reduction rate of the mass yield was higher than the rate of increase of the heating value as the reaction temperature increased, as observed in Figure 2a.
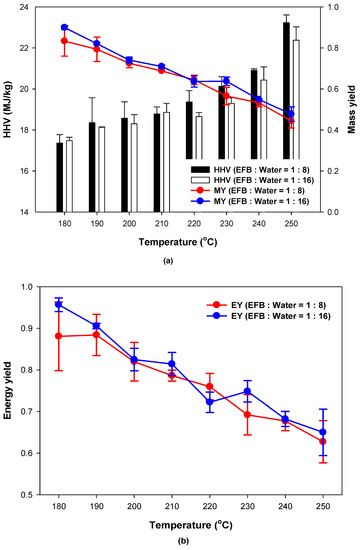
Figure 2.
(a) HHV, Mass yield (MY), and (b) energy yield (EY) of EFB according to reaction temperature and water content.
Figure 3 shows the reaction pressure according to the reaction temperature and water content. The critical temperature and pressure of water are 373.946 °C and 22.064 Mpa respectively. Therefore, the hydrothermal treatments were performed under subcritical water conditions, and as shown in the figure, the reaction pressure varied with the reaction temperature but exhibited minimal variation with water content. This indicates that the addition of more water than that required to immerse the sample does not significantly affect the hydrothermal treatment of EFB, as shown in Figure 2.
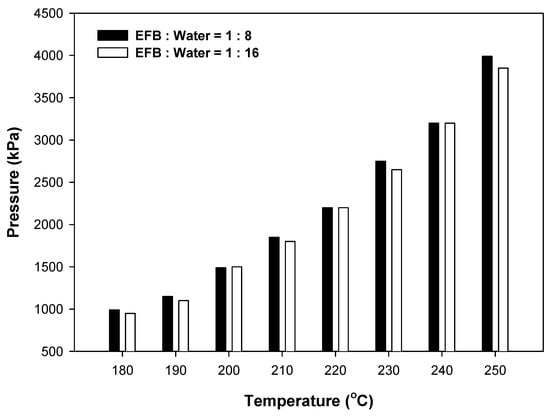
Figure 3.
Change in pressure with reaction temperature for different water contents.
3.2. Elemental and Proximate Analysis
The elemental and proximate analysis results for the reaction product and raw EFB according to reaction temperature and water content are shown in Figure 4 and Table 2 and Table 3. The oxygen content decreased while the carbon content increased as the reaction temperature increased (Figure 4a and Table 2 and Table 3), which confirmed that the carbon content increases during the hydrothermal reaction of EFB because the oxygen-containing components are decomposed first. On the other hand, there was little change in hydrogen and nitrogen contents. There was also minimal change in the elemental components with water content although there are differences in others, similar to the results for mass and energy yields (Figure 2). The proximate analysis results in Figure 4b show that the content of volatile matter (VM) decreased as the reaction temperature increased, thereby increasing the fixed carbon (FC) content. This is likely because VM, including oxygen, was decomposed as the reaction temperature increased, as can be seen from Figure 4a. No significant change was observed in ash content. The change in water content resulted in slight differences in VM, FC, and others in Table 2 and Table 3. This may reflect the analysis error caused by using a small amount of dry EFB sample due to the limited reactor capacity, which meant that only a small amount of reaction product was used in the proximate analysis. However, the overall trend was similar, suggesting that the water content had minimal influence.
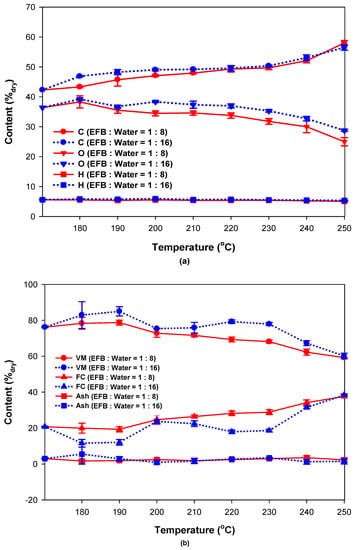
Figure 4.
(a) Elemental and (b) proximate analysis of EFB according to reaction temperature and water content.

Table 2.
Elemental and proximate analysis of EFB according to reaction temperature (EFB:water = 1:8).

Table 3.
Elemental and proximate analysis of EFB according to reaction temperature (EFB:water = 1:16).
3.3. Fuel Characteristics of EFB after Hydrothermal Treatment
In this study, the O/C and H/C ratios under different reaction temperatures and the correlations between carbon and hydrogen contents and the heating value were analyzed based on the elemental and heating value analysis results to investigate changes in the fuel characteristics of hydrothermally treated EFBs. Figure 5 compares the O/C and H/C ratios of EFB according to the reaction temperature and water content with those of the raw EFB and coal. As the reaction temperature increased, both the O/C and H/C ratios decreased and the characteristics of EFB became gradually more similar to those of coal. This is because the carbon content increased as the reaction temperature increased due to the increased decomposition of oxygen-containing VM, as mentioned previously. Therefore, although additional energy is required for the hydrothermal reaction, it can be seen that the hydrothermal treatment is effective to enhance the fuel characteristics of EFB because the pulverization and storage properties improve as it becomes similar to coal and the operation temperature is lower than torrefaction.
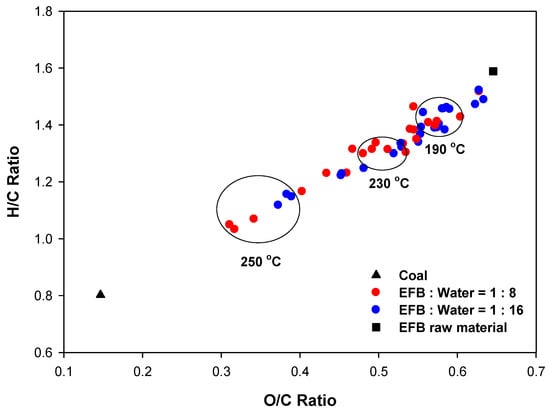
Figure 5.
O/C and H/C ratios of EFB according to the reaction temperature and water content.
Figure 6 shows the correlations between the carbon and hydrogen contents and the heating value of hydrothermally treated EFB. In Figure 6, the coefficient of determination, is a statistical measure of how close the data to the fitted linear regression line and it is calculated by the following equation:
is the actual value, is the predicted value of, is the mean of the y values.
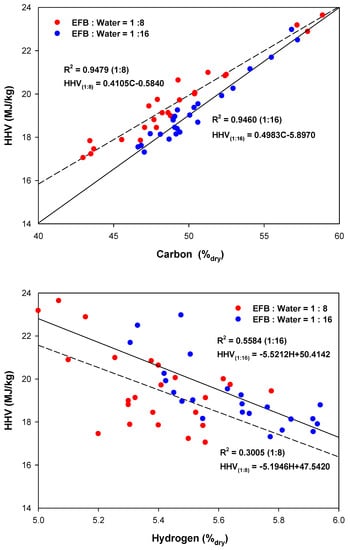
Figure 6.
Correlations between carbon and hydrogen contents and the heating value of hydrothermally treated EFB: (top) carbon–HHV; (bottom) hydrogen–HHV.
The of the linear regression analysis for the relationship between carbon content and HHV were 0.9479 and 0.9460 for ratios between the dry sample mass and water content of 1:8 and 1:16, respectively. In contrast, the values for the relationship between hydrogen and HHV were 0.3005 and 0.5584, respectively. Therefore, the heating value of hydrothermally treated EFB was significantly affected by the carbon content but less affected by hydrogen [29].
3.4. TGA and FT-IR Analysis
To investigate the effect of hydrothermal treatment on the thermal characteristics of EFB, the TGA and DTG analysis results for EFB according to the EFB raw material, reaction temperature, and water content are shown in Figure 7 and Figure 8. Figure 7a shows that hydrothermally treated EFB exhibited a larger amount of residue after decomposition than the raw EFB. Moreover, this amount increased as the reaction temperature increased. This is likely because the amount of substances that are difficult to decompose increased as the substances that are easily decomposed at low temperatures were decomposed and removed by hydrothermal treatment. As the hydrothermal reaction temperature increased, the DTG peak from 200 to 250 °C increased but the DTG peak from 300 to 350 °C decreased compared to the EFB raw material (Figure 7b). A previous TGA/DTG analysis indicated the pyrolysis of biomass hemicellulose between 200 °C and 250 °C and the pyrolysis of biomass cellulose between 300 °C and 350 °C [30,31]; therefore, the results of this study confirm that the cellulose of EFB was partially decomposed into hemicellulose as the hydrothermal reaction temperature increased. Moreover, a comparison of the results in Figure 7 and Figure 8 indicates that the water content had minimal influence.
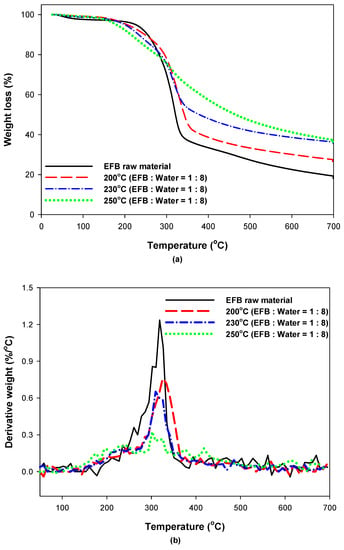
Figure 7.
(a) Thermogravimetric analysis (TGA) and (b) derivative thermogravimetry (DTG) curves of EFB according to reaction temperature (EFB:water = 1:8).
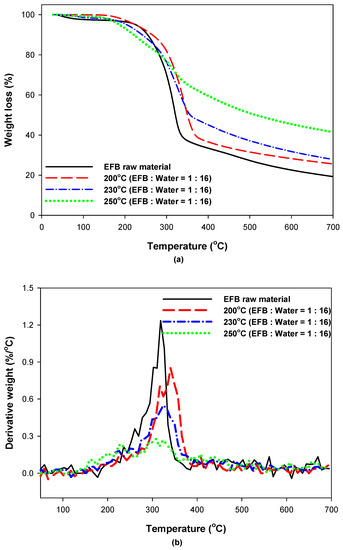
Figure 8.
(a) TGA and (b) DTG curves of EFB according to reaction temperature (EFB:water = 1:16).
Figure 9 shows the FT-IR spectra of the hydrothermally treated EFB under each experimental condition. The peaks between 3500 cm−1 and 3200 cm−1 represent the stretching of the O–H bonds of cellulose [32], whereas the peaks in the 2900 cm−1 and 2800 cm−1 sections represent stretching of the aliphatic C–H bonds of cellulose and hemicellulose [33]. In addition, the peaks in the 1700 cm−1 and 1600 cm−1 sections represent lignin-based C=C and C=O stretching, respectively [34]. Figure 9, however, shows that the peaks representing the O–H and C–H bonds of cellulose and hemicellulose hardly changed, but the peaks that represent lignin-based C=C and C=O stretching became more obvious compared to those of raw EFB after hydrothermal treatment. This appears to be due to the lignin content which decomposes at relatively high temperatures and relatively increases as the hydrothermal treatment temperature increased because of the decomposition of substances that decompose easily at low temperatures.
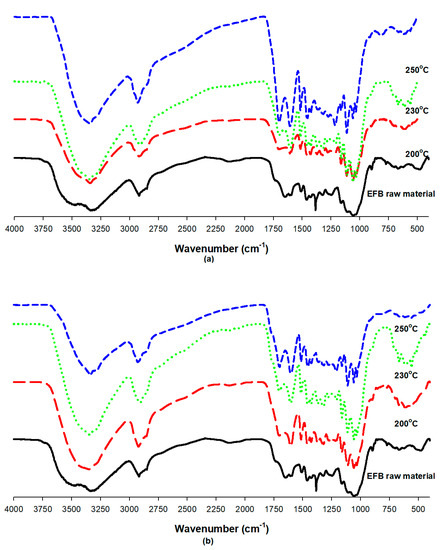
Figure 9.
Fourier transform infrared spectroscopy (FT-IR) spectra of EFB according to the reaction temperature and water content: (a) EFB:water = 1:8; (b) EFB:water = 1:16.
4. Conclusions
In this study, experiments were performed to determine the effect of various reaction temperatures and water content conditions during the hydrothermal treatment of empty fruit bunches (EFBs), which are a type of biomass waste generated in large quantities after palm oil extraction. The mass and energy yield results of hydrothermally treated EFB showed that the mass yield decreased as oxygen-containing volatile matter (VM) was decomposed and removed, but the heating value increased as the content of fixed carbon increased. The water content, however, exhibited minimal influence. Analysis of the O/C and H/C ratios was conducted to investigate the effect of hydrothermal treatment on the fuel characteristics of EFB, which also showed the minimal influence of water content. Conversely, both the O/C and H/C ratios decreased as the reaction temperature increased, which caused the characteristics of EFB to become gradually more similar to those of coal. The correlations between carbon, hydrogen and the heating value revealed that the heating value was predominantly affected by the carbon content and was less affected by hydrogen. Furthermore, thermogravimetric analysis, derivative thermogravimetry, and Fourier transform infrared spectroscopy of hydrothermally treated EFB confirmed that the amount of substances that are difficult to decompose relatively increased as the reaction temperature increased because substances that are easily decomposed at low temperatures were decomposed and removed.
Author Contributions
S.C.O. and H.J.K. are responsible for the idea formulation, project development and research preparation. H.J.K., C.P. and R.N. are responsible for the experimental work while all three authors are equally responsible for data analysis, manuscript preparation, proofreading and submission of the manuscript. S.C.O. is the corresponding author of the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research paper was funded by the Renewable Energy’s Technology Development Program (No. 20193010092990) of the Korea Institute of Energy Technology Evaluation and Planning (KETEP) funded by the Ministry of Trade, Industry and Energy.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
This work was supported by the Renewable Energy’s Technology Development Program (No. 20193010092990) of the Korea Institute of Energy Technology Evaluation and Planning (KETEP) funded by the Ministry of Trade, Industry and Energy.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviation
| EFBs | Empty fruit bunches |
| TGA | Thermogravimetric analysis |
| DTG | Derivative thermogravimetry |
| FT-IR | Fourier transform infrared spectroscopy |
| HHV | Higher heating value |
| VM | Volatile matter |
| FC | Fixed carbon |
References
- Field, C.B.; Campbell, J.E.; Lobell, D.B. Biomass energy: The scale of the potential resource. Trends Ecol. Evol. 2008, 23, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, S.D.; Trautmann, N.M.; Streets, D.G.; Roden, C.A.; Bond, T.C. Global biofuel use, 1850–2000. Glob. Biogeochem. Cycles 2007, 21. [Google Scholar] [CrossRef]
- Richter, D., Jr.; Jenkins, D.H.; Karakash, J.T.; Knight, J.; McCreery, L.R.; Nemestothy, K.P. Wood energy in America. Science 2009, 323, 1432–1433. [Google Scholar] [CrossRef]
- Poudel, J.; Ohm, T.-I.; Gu, J.H.; Shin, M.C.; Oh, S.C. Comparative study of torrefaction of empty fruit bunches and palm kernel shell. J. Mater. Cycles Waste Manag. 2016, 19, 917–927. [Google Scholar] [CrossRef]
- Jamari, S.S.; Howse, J.R. The effect of the hydrothermal carbonization process on palm oil empty fruit bunch. Biomass Bioenergy 2012, 47, 82–90. [Google Scholar] [CrossRef]
- Khatun, R.; Reza, M.I.H.; Moniruzzaman, M.; Yaakob, Z. Sustainable oil palm industry: The possibilities. Renew. Sustain. Energy Rev. 2017, 76, 608–619. [Google Scholar] [CrossRef]
- Poudel, J.; Oh, S.C. Effect of Torrefaction on the Properties of Corn Stalk to Enhance Solid Fuel Qualities. Energies 2014, 7, 5586–5600. [Google Scholar] [CrossRef]
- Nhuchhen, D.; Basu, P.; Acharya, B. A Comprehensive Review on Biomass Torrefaction. Int. J. Renew. Energy Biofuels 2014, 2014, 1–56. [Google Scholar] [CrossRef]
- Funke, A.; Ziegler, F. Hydrothermal carbonization of biomass: A summary and discussion of chemical mechanisms for process engineering. Biofuels Bioprod. Biorefining 2010, 4, 160–177. [Google Scholar] [CrossRef]
- Novianti, S.; Nurdiawati, A.; Zaini, I.N.; Sumida, H.; Yoshikawa, K. Hydrothermal treatment of palm oil empty fruit bunches: An investigation of the solid fuel and liquid organic fertilizer applications. Biofuels 2016, 7, 627–636. [Google Scholar] [CrossRef]
- Yan, M.; Hantoko, D.; Susanto, H.; Ardy, A.; Waluyo, J.; Weng, Z.; Lin, J. Hydrothermal treatment of empty fruit bunch and its pyrolysis characteristics. Biomass Convers. Biorefinery 2019, 9, 709–717. [Google Scholar] [CrossRef]
- Nonaka, M.; Hirajima, T.; Sasaki, K. Upgrading of low rank coal and woody biomass mixture by hydrothermal treatment. Fuel 2011, 90, 2578–2584. [Google Scholar] [CrossRef]
- Zhao, P.; Shen, Y.; Ge, S.; Chen, Z.; Yoshikawa, K. Clean solid biofuel production from high moisture content waste biomass employing hydrothermal treatment. Appl. Energy 2014, 131, 345–367. [Google Scholar] [CrossRef]
- Toor, S.S.; Rosendahl, L.; Rudolf, A. Hydrothermal liquefaction of biomass: A review of subcritical water technologies. Energy 2011, 36, 2328–2342. [Google Scholar] [CrossRef]
- Yang, T.; Liu, X.; Li, R.; Li, B.; Kai, X. Hydrothermal liquefaction of sewage sludge to produce bio-oil: Effect of co-pretreatment with subcritical water and mixed surfactants. J. Supercrit. Fluids 2019, 144, 28–38. [Google Scholar] [CrossRef]
- Lynam, J.G.; Coronella, C.J.; Yan, W.; Reza, M.T.; Vasquez, V.R. Acetic acid and lithium chloride effects on hydrothermal carbonization of lignocellulosic biomass. Bioresour. Technol. 2011, 102, 6192–6199. [Google Scholar] [CrossRef] [PubMed]
- Hägglund, S.; Vatkolning av Torv, A.B. Svensk Torvförädling; Lund University: Lund, Sweden, 1960; p. 188. [Google Scholar]
- Wang, Y.; Qiu, L.; Zhu, M.; Sun, G.; Zhang, T.; Kang, K. Comparative Evaluation of Hydrothermal Carbonization and Low Temperature Pyrolysis of Eucommia ulmoides Oliver for the Production of Solid Biofuel. Sci. Rep. 2019, 9, 5535. [Google Scholar] [CrossRef] [PubMed]
- Siskin, M.; Katritzky, A.R. Reactivity of Organic Compounds in Hot Water: Geochemical and Technological Implications. Science 1991, 254, 231–237. [Google Scholar] [CrossRef]
- Engel, M.H.; Macko, S.A. Organic Geochemistry: Principles and Applications; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2013; Volume 11. [Google Scholar]
- Bobleter, O. Hydrothermal degradation of polymers derived from plants. Prog. Polym. Sci. 1994, 19, 797–841. [Google Scholar] [CrossRef]
- Mok, W.S.H.; Antal, M.J., Jr.; Szabo, P.; Verhegyi, G.; Zelei, B. Formation of charcoal from biomass in a sealed reactor. Ind. Eng. Chem. Res. 1992, 31, 1162–1166. [Google Scholar] [CrossRef]
- Schwald, W.; Bobleter, O. Hydrothermolysis of Cellulose Under Static and Dynamic Conditions at High Temperatures. J. Carbohydr. Chem. 1989, 8, 565–578. [Google Scholar] [CrossRef]
- Siskin, M.; Katritzky, A.R. Reactivity of Organic Compounds in Superheated Water: General Background. Chem. Rev. 2001, 101, 825–836. [Google Scholar] [CrossRef] [PubMed]
- Léger, S.; Chornet, E.; Overend, R. Characterization and quantification of changes occurring in the low-severity dewatering of peat. Int. J. Coal Geol. 1987, 8, 135–146. [Google Scholar] [CrossRef]
- Parikh, J.; Channiwala, S.; Ghosal, G. A correlation for calculating elemental composition from proximate analysis of biomass materials. Fuel 2007, 86, 1710–1719. [Google Scholar] [CrossRef]
- Mohammad, I.N.; Ongkudon, C.M.; Misson, M. Physicochemical Properties and Lignin Degradation of Thermal-Pretreated Oil Palm Empty Fruit Bunch. Energies 2020, 13, 5966. [Google Scholar] [CrossRef]
- Kim, M.; Son, D.; Choi, J.-W.; Jae, J.; Suh, D.J.; Ha, J.-M.; Lee, K.-Y. Production of phenolic hydrocarbons using catalytic depolymerization of empty fruit bunch (EFB)-derived organosolv lignin on Hβ-supported Ru. Chem. Eng. J. 2017, 309, 187–196. [Google Scholar] [CrossRef]
- Uemura, Y.; Matsumoto, R.; Saadon, S.; Matsumura, Y. A study on torrefaction of Laminaria japonica. Fuel Process. Technol. 2015, 138, 133–138. [Google Scholar] [CrossRef]
- Yang, H.; Yan, R.; Chen, H.; Zheng, C.; Lee, A.D.H.; Liang, D.T. In-Depth Investigation of Biomass Pyrolysis Based on Three Major Components: Hemicellulose, Cellulose and Lignin. Energy Fuels 2006, 20, 388–393. [Google Scholar] [CrossRef]
- Gan, M.J.; Lim, W.S.; Ng, H.X.; Ong, M.H.; Gan, S.; Lee, L.Y.; Thangalazhy-Gopakumar, S. Enhancement of Palm Kernel Shell Fuel Properties via Wet Torrefaction: Response Surface, Optimization, and Combustion Studies. Energy Fuels 2019, 33, 11009–11020. [Google Scholar] [CrossRef]
- Stevanic, J.S.; Salmén, L. Characterizing wood polymers in the primary cell wall of Norway spruce (Picea abies (L.) Karst.) using dynamic FT-IR spectroscopy. Cellulose 2007, 15, 285–295. [Google Scholar] [CrossRef]
- Wang, P.; Zhang, J.; Shao, Q.; Wang, G. Physicochemical properties evolution of chars from palm kernel shell pyrolysis. J. Therm. Anal. Calorim. 2018, 133, 1271–1280. [Google Scholar] [CrossRef]
- Park, J.; Meng, J.; Lim, K.H.; Rojas, O.J.; Park, S. Transformation of lignocellulosic biomass during torrefaction. J. Anal. Appl. Pyrolysis 2013, 100, 199–206. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).