Hydrolysis-Based Hydrogen Generation Investigation of Aluminum System Adding Low-Melting Metals
Abstract
1. Introduction
2. Materials and Methods
2.1. Alloy Preparation
2.2. Observation of Phase Structure and Microstructure
2.3. Test on Hydrolysis Performance of Aluminum Alloy
3. Results and Discussion
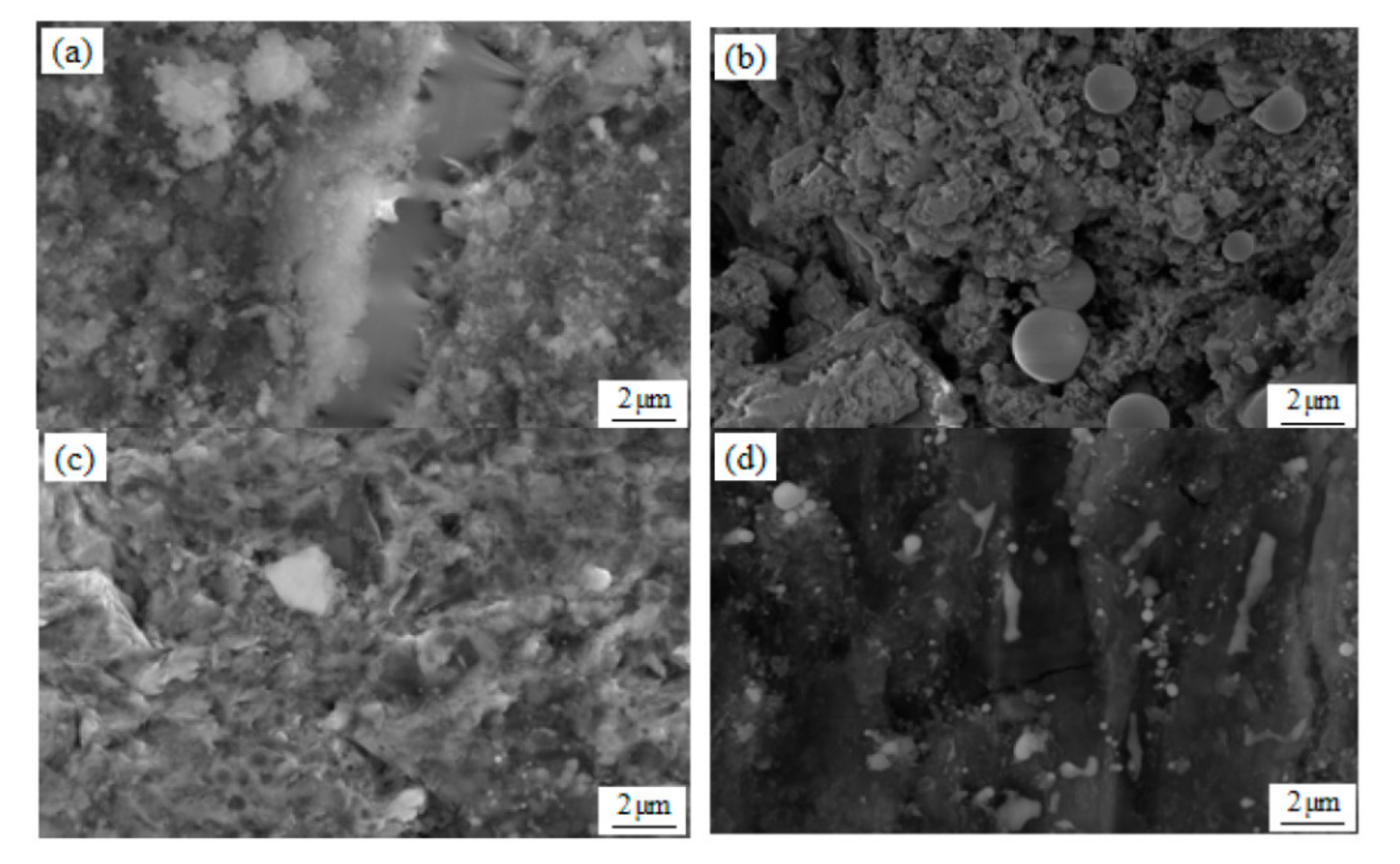
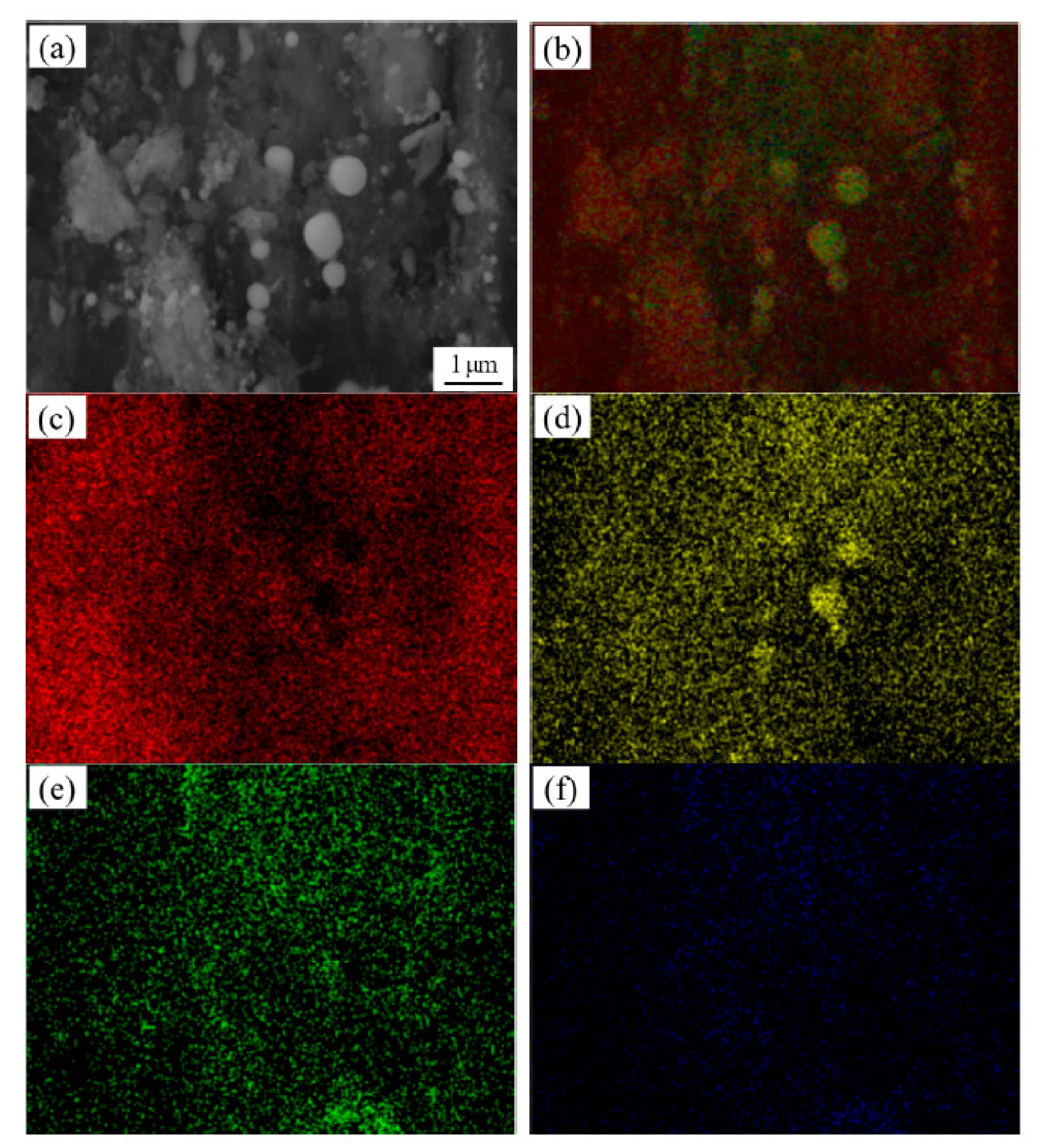
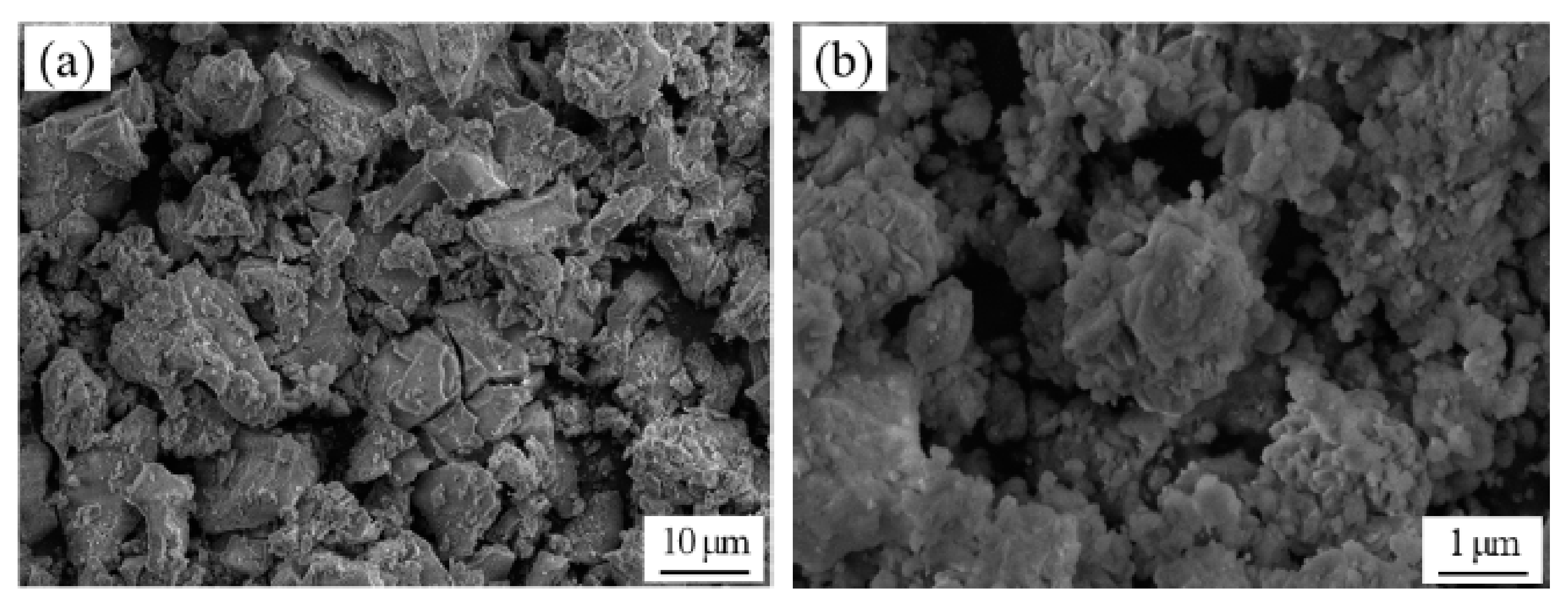
3.1. SEM Observation and Analysis
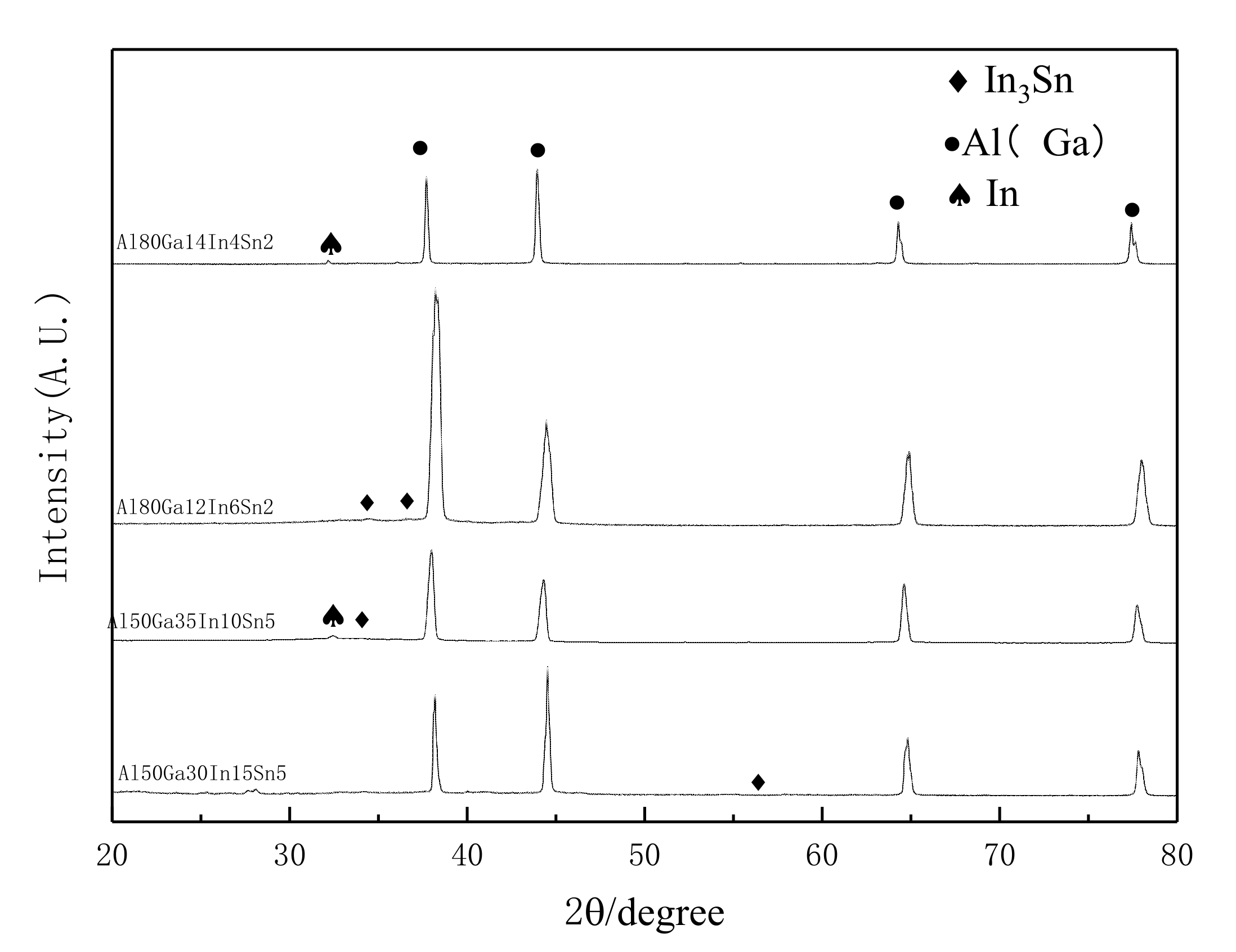
3.2. XRD Examination
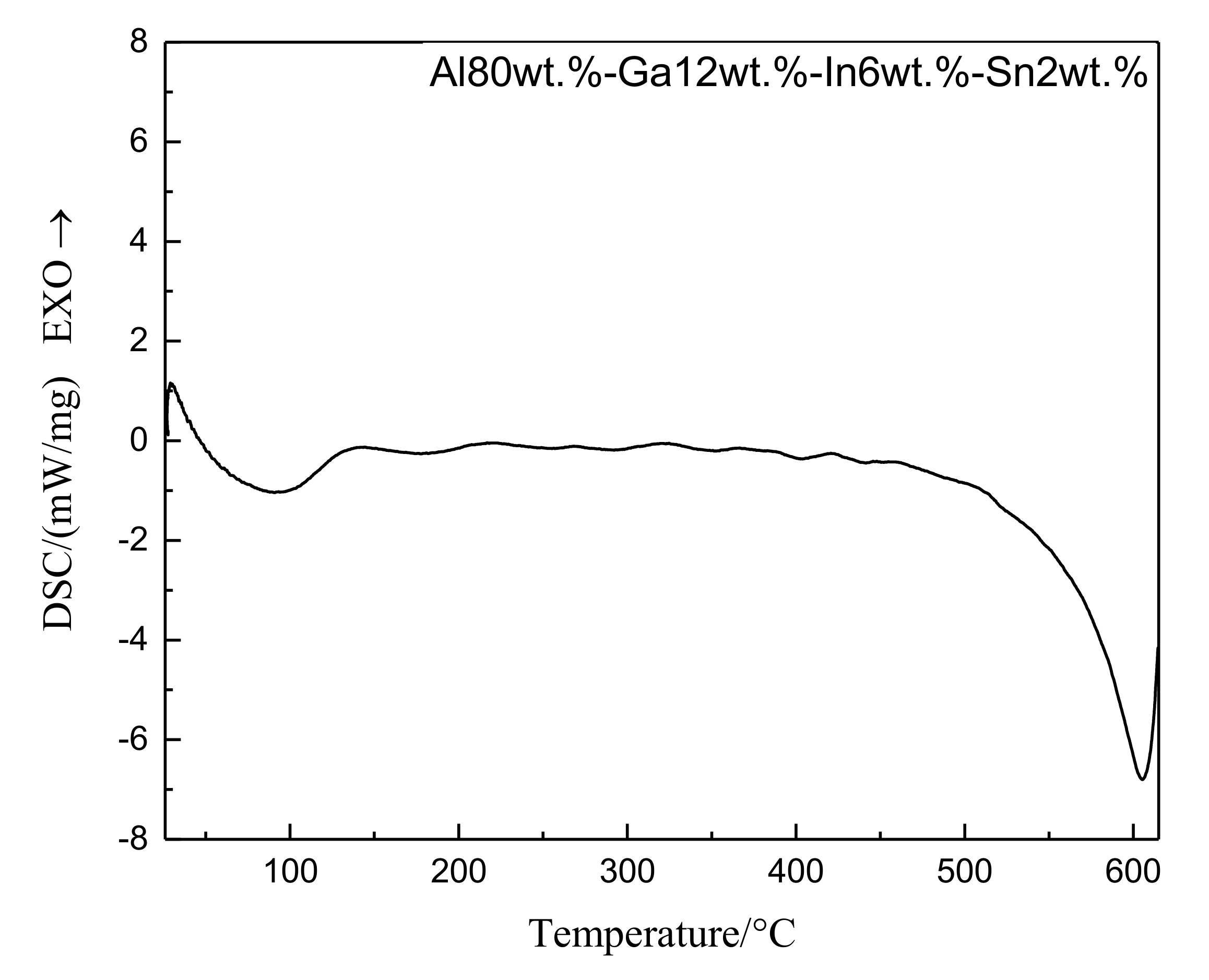
3.3. DSC Analysis of Alloy Ingot
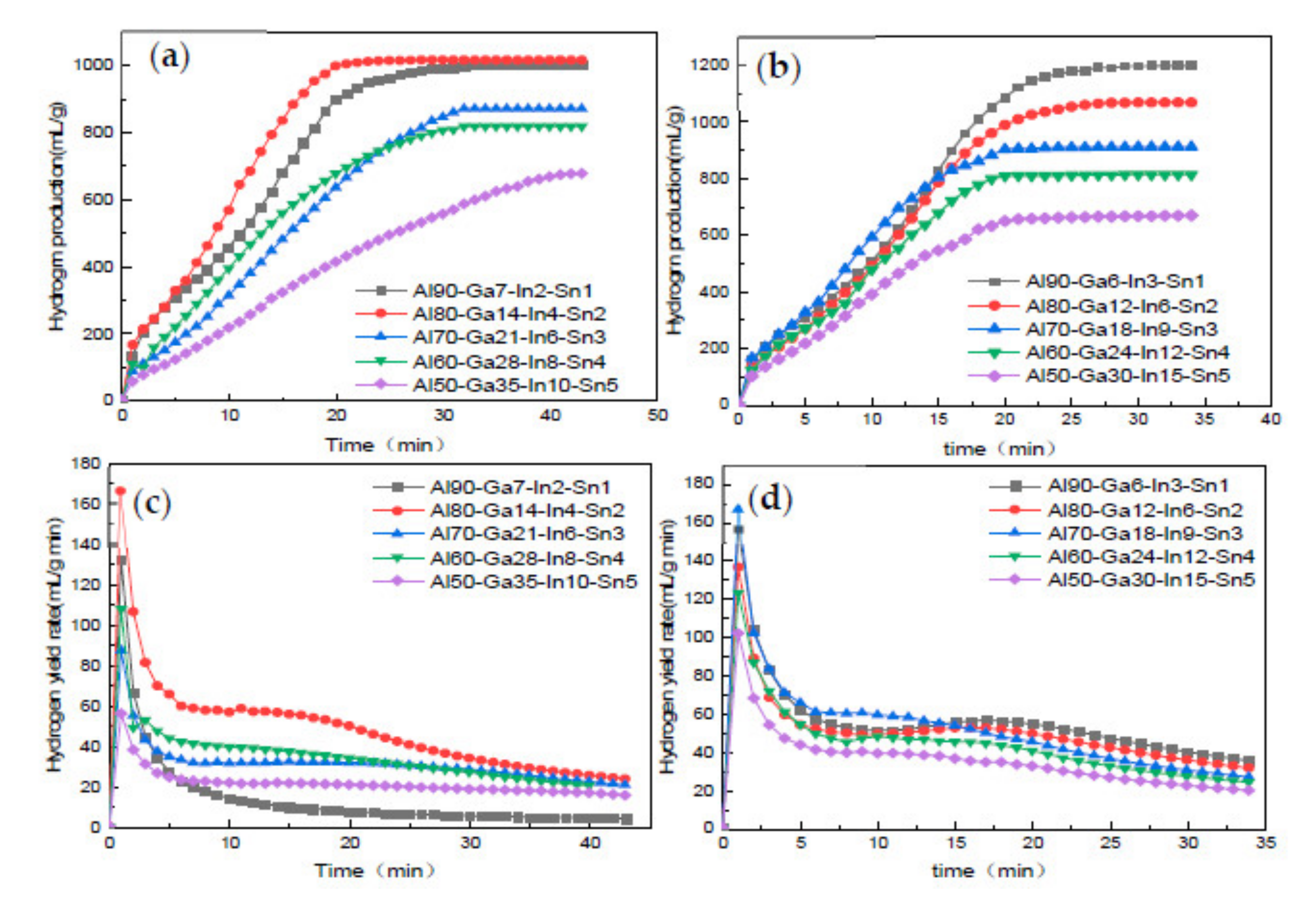
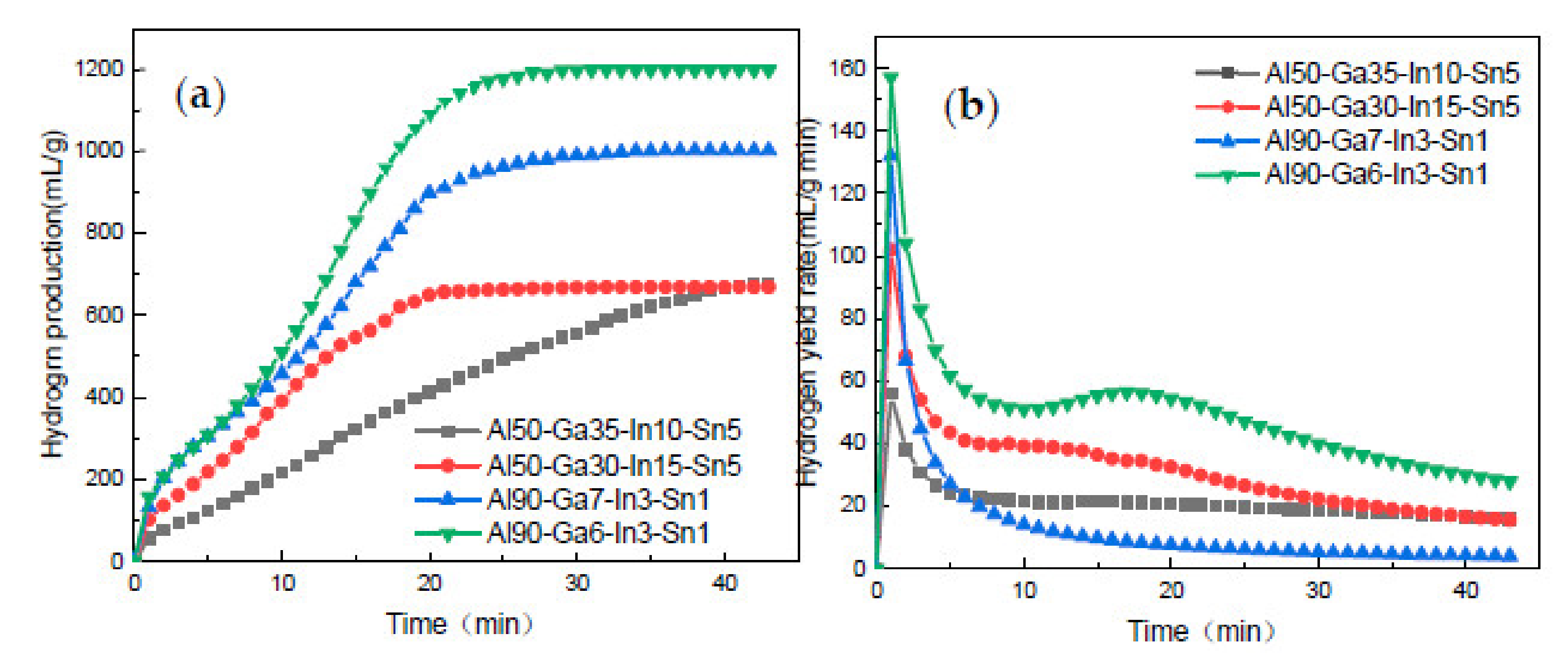
3.4. Analysis of Alloy Hydrogen Production Performance
4. Conclusions
- (1)
- While using low-melting-point metals Ga, In, and Sn to improve the hydrogen production performance of aluminum hydrolysis, changing the proportion degree of low-melting-point metals can effectively improve the hydrogen production amount and rate.
- (2)
- Combining the results of scanning electron microscopy and X-ray diffraction, the following conclusions can be drawn: when the ratio of In and Sn in the alloy is 3:1, the occurrence probability and content of the alloy phase In3Sn can be effectively improved.
- (3)
- When GIS is 7:2:1, because of the decrease in metal content at low melting point, the alloy phase that can promote hydrolysis reaction cannot be formed better, resulting in a reduction in hydrogen production far below the theoretical value. However, when GIS is 6:3:1, the maximum instantaneous hydrogen production rate is up to 157 mL/(g min) and the hydrogen production efficiency is very close to the theoretical value.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sun, H. Hydrogen energy is arousing great attention all over the world. Int. J. Hydrogen Energy 2020. [Google Scholar] [CrossRef]
- Von Jouanne, A.; Brekken, T.K.A. Ocean and Geothermal Energy Systems. Proc. IEEE 2017, 105, 2147–2165. [Google Scholar] [CrossRef]
- Srinivasan, M.; Velu, A.; Madhubabu, B. The Potential Impact of Solar Technology on the Environment. J. Energy Chem. 2019, 1. [Google Scholar]
- Dawood, F.; Anda, M.; Shafiullah, G.M. Hydrogen production for energy: An overview. Int. J. Hydrogen Energy 2020, 45, 3847–3869. [Google Scholar] [CrossRef]
- Dincer, I.; Acar, C. Innovation in hydrogen production. Int. J. Hydrogen Energy 2017, 42, 14843–14864. [Google Scholar] [CrossRef]
- Momirlan, M.; Veziroǧlu, T. Recent directions of world hydrogen production. Renew. Sust. Energ. Rev. 1999, 3, 219–231. [Google Scholar] [CrossRef]
- Mayakrishnan Gopiraman, D.D.S.G. Sustainable and Versatile CuO/GNS Nanocatalyst for Highly Efficient Base Free Coupling Reactions. ACS Sustain. Chem. Eng. 2015, 3, 150904102130008. [Google Scholar]
- Sathiskumar, C.; Ramakrishnan, S.; Vinothkannan, M.; Kim, A.R.; Karthikeyan, S.; Yoo, D.J. Nitrogen-Doped Porous Carbon Derived from Biomass Used as Trifunctional Electrocatalyst toward Oxygen Reduction, Oxygen Evolution and Hydrogen Evolution Reactions. Nanomaterials 2019, 10, 76. [Google Scholar] [CrossRef] [PubMed]
- Yaqiong, Z.; Jiawei, C.; Qiang, P.; Lingzhi, S.; Zhigang, W.; Zhongkai, W. Hydrogen bonding assisted toughness enhancement of poly(lactide blended with a bio-based polyamide elastomer of extremely low amounts. Appl. Surf. Sci. 2020, 506, 144684. [Google Scholar]
- Zhiznin, S.Z.; Vassilev, S.; Gusev, A.L. Economics of secondary renewable energy sources with hydrogen generation. Int. J. Hydrogen Energy 2019, 44, 11385–11393. [Google Scholar] [CrossRef]
- Irankhah, A.; Fattahi, S.M.S.; Salem, M. Hydrogen generation using activated aluminum/water reaction. Int. J. Hydrogen Energy 2018, 43, 15739–15748. [Google Scholar] [CrossRef]
- Dupiano, P.; Stamatis, D.; Dreizin, E.L. Hydrogen production by reacting water with mechanically milled composite aluminum-metal oxide powders. Int. J. Hydrogen Energy 2011, 36, 4781–4791. [Google Scholar] [CrossRef]
- Czech, E.; Troczynski, T. Hydrogen generation through massive corrosion of deformed aluminum in water. Int. J. Hydrogen Energy 2010, 35, 1029–1037. [Google Scholar] [CrossRef]
- Choi, G.; Ziebarth, J.T.; Woodall, J.M.; Kramer, R.; Allen, C.R. Mechanism of Hydrogen Generation via Water Reaction with Aluminum Alloys. In Proceedings of the 2010 18th Biennial University/Government/Industry Micro/Nano Symposium, West Lafayette, IN, USA, 28 June–1 July 2010; pp. 1–4. [Google Scholar]
- Saluena Berna, X.; Martinez Maezlu, R.; Borge Bravo, G.; Daga Monmany, J.M.; Martinez Lopez, J. Generating Hydrogen by Means of Reaction with Aluminium. U.S. Patent Application 14/765,062, 17 December 2015. [Google Scholar]
- Ilyukhina, A.V.; Ilyukhin, A.S.; Shkolnikov, E.I. Hydrogen generation from water by means of activated aluminum. Int. J. Hydrogen Energy 2012, 37, 16382–16387. [Google Scholar] [CrossRef]
- Parmuzina, A.V.; Kravchenko, O.V. Activation of aluminium metal to evolve hydrogen from water. Int. J. Hydrogen Energy 2008, 33, 3073–3076. [Google Scholar] [CrossRef]
- Fan, M.Q.; Sun, R.X.; Xu, R. Study of the controllable reactivity of aluminum alloys and their promising application for hydrogen generation. Energy Convers. Manag. 2010, 51, 594–599. [Google Scholar] [CrossRef]
- Reboul, M.C.; Gimenez, P.; Rameau, J.J. A Proposed Activation Mechanism for Al Anodes. Corrosion 2012, 40, 366–371. [Google Scholar] [CrossRef]
- Huang, X.N.; Wu, Z.H.; Cao, K.; Zeng, W.; Lv, C.J.; Huang, Y.X. Hydrogen Generation by Hydrolysis of the Ball Milled Al-C-KCl Composite Powder in Distilled Water. Key Eng. Mater. 2012, 519, 87–91. [Google Scholar] [CrossRef]
- Razali, H.; Sopian, K.; Mat, S. The Application of Aluminum and Hydrochloric Acid to Produce Hydrogen for Internal Combustion Engine Via Hydrogen Mixture with Gasoline based on Specific Fuel Consumption. In Advanced Materials Research; Trans Tech Publications Ltd.: Bäch, Switzerland, 2012; Volume 875, pp. 1804–1811. [Google Scholar]
- Gai, W.Z.; Fang, C.S.; Deng, Z.Y. Hydrogen generation by the reaction of Al with water using oxides as catalysts. Int. J. Energy Res. 2014, 38, 918–925. [Google Scholar] [CrossRef]
- Kunowsky, M.; Suárez García, F.; Linares Solano, Á. High pressure hydrogen storage. Investigació 2013. [Google Scholar] [CrossRef]
- Qiao, D.; Lu, Y.; Tang, Z.; Fan, X.; Wang, T.; Li, T.; Liaw, P.K. The superior hydrogen-generation performance of multi-component Al alloys by the hydrolysis reaction. Int. J. Hydrogen Energy 2019, 44, 3527–3537. [Google Scholar] [CrossRef]
- Wang, C.; Yang, T.; Liu, Y.; Ruan, J.; Yang, S.; Liu, X. Hydrogen generation by the hydrolysis of magnesium-aluminum-iron material in aqueous solutions. Int. J. Hydrogen Energy 2014, 39, 10843–10852. [Google Scholar] [CrossRef]
- Wang, D. Analyzing the Feasibility of Generating Hydrogen Al-Ga Alloy using Fly Ash as Raw Materials. Shenhua Sci. Technol. 2016, 14, 7–10. [Google Scholar]
- Yoo, H.; Ryu, H.; Cho, S.; Han, M.; Bae, K.; Lee, J. Effect of Si content on H 2 production using Al–Si alloy powders. Int. J. Hydrogen Energy 2011, 36, 15111–15118. [Google Scholar] [CrossRef]
- Evans, D.S.; Prince, A. Thermal analysis of Ga-In-Sn system. Met. Sci. 2013, 12, 411–414. [Google Scholar] [CrossRef]
- Woodall, J.M.; Ziebarth, J.; Allen, C.R. The Science and Technology of Al-Ga Alloys as a Material for Energy Storage, Transport and Splitting Water. In Proceedings of the Asme Energy Nanotechnology International Conference, Santa Clara, CA, USA, 5–7 September 2007. [Google Scholar]


| Materials | Al | Ga | In | Sn |
|---|---|---|---|---|
| melting point (°C) | 660.00 | 29.76 | 156.61 | 231.89 |
| Specimen No. | Element (wt.%) | |||
|---|---|---|---|---|
| Al | Ga | In | Sn | |
| 1# | 50 | 35 | 10 | 5 |
| 2# | 60 | 28 | 8 | 4 |
| 3# | 70 | 21 | 6 | 3 |
| 4# | 80 | 14 | 4 | 2 |
| 5# | 90 | 7 | 2 | 1 |
| 6# | 50 | 30 | 15 | 5 |
| 7# | 60 | 24 | 12 | 4 |
| 8# | 70 | 18 | 9 | 3 |
| 9# | 80 | 12 | 6 | 2 |
| 10# | 90 | 6 | 3 | 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, Z.; Ji, F.; Cheng, D.; Yin, C.; Niu, J.; Brnic, J. Hydrolysis-Based Hydrogen Generation Investigation of Aluminum System Adding Low-Melting Metals. Energies 2021, 14, 1433. https://doi.org/10.3390/en14051433
Gao Z, Ji F, Cheng D, Yin C, Niu J, Brnic J. Hydrolysis-Based Hydrogen Generation Investigation of Aluminum System Adding Low-Melting Metals. Energies. 2021; 14(5):1433. https://doi.org/10.3390/en14051433
Chicago/Turabian StyleGao, Zeng, Fei Ji, Dongfeng Cheng, Congxin Yin, Jitai Niu, and Josip Brnic. 2021. "Hydrolysis-Based Hydrogen Generation Investigation of Aluminum System Adding Low-Melting Metals" Energies 14, no. 5: 1433. https://doi.org/10.3390/en14051433
APA StyleGao, Z., Ji, F., Cheng, D., Yin, C., Niu, J., & Brnic, J. (2021). Hydrolysis-Based Hydrogen Generation Investigation of Aluminum System Adding Low-Melting Metals. Energies, 14(5), 1433. https://doi.org/10.3390/en14051433




.JPG)


