Analysis and Simulation of an Absorption Cooling System Using a Latent Heat Storage Tank and a Tempering Valve
Abstract
:1. Introduction
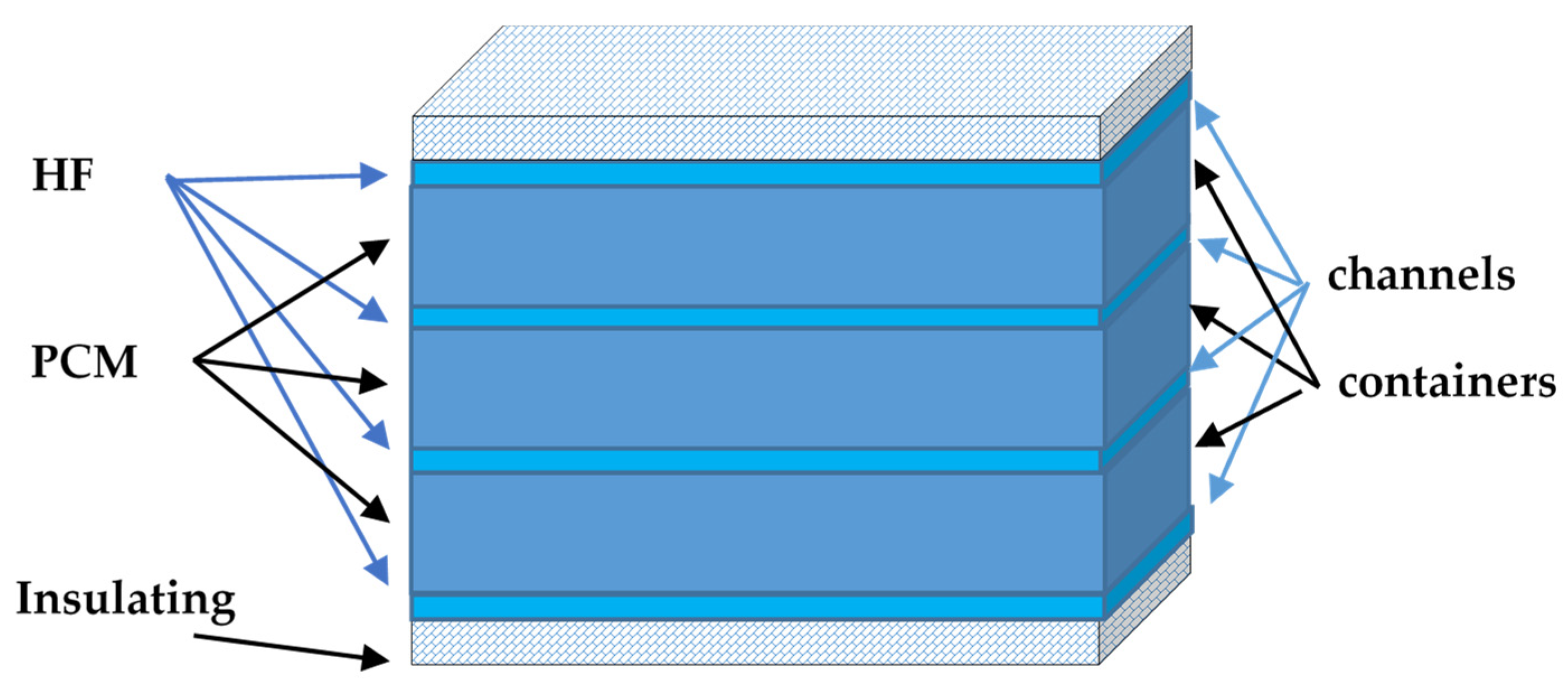
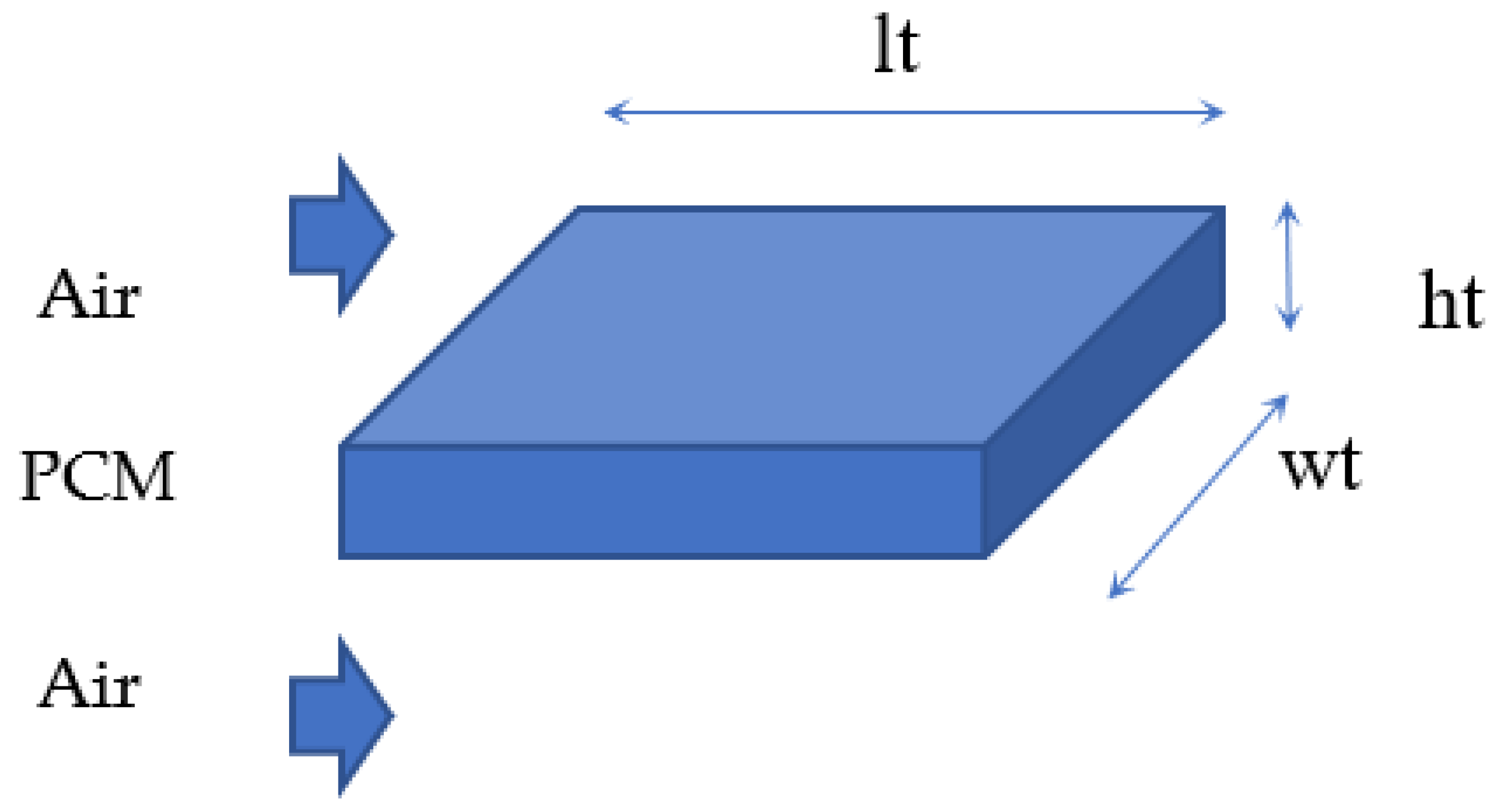
2. Latent Heat Storage Tank Model Development
- the PCM is homogeneous and isotropic;
- the thermophysical properties of the PCM are independent of temperature;
- the phase change of the PCM is assumed to be isothermal;
- the thermal resistance of the metal wall of the plates is insignificant;
- the temperatures of the input and output heating fluid are considered well mixed.
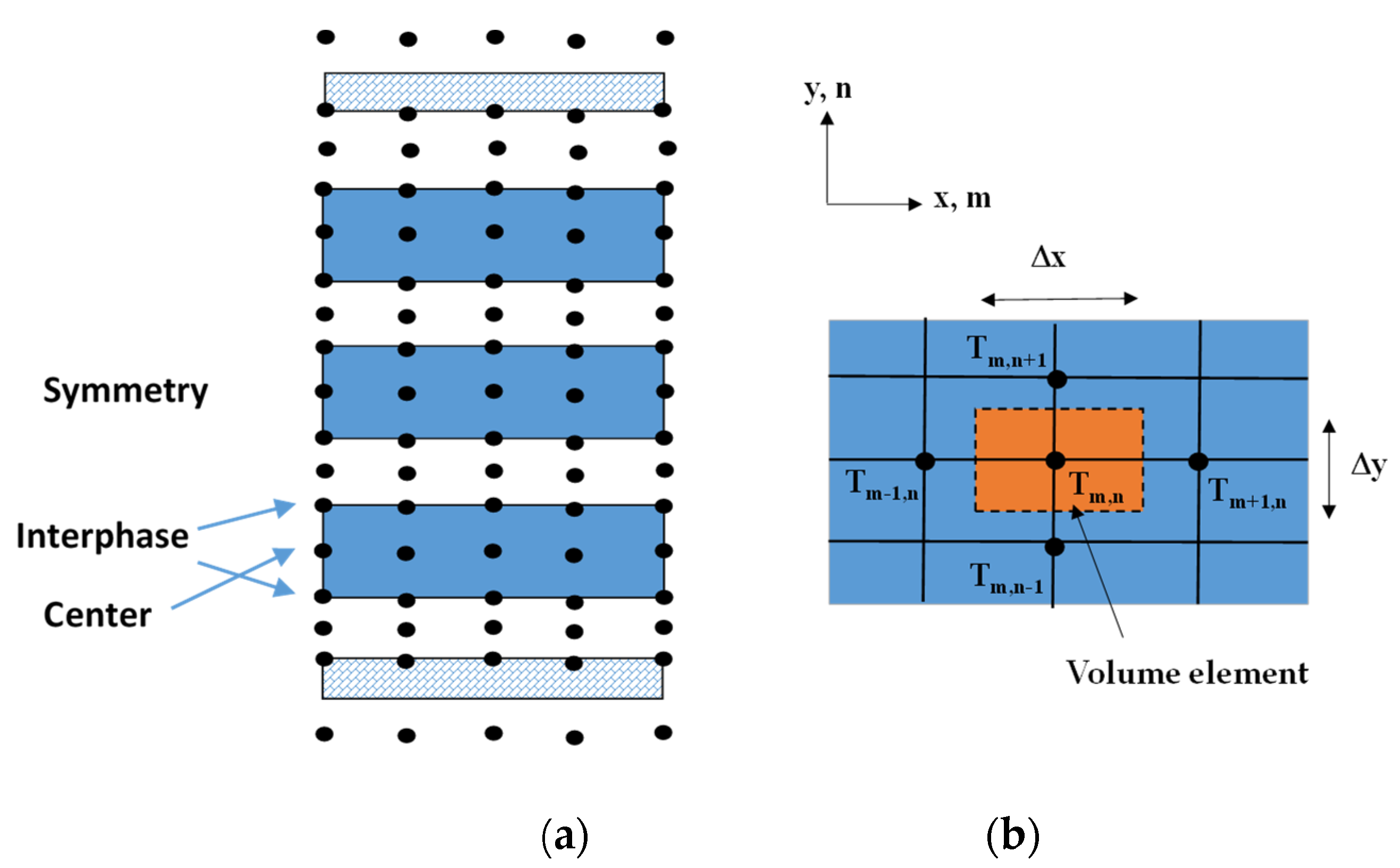
2.1. Governing Equations
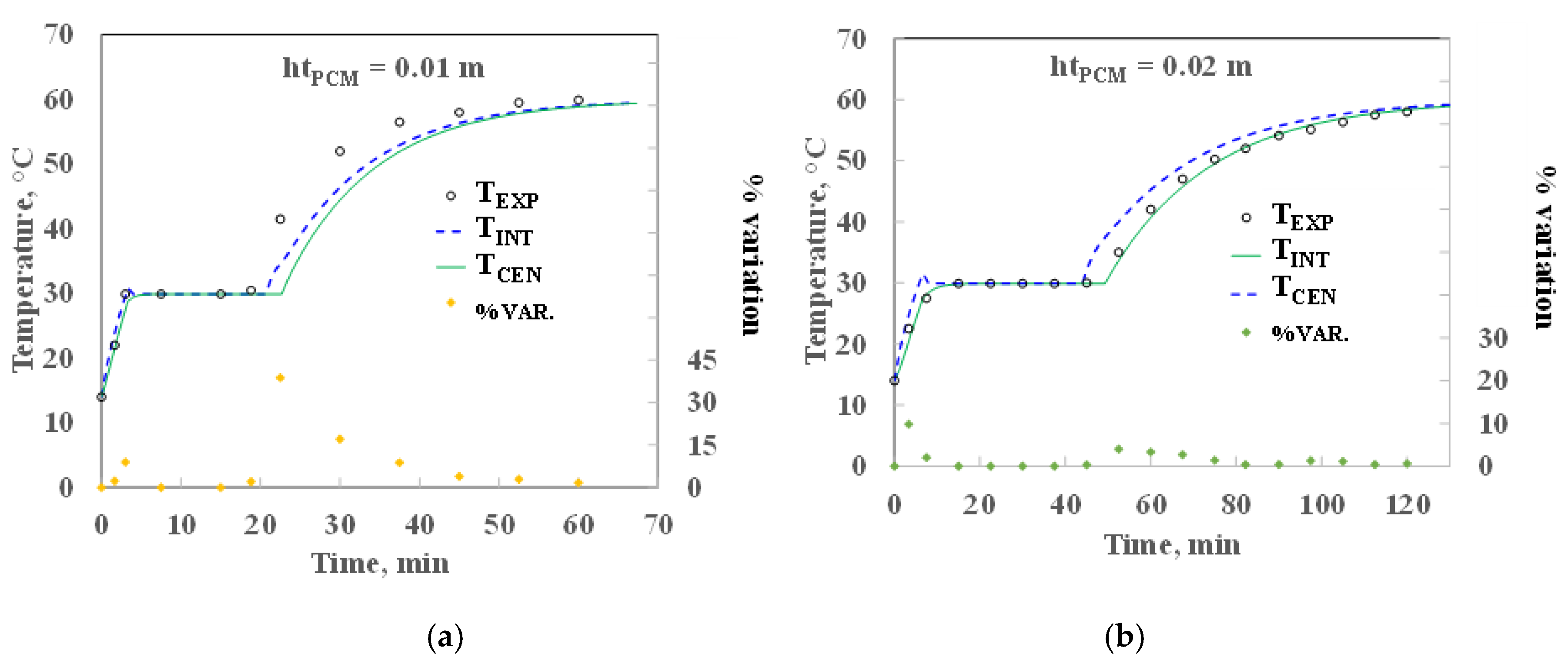
2.2. Mathematical Model Validation
3. Methodology
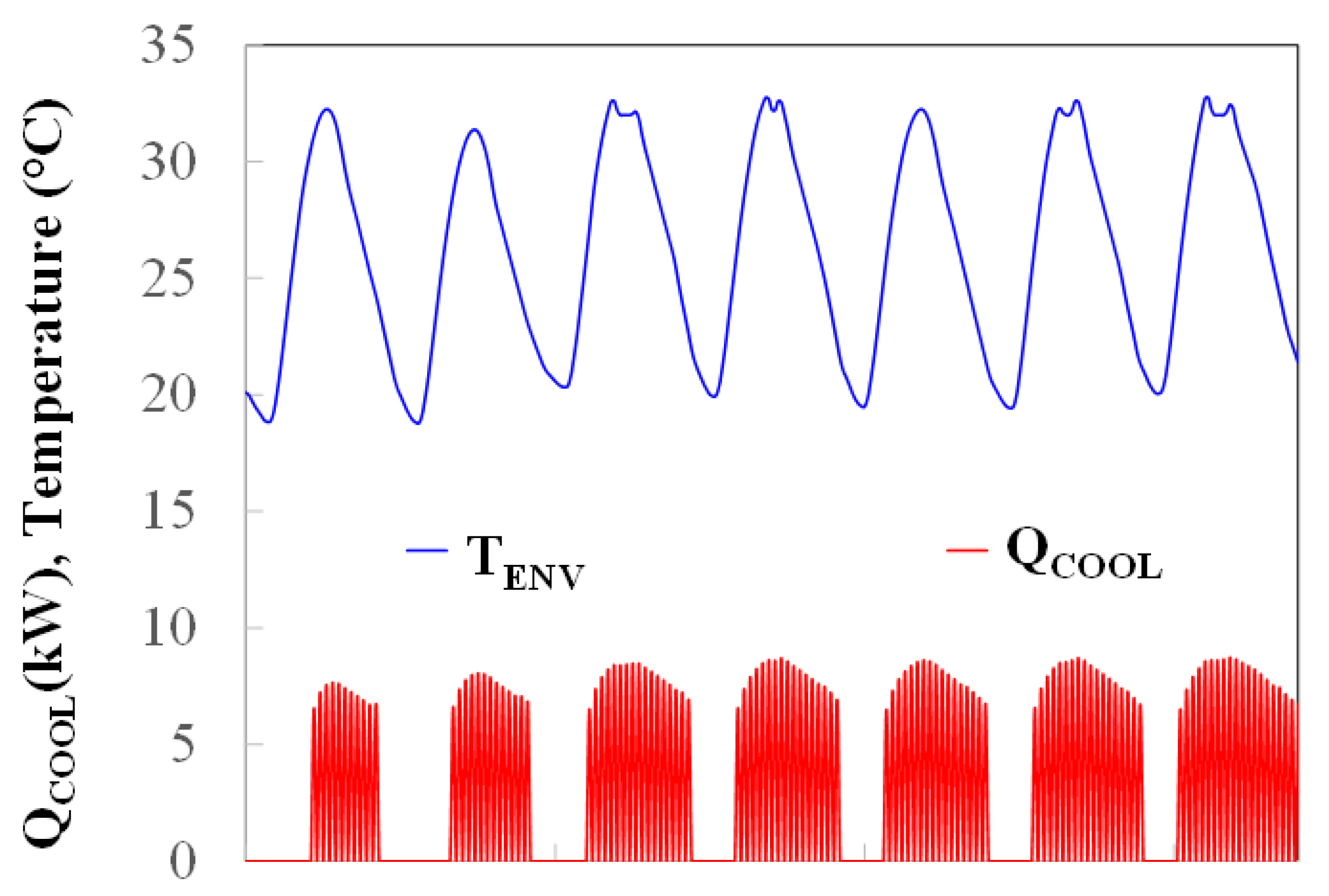
3.1. Operation Time
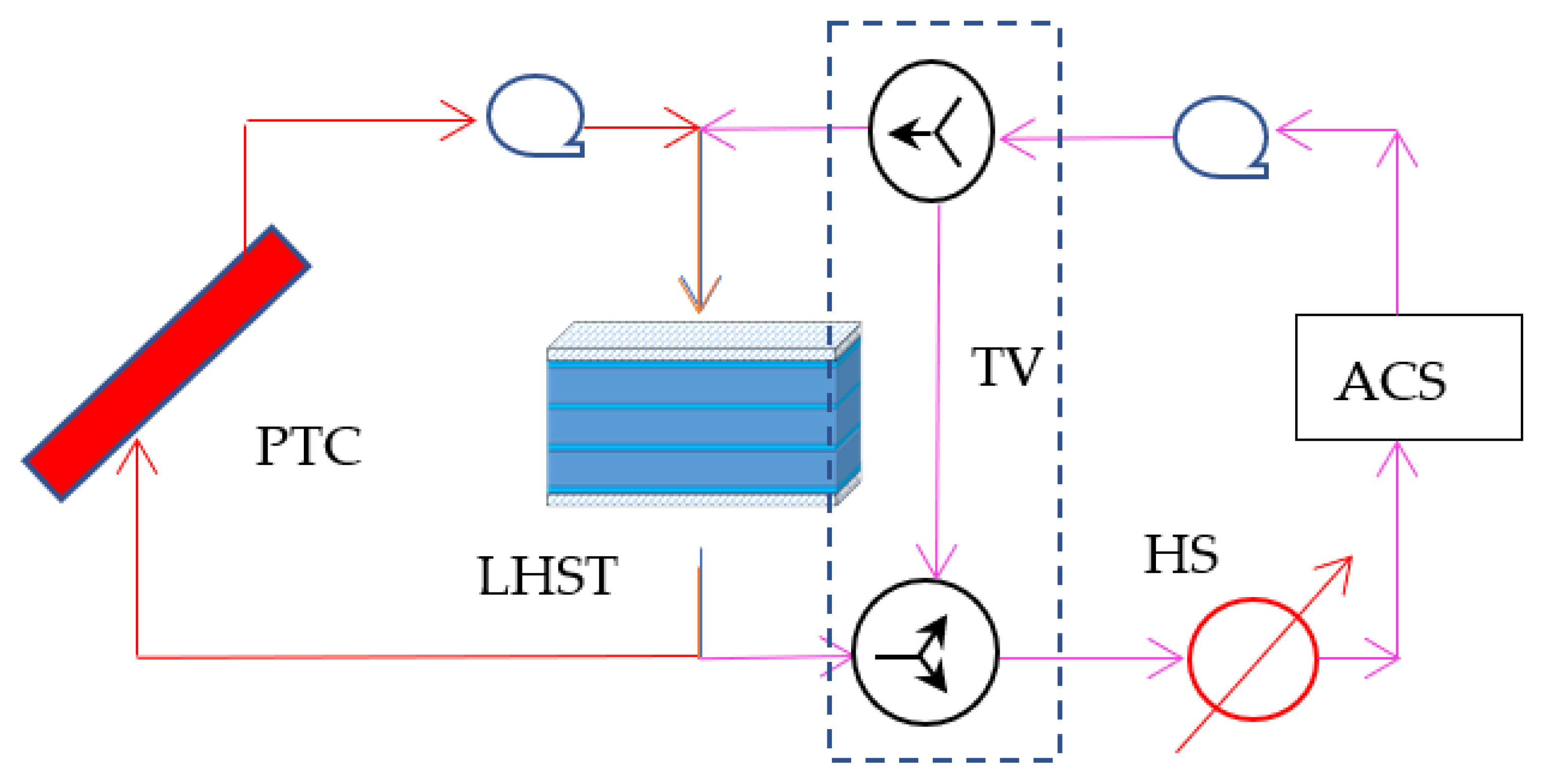
3.2. Solar Cooling System Simulation
4. Results
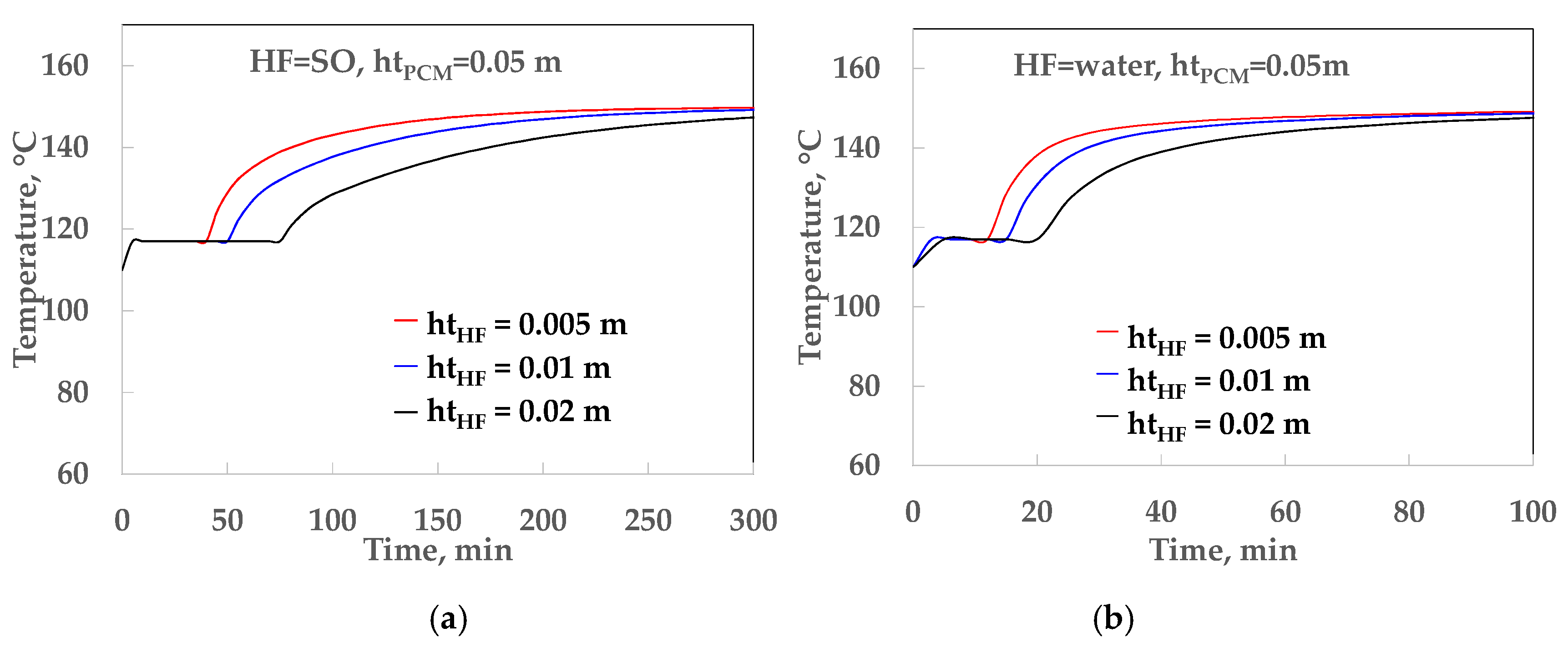
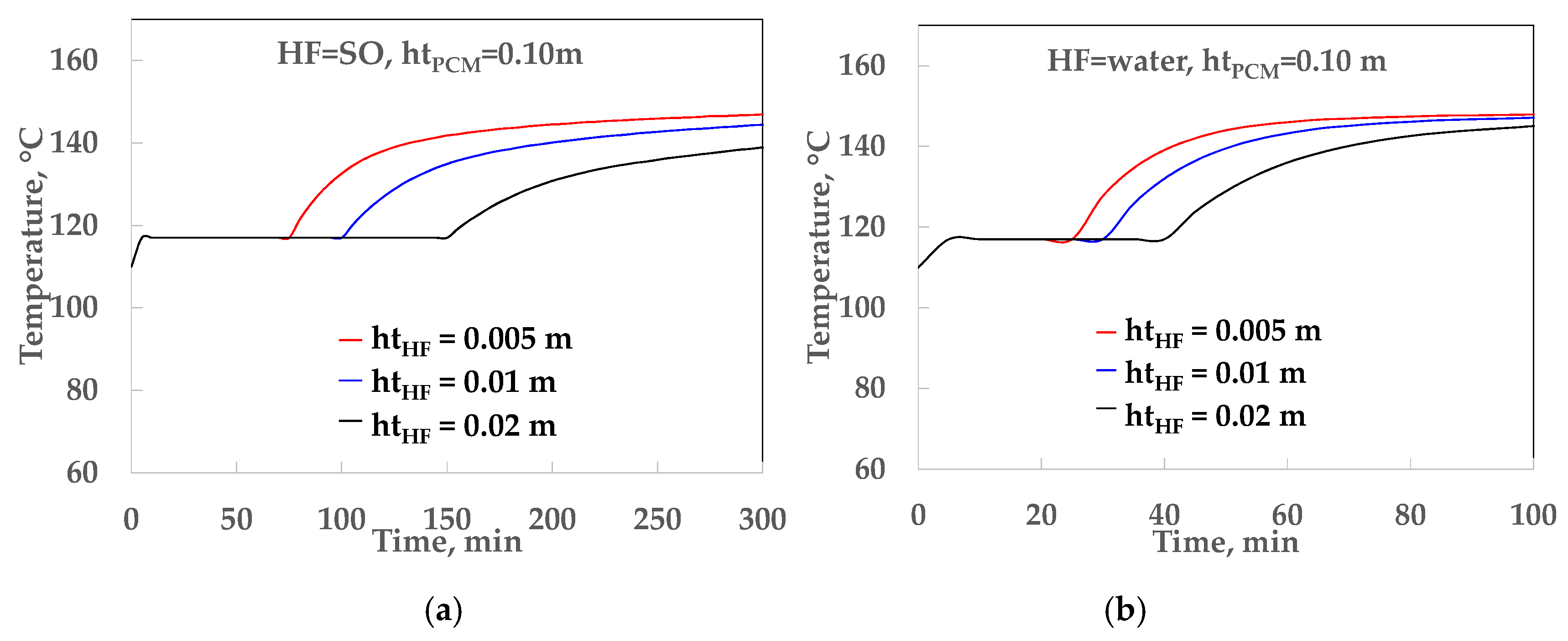
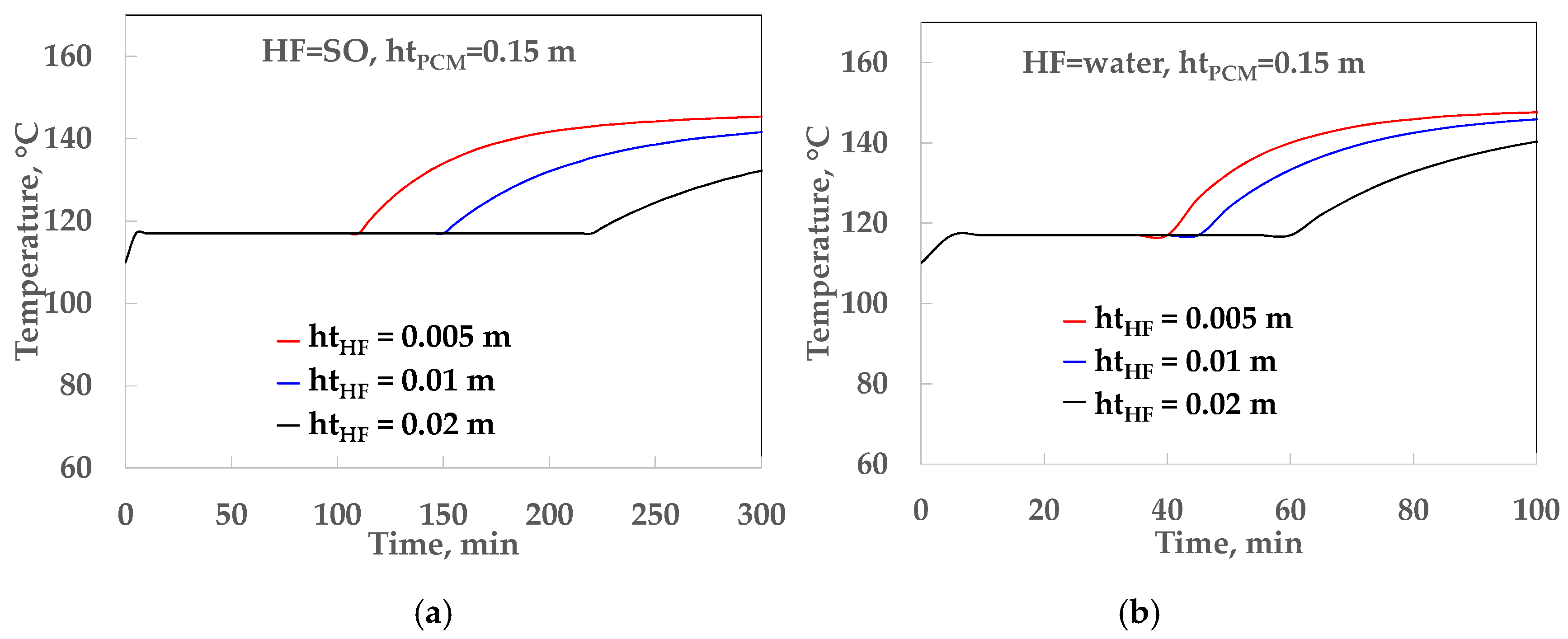
4.1. Thermal Analysis of the LHST
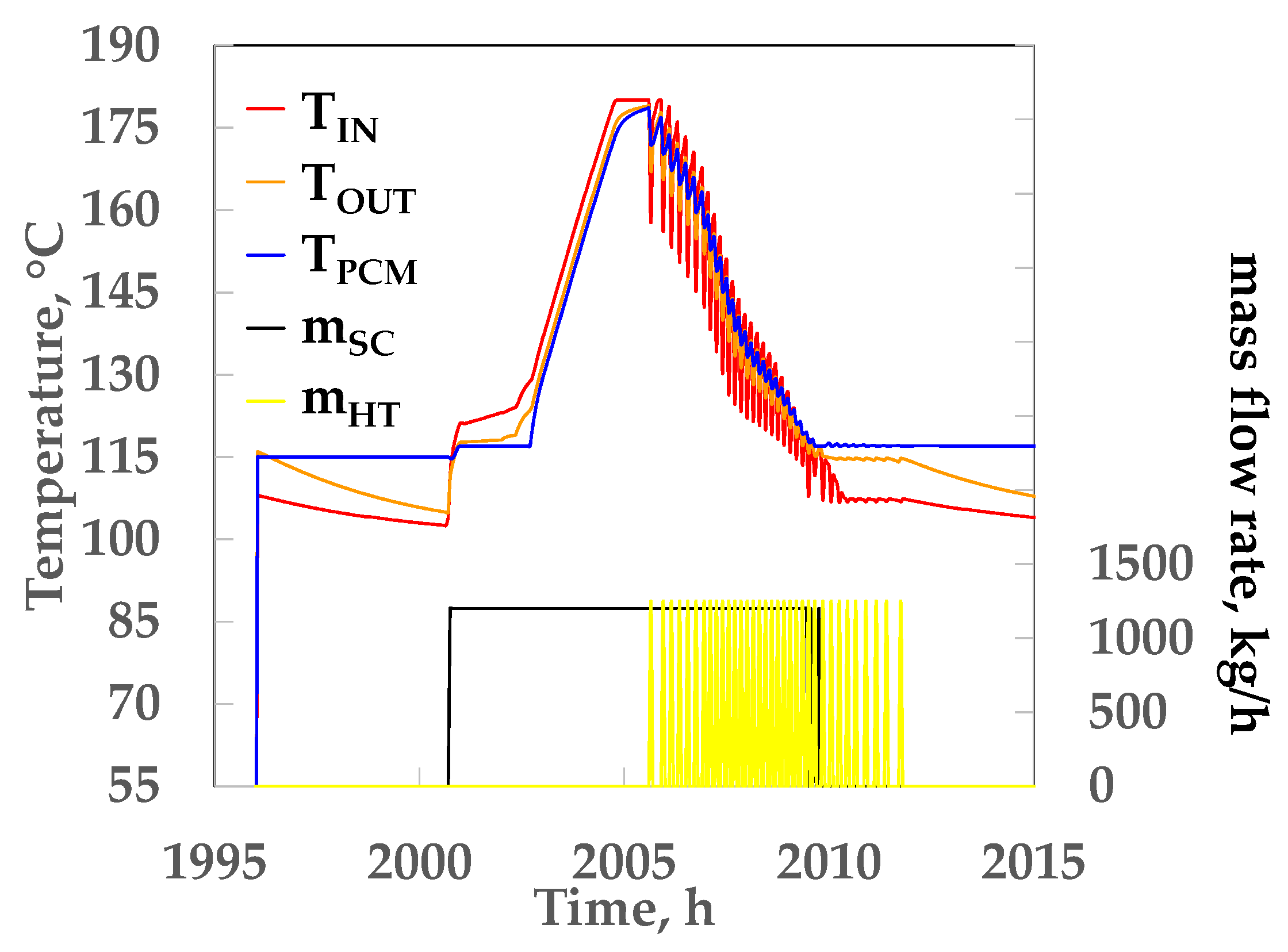
4.2. Thermal Analyses of the Absorption Cooling System
5. Discussion
6. Conclusions
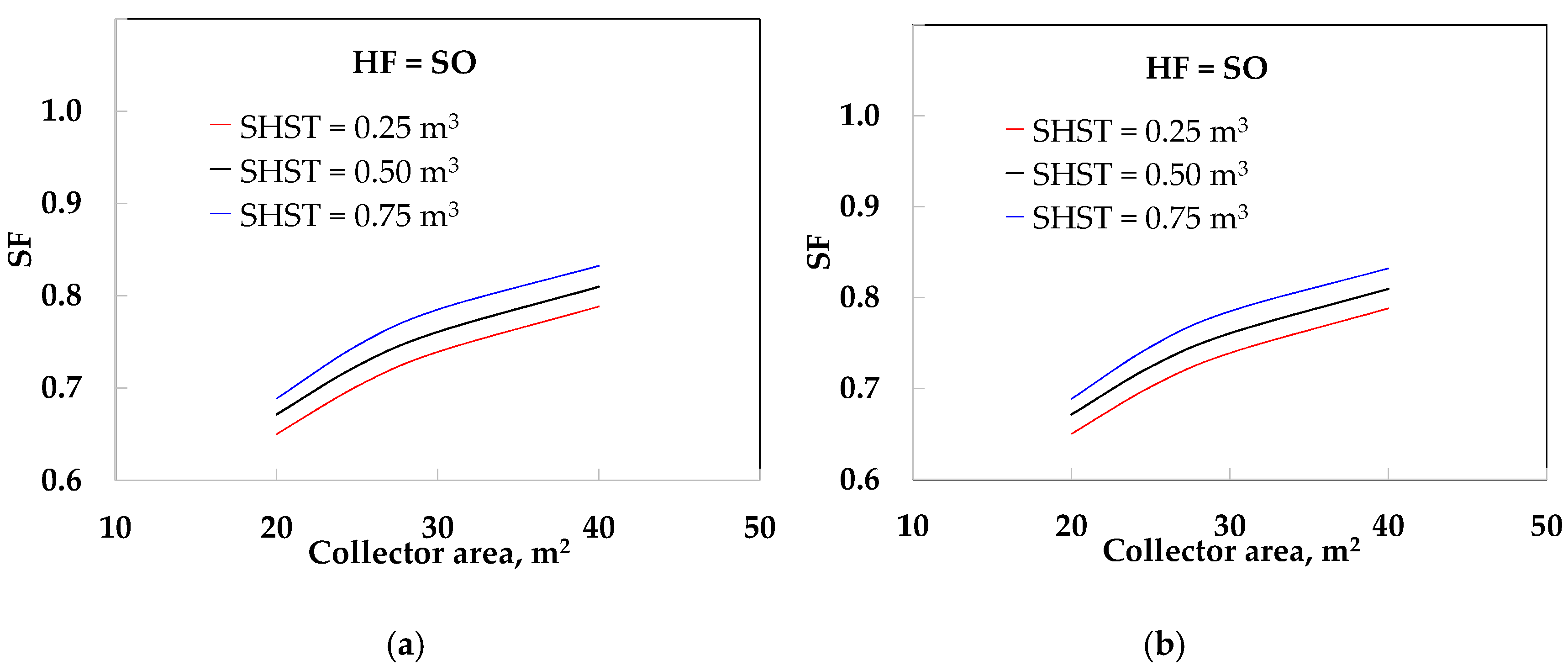
- The use of SHST (configuration 1) yielded a solar fraction ranging from 0.65 to 0.79 and 0.69 to 0.83 for SO at 0.25 and 0.75 m3 storage tank volume, respectively, from 20 to 40 m2 of solar collector area, while a solar fraction from 0.66 to 0.80 and 0.71 to 0.86 was obtained for water under similar conditions.
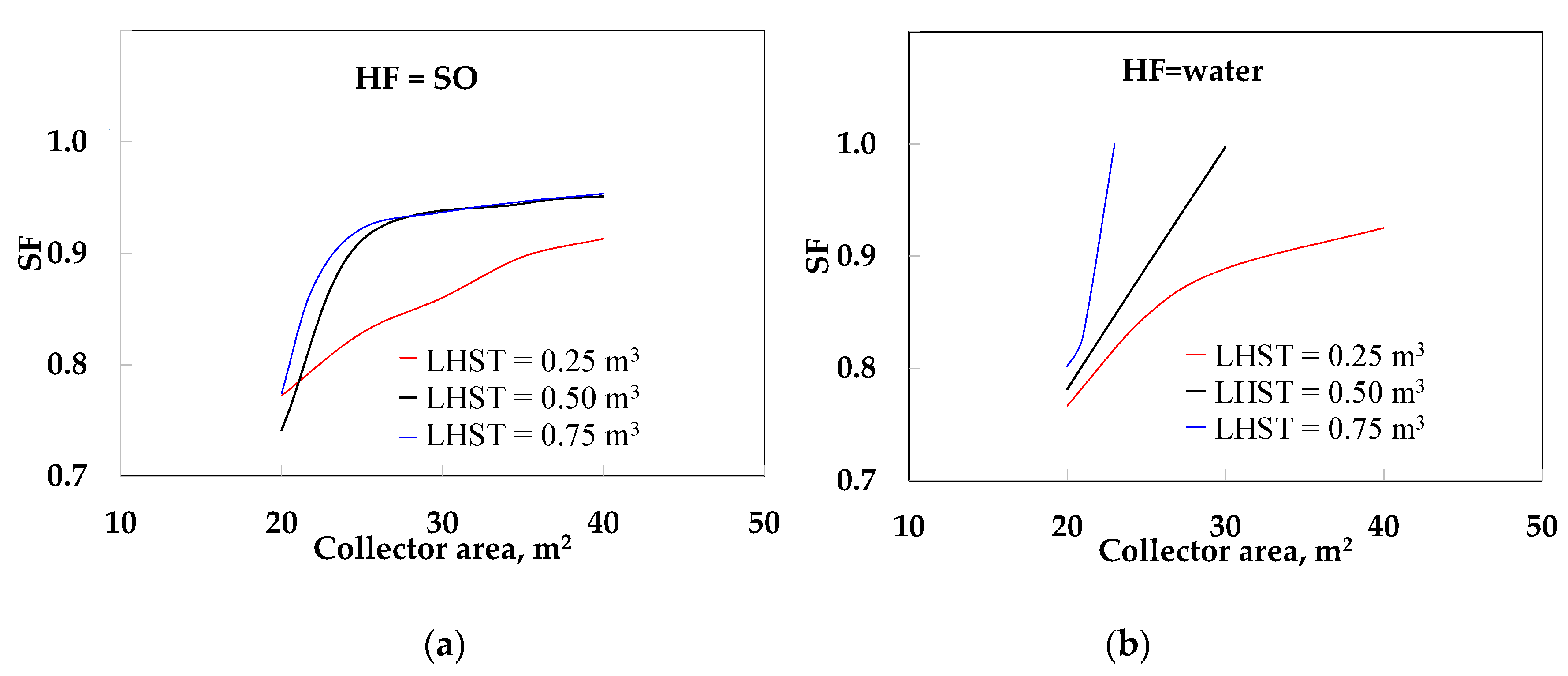
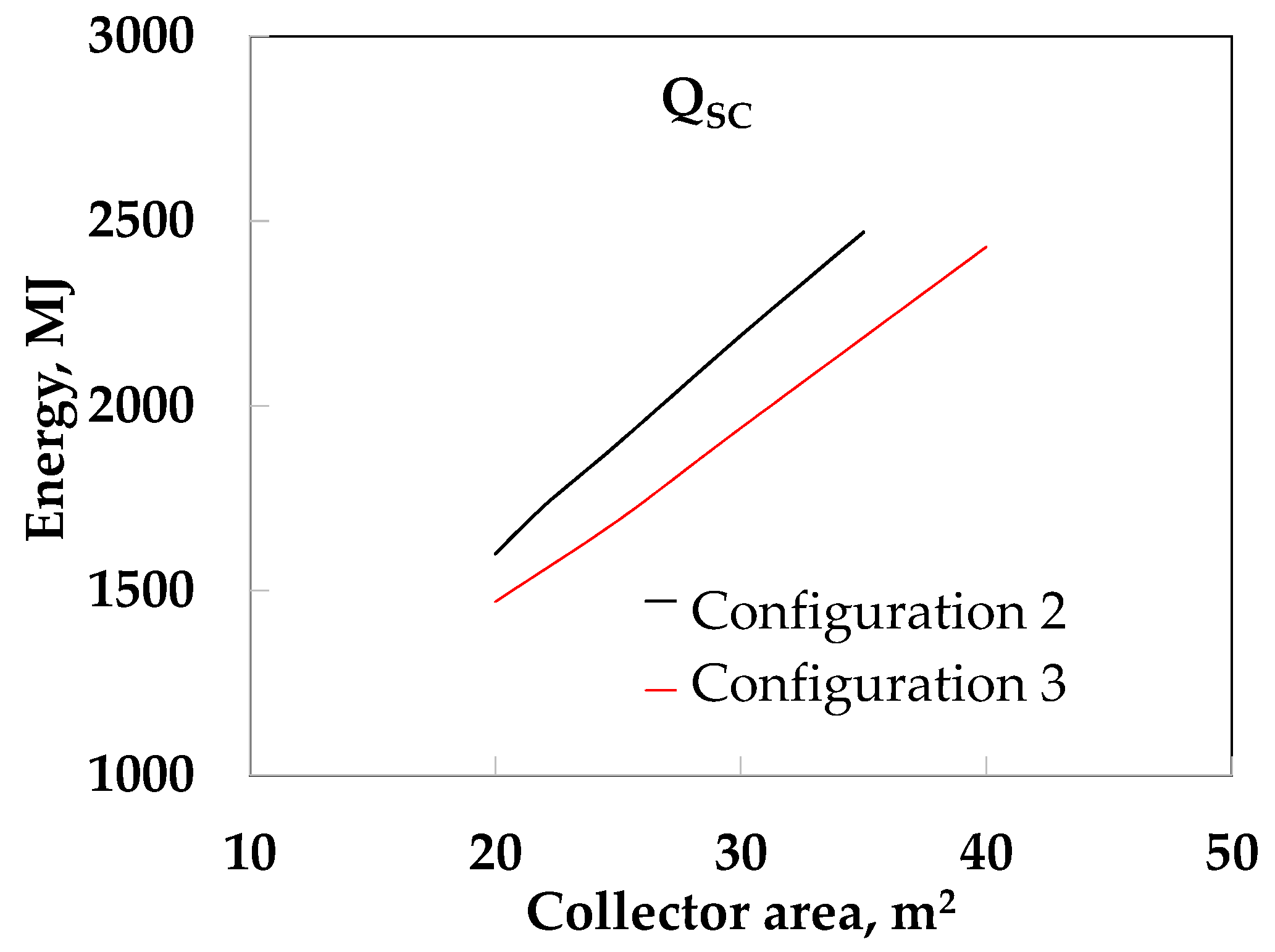
- The use of LHST (configuration 2) yielded better SF values than the use of SHST. When SO was used the SF increased almost linearly from 0.77 to 0.91 at 0.25 m3 from 20 to 40 m2, while the SF increased quickly at around 0.74 to 0.93 at 0.50 and 0.75 m3, respectively, from 20 to 30 m2. After this, similar values (from 0.93 to 0.95) were obtained, due to the high thermal resistance in both the PCM and the HF. When water was used, we obtained a maximum value of SF = 0.93 at 0.25 m3. However, the SF was 1.0 at 0.50 and 0. 75 m3 at 30 and 23 m2, respectively. This is because the thermal resistance in HF was reduced and improves the heat transfer of the LHST.
- Configuration 3 (LHST and TV) yielded better results than configuration 2 because it used the waste energy generated by the DS (configuration 2). When SO was used, the SF increased from 0.85 to 0.94 at 0.25 m3. The SF obtained similar values (0.90 to 0.96) at 0.50 and 0.75 m3. When water was used, the SF increased from 0.88 to 0.99 at 0.25 m3; however, the SF was 1.0 at 0.5 and 0.75 m3, when the collector area was more than 20 m2.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Nomenclature
| A | area: m2 |
| DS | dissipation system |
| D | diameter |
| F´(τα) | collector (F´) (transmittance) (absorptance) product |
| h | convective heat transfer coefficient, kW/m2 °C |
| HF | heating fluid |
| HS | heating system |
| Ht | height |
| k | thermal conductivity, kW/m °C |
| LHST | latent heat storage tank |
| lt | length |
| m | mass flow rate, kg/s |
| Nu | Nusselt number |
| PCM | phase change material |
| Pr | Prantl number |
| PTC | parabolic trough collector |
| Ra | Rayleigh number |
| Re | Reynolds number |
| SHST | sensible heat storage tank |
| T | temperature, °C |
| t | time, s |
| th | thickness |
| Q | energy |
| U | overall heat transfer coefficient, kW/m2 °C |
| V | volume, m3 |
| wt | width |
| Subscript | |
| CEN | center |
| ENV | environment |
| INS | insulating |
| INT | interphase between PCM container and HF |
| SC | solar collector |
| H | hydraulic |
References
- The Organization for Economic Co-Operation and Development. Available online: https://www.oecd.org/about/publishing/TheFutureofCooling2018Corrigendumpages.pdf (accessed on 2 February 2021).
- Mofijur, M.; Mahlia, T.M.; Silitonga, A.S.; Ong, H.C.; Silakhori, M.; Hasan, M.H.; Putra, N.; Ashrafur, S.M. Phase Change Materials (PCM) for Solar Energy Usages and Storage: An Overview. Energies 2019, 12, 3167. [Google Scholar] [CrossRef] [Green Version]
- Sarbu, I.; Sebarchievici, C. A Comprehensive Review of Thermal Energy Storage. Sustainability 2018, 10, 191. [Google Scholar] [CrossRef] [Green Version]
- Shirazia, A.; Taylor, R.A.; Morrison, G.L.; White, S.D. Solar-powered absorption chillers: A comprehensive and critical review. Energy Convers. Manag. 2018, 171, 59–81. [Google Scholar] [CrossRef]
- Fan, Z.; Infante, C.A.; Mosaffa, A.H. Numerical modelling of high temperature latent heat thermal storage for solar application combining with double-effect H2O/LiBr absorption refrigeration system. Sol. Energy 2014, 110, 398–409. [Google Scholar] [CrossRef]
- Pintaldi, S.; Sethuvenkatraman, S.; White, S.; Rosengarten, G. Energetic evaluation of thermal energy storage options for high efficiency solar cooling systems. Appl. Energy 2017, 188, 160–177. [Google Scholar] [CrossRef]
- Zhou, L.; Li, X.; Zhao, Y.; Dai, Y. Performance assessment of a single/double hybrid effect absorption cooling system driven by linear Fresnel solar collectors with latent thermal storage. Sol. Energy 2017, 151, 82–94. [Google Scholar] [CrossRef]
- Cengel, Y.A. Heat and Mass Transfer: A Practical Approach, 2nd ed.; Mc Graw-Hill Professional: New York, NY, USA, 2007. [Google Scholar]
- Cárdenas, B.; León, N. High temperature latent heat thermal energy storage: Phase change materials, design considerations and performance enhancement techniques. Renew. Sustain. Energy Rev. 2013, 27, 724–737. [Google Scholar] [CrossRef]
- Chejne, F. Solar Thermal Energy Storage Using Magnesium Chloride Hexahydrate. Ph.D. Thesis, Universidad Nacional de Colombia, Medellín, Colombia, 2007. (In Spanish). [Google Scholar]
- Kenisarin, M.; Mahkamov, K. Solar energy storage using phase change materials. Renew. Sustain. Energy 2007, 11, 1913–1965. [Google Scholar] [CrossRef]
- Höhlein, S.; König, A.; Brüggemann, D. Thermophysical characterization of MgCl2·6H2O, xylitol and erythritol as phase change materials (PCM) for Latent Heat Thermal Energy Storage (LHTES). Materials 2017, 10, 444. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zivkovic, B.; Fujii, I. An analysis of isothermal phase change of phase change material within rectangular and cylindrical containers. Sol. Energy 2001, 70, 51–61. [Google Scholar] [CrossRef]
- Cerezo, J.; Romero, R.J.; Ibarra, J.; Rodríguez, A.; Montero, G.; Acuña, A. Dynamic simulation of an absorption cooling system with different working mixtures. Energies 2018, 12, 259. [Google Scholar] [CrossRef] [Green Version]
- Solar Energy Laboratory. TRNSYS 18, A Transient System Simulation Program. Available online: http://sel.me.wisc.edu/trnsys (accessed on 2 February 2021).
- Klein, S.A. EES, Engineering Equation Solver; Professional Version; FChart Software: Madison, WI, USA, 2017; Available online: http://www.fchart.com/ees/ (accessed on 2 February 2021).
- Technical Data. Solar Concentration Model LH-3-2M, Solar Rating & Certification Corporation 2018. Available online: https://solar-rating.org/ (accessed on 2 February 2021).
- Dowthermtm Q Technical Data Sheet. Available online: https://www.dow.com/content/dam/dcc/documents/en-us/productdatasheet/176/176-01467-01-dowtherm-q-tds.pdf?iframe=true (accessed on 2 February 2021).



| Compound | Melting Temperature, °C | Heat of Fusion, kJ/kg | Thermal Conductivity, W/m °C | Density, kg/m3 |
|---|---|---|---|---|
| Inorganic | ||||
| MgCl2·6H2O | 117 | 168.6 | 0.69 (solid) 0.57 (liquid) | 1569 (solid) 1450 (liquid) |
| Mg(NO3)2·6H2O | 89 | 162.0 | 0.61 (solid) 0.49 (liquid) | 1636 (solid) 1550 (liquid) |
| CaCl2·6H2O | 29 | 190.8 | 1.09 (solid) 0.53 (liquid) | 1710 (solid) 1530 (liquid) |
| Organic | ||||
| Paraffin wax | 64 | 173.6 | 0.346 (solid) 0.167 (liquid) | 916 (solid) 790 (liquid) |
| Naphthalene | 80 | 147.7 | 0.341 (solid) 0.132 (liquid) | 1145 (solid) 976 (liquid) |
| Concept | Quantity |
|---|---|
| North and south wall | 35 m2 |
| Ceiling and floor | 75 m2 |
| West and east wall | 12.5 m2 |
| 4 windows | 1 m2 |
| Air change of ventilation | 6 h−1 |
| Parameter | Value |
|---|---|
| F’(τα) | 0.611 |
| Collector efficiency coefficient, C1 | 1.42 |
| Collector efficiency coefficient, C2 | 0.021 |
| Collector efficiency coefficient, C3 | 0.0 |
| Collector efficiency coefficient, C4 | 0.0 |
| Collector efficiency coefficient, C5 | 6.653 |
| Collector efficiency coefficient, C6 | 0.0 |
| Property at 120 °C | Water | Synthetic Organic |
|---|---|---|
| Heat capacity, kJ/kg °C | 4.25 | 1.97 |
| Thermal conductivity, kW/m °C | 0.67 | 0.11 |
| Pressure vapor, bar | 1.98 | 0.01 |
| Density, kg/m3 | 943.2 | 889.8 |
| Viscosity, centipoise | 0.23 | 0.62 |
| HF | hHF, kW/m2 °C | htPCM, m | TRHF, m2 °C/kW | TRPCM, m2 °C/kW | U, kW/m2 °C |
|---|---|---|---|---|---|
| SO | 0.09 | 0.05 | 11.11 | 8.77 | 0.050 |
| SO | 0.09 | 0.10 | 11.11 | 15.54 | 0.035 |
| SO | 0.09 | 0.15 | 11.11 | 26.32 | 0.027 |
| water | 0.52 | 0.05 | 1.92 | 8.77 | 0.094 |
| water | 0.52 | 0.10 | 1.92 | 17.54 | 0.051 |
| water | 0.52 | 0.15 | 1.92 | 26.32 | 0.035 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cerezo, J.; Lara, F.; Romero, R.J.; Rodríguez, A. Analysis and Simulation of an Absorption Cooling System Using a Latent Heat Storage Tank and a Tempering Valve. Energies 2021, 14, 1376. https://doi.org/10.3390/en14051376
Cerezo J, Lara F, Romero RJ, Rodríguez A. Analysis and Simulation of an Absorption Cooling System Using a Latent Heat Storage Tank and a Tempering Valve. Energies. 2021; 14(5):1376. https://doi.org/10.3390/en14051376
Chicago/Turabian StyleCerezo, Jesús, Fernando Lara, Rosenberg J. Romero, and Antonio Rodríguez. 2021. "Analysis and Simulation of an Absorption Cooling System Using a Latent Heat Storage Tank and a Tempering Valve" Energies 14, no. 5: 1376. https://doi.org/10.3390/en14051376









