Effects of Sugars and Degradation Products Derived from Lignocellulosic Biomass on Maleic Acid Production
Abstract
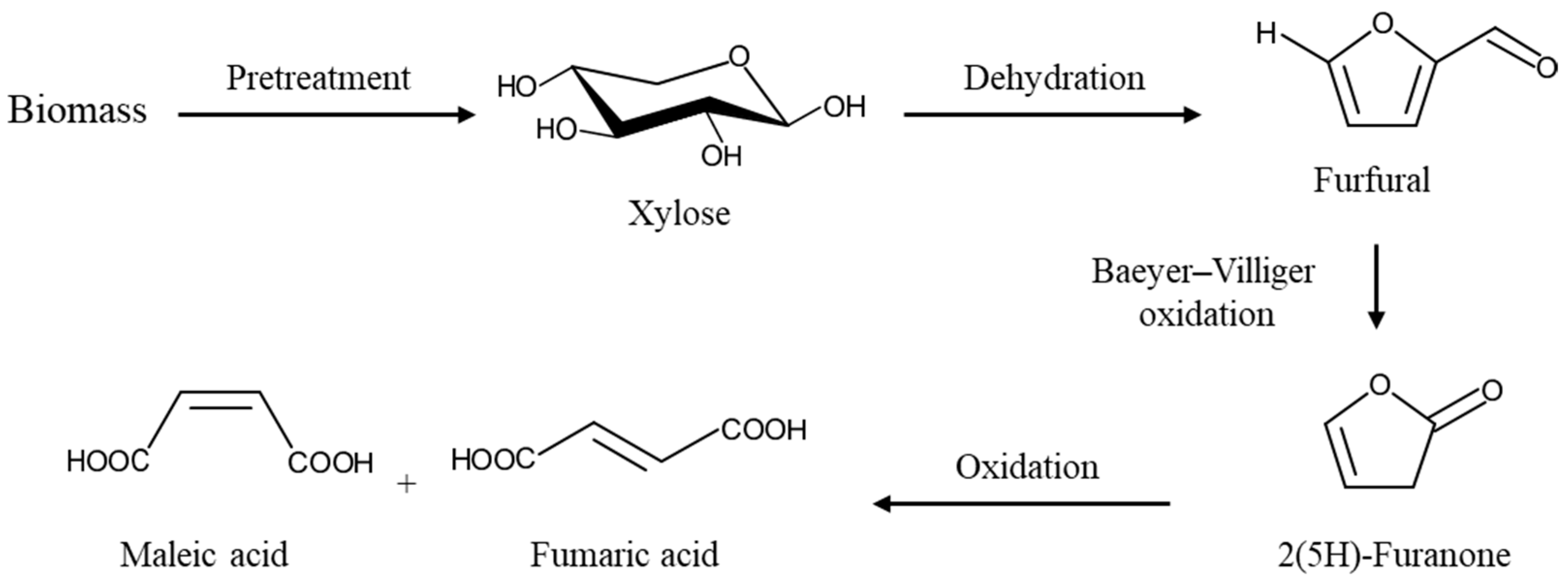
1. Introduction
2. Materials and Methods
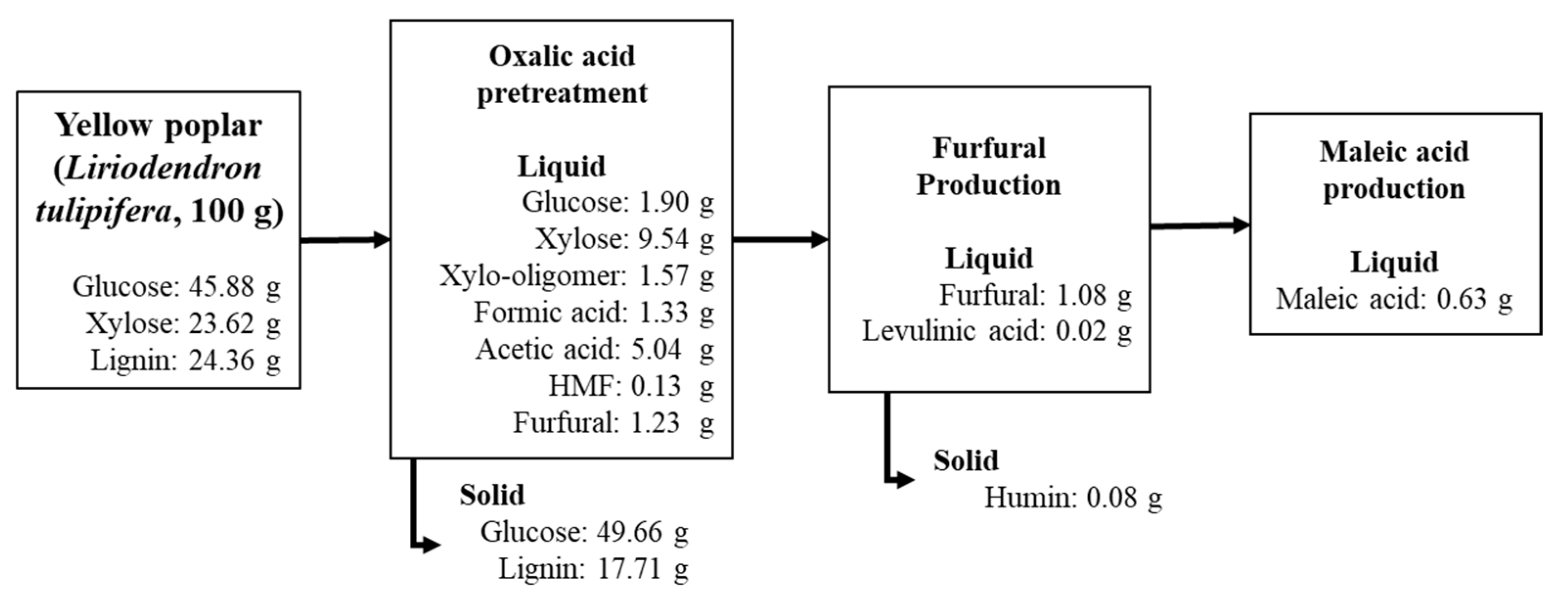
2.1. Hydrolysate Production from Yellow Poplar Pretreatment with Oxalic Acid
2.2. Furfural Production Using the First Hydrolysate
2.3. Maleic Acid Production Using the Second Hydrolysate
2.4. Analysis of Factors Affecting Maleic Acid Production
2.5. Analysis of the Hydrolysate
2.6. Statistical Analysis
3. Results and Discussion
3.1. Furfural Production from the First Hydrolysate
3.2. Maleic Acid Production from the Second Hydrolysate
3.3. Effects of Catalyst and Degradation Products on Maleic Acid Production from the Second Hydrolysate
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Jönsson, L.J.; Martín, C. Pretreatment of lignocellulose: Formation of inhibitory by-products and strategies for minimizing their effects. Bioresour. Technol. 2016, 199, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Amin, F.R.; Khalid, H.; Zhang, H.; u Rahman, S.; Zhang, R.; Liu, G.; Chen, C. Pretreatment methods of lignocellulosic biomass for anaerobic digestion. AMB Express 2017, 7, 72. [Google Scholar] [CrossRef]
- Bensah, E.C.; Mensah, M. Chemical pretreatment methods for the production of cellulosic ethanol: Technologies and innovations. Int. J. Chem. Eng. 2013, 2013. [Google Scholar] [CrossRef]
- Fei, X.; Jia, W.; Wang, J.; Chen, T.; Ling, Y. Study on enzymatic hydrolysis efficiency and physicochemical properties of cellulose and lignocellulose after pretreatment with electron beam irradiation. Int. J. Biol. Macromol. 2020, 145, 733–739. [Google Scholar] [CrossRef]
- Basak, B.; Jeon, B.-H.; Kim, T.H.; Lee, J.-C.; Chatterjee, P.K.; Lim, H. Dark fermentative hydrogen production from pretreated lignocellulosic biomass: Effects of inhibitory byproducts and recent trends in mitigation strategies. Renew. Sustain. Energy Rev. 2020, 133, 110338. [Google Scholar] [CrossRef]
- Jia, Q.; Teng, X.; Yu, S.; Si, Z.; Li, G.; Zhou, M.; Cai, D.; Qin, P.; Chen, B. Production of furfural from xylose and hemicelluloses using tin-loaded sulfonated diatomite as solid acid catalyst in biphasic system. Bioresour. Technol. Rep. 2019, 6, 145–151. [Google Scholar] [CrossRef]
- Luo, Y.; Li, Z.; Li, X.; Liu, X.; Fan, J.; Clark, J.H.; Hu, C. The production of furfural directly from hemicellulose in lignocellulosic biomass: A review. Catal. Today 2019, 319, 14–24. [Google Scholar] [CrossRef]
- Thiyagarajan, S.; Franciolus, D.; Bisselink, R.J.; Ewing, T.A.; Boeriu, C.G.; Van Haveren, J. Selective production of maleic acid from furfural via a cascade approach combining photochemistry and electro-or biochemistry. ACS Sustain. Chem. Eng. 2020, 8, 10626–10632. [Google Scholar] [CrossRef]
- López, F.; García, M.; Feria, M.; García, J.; De Diego, C.; Zamudio, M.A.; Díaz, M. Optimization of furfural production by acid hydrolysis of Eucalyptus globulus in two stages. Chem. Eng. J. 2014, 240, 195–201. [Google Scholar] [CrossRef]
- Mazar, A.; Jemaa, N.; Al Dajani, W.W.; Marinova, M.; Perrier, M. Furfural production from a pre-hydrolysate generated using aspen and maple chips. Biomass Bioenergy 2017, 104, 8–16. [Google Scholar] [CrossRef]
- Hu, L.; Lin, L.; Wu, Z.; Zhou, S.; Liu, S. Recent advances in catalytic transformation of biomass-derived 5-hydroxymethylfurfural into the innovative fuels and chemicals. Renew. Sustain. Energy Rev. 2017, 74, 230–257. [Google Scholar] [CrossRef]
- Ni, Y.; Bi, Z.; Su, H.; Yan, L. Deep eutectic solvent (DES) as both solvent and catalyst for oxidation of furfural to maleic acid and fumaric acid. Green Chem. 2019, 21, 1075–1079. [Google Scholar] [CrossRef]
- Kubota, S.R.; Choi, K.-S. Electrochemical Valorization of Furfural to Maleic Acid. ACS Sustain. Chem. Eng. 2018, 6, 9596–9600. [Google Scholar] [CrossRef]
- Teong, S.P.; Li, X.; Zhang, Y. Hydrogen peroxide as an oxidant in biomass-to-chemical processes of industrial interest. Green Chem. 2019, 21, 5753–5780. [Google Scholar] [CrossRef]
- Araji, N.; Madjinza, D.D.; Chatel, G.; Moores, A.; Jérôme, F.; Vigier, K.D.O. Synthesis of maleic and fumaric acids from furfural in the presence of betaine hydrochloride and hydrogen peroxide. Green Chem. 2017, 19, 98–101. [Google Scholar] [CrossRef]
- Deng, W.; Wang, Y.; Yan, N. Production of organic acids from biomass resources. Curr. Opin. Green Sustain. Chem. 2016, 2, 54–58. [Google Scholar] [CrossRef]
- Liu, L.; Chang, H.-M.; Jameel, H.; Park, S. Furfural production from biomass pretreatment hydrolysate using vapor-releasing reactor system. Bioresour. Technol. 2018, 252, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Qing, Q.; Guo, Q.; Zhou, L.; Wan, Y.; Xu, Y.; Ji, H.; Gao, X.; Zhang, Y. Catalytic conversion of corncob and corncob pretreatment hydrolysate to furfural in a biphasic system with addition of sodium chloride. Bioresour. Technol. 2017, 226, 247–254. [Google Scholar] [CrossRef]
- Wang, X.; Li, H.; Lin, Q.; Li, R.; Li, W.; Peng, F.; Ren, J. Efficient catalytic conversion of dilute-oxalic acid pretreated bagasse hydrolysate to furfural using recyclable ironic phosphates catalysts. Bioresour. Technol. 2019, 290, 121764. [Google Scholar] [CrossRef]
- Jeong, S.-Y.; Lee, J.-W. Analysis of Factors Affecting Ethanol Fermentation of Hydrolysate Derived from the Oxalic Acid Pretreatment of Yellow Poplar (Liriodendron tulipifera). New Renew. Energy 2019, 15, 75–85. [Google Scholar] [CrossRef]
- Li, W.-C.; Zhang, S.-J.; Xu, T.; Sun, M.-Q.; Zhu, J.-Q.; Zhong, C.; Li, B.-Z.; Yuan, Y.-J. Fractionation of corn stover by two-step pretreatment for production of ethanol, furfural, and lignin. Energy 2020, 195, 117076. [Google Scholar] [CrossRef]
- Li, X.; Ho, B.; Lim, D.S.; Zhang, Y. Highly efficient formic acid-mediated oxidation of renewable furfural to maleic acid with H2O2. Green Chem. 2017, 19, 914–918. [Google Scholar] [CrossRef]
- Alonso-Fagúndez, N.; Agirrezabal-Telleria, I.; Arias, P.; Fierro, J.; Mariscal, R.; Granados, M.L. Aqueous-phase catalytic oxidation of furfural with H2O2: High yield of maleic acid by using titanium silicalite-1. RSC Adv. 2014, 4, 54960–54972. [Google Scholar] [CrossRef]
- Sluiter, A.; Hames, B.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D. Determination of sugars, byproducts, and degradation products in liquid fraction process samples—Laboratory Analytical Procedure (LAP), NREL/TP-510-42623. 2006. Available online: https://www.nrel.gov/docs/gen/fy08/42623.pdf (accessed on 8 February 2021).
- Lee, C.B.T.L.; Wu, T.Y. A review on solvent systems for furfural production from lignocellulosic biomass. Renew. Sustain. Energy Rev. 2020, 110172. [Google Scholar] [CrossRef]
- Choi, J.-H.; Jang, S.-K.; Kim, J.-H.; Park, S.-Y.; Kim, J.-C.; Jeong, H.; Kim, H.-Y.; Choi, I.-G. Simultaneous production of glucose, furfural, and ethanol organosolv lignin for total utilization of high recalcitrant biomass by organosolv pretreatment. Renew. Energy 2019, 130, 952–960. [Google Scholar] [CrossRef]
- Kim, E.S.; Liu, S.; Abu-Omar, M.M.; Mosier, N.S. Selective conversion of biomass hemicellulose to furfural using maleic acid with microwave heating. Energy Fuels 2012, 26, 1298–1304. [Google Scholar] [CrossRef]
- Raman, J.K.; Gnansounou, E. Furfural production from empty fruit bunch–A biorefinery approach. Ind. Crops Prod. 2015, 69, 371–377. [Google Scholar] [CrossRef]
- Yu, Q.; Bai, R.; Wang, F.; Zhang, Q.; Sun, Y.; Zhang, Y.; Qin, L.; Wang, Z.; Yuan, Z. A sustainable system for maleic acid synthesis from biomass-derived sugar. J. Chem. Technol. Biotechnol. 2020, 95, 751–757. [Google Scholar] [CrossRef]
- Woiciechowski, A.L.; Neto, C.J.D.; de Souza Vandenberghe, L.P.; de Carvalho Neto, D.P.; Sydney, A.C.N.; Letti, L.A.J.; Karp, S.G.; Torres, L.A.Z.; Soccol, C.R. Lignocellulosic biomass: Acid and alkaline pretreatments and their effects on biomass recalcitrance–Conventional processing and recent advances. Bioresour. Technol. 2020, 304, 122848. [Google Scholar] [CrossRef]
- Chen, H.; Qin, L.; Yu, B. Furfural production from steam explosion liquor of rice straw by solid acid catalysts (HZSM-5). Biomass Bioenergy 2015, 73, 77–83. [Google Scholar] [CrossRef]
- Esteban, J.; Yustos, P.; Ladero, M. Catalytic processes from biomass-derived hexoses and pentoses: A recent literature overview. Catalysts 2018, 8, 637. [Google Scholar] [CrossRef]
- Mariscal, R.; Maireles-Torres, P.; Ojeda, M.; Sádaba, I.; Granados, M.L. Furfural: A renewable and versatile platform molecule for the synthesis of chemicals and fuels. Energy Environ. Sci. 2016, 9, 1144–1189. [Google Scholar] [CrossRef]

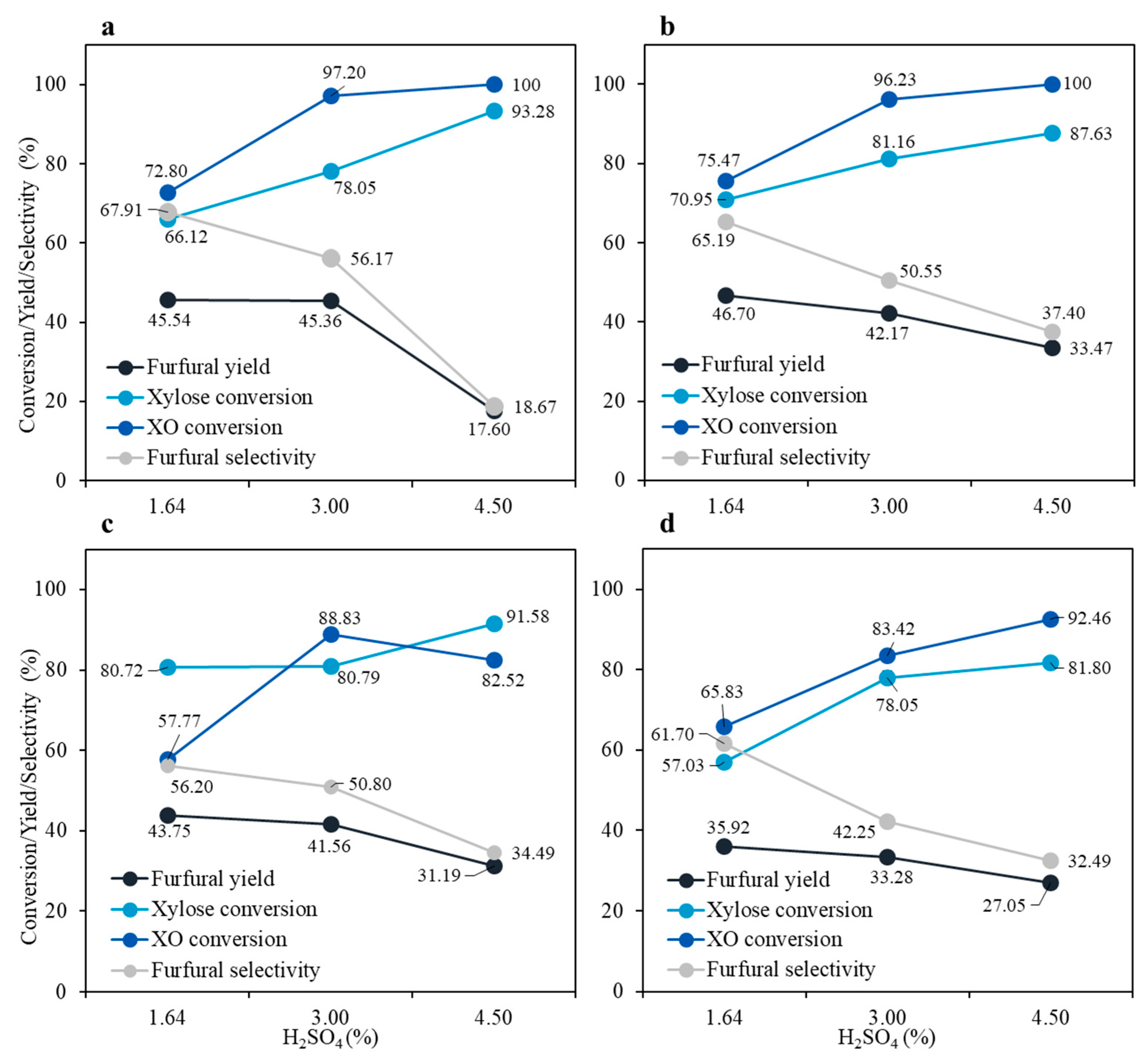


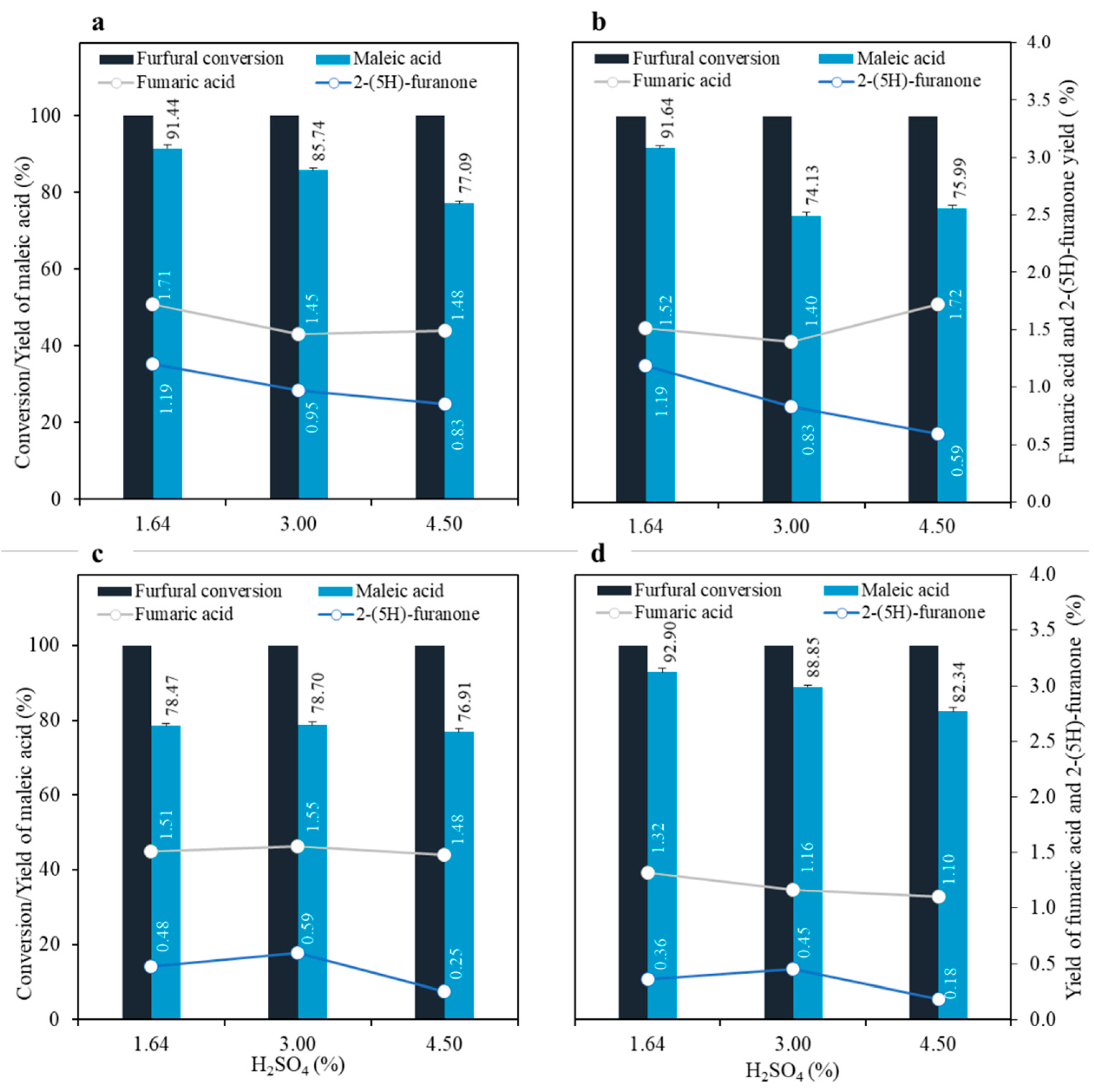
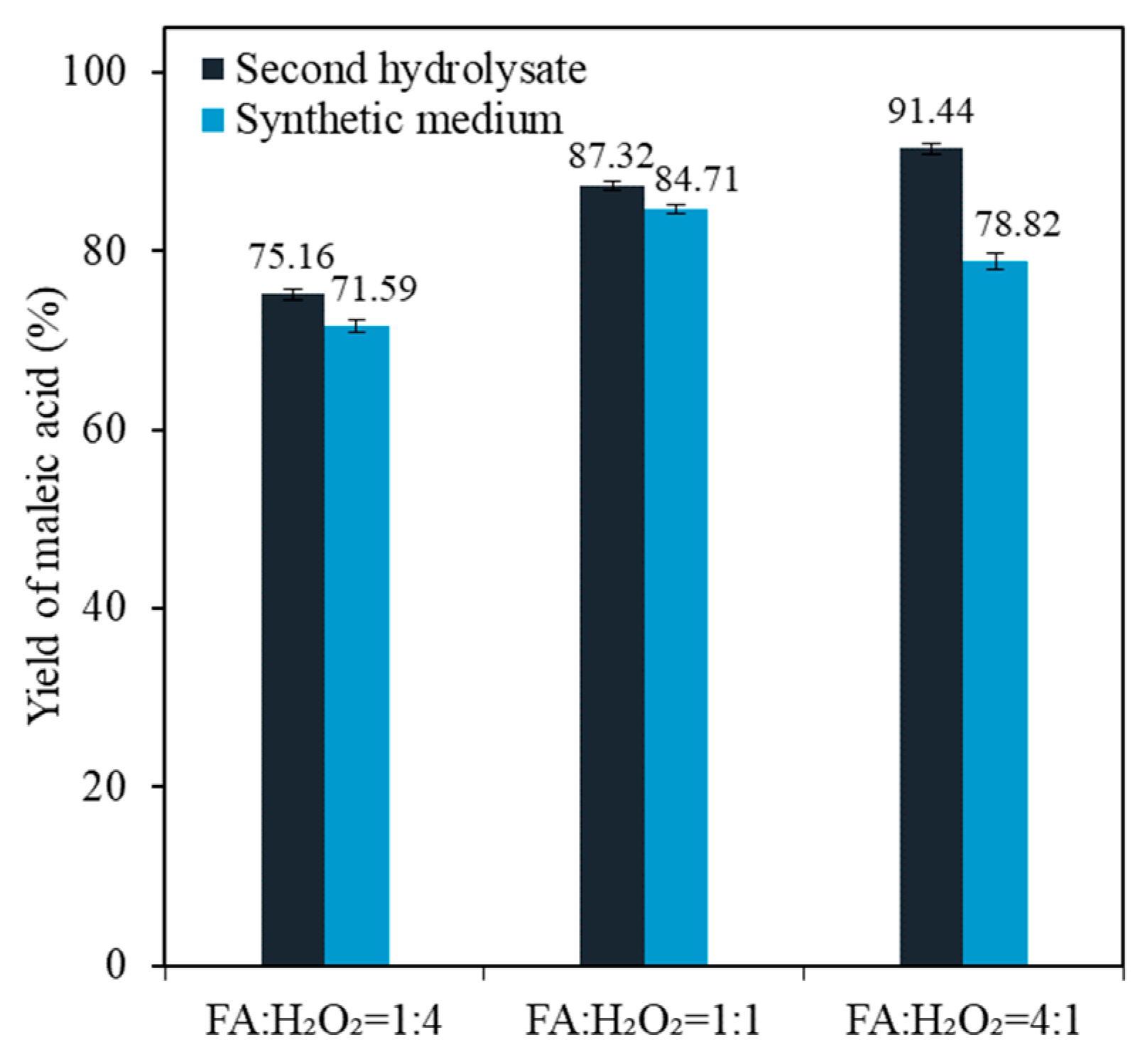
| Sample No. | Time (min) | Acid Concentration (mM) | CSF * |
|---|---|---|---|
| 1 | 30 | 82 | 1.89 |
| 2 | 139 | 2.08 | |
| 3 | 50 | 41 | 1.92 |
| 4 | 122 | 2.29 |
| CSF | Glu | Xyl | XO | FA | AA | HMF | Fur | pH |
|---|---|---|---|---|---|---|---|---|
| g/L | ||||||||
| 1.89 | 3.02 (0.22) | 15.17 (0.62) | 2.50 (0.25) | 2.12 (0.11) | 8.02 (0.14) | 0.21 (0.01) | 1.95 (0.05) | 2.10 (0.02) |
| 1.92 | 2.46 (0.16) | 14.95 (0.89) | 2.65 (0.18) | 1.33 (0.30) | 8.33 (0.33) | 0.22 (0.03) | 1.73 (0.12) | 2.19 (0.05) |
| 2.08 | 3.69 (0.19) | 14.37 (0.37) | 2.06 (0.19) | 2.67 (0.36) | 7.95 (0.12) | 0.27 (0.08) | 2.45 (0.45) | 1.84 (0.03) |
| 2.29 | 4.26 (0.02) | 12.80 (0.63) | 1.99 (0.34) | 2.59 (0.37) | 8.19 (0.14) | 0.38 (0.05) | 3.37 (0.32) | 2.00 (0.07) |
| H2SO4 (%) | Combined Severity Factors (CSF) | |||
|---|---|---|---|---|
| 1.89 | 1.92 | 2.08 | 2.29 | |
| 1.64 | 0.37 (0.05) | 0.50 (0.05) | 0.68 (0.10) | 0.57 (0.08) |
| 3.00 | 0.49 (0.09) | 0.79 (0.06) | 0.70 (0.07) | 0.90 (0.09) |
| 4.50 | 0.97 (0.07) | 1.10 (0.05) | 1.31 (0.07) | 1.09 (0.07) |
| Condition | Maleic Acid Yield (%) |
|---|---|
| Furfural | 78.82 (0.99) a |
| Furfural + acetic acid | 79.11 (0.08) b |
| Furfural + formic acid | 79.66 (0.25) b |
| Furfural + acetic acid + formic acid | 79.94 (0.78) c |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jeong, S.-Y.; Lee, J.-W. Effects of Sugars and Degradation Products Derived from Lignocellulosic Biomass on Maleic Acid Production. Energies 2021, 14, 918. https://doi.org/10.3390/en14040918
Jeong S-Y, Lee J-W. Effects of Sugars and Degradation Products Derived from Lignocellulosic Biomass on Maleic Acid Production. Energies. 2021; 14(4):918. https://doi.org/10.3390/en14040918
Chicago/Turabian StyleJeong, So-Yeon, and Jae-Won Lee. 2021. "Effects of Sugars and Degradation Products Derived from Lignocellulosic Biomass on Maleic Acid Production" Energies 14, no. 4: 918. https://doi.org/10.3390/en14040918
APA StyleJeong, S.-Y., & Lee, J.-W. (2021). Effects of Sugars and Degradation Products Derived from Lignocellulosic Biomass on Maleic Acid Production. Energies, 14(4), 918. https://doi.org/10.3390/en14040918





