Abstract
Plastic waste generation has increased dramatically every day. Indiscriminate disposal of plastic wastes can lead to several negative impacts on the environment, such as a significant increase in greenhouse gas emissions and water pollution. Therefore, it is wise to think of other alternatives to reduce plastic wastes without affecting the environment, including converting them into valuable products using effective methods such as pyrolysis. Products from the pyrolysis process encompassing of liquid, gas, and solid residues (char) can be turned into beneficial products, as the liquid product can be used as a commercial fuel and char can function as an excellent adsorbent. The char produced from plastic wastes could be modified to enhance carbon dioxide (CO2) adsorption performance. Therefore, this review attempts to compile relevant knowledge on the potential of adsorbents derived from waste plastic to capture CO2. This review was performed in accordance with PRISMA guidelines. The plastic-waste-derived activated carbon, as an adsorbent, could provide a promising method to solve the two environmental issues (CO2 emission and solid management) simultaneously. In addition, the future perspective on char derived from waste plastics is highlighted.
1. Introduction
The COVID-19 pandemic has had an unprecedented, harsh impact on the lives of people around the world. From another perspective, the pandemic has also changed the condition of the environment. For example, some parts of the world have seen improvements in air quality and a significant decrease in greenhouse gas emissions, which was hard to achieve before the COVID-19 pandemic. According to the International Energy Agency, the COVID-19 crisis in 2019–2020 has caused a significant drop in global carbon dioxide (CO2) emissions due to travel restrictions, closure of workplaces and other factors that have led to reduced energy consumption [1]. Table 1 shows the trends of the monthly global CO2 concentration in 2020 and 2021 [2,3]. It was confirmed that the global CO2 concentration was significantly reduced in 2020 due to the COVID-19 pandemic. For example, the global concentration of atmospheric CO2 was 411.66 ppm in October 2020, but increased to 413.93 ppm in October 2021 as a result of the economic recovery from the COVID-19 pandemic, increasing demand for residential electricity, and a lack of clean energy policies [2,3]. Besides that, there have been adverse impacts on solid waste management due to the COVID-19 pandemic. There is a drastic change in the nature of the wastes being generated. A significant increase in medical wastes, plastic wastes (single-use plastic), and food wastes has added an unprecedented load to the waste treatment facilities.

Table 1.
Global CO2 concentration in 2020 and 2021. Redrawn from the data of CO2 daily [2] and NOAA Earth System Research Laboratory, Global Monitoring Division [3].
CO2 emissions and water pollution caused by improper waste management such as plastic waste disposal are the two critical topics that need an urgent solution to protect and save the planet. CO2 released from the industries beyond power generation can be captured using pre-combustion CO2 capture, post-combustion CO2 capture, oxy-fuel combustion, and chemical looping technologies [4,5]. Among these CO2 capture technologies, post-combustion systems such as absorption (using solvent), adsorption (using solid material), cryogenic, and membrane separation have been recognized as mature technologies and have been developed at a large-scale application [5]. Absorption with aqueous alkanolamine solvent is the most promising technology and remains the dominant industrial technology for removal of gases such as CO2 [4]. However, the drawback of the absorption method is related to amine degradation, equipment corrosion, and the generation of volatile degradation compounds [4,5].
The adsorption of CO2 using solid material is an alternative method to amine-based absorption. This is because adsorption has several advantages, such as ease of operation, low energy consumption, good performance for the removal of gas and liquid, and ease of adsorbent regeneration [5,6]. There are several promising adsorbents developed for CO2 adsorption such as activated carbon [7], metal organic frameworks (MOFs) [8], zeolites [9], microporous organic polymer [10], mesoporous carbon material [11], nanoporous silica [12], and carbon nanotubes [13]. The most important characteristics of adsorbents include high surface area, high porosity, high stability, excellent recyclability, and high adsorption capacity [14]. However, the main challenges of some adsorbent materials such as MOFs and nanomaterials are that they are costly and difficult to produce on a large scale, thus causing some adsorbents to be less feasible for industrial applications [15]. Therefore, more research is needed in order to develop a low-cost material with an excellent adsorption capacity that can be used for commercial-scale production. Solid waste is an enormous problem in any part of the world, and conversion of the waste into porous carbon and adsorbents for CO2 capture is an alternative option to reduce waste generation. Due to sustainability considerations, there is a growing trend of using low-cost material as an adsorbent in which plastic waste has been categorized as the potential waste to be transformed as an effective adsorbent to capture CO2. It was proved that adsorbent derived from plastic waste has an excellent performance. For example, Machado et al. [16] investigated the potential of converting plastic waste (polystyrene foams) into magnetic activated carbon for redox supercapacitor application. They reported that magnetic activated carbon derived from plastic waste has a high surface area, excellent chemical and electrical properties and outstanding regeneration performance. In another study, Ilyas et al. [17] used activated carbon derived from waste polyethylene terephthalate and waste polystyrene for the removal of polycyclic aromatic hydrocarbons from industrial wastewater. The study reported that the activated carbon produced had a high surface area, highly porous structure material and produced excellent adsorption efficiency up to 95%.
However, there are very limited reports available in the literature on the potential of carbon-based material derived from waste plastic for CO2 capture. Waste polyethylene terephthalate (PET) is widely used as raw material to be converted into carbon-based material due to PET containing large amounts of carbon. In addition, functional groups such as the OH group found in PET-derived porous carbon will help in increasing CO2 adsorption capacity [18]. For example, Kaur et al. [19] used waste PET and carbonized it into carbon-based material using KOH as activating agent for CO2 capture. The produced carbon-based material has a high surface area (1690 m2/g) and the highest adsorption uptake of 1.31 mmol/g. To convert plastic waste into char (carbon-based material), the raw plastic waste was cut into small sizes and carbonized in a furnace at a temperature of 600 °C under an inert atmosphere (absence of oxygen) [18,19,20,21,22]. Next, the char was modified using physical activation such as CO2 or steam and chemical activation (such as KOH, NaOH, H3PO4 and K2CO3 as activating agent). The modified samples were heated to a temperature between 500 and 800 °C [22]. The physical and chemical activation will help in enhancing the surface properties of char, thus producing a highly porous structure carbon-based material [18,19].
Plastics have emerged to be an inseparable part of our daily lives and are a significant component of our economy due to them being cheap, durable, and lightweight, as well as the fact that they can be used in many applications. Therefore, plastic production has almost reached five hundred billion kilograms in 2018 [23]. The increasing plastic production causes severe negative impacts on the environment, especially in solid waste management [23,24]. An alternative solution to reduce the plastic waste problem is by converting plastic waste into char (solid material) using the pyrolysis process to produce porous material to be used for CO2 capture. Therefore, this review attempts to investigate the potential of plastic waste as an adsorbent for the removal of CO2 in which two critical environmental issues (CO2 emission and plastic waste) can be solved simultaneously. In this review, the latest scientific inventions on CO2 adsorption using plastic waste as an adsorbent from the literature will be analyzed, summarized, and compiled. These data are beneficial for researchers who are aiming to study relating to low-cost adsorbent, activated carbon, plastic waste, and CO2 adsorption.
This review has been structured in the following manner. The first section is the introductory section, which includes an overview of the waste plastic problem and the adopted solutions to resolve this issue. Next is the method used in these studies based on the PRISMA systematic review method applied in this study. Moving forward, a more in-depth discussion of the pyrolysis technique, physicochemical properties, and modification of plastic wastes as an adsorbent for CO2 capture is detailed. Finally, an overview of the main findings and a few suggestions for future studies in this area are proposed.
2. Overview of Plastic
Plastic is ubiquitous in our daily lives. Therefore, it is difficult to imagine a world without plastic. Plastic material has unique properties, such as being lightweight, flexibility, resistance to corrosion, and the ability to be mixed with different colors, making plastic suitable to be applied in various applications [24,25]. All plastics consist of large molecules of substances called polymers. They consist of many identical small particles that are strong and bound together like a chain. In general, a monomer is a molecule that forms the basic unit of polymers. Polymers, either being produced from natural or synthetic materials, are formed using the polymerization process of many small molecules known as monomers. Specific polymers normally comprise one or two types of monomers [25]. The polymers are formed by joining together many monomers like a long chain of paperclips to form one long molecule [25,26].
Due to the increase in the mass production of synthetic plastics since the 1950s [10], plastic waste disposal has drastically increased, tripling since the 1990s [27]. Plastics are difficult to be broken down into small components and usually can take up to 1000 years to decompose in landfills [28]. People around the world become addicted to single-use plastics, of which 60% end up in landfills thus causing severe environmental problems [29,30]. Every year, there is an increase in plastic waste generation to a current total of three hundred billion kilograms, which is equivalent to the total number of the entire human population [31]. Most plastics will not be destroyed or disappear, but will be broken down into smaller particles. Many of these tiny plastic particles are swallowed by farm animals or fish who mistake them for food, and thus these particles find their way onto our dinner plates. If the current trends continue, our oceans could contain more plastic than fish by 2050 [32,33]. Plastic waste has become a pressing issue of discussion in many countries, such as Malaysia, Thailand, the Philippines, and Indonesia. Many countries have restricted plastic waste imports and introduced several policies to address this problem [34]. Over the year, plastic pollution has become one of the most pressing environmental issues, since the rapid increase in the production of disposable plastic products overwhelms the world’s ability to deal with them. Plastics have surprisingly carbon-intense life cycles. The carbon footprint of plastics continues even after being disposed of. The dumping, incinerating, recycling, and composting of these plastic materials (for certain plastics) release CO2 [35]. Therefore, it is important to discuss research on the life cycle of plastics [36,37]. Only 9% of plastic was recycled, 12% was incinerated, and a huge amount of plastic (79%) was gathered in the landfills. Recycling offers the simplest solution, and it has plenty of room for improvement [38]. The public must understand the magnitude of the challenges that each country is facing to overcome this issue [39]. There is a lot of efforts that have been carried out but the more sustainable effort is required to reduce plastic waste and environmental pollution.
2.1. Types of Plastic
There are two broad categories of synthetic polymer that are important to be familiarized as [25,26,27]:
- Thermoplastics are a class of polymer that can be softened and melted by the application of heat and can be processed in a heat-softened state. They can be remolded and recycled without negatively affecting the material’s physical properties. The examples include polyethylene (PE), polypropylene (PP), polytetrafluoroethylene (Teflon), polyethylene terephthalate (PET), polyamide (PA), polyvinyl chloride (PVC) and polystyrene (PS) (Table 2).
- Thermoset plastics comprise polymers that are cross-linked together to form an irreversible chemical bond. The cross-linking process makes thermosets ideal for high-heat applications such as epoxy resins, polyurethane (PU), polyester resins, and Bakelite.

Table 2.
Summary of commonly used synthetic polymer and their application [40,41,42,43].
Table 2.
Summary of commonly used synthetic polymer and their application [40,41,42,43].
| Types of Synthetic Polymers | Abbreviation | Advantages | Application |
|---|---|---|---|
| Polyethylene terephthalate | PET or PETE | Hard and flexible Absorbs odours and flavours from foods and drinks | Beverage bottles, food packaging, carpet fibre, electrical parts, and films |
| High density polyethylene | HDPE | Rigid Not transmit any chemical into foods and drinks | Grocery bag, harder bottles, piping, toys, window shades and board for building |
| Polyvinyl chloride | PVC | Flame resistance Flexibility Lightweight | Blister wrap, window and door frames, blood bags, medical tubing, drainage pipes, electrice wire and cable. |
| Low density polyethylene | LDPE | Durable and flexible | Packaging films, soft bottles, soft tubing, carrier bag, molded materials of laboratory equipment. |
| Polypropylene | PP | Resistant to high temperature Hard and flexible | Microwaveable meal trays, disposable cups and bowls, drinking bottles and straws. |
| Polystyrene | PS | Hard and brittle | Food containers, yoghurt pots, protective packaging, electrical appliances and building insulation. |
2.2. Proximate Analysis of Plastic
Proximate analysis shows that plastics are different, as each one of them is made of different types of monomers [44]. Proximate analysis is a method to determine the chemical properties of plastic based on four specific elements, which are moisture content, fixed carbon, volatile matter, and ash content [44]. The percentage of moisture and ash content varies for all types of plastic [45]. The yield of liquid is related to the volatile matter and ash content, as the amount of liquid oil will increase if the volatile matter is high, while the amount of liquid oil will decrease if the ash content is high [46,47]. As stated by Zhou et al. [45], heating value is influenced by moisture and ash content. The volatile matter will affect the ignition of plastic incineration in which higher content of volatile matter will accelerate the ignition. A high volatile matter content (above 90 wt%) and high carbon and hydrogen content make plastic waste an excellent candidate for the pyrolysis process. Table 3 encapsulates the proximate analysis of different plastics. The Resin Identification Code (RIC) was introduced by the Society of the Plastics Industry (SPI) in 1988. The RIC symbol is three arrows in a clockwise rotation forming a triangle shape with a code number in the center. This RIC code is used by manufacturers and recyclers to easily differentiate different types of polymer product [48]. There are seven types of synthetic polymer with seven codes, namely: code (1) polyethylene terephthalate (PET or PETE), code (2) high-density polyethylene (HDPE), code (3) polyvinyl chloride (PVC), code (4) low-density polyethylene (LDPE), code (5) polypropylene (PP), code (6) polystyrene (PS), and code (7) others (such as polycarbonate (PC) and ABS) or mixed plastics. Not all types of polymers can be recycled or reused. In addition, the RIC code does not indicate whether the product is recyclable [49].

Table 3.
Proximate analysis for plastics [46,47,50,51,52].
3. Search Strategy and Methodology
This review was performed in accordance with Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guidelines. A search for keywords of “Plastic waste AND adsorbent”, “Plastic waste AND CO2 capture”, Plastic waste AND CO2 adsorption” and “Plastic waste AND adsorbent AND pyrolysis”, Plastic waste AND adsorbent AND pyrolysis AND CO2 capture” on Web of Science were applied to retrieve the relevant articles. The analysis results showed that for the keywords of “Plastic waste AND CO2 capture”, Plastic waste AND CO2 adsorption” and “Plastic waste AND adsorbent AND pyrolysis”, Plastic waste AND adsorbent AND pyrolysis AND CO2 capture” a total of 187, 61, 15, 39 and 3 records, respectively were identified in the initial literature search. After the removal of duplicates and full-text screening, only 20 journal articles under the keywords of “Plastic waste AND CO2 capture”, and “Plastic waste AND adsorbent AND pyrolysis” were included for data extraction. For literature search, only full-text articles written in English language were included. All articles were searched from 2010 to 2021. Records and full texts were screened independently. PRISMA is an important method to produce effective review articles. However, only a few review articles used the PRISMA method specifically in the field of engineering and environmental management. In 2018, Haddaway and the team [53] developed a specific guideline and specific methodology for the environmental field in conducting a systematic review known as Reporting of Strategies in Systematic Evidence Syntheses (ROSES). They summarized a detailed set of reporting standards for systematic review. They proved that for conducting a systematic review in the environmental field, the ROSES guideline standard was more appropriate to be used compared to PRISMA. However, other researchers claimed that PRISMA is also suitable for environment management and other engineering fields because it clearly defines the aim of the research and provides clear and details information to the reader. PRISMA has provided guidelines and a checklist from the flow diagram to prepare a high-quality review article [54]. Recently, Page et al. [55] published an updated reporting guidance for systematic reviews to replace the PRISMA 2009 statement and includes new reporting guidance that reflects advances in methods to identify, select, appraise, and synthesize studies. They provided 27 item checklists with detail reporting recommendations for each item. Therefore, this study used PRISMA method guidelines such as search specific keywords using various databases, data collection process, selection process (analyze the abstract and full article), the general interpretation of the results, and discussion.
4. Method for Management and Reducing Plastic Waste
As plastic waste has increased quite rapidly every year, the course of actions to mitigate or recycle plastic waste should be determined immediately. Therefore, by implementing this action, the negative impacts on the environment, either the land or the water, will decrease. Hence, waste management plays an important role in the protection of the environment, covering parts of the collection, transport, recovery, and neutralization of plastic waste [56]. Due to urbanization, the demand for plastic production is increasing because the properties of plastic have advantageous in the food packaging industries, manufacturing industries, the medical world, and other fields [25,26]. The demand for plastic in industries, as well as the number of humans, is growing rapidly, leading to an increase in the utilization of plastic and demand for energy, as they are made from exhaustible fossil fuels such as coal, natural gas or oil [57]. The conventional way has been used to minimize the number of plastic wastes involving landfilling whereby plastics are dumped at a specific land site. This is an ineffective way of reducing plastic waste that causes severe problems to the environment. Landfill sites occupy large areas of land with expensive taxes or tariffs in place to discourage sending waste to these lands [56,57]. This method is quite disadvantageous, as plastic takes time to self-biodegrade since this process uses heat from the sunlight [58]. Therefore, the rate of landfilling keeps growing, while the rate of biodegradable waste increases very slowly. The process of plastic degradation in nature is partitioned into several parts, which are physical, biological and chemical processes [57]. These processes require pressure, humidity, and heat from the sun for physical degradation. Plastic build-up from a chemical compound, which is a hydrocarbon compound procured from petroleum refining, hardens the bond between the monomers. Thus, the degradation process has difficulty proceeding at the ambient temperature (32 °C). Biological degradation by enzymes and bacteria is difficult, and this process takes quite a long time to be completed [59]. Incineration is another method used for plastic waste management. However, incineration is not considered an eco-friendly approach, since hazardous materials are released during this process. The cost is also high for constructing the incineration unit [60].
Furthermore, a recycling method can be applied to reduce the plastic waste problem since many plastics can be recycled. Plastics such as PET and HDPE are recyclable but it is quite costly to recycle them. Hannah and Max [61] stated that only 20 percent of the plastic waste is recycled globally. This indicates that the recycle method has not been receiving attention widely enough to be used as a strategy to reduce plastic waste in many countries. For example, the recycling rate of plastic in Malaysia is about 28%, which is lower than other countries. Awareness regarding plastic waste should be increased and the importance of the 3Rs that is “reuse, reduce and recycle” should be taken seriously to reduce the rate at which plastic waste is polluting the environment [34].
The utilization of plastic waste for recycling is one of the efficient ways to overcome plastic wastage, and it has been delivering a good result as it reduces the production cost and other factors. Plastic waste can be utilized by converting them through the treatment process. These treatments of plastic waste and recycling would be partitioned into four major types, such as primary or re-extrusion, secondary or mechanical based, tertiary or chemically oriented, and quaternary or energy recovery [62,63]. However, each of these offers its own benefits and disadvantages. Primary recycling is known as closed-loop recycling, which is the process of recycling the waste into similar products to the original and use it back in the original application [62]. These newly produced products will have similar characteristics to the original. One of the examples of primary recycling is injection molding to produce low-density polypropylene (LDPE) products. Plastic wastes are mainly recycled from the industry via this technique [62,63]. The secondary recycling technique is mechanical-based, which is recovering plastic waste in plastic manufacturing via physical modification, and it only can be done on single-polymer plastic [64]. Contaminated plastic will cause difficulty in recycling using mechanical techniques due to the degradation and heterogeneity of plastic waste [62,63,64]. In addition, mechanical recycling is expensive and requires a high-energy process as it involves several treatment and preparation steps [65]. One of the examples of mechanical recycling techniques is the construction of pavement blocks using plastic waste such as PE and PP [66]. In addition, tertiary recycling is using plastic waste as feedstock to generate fuels and solid residues [60]. It is an advanced process that converts plastic waste into smaller molecules, which are mostly in liquid or gas forms [62,63]. These molecules are fit to be utilized as a feedstock for the new chemical and plastic production. It is believed that the product that is produced through chemical recycling is beneficial to be used as fuel as it has a high yield of product and is able to minimize waste [62]. The effective way of plastic waste utilization is chemical recycling whereby various products are being produced, such as gases, liquid, and char [65]. Thus, it will be a profitable and sustainable industrial plan of action. The main benefit of chemical recycling is the likelihood of treating heterogeneous and contaminated polymers with a finite use of pre-treatment [64]. Chemical recycling requires hydrogenation, pyrolysis, and gasification techniques, which are the most effective ways to recycle plastic [62,63,64,65].
Pyrolysis is an alternative technique in chemical recycling, as this process takes place in a zero-oxygen condition, leading to thermal cracking and condensation resulting in an increased production of numerous liquids, gases, and solid fractions [46,47]. The difference between pyrolysis with the hydrogenation of plastic and pyrolysis with the gasification of plastic depends on the carbonization method, temperature, and product formed [46,47,66]. The profit of incineration, gasification or decomposition in converting plastic waste will reduce the amount of heat released into the environment. An example of this process is the utilization of waste plastic oil in a diesel engine by catalytic pyrolysis [66,67]. Quaternary recycling involves energy recovery [35]. This refers to the burning of waste to yield energy in the form of heat, steam, and electricity [35]. It is believed that this method is a practical means of waste treatment when the material recovery process fails due to economic limitations. Plastic material has a very high calorific value when it is burned, as it is derived from crude oil, making it a useful energy source [64]. Therefore, heat that is one of the forms of energy recovered by this process can be used for power generation [65]. The pyrolysis oil obtained from the catalytic treatment of polystyrene results in better engine power with a comparable temperature of an engine with a lower amount of carbon monoxide and carbon monoxide emissions, as compared to the uncatalyzed oil and mercantile fuel in gasoline engine [68]. Besides that, char produced from the pyrolysis process of mixed waste plastic will form a porous carbon that can be used in the adsorption of liquid or gas or other applications [69].
4.1. Conversion of Plastic Waste into a Carbon-Based Material
In order to reduce the environmental pollution generated from the disposal of used plastic materials, a growing interest has been focused on the conversion of plastic waste into valuable materials [70,71]. Numerous studies have successfully converted plastic waste into porous carbon in various applications, such as that applied in organic and inorganic removal from synthetic and real wastewater [72,73], used in natural gas application [74], nanoporous adsorbents used as electrode material in supercapacitors [75], and porous carbon nanosheets with excellent performance applied in hydrogen storage [76]. However, very limited reports are available on their application in capturing CO2. There are different methods that have been used to transform plastic waste into porous carbons such as using gasification, direct carbonization (physical or chemical activation), hydrothermal carbonization and pyrolysis [18,46]. Among the various technologies available for plastics waste treatment, pyrolysis is one of the most promising methods and the process can be carried out with or without catalysts [77]. Plastics are among the most valuable types of waste, and it is possible to convert plastics directly into useful forms of new adsorbent material, energy, and chemicals for an industry, known as “pyrolysis”. Pyrolysis is normally used to generate energy in the form of heat, electricity or fuels, but it could also help to recover other chemicals and materials. Roberts et al. [78] stated that pyrolysis is a thermochemical method in the absence of oxygen to convert biomass or waste material into valuable char or carbon materials, biochar, bio-oil, and syngas. Char or carbon-based material produced from the pyrolysis process is stable charcoal with high carbon content and can be used as a soil amendment. Pyrolysis systems that produce char or carbon-based material and energy (fuel or oil) do not result in pollution, contaminate water supplies or create waste disposal problems [31]. In another study, Scott et al. [79] reported that pyrolysis is a decomposition process of long-chain hydrocarbon (polymer) molecules into smaller sizes (monomer) with the use of high heat (450–800 °C) in a shorter duration. This process, as claimed by the same study, generates products in the form of carbon or char as the residues, as well as volatile hydrocarbons in the form of condensed and non-condensable fuel and as a gaseous fuel. The pyrolysis technique has been investigated by many researchers mainly on the conversion of liquid pyrolysis products into products similar to crude oil [80]. However, there is a limited study exploring the char by-product produced from pyrolysis. To the best of our knowledge, a systematic and comprehensive review on the utilization of plastic waste as a potential adsorbent for CO2 capture is yet to be reported.
4.2. Pyrolysis of Plastic Waste
The pyrolysis process is a process of converting organic material into three phases, which are solid (char), liquid (tar), and gas [81] without involving reaction with oxygen, water and other reagents. As stated by Fakhrhoseini and Dastanian [81], all these phases are immiscible and unprompted. There are several types of pyrolysis of biomass, including fast pyrolysis, slow pyrolysis [82], and microwave pyrolysis. These processes are differentiated by the pyrolysis method, processing time, pyrolysis temperature and the catalyst [83]. However, the processes can be done with or without a catalyst since they only act to increase the reaction rate. The optimum amount of bio-oil yield is obtained when high temperature and high heating rates are applied. Hence, this is a fast pyrolysis process. For slow pyrolysis, a slower heating rate will be applied and the major product for this process is the char [84,85]. Char is a good adsorbent because of its highly porous carbon. As mentioned by Al-Salem et al. [84], pyrolysis is a thermo-chemical plastic waste treatment method that does not contribute to pollution and required less energy and product such as oil and gas. The large-scale experimental set-up of the pyrolysis process involves the process flow to a heat exchanger, condenser, and exhaust stream. For the first side of the exchanger, the condenser with cooling silicon oil in the shell is used while the second side of the exchanger uses water. As the reaction starts, the pyrolysis product enters the tube side of the heat exchanger and the liquid sample bottle is used to accumulate the liquid product while char as the solid product remains in the reactor [81]. An example of a pyrolyzer used is the fluidized bed pyrolyzer with an electrostatic precipitator or with a circulating heat carrier. This pyrolyzer has a good heat transfer rate and a uniform temperature. Moreover, a high amount of bio-oil will be produced, which is up to 75% [86]. However, it requires a small particle size, a large amount of inert gas, and a high operating cost. The other two pyrolyzers are rotating cones and Auger pyrolyzer. For the rotating cone, it has a comparatively simple construction and operation, and a low heat carrier but with a limited capacity. Lastly, the Auger purolyzer is compact, has a simple construction and hence is easy to operate and can be operated at a lower temperature. The disadvantages of the Auger pyrolyzer is that the residence time is long, it has a low bio-oil yield, high yield of char, and limitation for scaling up due to the heat transfer [87]. Table 4 shows the summary of studies on plastic waste using pyrolysis process. Only a few data reported on the product yields acquired from the thermal treatment at 400–800 °C.

Table 4.
Summary of studies on plastic waste using pyrolysis process.
4.3. Modification of Plastic Wastes for CO2 Capture
For the pyrolysis process, the most important parameters that need to be considered are the pyrolysis temperature, treatment (heating) time, and heating rate. After the first cycle of pyrolysis, the produced sample (char) needs to be modified with a chemical activation treatment to improve the surface properties of the material. Then, the modified sample needs to be heated again using a pyrolysis technique. The physicochemical properties of the char produced from pyrolysis, such as elemental analysis (carbon, hydrogen, nitrogen, and oxygen content), surface area and pore size, functional groups, surface morphology, and thermal stability, are important to monitor [46]. From the results of the physicochemical properties, the adsorbent material that produces high performance in terms of high carbon content, large surface area and pore size, and high thermal stability has the potential to be used to study CO2 adsorption. In general, the overall adsorption process using plastic waste as an adsorbent is illustrated in Figure 1.
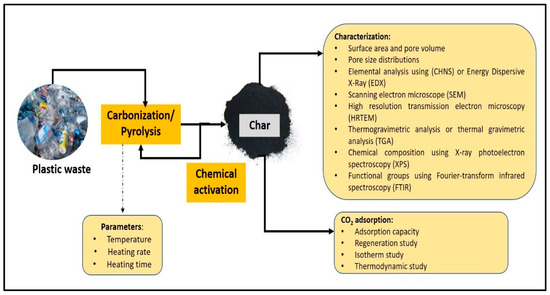
Figure 1.
Overall adsorption process using plastic waste as an adsorbent.
4.3.1. Characterization of Char
The different factors that affect the high CO2 adsorption capacity are the pyrolysis method, pyrolysis temperature, activation process, activating agent, impregnation ratio, physicochemical properties of the adsorbent, adsorption temperature, and adsorption pressure. The physicochemical properties, such as surface area and porosity, surface chemistry determined from X-ray photoelectron spectroscopy (XPS) spectra, surface morphology, surface functional groups, and the elemental composition of carbonized char produced from plastic waste, are discussed in the following sub-sections.
BET Surface Area and Porosity
Researchers identified that the shape of the adsorption isotherm can provide qualitative information on the adsorption process and the amount of surface area available to the adsorbate. Based on the N2 adsorption isotherms, adsorbents from plastic wastes can be categorized as microporous solid, whereby the shape of the isotherms followed a typical behavior of Type I of the International Union of Pure and Applied Chemistry (IUPAC) classification [89]. They found that all modified plastic samples are categorized as Type I of the IUPAC classification suggesting that the prepared samples belong to typical microporous carbons. The surface area and total pore volume of the adsorbent will increase due to several factors such as the pyrolysis method, pyrolysis temperature, activation process, impregnation or activation ratio (mass ratio of activating agent and carbonized char), and the type of activating agent. However, the surface area and total pore volume may decrease after chemical activation depending on the type of the activating agent and the impregnation ratio of the activating agent. At a high concentration or ratio of the activating agent’s solution, it may block the tiny pores and reduce the surface area and total pore volume performance. For physical activation, the microporosity of the adsorbent increased, thus increasing the surface area and total pore volume because of pyrolysis temperature. For example, the values of the surface area increased from 1812 to 2006 m2/g with increasing pyrolysis temperature from 700 to 800 °C. However, at 900 to 1000 °C activation temperature, the surface area was significantly reduced from 1860 to 1689 m2/g [19]. By increasing the activation temperature (during the pyrolysis process), the values of the pore volume show a decreasing trend, suggesting that a higher activation temperature is not favorable for developing narrow microporous structures. It was observed that surface area and pore volume dramatically decreased when the activation temperature increased above 800 °C [18,19,21]. In addition, at high activation temperature (800–1100 °C), char and pyrolytic intermediates could directly decompose, thus decreasing the char yield performance [35]. For chemical activation, KOH reacted with char to convert some carbon and oxygen from char into organic species (K2CO3). Meanwhile, at higher temperatures (800–1100 °C), certain oxygen and K metals from KOH were released to become gas or bio-oil by-products [19,90]. It can be concluded that if the activation temperature is too high, the micropore structure will be destroyed, thus decreasing the char yield.
The possible reactions between KOH and char or carbon samples are presented as below (Equations (1)–(9)) [19,91]:
As reported by Wang and Kaskel, [91] and Liu et al. [92], the three important chemical activation mechanisms that occurred between KOH activation and char or solid carbon are described below:
- (a)
- The redox reactions between various potassium species with carbon (in Equations (5)–(9)). This process or reaction is responsible for creating a network of porosity.
- (b)
- The formation of H2O and CO2 (in Equations (1)–(7)) positively contributes to the development of porosity by physical activation.
- (c)
- The intermediate potassium oxide species such as K2CO3 or K2O are reduced by carbon to produce metallic K at temperatures over 700 °C (in Equations (8) and (9)). The metallic K intercalates into the carbon surface, thereby expanding the lattice. This led to the formation of a larger pore surface. After activation, the sample underwent a washing process to remove the intercalated metallic K and other K compounds. Carbon with high porosity and a large surface area was obtained. The reaction mechanism is highly dependent on the activation temperature, activating agent ratio, and the type of feedstock (type of plastic waste) [15].
Similarly, the total pore volume significantly decreased with an increase in the impregnation ratio due to the high mass ratio resulting in the collapse of the pore wall and the reduction in the adsorption force [19]. Pore size can be classified into the following groups namely (1) macropores (>50 nm), (2) mesopores (in the range of 2.0–50 nm), (3) micropores (<2.0 nm), (4) supermicropores (0.7–2.0 nm), (5) ultramicropores (<0.7 nm), and (6) submi-cropores (<0.4 nm) [93]. The main pore sizes of an adsorbent from plastic wastes were smaller than 2.0 nm (usually micropores in the range 1.0–2.0 nm), especially after modification with KOH activation, which plays an important role in enhancing adsorption capacities and effective CO2 capturing [19]. As it can be seen in Table 5, the porous carbon shows good regeneration performance and excellent stability over 10 repetitive cycles with no significant reduction in adsorption capacity. Table 5 shows the summary of the textural properties of chars derived from plastic pyrolysis.

Table 5.
Textural properties of chars derived from plastic pyrolysis.
X-ray Photoelectron Spectroscopy (XPS)
XPS is a surface measurement technique used to both determine the chemical composition of the surfaces of solid materials and assess the surface chemistry of a material. For example, to determine the chemical bonding states (or oxidation state) of the elements and the compounds [94]. The high oxygen functional groups provide basic active sites on the adsorbent and thus enhance the affinity towards CO2 binding, resulting in high CO2 adsorption and effective CO2 capturing [18,19,95]. This can be confirmed based on the XPS spectra whereby a peak at 532 eV is associated with oxygen atoms in the carboxylic groups (O1s) that form Lewis base sites on the surface of the pores and beneficial to CO2 binding [19]. The O2 and O3 peaks in Table 6 explain the basic groups existing in the porous carbon (char produced from plastic waste). The presence of oxygen species based on XPS spectra is summarized in Table 6.

Table 6.
XPS result of oxygen species of char derived from plastic waste.
Surface Morphology
Scanning electron microscopy (SEM) was used to examine the surface morphology of carbon samples. Based on the SEM images in Figure 2a, at an activation temperature of 700 °C, many small holes with voids on the surfaces and well-developed porous structures were observed, making porous carbon feasible for capturing CO2, whereas irregular shapes and smooth surfaces were found in the samples at a high temperature of 1000 °C (Figure 2b) [18]. Similarly, Kaur et al. [19] reported that the morphology of the carbonized PET-AC shows a smooth surface with very few pores (Figure 2c). However, after activation with KOH, PET-AC showed improvement in surface morphology. The samples had irregular shapes, consisting of many small pores (Figure 2d). This proves the effective function of KOH activating agent to produce high porosity. Furthermore, Transmission Electron Microscopy (TEM) was also used to observe the structure of carbon-based material. TEM is a technique of imaging the internal structure of porous carbon using a beam of high-energy electrons transmitted through the solid. The TEM images are usually more difficult to be used to interpret the structure of the solid compared to SEM images [20]. As shown in Figure 2e, TEM images of PET-AC exhibit a uniform micropore distribution, thus indicating that pyrolysis and chemical activation formed well-developed porous carbon with a micropore structure.
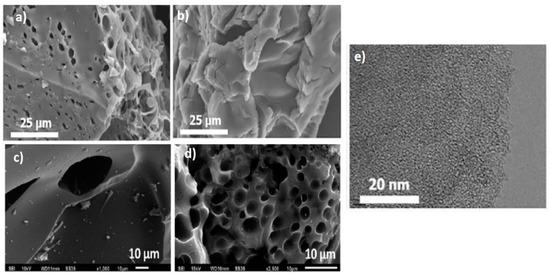
Figure 2.
SEM images of (a) modified PET-AC with KOH at 700 °C, (b) modified PET-AC with KOH at 1000 °C, (c) carbonized PET-AC at 700 °C, and (d) modified PET-AC with KOH at 700 °C. TEM images of (e) modified PET-AC with KOH at 700 °C. (Adapted from Yuan et al. [18] and Kaur et al. [19] with permission from Elsevier).
Surface Functional Groups
Fourier-transform infrared (FTIR) spectroscopy is employed as a powerful tool and was applied for the qualitative and quantitative identification of chemical species or functional groups located on the adsorbent surface with the assistance of infrared spectra shone on the sample. FTIR is also used to determine and identify track changes in the functional groups that are present in the carbonized char produced from plastic waste before and after chemical activation. FTIR spectra of carbonized char samples were studied to investigate the presence of functional groups on the carbonized char before and after a chemical activation using KOH as the activating agent [19]. Kaur et al. [19] reported that the highest absorption was observed at 3440, 1620, and 1079 cm−1 for modified char derived from plastic waste. The bands observed at 3440 cm−1 could be associated with the O-H stretching vibration of the hydroxyl functional groups due to the breaking hydrogen bonds, which released alcohol and water. The bands at around 1620–1640 cm−1 are probably associated with carboxylate ion (COO-) and ester carbonyl groups. The adsorption peak at 1079 cm−1 corresponded to the C-O-C stretching and shows the presence of aromatic compounds and the existence of oxygen. In addition, the weak band at around 2935, 2300, 1440, 780, and 680 cm−1 corresponded to the C-H stretching of the hydrocarbon compound (aliphatic and aromatic structure) in which these peaks significantly decreased after pyrolysis because of the temperature of the pyrolysis process [96,97,98].
Elemental Composition
Elemental analysis of chars derived from plastic waste using pyrolysis is shown in Table 7. The pyrolysis temperature may influence the physicochemical properties of char. For example, the pyrolysis temperature may affect the carbon content of the char derived from plastic waste whereby the carbon content increased with increasing pyrolysis temperature [98,99]. Increasing pyrolysis temperature had a significant effect on the elemental compositions, especially on the contents of carbon, oxygen, and nitrogen. However, based on the elemental composition of chars, as presented in Table 7, most of the studies used PET waste to produce char and produce a high performance at a pyrolysis temperature of 700 °C. Kaur et al. [19] prepared nanoporous carbon adsorbents from PET by carbonizing at 700 °C in a tubular furnace followed by a chemical activation at 700 °C for 2 h before assessing their CO2 uptake. The compositions of carbon, hydrogen, oxygen and nitrogen both before and after the activation process with different impregnation ratios were determined using an elemental analyzer. The results show that the impregnation ratio affects the percentage composition of carbon and oxygen content with a reduced carbon content percentage with an increased impregnation ratio. For example, the percentage of carbon content for the 1:1 impregnation ratio of KOH to carbon and the 3:3 impregnation ratio was 80.38% and 65.10%, respectively. Meanwhile, the percentage of oxygen content increased with an increase in the impregnation ratio caused by the activation process. Oxygen functional groups led to higher CO2 adsorption capacity whereby oxygen functionality can increase the attraction towards CO2 binding due to the importance of the basic sites on the adsorbent. An adsorbent with basic surface groups possesses more active sites for the adsorption of CO2 [100,101,102]. The presence of a high percentage composition of oxygen on the nanoporous carbon adsorbents may affect the adsorption capacity of CO2 [80]. Similarly, Kaur et al. [96] also highlighted that PET carbonized samples without a chemical activation showed a low amount of oxygen and low adsorption capacity, thus decreasing its attraction towards acidic CO2 gas. Yuan et al. [95] prepared a PET-derived microporous carbon using two activation methods of one-pot and two-pot synthesis. For one-pot synthesis, the carbonized PET was impregnated with KOH and urea simultaneously. Meanwhile, for two-pot synthesis, the activation process was performed separately. First, the carbonized PET was mixed with KOH and the modified sample was mixed with urea. The study found that one-pot synthesis has higher oxygen content (18.8%) compared to two-pot synthesis (6.68%). This confirms that oxygen functional groups help to increase CO2 uptake.

Table 7.
Elemental composition of chars derived from plastic pyrolysis.
4.4. CO2 Adsorption Performance
There are only a few literature studies reported on the use of porous carbon derived from plastic waste in the application of CO2 (Table 8). PET plastic waste is considered a low-cost material and effective to capture CO2 due to the high carbon and oxygen content confirmed by the elemental composition of XPS analysis. Table 8 shows the previously published studies that used PET plastic waste as a raw material to produce porous carbon material. For example, Arenillas et al. [103] investigated the potential of carbon material derived from PET bottles as an adsorbent to capture CO2 using the carbonization process at 500 °C. The study reported that the highest CO2 uptake was achieved at 1.09 mmol/g using adsorption temperature at 25 °C. Adibfar et al. [104] modified the PET waste with various types of activating agents, such as KOH, H3PO4, ZnCl2, and H2SO4, and compared their adsorption capacity performances. The team reported that the porous carbon modified with alkaline hydroxides as activating agents showed the highest adsorption capacity, while the acidic activating agents showed the lowest adsorption capacity. This is because KOH activation significantly increases the surface area and porosity of the porous carbon, thus enhancing the adsorption capacity. In another study, Moura et al. [105] studied the potential of nanocarbons derived from PET waste by physical activation to remove CO2. The porous carbons mainly contain micropores and showed a high surface area, high pore volume, and a well-developed porous structure. Using a similar method, Parra et al. [106] used PET waste to convert the waste into valuable products using the pyrolysis process. The product obtained after the pyrolysis process is gas (58%), terephthalic acid (20%), and char (22%). The char produced from pyrolysis was used as an adsorbent. The highest surface area was found to be at 2468 m2/g, which was obtained at an optimum pyrolysis temperature of 925 °C and a flow rate of 10 mL/min. The highest adsorption capacity of 4.04 mmol/g was achieved at an optimum adsorption temperature of 25 °C. Kaur et al. [19] conducted a study to convert waste PET into carbonized char and then modified the surface properties of the material using KOH to enhance the CO2 adsorption capacity. The maximum adsorption capacity of 1.31 mmol/g was obtained at an optimum adsorption temperature of 30 °C and a CO2 concentration of 12.5%. The regeneration study showed that the modified adsorbent can be regenerated up to 4 cycles without reducing the adsorption capacity. The adsorption kinetics and the isotherm data follow the fractional-order kinetic model and Freundlich isotherm model. In addition, the negative values of enthalpy and Gibbs free energy suggest that these adsorption processes were exothermic and thus confirm the feasibility of the process and the spontaneous nature of the adsorption. Isosteric heat of adsorption (Qst) is an important element in thermodynamics and is used to measure the heat released upon the adsorption [90,107]. For the carbonized PET-KOH sample, the average Qst value obtained was at −12.08 kJ/mol, and the value tends to be more negative with changes in the temperature, demonstrating the exothermic nature of the adsorption process [90].

Table 8.
Summary of plastic waste as adsorbent for CO2 capture.
Yuan et al. [95] also used waste PET to convert the waste into char using the carbonization process to produce porous carbon material and then modified the surface properties of the porous carbon with a mixture of KOH and urea. The modified sample was used as an adsorbent to capture CO2. The highest adsorption capacity was achieved using modified carbonized PET with a mixture of KOH and urea due to the high oxygen content (18.80%) and high surface basicity that was determined using XPS analysis. The highest CO2 adsorption capacity of 4.58 mmol/g was obtained at an optimum pyrolysis temperature of 700 °C, an adsorption temperature of 25 °C, and 1 atmosphere of pressure. The Langmuir and pseudo second-order models demonstrated well-fitted relations with the experimental data and models. In a recent study, Yuan et al. [18] developed a porous carbon adsorbent derived from PET plastic bottles to capture CO2. They reported that a maximum CO2 adsorption capacity of 4.42 mol/kg was achieved at an adsorption temperature of 25 °C using a low-pressure adsorption system. The isotherm and kinetics data best fitted with the Langmuir isotherm and pseudo second-order kinetic models. The porous carbon has high adsorption performance due to the high surface area and pore volume as well as the high oxygen content confirmed by the XPS results. In addition, the authors suggested that the grand canonical Monte Carlo (GCMC) simulation is the best technique to determine and understand the effect of pore size on CO2 capture. Furthermore, a density-functional theory (DFT), which is a computational method, can be used to understand the in-depth adsorption energies for CO2 in each functional group [18]. Therefore, the relationship between pore size and interaction on the functional group are important properties to be studied to understand the CO2 adsorption performance.
5. Challenges and Future Prospective on Life Cycle Analysis
Based on a study of the literature, the pyrolysis process was chosen by most researchers due to its potential to convert plastic waste into valuable products such as liquid oil and solid char. However, there are several challenges or limitations to plastic waste pyrolysis and more research needs to be developed to solve this issue. The main challenge of the plastic waste pyrolysis process is selecting suitable feedstock. This is because not all types of plastic are suitable for pyrolysis. For example, as reported by Papari et al. [109], waste PVC produces a significant amount of benzoic acid and hydrochloric acid during the pyrolysis process, which can cause corrosion problems to the equipment. The pyrolysis process can generate high aromatic contents of oil, but some of the aromatic hydrocarbons are toxic and can have a negative impact on the environment and cause serious human health issues. In addition, liquid fuel produced from plastic pyrolysis may not be suitable for commercial application due to high sulfur content. The pyrolysis process also produces a solid by-product known as char. However, to get a high solid yield, activation temperature plays a key role in the pyrolysis process. An optimum activation temperature is needed to produce a high solid yield.
The outstanding results of adsorption capacity resulting from porous carbon derived from plastic indicates that porous carbon adsorbents have great potential to be used to capture CO2. In order to apply this adsorbent material in a large-scale application, there are several aspects that need to be considered, such as life cycle analysis or assessment (LCA), the impact of adsorbent (raw material), and the adsorption system on the environment and the economic analysis. LCA is regarded as an important tool to understand the environmental impact of materials, processes, economy, technology, and investment in appropriate new technologies [110]. This process involves comprehensive energy and material balance calculations at each stage of the process, such as the raw material, processing, manufacturing, product distribution, maintenance, and disposal or recycling stages. Besides that, sensitivity analysis is a significant tool in providing an in-depth analysis of the factor and selection of assumptions in the proposed LCA model. Therefore, more work is needed to understand the economic and environmental impacts of this technology. However, when it is expanded to a large-scale application, energy consumption should also be considered. The results of LCA can be helpful to produce an effective and sustainable process [111].
6. Conclusions
Higher demand in plastic production due to the ease of usage will lead to more plastic waste, which will have a negative impact on the environment. However, there are some methods for reducing the amount of plastic waste by converting the plastic into beneficial products such as through the pyrolysis process. The pyrolysis process will yield bio-oil, char, and gas as the products, and the percentage of the product is dependent on the type of plastic used, temperature, and other factors. Bio-oil product might replace fuel, while char will be converted into an adsorbent for CO2 capture. Char from the pyrolysis product shows a good characteristic of being adsorbent as it is highly porous and has a high oxygen content that helps in enhancing CO2 adsorption. This can be observed from the physicochemical properties of the porous carbon derived from plastic waste. This porous carbon produced from low-cost waste material showed high adsorption performance and an alternative option to reduce the plastic waste problem. From this review, several important outcomes obtained from char derived from plastic waste as a potential porous carbon to capture CO2 can be summarized as below:
- Converting plastic waste into carbon-based material is an alternative option to reduce the solid waste problem. Plastic waste is suitable to be converted into valuable products such as carbon-based material rather than being thrown away.
- This review addresses the important elements in the United Nations (UN) Sustainable Development Goal under the category of climate action of SDG 13 and SDG 12 aims for sustainable consumption and production.
- This review compiles the important information to manage plastic waste and control CO2 emission. This review can cater to the environmental issues as well as provide a long-term sustainability solution on solid waste management and CO2 emission.
- Converting plastic waste into char (porous carbon material) using the pyrolysis method shows high surface area, high pore volume, high oxygen content, and enhanced adsorption capacity. This adsorbent is suitable to be used to capture CO2.
Author Contributions
Writing—original draft preparation, F.H.; Review and editing, M.K.A.; Collecting data and editing, M.A.K.; Checking the final draft, U.F.M.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Sunway University Sdn Bhd, grant number GRTIN-IRG-33-2021. The APC was sponsored by Sunway University Sdn Bhd.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All numbers used in this article are appropriately referenced.
Acknowledgments
The author would like to thank Sunway University Sdn Bhd for the research funding provided (grant no. GRTIN-IRG-33-2021).
Conflicts of Interest
The authors declare no conflict of interest.
References
- IEA. After Steep Drop in Early 2020, Global Carbon Dioxide Emissions Have Rebounded Strongly. 2021. Available online: https://www.iea.org/news/after-steep-drop-in-early-2020-global-carbon-dioxide-emissions-have-rebounded-strongly (accessed on 1 August 2021).
- Daily CO2. Available online: https://www.co2.earth/daily-co2 (accessed on 12 October 2021).
- NOAA. Trends in Atmospheric Carbon Dioxide. Global Monitoring Laboratory Earth System Research Laboratories. National Oceanic & Atmospheric Administration (NOAA Research). 2021. Available online: https://gml.noaa.gov/ccgg/trends/ (accessed on 6 November 2021).
- Lisbona, P.; Bailera, M.; Peña, B.; Romeo, L.M. Chapter 22—Integration of CO2 capture and conversion. In Advances in Carbon Capture; Rahimpour, M.R., Farsi, M., Makarem, M.A., Eds.; Woodhead Publishing: Sawston, UK, 2020; pp. 503–522. [Google Scholar]
- Leung, D.Y.C.; Caramanna, G.; Maroto-Valer, M.M. An overview of current status of carbon dioxide capture and storage technologies. Renew. Sustain. Energy Rev. 2014, 39, 426–443. [Google Scholar] [CrossRef] [Green Version]
- Dissanayake, P.D.; You, S.; Igalavithana, A.D.; Xia, Y.; Bhatnagar, A.; Gupta, S.; Kua, H.W.; Kim, S.; Kwon, J.H.; Tsang, D.C.W.; et al. Biochar-based adsorbents for carbon dioxide capture: A critical review. Renew. Sustain. Energy Rev. 2020, 119, 109582. [Google Scholar] [CrossRef]
- Mallesh, D.; Anbarasan, J.; Mahesh Kumar, P.; Upendar, K.; Chandrashekar, P.; Rao, B.V.S.K.; Lingaiah, N. Synthesis, characterization of carbon adsorbents derived from waste biomass and its application to CO2 capture. Appl. Surf. Sci. 2020, 530, 147226. [Google Scholar] [CrossRef]
- Qasem, N.A.A.; Ben-Mansour, R. Adsorption breakthrough and cycling stability of carbon dioxide separation from CO2/N2/H2O mixture under ambient conditions using 13X and Mg-MOF-74. Appl. Energy 2018, 230, 1093–1107. [Google Scholar] [CrossRef]
- Davarpanah, E.; Armandi, M.; Hernández, S.; Fino, D.; Arletti, R.; Bensaid, S.; Piumetti, M. CO2 capture on natural zeolite clinoptilolite: Effect of temperature and role of the adsorption sites. J. Environ. Manag. 2020, 275, 111229. [Google Scholar] [CrossRef]
- Xu, C.; Yu, G.; Yuan, J.; Strømme, M.; Hedin, N. Microporous organic polymers as CO2 adsorbents: Advances and challenges. Mater. Today Adv. 2020, 6, 100052. [Google Scholar] [CrossRef]
- Zhao, P.; Yin, Y.; Cheng, W.; Xu, X.; Yang, D.; Yuan, W. Development of facile synthesized mesoporous carbon composite adsorbent for efficient CO2 capture. J. CO2 Util. 2021, 50, 101612. [Google Scholar] [CrossRef]
- Chen, C.; Bhattacharjee, S. Trimodal nanoporous silica as a support for amine-based CO2 adsorbents: Improvement in adsorption capacity and kinetics. Appl. Surf. Sci. 2017, 396, 1515–1519. [Google Scholar] [CrossRef]
- Rahimi, K.; Riahi, S.; Abbasi, M.; Fakhroueian, Z. Modification of multi-walled carbon nanotubes by 1,3-diaminopropane to increase CO2 adsorption capacity. J. Environ. Manag. 2019, 242, 81–89. [Google Scholar] [CrossRef]
- Kamran, U.; Park, S.J. Chemically modified carbonaceous adsorbents for enhanced CO2 capture: A review. J. Clean. Prod. 2021, 290, 125776. [Google Scholar] [CrossRef]
- Petrovic, B.; Gorbounov, M.; Masoudi Soltani, S. Influence of surface modification on selective CO2 adsorption: A technical review on mechanisms and methods. Microporous Mesoporous Mater. 2021, 312, 110751. [Google Scholar] [CrossRef]
- Machado, N.C.F.; de Jesus, L.A.M.; Pinto, P.S.; de Paula, F.G.F.; Alves, M.O.; Mendes, K.H.A.; Mambrini, R.V.; Barrreda, D.; Rocha, V.; Santamaría, R.; et al. Waste-polystyrene foams-derived magnetic carbon material for adsorption and redox supercapacitor applications. J. Clean. Prod. 2021, 313, 127903. [Google Scholar] [CrossRef]
- Ilyas, M.; Ahmad, W.; Khan, H. Utilization of activated carbon derived from waste plastic for decontamination of polycyclic aromatic hydrocarbons laden wastewater. Water Sci. Technol. 2021, 84, 609–631. [Google Scholar] [CrossRef]
- Yuan, X.; Lee, J.G.; Yun, H.; Deng, S.; Kim, Y.J.; Lee, J.E.; Kwak, S.K.; Lee, K.B. Solving two environmental issues simultaneously: Waste polyethylene terephthalate plastic bottle-derived microporous carbons for capturing CO2. Chem. Eng. J. 2020, 397, 125350. [Google Scholar] [CrossRef]
- Kaur, B.; Gupta, R.K.; Bhunia, H. Chemically activated nanoporous carbon adsorbents from waste plastic for CO2 capture: Breakthrough adsorption study. Microporous Mesoporous Mater. 2019, 282, 146–158. [Google Scholar] [CrossRef]
- Minakshi, M.; Meyrick, D.; Appadoo, D. Maricite (NaMn1/3Ni1/3Co1/3PO4)/activated carbon: Hybrid capacitor. Energy Fuels 2013, 27, 3516–3522. [Google Scholar] [CrossRef]
- Sajjadi, B.; Chen, W.Y.; Egiebor, N.O. A comprehensive review on physical activation of biochar for energy and environmental applications. Rev. Chem. Eng. 2019, 35, 735–776. [Google Scholar] [CrossRef]
- Wickramaarachchi, W.A.M.K.P.; Minakshi, M.; Gao, X.; Dabare, R.; Wong, K.W. Hierarchical porous carbon from mango seed husk for electro-chemical energy storage. Chem. Eng. J. Adv. 2021, 8, 100158. [Google Scholar] [CrossRef]
- Shrivastava, A. 7—Environmental aspects of plastics. In Introduction to Plastics Engineering; Shrivastava, A., Ed.; William Andrew Publishing: Burlington, MA, USA, 2018; pp. 207–232. [Google Scholar]
- Shrivastava, A. 3—Plastic properties and testing. In Introduction to Plastics Engineering; Shrivastava, A., Ed.; William Andrew Publishing: Burlington, MA, USA, 2018; pp. 49–110. [Google Scholar]
- Flores-Tlacuahuac, A.; Saldívar-Guerra, E.; Guerrero-Santos, R. Dynamic modelling, nonlinear parameter fitting and sensitivity analysis of a living free-radical polymerization reactor. In Computer Aided Chemical Engineering; Asprey, S.P., Macchietto, S., Eds.; Elsevier: Amsterdam, The Netherlands, 2003; pp. 21–39. [Google Scholar]
- Evode, N.; Qamar, S.A.; Bilal, M.; Barceló, D.; Iqbal, H.M.N. Plastic waste and its management strategies for environmental sustainability. Case Stud. Chem. Environ. Eng. 2021, 4, 100142. [Google Scholar] [CrossRef]
- Tuladhar, R.; Yin, S. 21—Sustainability of using recycled plastic fiber in concrete. In Use of Recycled Plastics in Eco-efficient Concrete; Pacheco-Torgal, F., Khatib, J., Colangelo, F., Tuladhar, R., Eds.; Woodhead Publishing: Burlington, MA, USA, 2019; pp. 441–460. [Google Scholar]
- UN. Our Planet Is Drowning in Plastic Pollution, UN Environment. 2018. Available online: https://www.unep.org/interactive/beat-plastic-pollution/ (accessed on 1 September 2021).
- Szaky, T. Outsmart Waste: The Modern Idea of Garbage and How to Think Our Way Out of It; Berrett-Koehler Publishers: Oakland, CA, USA, 2014; p. 168. [Google Scholar]
- Thiounn, T.; Smith, R.C. Advances and approaches for chemical recycling of plastic waste. J. Polym. Sci. 2020, 58, 1347–1364. [Google Scholar] [CrossRef] [Green Version]
- Williams, P.T.; Slaney, E. Analysis of products from the pyrolysis and liquefaction of single plastics and waste plastic mixtures. Resour. Conserv. Recycl. 2007, 51, 754–769. [Google Scholar] [CrossRef]
- Ellen MacArthur Foundation. The New Plastics Economy, Rethinking the Future of Plastics. Available online: http://www3.weforum.org/docs/WEF_The_New_Plastics_Economy.pdf (accessed on 1 September 2021).
- Kosior, E.; Crescenzi, I. Chapter 16—Solutions to the plastic waste problem on land and in the oceans. In Plastic Waste and Recycling; Letcher, T.M., Ed.; Academic Press: Cambridge, MA, USA, 2020; pp. 415–446. [Google Scholar]
- Asmuni, S.; Hussin, N.B.; Khalili, J.M.; Zain, Z.M. Public participation and effectiveness of the no plastic bag day program in Malaysia. Procedia Soc. Behav. Sci. 2015, 168, 328–340. [Google Scholar] [CrossRef] [Green Version]
- Merrington, A. 9—Recycling of Plastics. In Applied Plastics Engineering Handbook, 2nd ed.; Kutz, M., Ed.; William Andrew Publishing: Burlington, MA, USA, 2017; pp. 167–189. [Google Scholar]
- Bernardo, C.A.; Simões, C.L.; Pinto, L.M.C. Environmental and economic life cycle analysis of plastic waste management options: A review. AIP Conf. Proc. 2016, 1779, 140001. [Google Scholar]
- Khoo, H.H. LCA of plastic waste recovery into recycled materials, energy and fuels in Singapore. Resour. Conserv. Recycl. 2019, 145, 67–77. [Google Scholar] [CrossRef]
- Barbara, U.O.C.-S. Plastic’s Carbon Footprint: Researchers Conduct First Global Assessment of the Life Cycle Greenhouse Gas Emissions from Plastics; ScienceDaily: Rockville, MD, USA, 2019. [Google Scholar]
- Jayaraman, K.; Haron, H.; Sung, G.B.; Lin, S.K. Consumer reflections on the usage of plastic bags to parcel hot edible items: An empirical study in Malaysia. J. Clean. Prod. 2011, 19, 1527–1535. [Google Scholar] [CrossRef]
- Awoyera, P.O.; Adesina, A. Plastic wastes to construction products: Status, limitations and future perspective. Case Stud. Constr. Mater. 2020, 12, e00330. [Google Scholar] [CrossRef]
- Sharma, R. Plastics: Issues challenges and remediation. Int. J. Waste Resour. 2014. [Google Scholar] [CrossRef] [Green Version]
- Andrady, A.; Neal, M. Applications and societal benefits of plastics, philosophical transactions of the royal society of London. Ser. B Biol. Sci. 2009, 364, 1977–1984. [Google Scholar] [CrossRef]
- Wang, Z.; Burra, K.G.; Lei, T.; Gupta, A.K. Co-pyrolysis of waste plastic and solid biomass for synergistic production of biofuels and chemicals-A review. Prog. Energy Combust. Sci. 2021, 84, 100899. [Google Scholar] [CrossRef]
- Jouhara, H.; Ahmad, D.; van den Boogaert, I.; Katsou, E.; Simons, S.; Spencer, N. Pyrolysis of domestic based feedstock at temperatures up to 300 °C. Therm. Sci. Eng. Prog. 2018, 5, 117–143. [Google Scholar] [CrossRef]
- Zhou, H.; Meng, A.; Long, Y.; Li, Q.; Zhang, Y. An overview of characteristics of municipal solid waste fuel in China: Physical, chemical composition and heating value. Renew. Sustain. Energy Rev. 2014, 36, 107–122. [Google Scholar] [CrossRef]
- Anuar Sharuddin, S.D.; Abnisa, F.; Wan Daud, W.M.A.; Aroua, M.K. A review on pyrolysis of plastic waste. Energy Convers. Manag. 2016, 115, 308–326. [Google Scholar] [CrossRef]
- Abnisa, F.; Wan Daud, W.M.A. A review on co-pyrolysis of biomass: An optional technique to obtain a high-grade pyrolysis oil. Energy Convers. Manag. 2014, 87, 71–85. [Google Scholar] [CrossRef]
- Niaounakis, M. 4—Assessment. In Management of Marine Plastic Debris; Niaounakis, M., Ed.; William Andrew Publishing: Burlington, MA, USA, 2017; pp. 143–214. [Google Scholar]
- Meert, J.; Izzo, A.; Atkinson, J.D. Impact of plastic bag bans on retail return polyethylene film recycling contamination rates and speciation. Waste Manag. 2021, 135, 234–242. [Google Scholar] [CrossRef]
- Heikkinen, J.M.; Hordijk, J.C.; de Jong, W.; Spliethoff, H. Thermogravimetry as a tool to classify waste components to be used for energy generation. J. Anal. Appl. Pyrolysis 2004, 71, 883–900. [Google Scholar] [CrossRef]
- Park, S.S.; Seo, D.K.; Lee, S.H.; Yu, T.U.; Hwang, J. Study on pyrolysis characteristics of refuse plastic fuel using lab-scale tube furnace and thermogravimetric analysis reactor. J. Anal. Appl. Pyrolysis 2012, 97, 29–38. [Google Scholar] [CrossRef]
- Jung, S.H.; Cho, M.H.; Kang, B.S.; Kim, J.S. Pyrolysis of a fraction of waste polypropylene and polyethylene for the recovery of BTX aromatics using a fluidized bed reactor. Fuel Process. Technol. 2010, 91, 277–284. [Google Scholar] [CrossRef]
- Haddaway, N.R.; Macura, B.; Whaley, P.; Pullin, A.S. ROSES Reporting standards for systematic evidence syntheses: Pro forma, flow-diagram and descriptive summary of the plan and conduct of environmental systematic reviews and systematic maps. Environ. Evid. 2018, 7, 7. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Br. Med. J. 2009, 339, b2535. [Google Scholar] [CrossRef] [Green Version]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. J. Clin. Epidemiol. 2021, 134, 178–189. [Google Scholar] [CrossRef]
- Bujak, J.W. Thermal utilization (treatment) of plastic waste. Energy 2015, 90, 1468–1477. [Google Scholar] [CrossRef]
- Quesada, L.; Calero, M.; Martín-Lara, M.A.; Pérez, A.; Blázquez, G. Characterization of fuel produced by pyrolysis of plastic film obtained of municipal solid waste. Energy 2019, 186, 115874. [Google Scholar] [CrossRef]
- Scalenghe, R. Resource or waste? A perspective of plastics degradation in soil with a focus on end-of-life options. Heliyon 2018, 4, e00941. [Google Scholar] [CrossRef] [Green Version]
- Thahir, R.; Altway, A.; Susianto, S.R.J. Production of liquid fuel from plastic waste using integrated pyrolysis method with refinery distillation bubble cap plate column. Energy Rep. 2019, 5, 70–77. [Google Scholar] [CrossRef]
- Abdel-Shafy, H.I.; Mansour, M.S.M. Solid waste issue: Sources, composition, disposal, recycling, and valorization. Egypt. J. Pet. 2018, 27, 1275–1290. [Google Scholar] [CrossRef]
- Hannah, R.; Max, R. Plastic Pollution. 2018. Available online: https://ourworldindata.org/plastic-pollution (accessed on 10 September 2021).
- Al-Salem, S.M.; Lettieri, P.; Baeyens, J. The valorization of plastic solid waste (PSW) by primary to quaternary routes: From re-use to energy and chemicals. Prog. Energy Combust. Sci. 2010, 36, 103–129. [Google Scholar] [CrossRef]
- Singh, N.; Hui, D.; Singh, R.; Ahuja, I.P.S.; Feo, L.; Fraternali, F. Recycling of plastic solid waste: A state of art review and future applications. Compos. Part B: Eng. 2017, 115, 409–422. [Google Scholar] [CrossRef]
- Al-Salem, S.M.; Lettieri, P.; Baeyens, J. Recycling and recovery routes of plastic solid waste (PSW): A review. Waste Manag. 2009, 29, 2625–2643. [Google Scholar] [CrossRef] [PubMed]
- Awasthi, A.; Shivashankar, M.; Majumder, S. Plastic solid waste utilization technologies: A Review. IOP Conf. Ser. Mater. Sci. Eng. 2017, 263, 022024. [Google Scholar] [CrossRef]
- Tulashie, S.K.; Boadu, E.K.; Kotoka, F.; Mensah, D. Plastic wastes to pavement blocks: A significant alternative way to reducing plastic wastes generation and accumulation in Ghana. Constr. Build. Mater. 2020, 241, 118044. [Google Scholar] [CrossRef]
- Dillikannan, D.; Gopal, R.B.K.; Poures, M.; Sethuramasamyraja, B. Utilization of waste plastic oil in diesel engines: A review. Rev. Environ. Sci. Bio/Technol. 2019, 18, 681–697. [Google Scholar]
- Budsaereechai, S.; Hunt, A.; Ngernyen, Y. Catalytic pyrolysis of plastic waste for the production of liquid fuels for engines. RSC Adv. 2019, 9, 5844–5857. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Chen, X.; Jiang, Z.; Wen, X.; Mijowska, E.; Tang, T. Converting real-world mixed waste plastics into porous carbon nanosheets with excellent performance in the adsorption of an organic dye from wastewater. J. Mater. Chem. A: Mater. Energy Sustain. 2015, 3, 341–351. [Google Scholar]
- Mishra, R.K.; Maria, H.J.; Joseph, K.; Thomas, S. 1—Basic structural and properties relationship of recyclable microfibrillar composite materials from immiscible plastics blends: An introduction. In Micro and Nano Fibrillar Composites (MFCs and NFCs) from Polymer Blends; Mishra, R.K., Thomas, S., Kalarikkal, N., Eds.; Woodhead Publishing: Burlington, MA, USA, 2017; pp. 1–25. [Google Scholar]
- Parra, J.B.; Ania, C.O.; Arenillas, A.; Rubiera, F.; Pis, J.J. High value carbon materials from PET recycling. Appl. Surf. Sci. 2004, 238, 304–308. [Google Scholar] [CrossRef] [Green Version]
- Wankasi, D.; Dikio, E.D. Polyvinyl chloride waste as an adsorbent for the sorption of from aqueous solution. J. Chem. 2014, 2014, 817527. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Pap, S.; Taggart, M.A.; Boyd, K.G.; James, N.A.; Gibb, S.W. A review of the potential utilisation of plastic waste as adsorbent for removal of hazardous priority contaminants from aqueous environments. Environ. Pollut. 2020, 258, 113698. [Google Scholar] [CrossRef]
- Yuliusman, Y.; Nasruddin, N.; Sanal, A.; Bernama, A.; Haris, F.; Ramadhan, I.T. Preparation of activated carbon from waste plastics polyethylene terephthalate as adsorbent in natural gas storage. IOP Conf. Ser. Mater. Sci. Eng. 2017, 176, 012055. [Google Scholar] [CrossRef] [Green Version]
- Utetiwabo, W.; Yang, L.; Tufail, M.K.; Zhou, L.; Chen, R.; Lian, Y.; Yang, W. Electrode materials derived from plastic wastes and other industrial wastes for supercapacitors. Chin. Chem. Lett. 2020, 31, 1474–1489. [Google Scholar] [CrossRef]
- Gong, J.; Michalkiewicz, B.; Chen, X.; Mijowska, E.; Liu, J.; Jiang, Z.; Wen, X.; Tang, T. Sustainable conversion of mixed plastics into porous carbon nanosheets with high performances in uptake of carbon dioxide and storage of hydrogen. ACS Sustain. Chem. Eng. 2014, 2, 2837–2844. [Google Scholar] [CrossRef]
- Singh, T.S.; Verma, T.N.; Singh, H.N. A lab scale waste to energy conversion study for pyrolysis of plastic with and without catalyst: Engine emissions testing study. Fuel 2020, 277, 118176. [Google Scholar] [CrossRef]
- Roberts, K.G.; Gloy, B.A.; Joseph, S.; Scott, N.R.; Lehmann, J. Life cycle assessment of biochar systems: Estimating the energetic, economic, and climate change potential. Environ. Sci. Technol. 2010, 44, 827–833. [Google Scholar] [CrossRef]
- Scott, D.S.; Czernik, S.R.; Piskorz, J.; Radlein, D.S.A.G. Fast pyrolysis of plastic waste. Energy Fuels 1990, 4, 407–411. [Google Scholar] [CrossRef]
- Miskolczi, N.; Angyal, A.; Bartha, L.; Valkai, I. Fuels by pyrolysis of waste plastics from agricultural and packaging sectors in a pilot scale reactor. Fuel Process. Technol. 2009, 90, 1032–1040. [Google Scholar] [CrossRef]
- Fakhrhoseini, S.; Dastanian, M. Predicting pyrolysis products of PE, PP, and PET using NRTL activity coefficient model. J. Chem. 2013, 2013, 487676. [Google Scholar] [CrossRef]
- Caldwell, S.J.; Al-Duri, B.; Sun, N.; Sun, C.G.; Snape, C.E.; Li, K.; Wood, J. Carbon dioxide separation from nitrogen/hydrogen mixtures over activated carbon beads: Adsorption isotherms and breakthrough studies. Energy Fuels 2015, 29, 3796–3807. [Google Scholar] [CrossRef] [Green Version]
- Aishwarya, K.N.; Sindhu, N. Microwave assisted pyrolysis of plastic waste. Procedia Technol. 2016, 25, 990–997. [Google Scholar] [CrossRef] [Green Version]
- Al-Salem, S.M.; Antelava, A.; Constantinou, A.; Manos, G.; Dutta, A. A review on thermal and catalytic pyrolysis of plastic solid waste (PSW). J. Environ. Manag. 2017, 197, 177–198. [Google Scholar] [CrossRef]
- Lehmann, J.; Gaunt, J.; Rondon, M. Bio-char sequestration in terrestrial ecosystems—A review. Mitig. Adapt. Strateg. Glob. Chang. 2006, 11, 403–427. [Google Scholar] [CrossRef]
- Yang, J.; Yue, L.; Hu, X.; Wang, L.; Zhao, Y.; Lin, Y.; Sun, Y.; DaCosta, H.; Guo, L. Efficient CO2 capture by porous carbons derived from coconut shell. Energy Fuels 2017, 31, 4287–4293. [Google Scholar] [CrossRef]
- Kunwar, B.; Cheng, H.N.; Chandrashekaran, S.R.; Sharma, B.K. Plastics to fuel: A review. Renew. Sustain. Energy Rev. 2016, 54, 421–428. [Google Scholar] [CrossRef]
- Uemichi, Y.; Nakamura, J.; Itoh, T.; Sugioka, M.; Garforth, A.A.; Dwyer, J. Conversion of Polyethylene into Gasoline-Range Fuels by Two-Stage Catalytic Degradation using silica−alumina and HZSM-5 zeolite. Ind. Eng. Chem. Res. 1999, 38, 385–390. [Google Scholar] [CrossRef]
- Rouquerol, J.; Rouquerol, F.; Llewellyn, P.; Maurin, G.; Sing, K. Adsorption by Powders and Porous Solids. Principles, Methodology and Applications; Academic Press: London, UK, 2013. [Google Scholar]
- Cen, Q.; Fang, M.; Wang, T.; Majchrzak-Kucęba, I.; Wawrzyńczak, D.; Luo, Z. Thermodynamics and regeneration studies of CO2 adsorption on activated carbon. Greenh. Gases: Sci. Technol. 2016, 6, 787–796. [Google Scholar] [CrossRef]
- Wang, J.; Kaskel, S. KOH activation of carbon-based materials for energy storage. J. Mater. Chem. 2012, 22, 23710–23725. [Google Scholar] [CrossRef]
- Liu, W.J.; Jiang, H.; Yu, H.Q. Development of biochar-based functional materials: Toward a sustainable platform carbon material. Chem. Rev. 2015, 115, 12251–12285. [Google Scholar] [CrossRef] [PubMed]
- Zdravkov, B.D.; Čermák, J.J.; Šefara, M.; Janků, J. Pore classification in the characterization of porous materials: A perspective. Cent. Eur. J. Chem. 2007, 5, 385–395. [Google Scholar]
- Huang, J.; Best, S. 1—Ceramic biomaterials for tissue engineering. In Tissue Engineering Using Ceramics and Polymers, 2nd ed.; Boccaccini, A.R., Ma, P.X., Eds.; Woodhead Publishing: Burlington, MA, USA, 2014; pp. 3–34. [Google Scholar]
- Yuan, X.; Li, S.; Jeon, S.; Deng, S.; Zhao, L.; Lee, K.B. Valorization of waste polyethylene terephthalate plastic into N-doped microporous carbon for CO2 capture through a one-pot synthesis. J. Hazard. Mater. 2020, 399, 123010. [Google Scholar] [CrossRef]
- Kaur, B.; Singh, J.; Gupta, R.K.; Bhunia, H. Porous carbons derived from polyethylene terephthalate (PET) waste for CO2 capture studies. J. Environ. Manag. 2019, 242, 68–80. [Google Scholar] [CrossRef] [PubMed]
- Kaur, B.; Gupta, R.K.; Bhunia, H. CO2 capture on activated carbon from PET (polyethylene terephthalate) waste: Kinetics and modeling studies. Chem. Eng. Commun. 2019, 207, 1031–1047. [Google Scholar] [CrossRef]
- Buah, W.K.; Cunliffe, A.M.; Williams, P.T. Characterization of products from the pyrolysis of municipal solid waste. Process. Saf. Environ. Prot. 2007, 85, 450–457. [Google Scholar]
- Jamradloedluk, J.; Lertsatitthanakorn, C. Characterization and utilization of char derived from fast pyrolysis of plastic wastes. Procedia Eng. 2014, 69, 1437–1442. [Google Scholar]
- Tiwari, D.; Goel, C.; Bhunia, H.; Bajpai, P.K. Melamine-formaldehyde derived porous carbons for adsorption of CO2 capture. J. Environ. Manag. 2017, 197, 415–427. [Google Scholar] [CrossRef]
- Georgiadis, A.G.; Charisiou, N.D.; Goula, M.A. Removal of hydrogen sulfide from various industrial gases: A review of the most promising adsorbing materials. Catalysts 2020, 10, 521. [Google Scholar] [CrossRef]
- Petrovic, B.; Gorbounov, M.; Lahiri, A.; Soltani, S.M. Biomass combustion fly ash-derived nanoporous zeolites for post-combustion carbon capture. In Proceedings of the 2021 IEEE 21st International Conference on Nanotechnology (NANO), Montreal, QC, Canada, 28–30 July 2021; pp. 233–236. [Google Scholar]
- Arenillas, A.; Rubiera, F.; Parra, J.B.; Ania, C.O.; Pis, J.J. Surface modification of low-cost carbons for their application in the environmental protection. Appl. Surf. Sci. 2005, 252, 619–624. [Google Scholar] [CrossRef] [Green Version]
- Adibfar, M.; Kaghazchi, T.; Asasian, N.; Soleimani, M. Conversion of Poly (Ethylene Terephthalate) waste into activated carbon: Chemical activation and characterization. Chem. Eng. Technol. 2014, 37, 979–986. [Google Scholar] [CrossRef]
- Moura, P.A.S.; Vilarrasa-Garcia, E.; Maia, D.A.S.; Bastos-Neto, M.; Ania, C.O.; Parra, J.B.; Azevedo, D.S.C. Assessing the potential of nanoporous carbon adsorbents from polyethylene terephthalate (PET) to separate CO2 from flue gas. Adsorption 2018, 24, 279–291. [Google Scholar] [CrossRef]
- Parra, J.B.; Ania, C.O.; Arenillas, A.; Pis, J.J. Textural characterisation of activated carbons obtained from poly (ethylene terephthalate) by carbon dioxide activation. In Studies in Surface Science and Catalysis; Rodriguez-Reinoso, F., McEnaney, B., Rouquerol, J., Unger, K., Eds.; Elsevier: Amsterdam, The Netherlands, 2002; pp. 537–543. [Google Scholar]
- Mukherjee, S.; Kumar, A.; Zaworotko, M.J. 2—Metal-organic framework-based carbon capture and purification technologies for clean environment. In Metal-Organic Frameworks (MOFs) for Environmental Applications; Ghosh, S.K., Ed.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 5–61. [Google Scholar]
- Fu, Z.; Mohamed, I.M.A.; Li, J.; Liu, C. Novel adsorbents derived from recycled waste polystyrene via cross-linking reaction for enhanced adsorption capacity and separation selectivity of CO2. J. Taiwan Inst. Chem. Eng. 2019, 97, 381–388. [Google Scholar] [CrossRef]
- Papari, S.; Bamdad, H.; Berruti, F. Pyrolytic conversion of plastic waste to value-added products and fuels: A review. Materials 2021, 14, 2586. [Google Scholar] [CrossRef] [PubMed]
- Rouwenhorst, K.; Valera-Medina, A.; Ramirez, A.D. Environmental life cycle assessment of ammonia-based electricity. Energies 2021, 14, 6721. [Google Scholar]
- Miandad, R.; Rehan, M.; Barakat, M.A.; Aburiazaiza, A.S.; Khan, H.; Ismail, I.M.I.; Dhavamani, J.; Gardy, J.; Hassanpour, A.; Nizami, A.S. Catalytic Pyrolysis of Plastic Waste: Moving Toward Pyrolysis Based Biorefineries. Front. Energy Res. 2019, 7, 27. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).






