Transforming Plastic Waste into Porous Carbon for Capturing Carbon Dioxide: A Review
Abstract
:1. Introduction
2. Overview of Plastic
2.1. Types of Plastic
- Thermoplastics are a class of polymer that can be softened and melted by the application of heat and can be processed in a heat-softened state. They can be remolded and recycled without negatively affecting the material’s physical properties. The examples include polyethylene (PE), polypropylene (PP), polytetrafluoroethylene (Teflon), polyethylene terephthalate (PET), polyamide (PA), polyvinyl chloride (PVC) and polystyrene (PS) (Table 2).
- Thermoset plastics comprise polymers that are cross-linked together to form an irreversible chemical bond. The cross-linking process makes thermosets ideal for high-heat applications such as epoxy resins, polyurethane (PU), polyester resins, and Bakelite.
| Types of Synthetic Polymers | Abbreviation | Advantages | Application |
|---|---|---|---|
| Polyethylene terephthalate | PET or PETE | Hard and flexible Absorbs odours and flavours from foods and drinks | Beverage bottles, food packaging, carpet fibre, electrical parts, and films |
| High density polyethylene | HDPE | Rigid Not transmit any chemical into foods and drinks | Grocery bag, harder bottles, piping, toys, window shades and board for building |
| Polyvinyl chloride | PVC | Flame resistance Flexibility Lightweight | Blister wrap, window and door frames, blood bags, medical tubing, drainage pipes, electrice wire and cable. |
| Low density polyethylene | LDPE | Durable and flexible | Packaging films, soft bottles, soft tubing, carrier bag, molded materials of laboratory equipment. |
| Polypropylene | PP | Resistant to high temperature Hard and flexible | Microwaveable meal trays, disposable cups and bowls, drinking bottles and straws. |
| Polystyrene | PS | Hard and brittle | Food containers, yoghurt pots, protective packaging, electrical appliances and building insulation. |
2.2. Proximate Analysis of Plastic
3. Search Strategy and Methodology
4. Method for Management and Reducing Plastic Waste
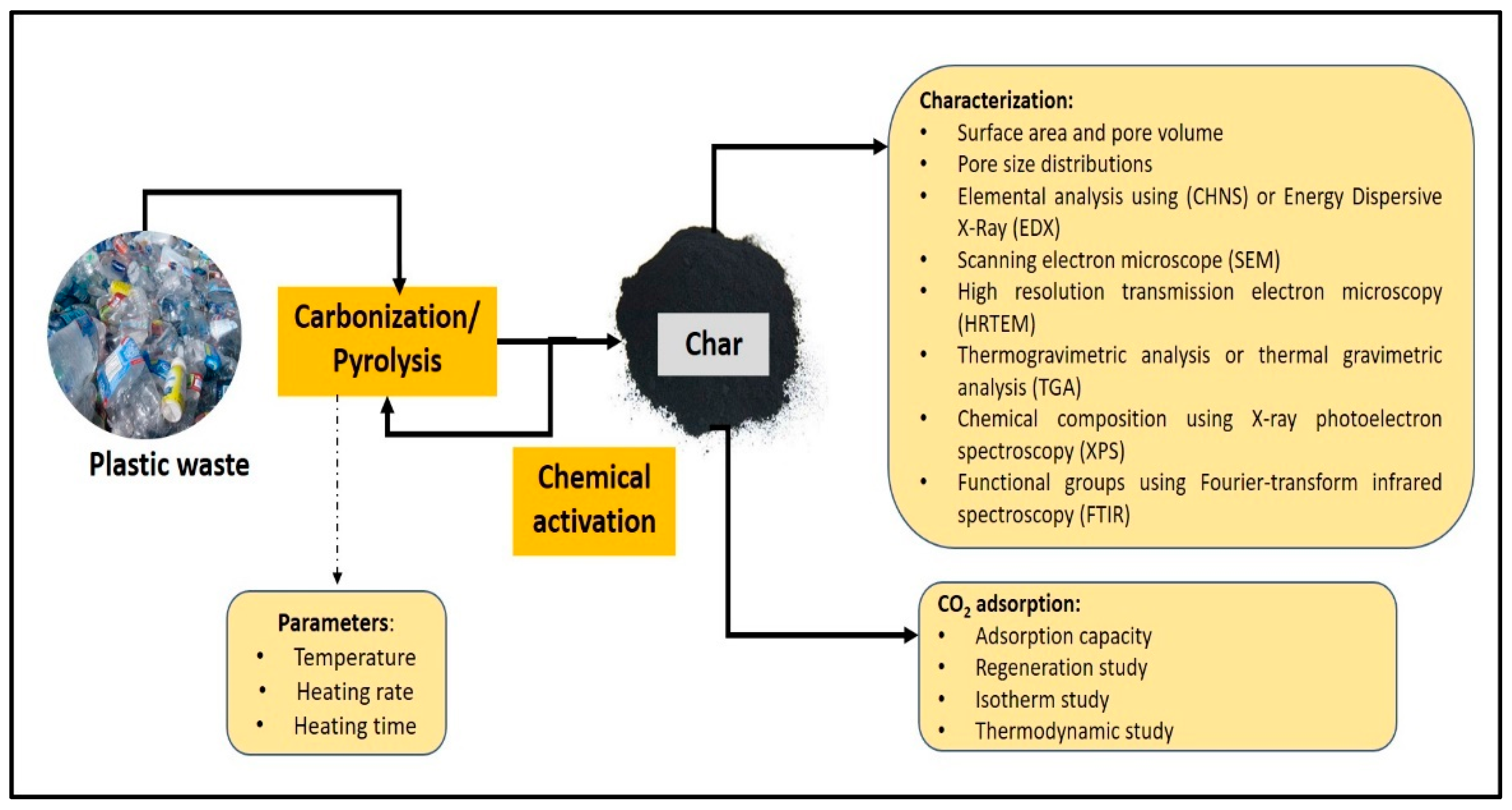
4.1. Conversion of Plastic Waste into a Carbon-Based Material
4.2. Pyrolysis of Plastic Waste
4.3. Modification of Plastic Wastes for CO2 Capture
4.3.1. Characterization of Char
BET Surface Area and Porosity
- (a)
- The redox reactions between various potassium species with carbon (in Equations (5)–(9)). This process or reaction is responsible for creating a network of porosity.
- (b)
- The formation of H2O and CO2 (in Equations (1)–(7)) positively contributes to the development of porosity by physical activation.
- (c)
- The intermediate potassium oxide species such as K2CO3 or K2O are reduced by carbon to produce metallic K at temperatures over 700 °C (in Equations (8) and (9)). The metallic K intercalates into the carbon surface, thereby expanding the lattice. This led to the formation of a larger pore surface. After activation, the sample underwent a washing process to remove the intercalated metallic K and other K compounds. Carbon with high porosity and a large surface area was obtained. The reaction mechanism is highly dependent on the activation temperature, activating agent ratio, and the type of feedstock (type of plastic waste) [15].
X-ray Photoelectron Spectroscopy (XPS)
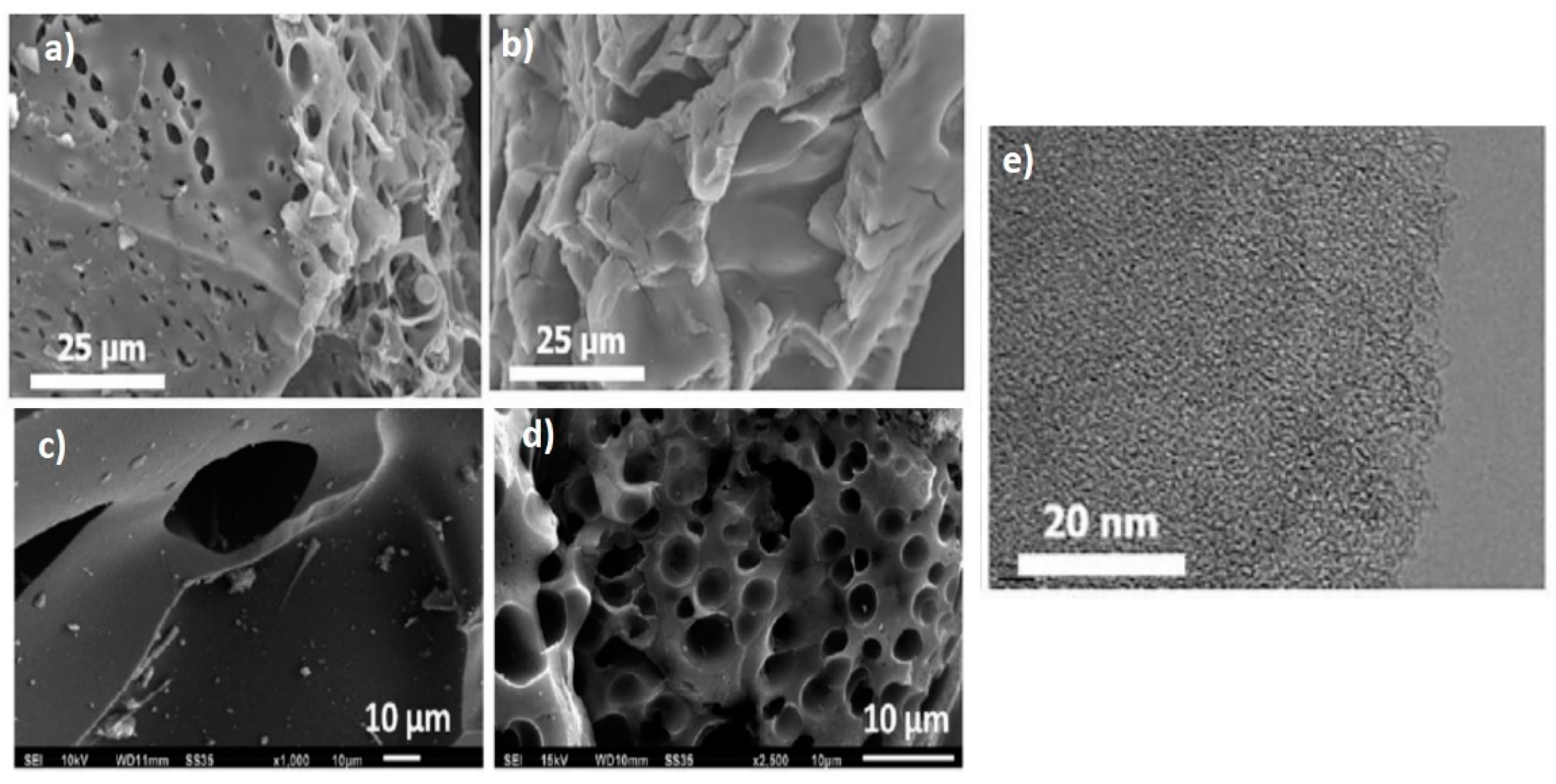
Surface Morphology
Surface Functional Groups
Elemental Composition
4.4. CO2 Adsorption Performance
5. Challenges and Future Prospective on Life Cycle Analysis
6. Conclusions
- Converting plastic waste into carbon-based material is an alternative option to reduce the solid waste problem. Plastic waste is suitable to be converted into valuable products such as carbon-based material rather than being thrown away.
- This review addresses the important elements in the United Nations (UN) Sustainable Development Goal under the category of climate action of SDG 13 and SDG 12 aims for sustainable consumption and production.
- This review compiles the important information to manage plastic waste and control CO2 emission. This review can cater to the environmental issues as well as provide a long-term sustainability solution on solid waste management and CO2 emission.
- Converting plastic waste into char (porous carbon material) using the pyrolysis method shows high surface area, high pore volume, high oxygen content, and enhanced adsorption capacity. This adsorbent is suitable to be used to capture CO2.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- IEA. After Steep Drop in Early 2020, Global Carbon Dioxide Emissions Have Rebounded Strongly. 2021. Available online: https://www.iea.org/news/after-steep-drop-in-early-2020-global-carbon-dioxide-emissions-have-rebounded-strongly (accessed on 1 August 2021).
- Daily CO2. Available online: https://www.co2.earth/daily-co2 (accessed on 12 October 2021).
- NOAA. Trends in Atmospheric Carbon Dioxide. Global Monitoring Laboratory Earth System Research Laboratories. National Oceanic & Atmospheric Administration (NOAA Research). 2021. Available online: https://gml.noaa.gov/ccgg/trends/ (accessed on 6 November 2021).
- Lisbona, P.; Bailera, M.; Peña, B.; Romeo, L.M. Chapter 22—Integration of CO2 capture and conversion. In Advances in Carbon Capture; Rahimpour, M.R., Farsi, M., Makarem, M.A., Eds.; Woodhead Publishing: Sawston, UK, 2020; pp. 503–522. [Google Scholar]
- Leung, D.Y.C.; Caramanna, G.; Maroto-Valer, M.M. An overview of current status of carbon dioxide capture and storage technologies. Renew. Sustain. Energy Rev. 2014, 39, 426–443. [Google Scholar] [CrossRef] [Green Version]
- Dissanayake, P.D.; You, S.; Igalavithana, A.D.; Xia, Y.; Bhatnagar, A.; Gupta, S.; Kua, H.W.; Kim, S.; Kwon, J.H.; Tsang, D.C.W.; et al. Biochar-based adsorbents for carbon dioxide capture: A critical review. Renew. Sustain. Energy Rev. 2020, 119, 109582. [Google Scholar] [CrossRef]
- Mallesh, D.; Anbarasan, J.; Mahesh Kumar, P.; Upendar, K.; Chandrashekar, P.; Rao, B.V.S.K.; Lingaiah, N. Synthesis, characterization of carbon adsorbents derived from waste biomass and its application to CO2 capture. Appl. Surf. Sci. 2020, 530, 147226. [Google Scholar] [CrossRef]
- Qasem, N.A.A.; Ben-Mansour, R. Adsorption breakthrough and cycling stability of carbon dioxide separation from CO2/N2/H2O mixture under ambient conditions using 13X and Mg-MOF-74. Appl. Energy 2018, 230, 1093–1107. [Google Scholar] [CrossRef]
- Davarpanah, E.; Armandi, M.; Hernández, S.; Fino, D.; Arletti, R.; Bensaid, S.; Piumetti, M. CO2 capture on natural zeolite clinoptilolite: Effect of temperature and role of the adsorption sites. J. Environ. Manag. 2020, 275, 111229. [Google Scholar] [CrossRef]
- Xu, C.; Yu, G.; Yuan, J.; Strømme, M.; Hedin, N. Microporous organic polymers as CO2 adsorbents: Advances and challenges. Mater. Today Adv. 2020, 6, 100052. [Google Scholar] [CrossRef]
- Zhao, P.; Yin, Y.; Cheng, W.; Xu, X.; Yang, D.; Yuan, W. Development of facile synthesized mesoporous carbon composite adsorbent for efficient CO2 capture. J. CO2 Util. 2021, 50, 101612. [Google Scholar] [CrossRef]
- Chen, C.; Bhattacharjee, S. Trimodal nanoporous silica as a support for amine-based CO2 adsorbents: Improvement in adsorption capacity and kinetics. Appl. Surf. Sci. 2017, 396, 1515–1519. [Google Scholar] [CrossRef]
- Rahimi, K.; Riahi, S.; Abbasi, M.; Fakhroueian, Z. Modification of multi-walled carbon nanotubes by 1,3-diaminopropane to increase CO2 adsorption capacity. J. Environ. Manag. 2019, 242, 81–89. [Google Scholar] [CrossRef]
- Kamran, U.; Park, S.J. Chemically modified carbonaceous adsorbents for enhanced CO2 capture: A review. J. Clean. Prod. 2021, 290, 125776. [Google Scholar] [CrossRef]
- Petrovic, B.; Gorbounov, M.; Masoudi Soltani, S. Influence of surface modification on selective CO2 adsorption: A technical review on mechanisms and methods. Microporous Mesoporous Mater. 2021, 312, 110751. [Google Scholar] [CrossRef]
- Machado, N.C.F.; de Jesus, L.A.M.; Pinto, P.S.; de Paula, F.G.F.; Alves, M.O.; Mendes, K.H.A.; Mambrini, R.V.; Barrreda, D.; Rocha, V.; Santamaría, R.; et al. Waste-polystyrene foams-derived magnetic carbon material for adsorption and redox supercapacitor applications. J. Clean. Prod. 2021, 313, 127903. [Google Scholar] [CrossRef]
- Ilyas, M.; Ahmad, W.; Khan, H. Utilization of activated carbon derived from waste plastic for decontamination of polycyclic aromatic hydrocarbons laden wastewater. Water Sci. Technol. 2021, 84, 609–631. [Google Scholar] [CrossRef]
- Yuan, X.; Lee, J.G.; Yun, H.; Deng, S.; Kim, Y.J.; Lee, J.E.; Kwak, S.K.; Lee, K.B. Solving two environmental issues simultaneously: Waste polyethylene terephthalate plastic bottle-derived microporous carbons for capturing CO2. Chem. Eng. J. 2020, 397, 125350. [Google Scholar] [CrossRef]
- Kaur, B.; Gupta, R.K.; Bhunia, H. Chemically activated nanoporous carbon adsorbents from waste plastic for CO2 capture: Breakthrough adsorption study. Microporous Mesoporous Mater. 2019, 282, 146–158. [Google Scholar] [CrossRef]
- Minakshi, M.; Meyrick, D.; Appadoo, D. Maricite (NaMn1/3Ni1/3Co1/3PO4)/activated carbon: Hybrid capacitor. Energy Fuels 2013, 27, 3516–3522. [Google Scholar] [CrossRef]
- Sajjadi, B.; Chen, W.Y.; Egiebor, N.O. A comprehensive review on physical activation of biochar for energy and environmental applications. Rev. Chem. Eng. 2019, 35, 735–776. [Google Scholar] [CrossRef]
- Wickramaarachchi, W.A.M.K.P.; Minakshi, M.; Gao, X.; Dabare, R.; Wong, K.W. Hierarchical porous carbon from mango seed husk for electro-chemical energy storage. Chem. Eng. J. Adv. 2021, 8, 100158. [Google Scholar] [CrossRef]
- Shrivastava, A. 7—Environmental aspects of plastics. In Introduction to Plastics Engineering; Shrivastava, A., Ed.; William Andrew Publishing: Burlington, MA, USA, 2018; pp. 207–232. [Google Scholar]
- Shrivastava, A. 3—Plastic properties and testing. In Introduction to Plastics Engineering; Shrivastava, A., Ed.; William Andrew Publishing: Burlington, MA, USA, 2018; pp. 49–110. [Google Scholar]
- Flores-Tlacuahuac, A.; Saldívar-Guerra, E.; Guerrero-Santos, R. Dynamic modelling, nonlinear parameter fitting and sensitivity analysis of a living free-radical polymerization reactor. In Computer Aided Chemical Engineering; Asprey, S.P., Macchietto, S., Eds.; Elsevier: Amsterdam, The Netherlands, 2003; pp. 21–39. [Google Scholar]
- Evode, N.; Qamar, S.A.; Bilal, M.; Barceló, D.; Iqbal, H.M.N. Plastic waste and its management strategies for environmental sustainability. Case Stud. Chem. Environ. Eng. 2021, 4, 100142. [Google Scholar] [CrossRef]
- Tuladhar, R.; Yin, S. 21—Sustainability of using recycled plastic fiber in concrete. In Use of Recycled Plastics in Eco-efficient Concrete; Pacheco-Torgal, F., Khatib, J., Colangelo, F., Tuladhar, R., Eds.; Woodhead Publishing: Burlington, MA, USA, 2019; pp. 441–460. [Google Scholar]
- UN. Our Planet Is Drowning in Plastic Pollution, UN Environment. 2018. Available online: https://www.unep.org/interactive/beat-plastic-pollution/ (accessed on 1 September 2021).
- Szaky, T. Outsmart Waste: The Modern Idea of Garbage and How to Think Our Way Out of It; Berrett-Koehler Publishers: Oakland, CA, USA, 2014; p. 168. [Google Scholar]
- Thiounn, T.; Smith, R.C. Advances and approaches for chemical recycling of plastic waste. J. Polym. Sci. 2020, 58, 1347–1364. [Google Scholar] [CrossRef] [Green Version]
- Williams, P.T.; Slaney, E. Analysis of products from the pyrolysis and liquefaction of single plastics and waste plastic mixtures. Resour. Conserv. Recycl. 2007, 51, 754–769. [Google Scholar] [CrossRef]
- Ellen MacArthur Foundation. The New Plastics Economy, Rethinking the Future of Plastics. Available online: http://www3.weforum.org/docs/WEF_The_New_Plastics_Economy.pdf (accessed on 1 September 2021).
- Kosior, E.; Crescenzi, I. Chapter 16—Solutions to the plastic waste problem on land and in the oceans. In Plastic Waste and Recycling; Letcher, T.M., Ed.; Academic Press: Cambridge, MA, USA, 2020; pp. 415–446. [Google Scholar]
- Asmuni, S.; Hussin, N.B.; Khalili, J.M.; Zain, Z.M. Public participation and effectiveness of the no plastic bag day program in Malaysia. Procedia Soc. Behav. Sci. 2015, 168, 328–340. [Google Scholar] [CrossRef] [Green Version]
- Merrington, A. 9—Recycling of Plastics. In Applied Plastics Engineering Handbook, 2nd ed.; Kutz, M., Ed.; William Andrew Publishing: Burlington, MA, USA, 2017; pp. 167–189. [Google Scholar]
- Bernardo, C.A.; Simões, C.L.; Pinto, L.M.C. Environmental and economic life cycle analysis of plastic waste management options: A review. AIP Conf. Proc. 2016, 1779, 140001. [Google Scholar]
- Khoo, H.H. LCA of plastic waste recovery into recycled materials, energy and fuels in Singapore. Resour. Conserv. Recycl. 2019, 145, 67–77. [Google Scholar] [CrossRef]
- Barbara, U.O.C.-S. Plastic’s Carbon Footprint: Researchers Conduct First Global Assessment of the Life Cycle Greenhouse Gas Emissions from Plastics; ScienceDaily: Rockville, MD, USA, 2019. [Google Scholar]
- Jayaraman, K.; Haron, H.; Sung, G.B.; Lin, S.K. Consumer reflections on the usage of plastic bags to parcel hot edible items: An empirical study in Malaysia. J. Clean. Prod. 2011, 19, 1527–1535. [Google Scholar] [CrossRef]
- Awoyera, P.O.; Adesina, A. Plastic wastes to construction products: Status, limitations and future perspective. Case Stud. Constr. Mater. 2020, 12, e00330. [Google Scholar] [CrossRef]
- Sharma, R. Plastics: Issues challenges and remediation. Int. J. Waste Resour. 2014. [Google Scholar] [CrossRef] [Green Version]
- Andrady, A.; Neal, M. Applications and societal benefits of plastics, philosophical transactions of the royal society of London. Ser. B Biol. Sci. 2009, 364, 1977–1984. [Google Scholar] [CrossRef]
- Wang, Z.; Burra, K.G.; Lei, T.; Gupta, A.K. Co-pyrolysis of waste plastic and solid biomass for synergistic production of biofuels and chemicals-A review. Prog. Energy Combust. Sci. 2021, 84, 100899. [Google Scholar] [CrossRef]
- Jouhara, H.; Ahmad, D.; van den Boogaert, I.; Katsou, E.; Simons, S.; Spencer, N. Pyrolysis of domestic based feedstock at temperatures up to 300 °C. Therm. Sci. Eng. Prog. 2018, 5, 117–143. [Google Scholar] [CrossRef]
- Zhou, H.; Meng, A.; Long, Y.; Li, Q.; Zhang, Y. An overview of characteristics of municipal solid waste fuel in China: Physical, chemical composition and heating value. Renew. Sustain. Energy Rev. 2014, 36, 107–122. [Google Scholar] [CrossRef]
- Anuar Sharuddin, S.D.; Abnisa, F.; Wan Daud, W.M.A.; Aroua, M.K. A review on pyrolysis of plastic waste. Energy Convers. Manag. 2016, 115, 308–326. [Google Scholar] [CrossRef]
- Abnisa, F.; Wan Daud, W.M.A. A review on co-pyrolysis of biomass: An optional technique to obtain a high-grade pyrolysis oil. Energy Convers. Manag. 2014, 87, 71–85. [Google Scholar] [CrossRef]
- Niaounakis, M. 4—Assessment. In Management of Marine Plastic Debris; Niaounakis, M., Ed.; William Andrew Publishing: Burlington, MA, USA, 2017; pp. 143–214. [Google Scholar]
- Meert, J.; Izzo, A.; Atkinson, J.D. Impact of plastic bag bans on retail return polyethylene film recycling contamination rates and speciation. Waste Manag. 2021, 135, 234–242. [Google Scholar] [CrossRef]
- Heikkinen, J.M.; Hordijk, J.C.; de Jong, W.; Spliethoff, H. Thermogravimetry as a tool to classify waste components to be used for energy generation. J. Anal. Appl. Pyrolysis 2004, 71, 883–900. [Google Scholar] [CrossRef]
- Park, S.S.; Seo, D.K.; Lee, S.H.; Yu, T.U.; Hwang, J. Study on pyrolysis characteristics of refuse plastic fuel using lab-scale tube furnace and thermogravimetric analysis reactor. J. Anal. Appl. Pyrolysis 2012, 97, 29–38. [Google Scholar] [CrossRef]
- Jung, S.H.; Cho, M.H.; Kang, B.S.; Kim, J.S. Pyrolysis of a fraction of waste polypropylene and polyethylene for the recovery of BTX aromatics using a fluidized bed reactor. Fuel Process. Technol. 2010, 91, 277–284. [Google Scholar] [CrossRef]
- Haddaway, N.R.; Macura, B.; Whaley, P.; Pullin, A.S. ROSES Reporting standards for systematic evidence syntheses: Pro forma, flow-diagram and descriptive summary of the plan and conduct of environmental systematic reviews and systematic maps. Environ. Evid. 2018, 7, 7. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Br. Med. J. 2009, 339, b2535. [Google Scholar] [CrossRef] [Green Version]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. J. Clin. Epidemiol. 2021, 134, 178–189. [Google Scholar] [CrossRef]
- Bujak, J.W. Thermal utilization (treatment) of plastic waste. Energy 2015, 90, 1468–1477. [Google Scholar] [CrossRef]
- Quesada, L.; Calero, M.; Martín-Lara, M.A.; Pérez, A.; Blázquez, G. Characterization of fuel produced by pyrolysis of plastic film obtained of municipal solid waste. Energy 2019, 186, 115874. [Google Scholar] [CrossRef]
- Scalenghe, R. Resource or waste? A perspective of plastics degradation in soil with a focus on end-of-life options. Heliyon 2018, 4, e00941. [Google Scholar] [CrossRef] [Green Version]
- Thahir, R.; Altway, A.; Susianto, S.R.J. Production of liquid fuel from plastic waste using integrated pyrolysis method with refinery distillation bubble cap plate column. Energy Rep. 2019, 5, 70–77. [Google Scholar] [CrossRef]
- Abdel-Shafy, H.I.; Mansour, M.S.M. Solid waste issue: Sources, composition, disposal, recycling, and valorization. Egypt. J. Pet. 2018, 27, 1275–1290. [Google Scholar] [CrossRef]
- Hannah, R.; Max, R. Plastic Pollution. 2018. Available online: https://ourworldindata.org/plastic-pollution (accessed on 10 September 2021).
- Al-Salem, S.M.; Lettieri, P.; Baeyens, J. The valorization of plastic solid waste (PSW) by primary to quaternary routes: From re-use to energy and chemicals. Prog. Energy Combust. Sci. 2010, 36, 103–129. [Google Scholar] [CrossRef]
- Singh, N.; Hui, D.; Singh, R.; Ahuja, I.P.S.; Feo, L.; Fraternali, F. Recycling of plastic solid waste: A state of art review and future applications. Compos. Part B: Eng. 2017, 115, 409–422. [Google Scholar] [CrossRef]
- Al-Salem, S.M.; Lettieri, P.; Baeyens, J. Recycling and recovery routes of plastic solid waste (PSW): A review. Waste Manag. 2009, 29, 2625–2643. [Google Scholar] [CrossRef] [PubMed]
- Awasthi, A.; Shivashankar, M.; Majumder, S. Plastic solid waste utilization technologies: A Review. IOP Conf. Ser. Mater. Sci. Eng. 2017, 263, 022024. [Google Scholar] [CrossRef]
- Tulashie, S.K.; Boadu, E.K.; Kotoka, F.; Mensah, D. Plastic wastes to pavement blocks: A significant alternative way to reducing plastic wastes generation and accumulation in Ghana. Constr. Build. Mater. 2020, 241, 118044. [Google Scholar] [CrossRef]
- Dillikannan, D.; Gopal, R.B.K.; Poures, M.; Sethuramasamyraja, B. Utilization of waste plastic oil in diesel engines: A review. Rev. Environ. Sci. Bio/Technol. 2019, 18, 681–697. [Google Scholar]
- Budsaereechai, S.; Hunt, A.; Ngernyen, Y. Catalytic pyrolysis of plastic waste for the production of liquid fuels for engines. RSC Adv. 2019, 9, 5844–5857. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Chen, X.; Jiang, Z.; Wen, X.; Mijowska, E.; Tang, T. Converting real-world mixed waste plastics into porous carbon nanosheets with excellent performance in the adsorption of an organic dye from wastewater. J. Mater. Chem. A: Mater. Energy Sustain. 2015, 3, 341–351. [Google Scholar]
- Mishra, R.K.; Maria, H.J.; Joseph, K.; Thomas, S. 1—Basic structural and properties relationship of recyclable microfibrillar composite materials from immiscible plastics blends: An introduction. In Micro and Nano Fibrillar Composites (MFCs and NFCs) from Polymer Blends; Mishra, R.K., Thomas, S., Kalarikkal, N., Eds.; Woodhead Publishing: Burlington, MA, USA, 2017; pp. 1–25. [Google Scholar]
- Parra, J.B.; Ania, C.O.; Arenillas, A.; Rubiera, F.; Pis, J.J. High value carbon materials from PET recycling. Appl. Surf. Sci. 2004, 238, 304–308. [Google Scholar] [CrossRef] [Green Version]
- Wankasi, D.; Dikio, E.D. Polyvinyl chloride waste as an adsorbent for the sorption of from aqueous solution. J. Chem. 2014, 2014, 817527. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Pap, S.; Taggart, M.A.; Boyd, K.G.; James, N.A.; Gibb, S.W. A review of the potential utilisation of plastic waste as adsorbent for removal of hazardous priority contaminants from aqueous environments. Environ. Pollut. 2020, 258, 113698. [Google Scholar] [CrossRef]
- Yuliusman, Y.; Nasruddin, N.; Sanal, A.; Bernama, A.; Haris, F.; Ramadhan, I.T. Preparation of activated carbon from waste plastics polyethylene terephthalate as adsorbent in natural gas storage. IOP Conf. Ser. Mater. Sci. Eng. 2017, 176, 012055. [Google Scholar] [CrossRef] [Green Version]
- Utetiwabo, W.; Yang, L.; Tufail, M.K.; Zhou, L.; Chen, R.; Lian, Y.; Yang, W. Electrode materials derived from plastic wastes and other industrial wastes for supercapacitors. Chin. Chem. Lett. 2020, 31, 1474–1489. [Google Scholar] [CrossRef]
- Gong, J.; Michalkiewicz, B.; Chen, X.; Mijowska, E.; Liu, J.; Jiang, Z.; Wen, X.; Tang, T. Sustainable conversion of mixed plastics into porous carbon nanosheets with high performances in uptake of carbon dioxide and storage of hydrogen. ACS Sustain. Chem. Eng. 2014, 2, 2837–2844. [Google Scholar] [CrossRef]
- Singh, T.S.; Verma, T.N.; Singh, H.N. A lab scale waste to energy conversion study for pyrolysis of plastic with and without catalyst: Engine emissions testing study. Fuel 2020, 277, 118176. [Google Scholar] [CrossRef]
- Roberts, K.G.; Gloy, B.A.; Joseph, S.; Scott, N.R.; Lehmann, J. Life cycle assessment of biochar systems: Estimating the energetic, economic, and climate change potential. Environ. Sci. Technol. 2010, 44, 827–833. [Google Scholar] [CrossRef]
- Scott, D.S.; Czernik, S.R.; Piskorz, J.; Radlein, D.S.A.G. Fast pyrolysis of plastic waste. Energy Fuels 1990, 4, 407–411. [Google Scholar] [CrossRef]
- Miskolczi, N.; Angyal, A.; Bartha, L.; Valkai, I. Fuels by pyrolysis of waste plastics from agricultural and packaging sectors in a pilot scale reactor. Fuel Process. Technol. 2009, 90, 1032–1040. [Google Scholar] [CrossRef]
- Fakhrhoseini, S.; Dastanian, M. Predicting pyrolysis products of PE, PP, and PET using NRTL activity coefficient model. J. Chem. 2013, 2013, 487676. [Google Scholar] [CrossRef]
- Caldwell, S.J.; Al-Duri, B.; Sun, N.; Sun, C.G.; Snape, C.E.; Li, K.; Wood, J. Carbon dioxide separation from nitrogen/hydrogen mixtures over activated carbon beads: Adsorption isotherms and breakthrough studies. Energy Fuels 2015, 29, 3796–3807. [Google Scholar] [CrossRef] [Green Version]
- Aishwarya, K.N.; Sindhu, N. Microwave assisted pyrolysis of plastic waste. Procedia Technol. 2016, 25, 990–997. [Google Scholar] [CrossRef] [Green Version]
- Al-Salem, S.M.; Antelava, A.; Constantinou, A.; Manos, G.; Dutta, A. A review on thermal and catalytic pyrolysis of plastic solid waste (PSW). J. Environ. Manag. 2017, 197, 177–198. [Google Scholar] [CrossRef]
- Lehmann, J.; Gaunt, J.; Rondon, M. Bio-char sequestration in terrestrial ecosystems—A review. Mitig. Adapt. Strateg. Glob. Chang. 2006, 11, 403–427. [Google Scholar] [CrossRef]
- Yang, J.; Yue, L.; Hu, X.; Wang, L.; Zhao, Y.; Lin, Y.; Sun, Y.; DaCosta, H.; Guo, L. Efficient CO2 capture by porous carbons derived from coconut shell. Energy Fuels 2017, 31, 4287–4293. [Google Scholar] [CrossRef]
- Kunwar, B.; Cheng, H.N.; Chandrashekaran, S.R.; Sharma, B.K. Plastics to fuel: A review. Renew. Sustain. Energy Rev. 2016, 54, 421–428. [Google Scholar] [CrossRef]
- Uemichi, Y.; Nakamura, J.; Itoh, T.; Sugioka, M.; Garforth, A.A.; Dwyer, J. Conversion of Polyethylene into Gasoline-Range Fuels by Two-Stage Catalytic Degradation using silica−alumina and HZSM-5 zeolite. Ind. Eng. Chem. Res. 1999, 38, 385–390. [Google Scholar] [CrossRef]
- Rouquerol, J.; Rouquerol, F.; Llewellyn, P.; Maurin, G.; Sing, K. Adsorption by Powders and Porous Solids. Principles, Methodology and Applications; Academic Press: London, UK, 2013. [Google Scholar]
- Cen, Q.; Fang, M.; Wang, T.; Majchrzak-Kucęba, I.; Wawrzyńczak, D.; Luo, Z. Thermodynamics and regeneration studies of CO2 adsorption on activated carbon. Greenh. Gases: Sci. Technol. 2016, 6, 787–796. [Google Scholar] [CrossRef]
- Wang, J.; Kaskel, S. KOH activation of carbon-based materials for energy storage. J. Mater. Chem. 2012, 22, 23710–23725. [Google Scholar] [CrossRef]
- Liu, W.J.; Jiang, H.; Yu, H.Q. Development of biochar-based functional materials: Toward a sustainable platform carbon material. Chem. Rev. 2015, 115, 12251–12285. [Google Scholar] [CrossRef] [PubMed]
- Zdravkov, B.D.; Čermák, J.J.; Šefara, M.; Janků, J. Pore classification in the characterization of porous materials: A perspective. Cent. Eur. J. Chem. 2007, 5, 385–395. [Google Scholar]
- Huang, J.; Best, S. 1—Ceramic biomaterials for tissue engineering. In Tissue Engineering Using Ceramics and Polymers, 2nd ed.; Boccaccini, A.R., Ma, P.X., Eds.; Woodhead Publishing: Burlington, MA, USA, 2014; pp. 3–34. [Google Scholar]
- Yuan, X.; Li, S.; Jeon, S.; Deng, S.; Zhao, L.; Lee, K.B. Valorization of waste polyethylene terephthalate plastic into N-doped microporous carbon for CO2 capture through a one-pot synthesis. J. Hazard. Mater. 2020, 399, 123010. [Google Scholar] [CrossRef]
- Kaur, B.; Singh, J.; Gupta, R.K.; Bhunia, H. Porous carbons derived from polyethylene terephthalate (PET) waste for CO2 capture studies. J. Environ. Manag. 2019, 242, 68–80. [Google Scholar] [CrossRef] [PubMed]
- Kaur, B.; Gupta, R.K.; Bhunia, H. CO2 capture on activated carbon from PET (polyethylene terephthalate) waste: Kinetics and modeling studies. Chem. Eng. Commun. 2019, 207, 1031–1047. [Google Scholar] [CrossRef]
- Buah, W.K.; Cunliffe, A.M.; Williams, P.T. Characterization of products from the pyrolysis of municipal solid waste. Process. Saf. Environ. Prot. 2007, 85, 450–457. [Google Scholar]
- Jamradloedluk, J.; Lertsatitthanakorn, C. Characterization and utilization of char derived from fast pyrolysis of plastic wastes. Procedia Eng. 2014, 69, 1437–1442. [Google Scholar]
- Tiwari, D.; Goel, C.; Bhunia, H.; Bajpai, P.K. Melamine-formaldehyde derived porous carbons for adsorption of CO2 capture. J. Environ. Manag. 2017, 197, 415–427. [Google Scholar] [CrossRef]
- Georgiadis, A.G.; Charisiou, N.D.; Goula, M.A. Removal of hydrogen sulfide from various industrial gases: A review of the most promising adsorbing materials. Catalysts 2020, 10, 521. [Google Scholar] [CrossRef]
- Petrovic, B.; Gorbounov, M.; Lahiri, A.; Soltani, S.M. Biomass combustion fly ash-derived nanoporous zeolites for post-combustion carbon capture. In Proceedings of the 2021 IEEE 21st International Conference on Nanotechnology (NANO), Montreal, QC, Canada, 28–30 July 2021; pp. 233–236. [Google Scholar]
- Arenillas, A.; Rubiera, F.; Parra, J.B.; Ania, C.O.; Pis, J.J. Surface modification of low-cost carbons for their application in the environmental protection. Appl. Surf. Sci. 2005, 252, 619–624. [Google Scholar] [CrossRef] [Green Version]
- Adibfar, M.; Kaghazchi, T.; Asasian, N.; Soleimani, M. Conversion of Poly (Ethylene Terephthalate) waste into activated carbon: Chemical activation and characterization. Chem. Eng. Technol. 2014, 37, 979–986. [Google Scholar] [CrossRef]
- Moura, P.A.S.; Vilarrasa-Garcia, E.; Maia, D.A.S.; Bastos-Neto, M.; Ania, C.O.; Parra, J.B.; Azevedo, D.S.C. Assessing the potential of nanoporous carbon adsorbents from polyethylene terephthalate (PET) to separate CO2 from flue gas. Adsorption 2018, 24, 279–291. [Google Scholar] [CrossRef]
- Parra, J.B.; Ania, C.O.; Arenillas, A.; Pis, J.J. Textural characterisation of activated carbons obtained from poly (ethylene terephthalate) by carbon dioxide activation. In Studies in Surface Science and Catalysis; Rodriguez-Reinoso, F., McEnaney, B., Rouquerol, J., Unger, K., Eds.; Elsevier: Amsterdam, The Netherlands, 2002; pp. 537–543. [Google Scholar]
- Mukherjee, S.; Kumar, A.; Zaworotko, M.J. 2—Metal-organic framework-based carbon capture and purification technologies for clean environment. In Metal-Organic Frameworks (MOFs) for Environmental Applications; Ghosh, S.K., Ed.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 5–61. [Google Scholar]
- Fu, Z.; Mohamed, I.M.A.; Li, J.; Liu, C. Novel adsorbents derived from recycled waste polystyrene via cross-linking reaction for enhanced adsorption capacity and separation selectivity of CO2. J. Taiwan Inst. Chem. Eng. 2019, 97, 381–388. [Google Scholar] [CrossRef]
- Papari, S.; Bamdad, H.; Berruti, F. Pyrolytic conversion of plastic waste to value-added products and fuels: A review. Materials 2021, 14, 2586. [Google Scholar] [CrossRef] [PubMed]
- Rouwenhorst, K.; Valera-Medina, A.; Ramirez, A.D. Environmental life cycle assessment of ammonia-based electricity. Energies 2021, 14, 6721. [Google Scholar]
- Miandad, R.; Rehan, M.; Barakat, M.A.; Aburiazaiza, A.S.; Khan, H.; Ismail, I.M.I.; Dhavamani, J.; Gardy, J.; Hassanpour, A.; Nizami, A.S. Catalytic Pyrolysis of Plastic Waste: Moving Toward Pyrolysis Based Biorefineries. Front. Energy Res. 2019, 7, 27. [Google Scholar] [CrossRef] [Green Version]
| Month | Global CO2 Concentration (Year, 2020) | Global CO2 Concentration (Year, 2021) |
|---|---|---|
| January | 412.43 | 414.77 |
| February | 412.95 | 415.27 |
| March | 413.44 | 415.60 |
| April | 413.86 | 415.92 |
| May | 413.81 | 416.11 |
| June | 412.88 | 415.35 |
| July | 411.17 | 416.96 |
| August | 409.73 | 414.77 |
| September | 410.00 | 413.30 |
| October | 411.66 | 413.93 |
| November | 413.25 | 414.26 |
| General Properties | Plastic Coding Marks | Moisture (wt%) | Fixed Carbon (wt%) | Volatile (wt%) | Ash (wt%) | Recyclable |
|---|---|---|---|---|---|---|
| Type of Synthetic Polymer | ||||||
| Polyethylene terephthalate (PETE) |  | 0.61 | 13.17 | 86.83 | 0.00 | Yes, can be recycled |
| High density polyethylene (HDPE) |  | 0.00 | 0.03 | 98.57 | 1.40 | Yes, can be recycled |
| Polyvinyl chloride (PVC) |  | 0.74 | 5.19 | 94.82 | 0.00 | Not recommended |
| Low density polyethylene (LDPE) |  | 0.30 | 0.00 | 99.70 | 0.00 | Not recommended |
| Polypropylene (PP) |  | 0.15 | 1.22 | 95.08 | 3.55 | Not recommended |
| Polystyrene (PS) |  | 0.30 | 0.20 | 99.50 | 0.00 | Not recommended |
| Polyethylene Acrylonitrile Polybutylene terephthalate (PBT) |  | 0.10 0.16 | 0.04 2.88 | 98.87 97.12 | 0.99 0.00 | Not recommended |
| Type of Plastic | Reactor | Pyrolysis Temperature (°C) | Catalyst | Crude Oil(wt%) | Solid Residue (wt%) | Gas (wt%) | Reference |
|---|---|---|---|---|---|---|---|
| PE | Parr mini bench top | 500 | None | 93 | 0 | 7 | [31,87] |
| PP | 95 | 5 | 5 | ||||
| PS | 71 | 27 | 2 | ||||
| PET | 15 | 53 | 32 | ||||
| Mixed | 90 | 5 | 5 | ||||
| PE | Activated carbon bed | 515–795 | None | 88–96 | 5.5 | 2 | [79,87] |
| LDPE | Fixed-bed tubular flow reactor | 425 | HZSM-5 SiO2-Al2O3 | [87,88] | |||
| HDPE | Continuous reactor | 520 | HZSM-5 | [80,87] | |||
| PET | Fixed bed | 500 | - | 23.1 | 0 | 76.9 | [46] |
| References | Type of Plastic | Activating Agent | Optimum Pyrolysis Temperature | Surface Area (m2/g) | Total Pore Volume (cm3/g) | Micropore Volume (cm3/g) | Regeneration and Cyclic Stability |
|---|---|---|---|---|---|---|---|
| [56] | PET | KOH | 700 | 1690 | 0.83 | 0.78 | 4 cycles |
| [71] | PET | KOH | 700 | 1812 | 0.75 | 0.71 | 10 cycles |
| [81] | PET | Mixture of KOH and urea | 700 | 1165 | 0.469 | 0.460 | 6 cycles |
| Functional Groups | Ketone (O1 Peak) | Carbonyl (O2 Peak) | Hydroxyl (O3 Peak) | Carboxyl (O4 Peak) | References |
|---|---|---|---|---|---|
| Binding Energy (eV) | 530.37 | 531.14 | 532.29 | 533.79 | [19] |
| 530.4 | 532.1 | [95] | |||
| 530.6 | 532.4 | 534.4 | [18] |
| References | Type of Plastic | Pyrolysis Temperature | C | H | O | N | S | Ash |
|---|---|---|---|---|---|---|---|---|
| [19] | PET | 700 | 65.10 | 0.57 | 34.33 | - | - | - |
| [18] | PET | 700 | 65.10 | 0.57 | 34.33 | - | - | - |
| [95] | PET | 700 | 77.97 | - | 18.80 | 3.23 | - | - |
| Type of Plastic | Activating Agent | Characterization | Surface Area, m2/g | CO2 Adsorption Capacity, mmol/ g | Optimum Operating Condition | References |
|---|---|---|---|---|---|---|
| Polyethylene terephthalate (PET) | KOH | CHN, FTIR, XRD, SEM, HRTEM, BET and XPS techniques | 1690 | 1.31 | Temperature, 30 °C and 12.5% CO2 concentration | [19] |
| PET | KOH or NaOH | XRD, SEM, HRTEM, BET and XPS techniques | 1812 | 4.42 | Temperature, 25 °C | [18] |
| PET | KOH | BET, XPS, FTIR, and EDX analysis | 1690 | 1.35 | Temperature, 30 °C and 12.5% CO2 concentration | [95] |
| PET | KOH | BET, XPS, SEM, HRTEM, TPD and CHN analysis | 1690 | 2.31 | Temperature, 30 °C and 12.5% CO2 concentration | [96] |
| WEPS (waste expanded polystyrene) | - | BET, XPS, SEM, HRTEM, TPD and CHN analysis | 777 | 2.52 | Temperature, 30 °C | [108] |
| PET | KOH | BET, XPS, SEM, HRTEM, FTIR analysis | 1165 | 4.58 | Temperature, 25 °C | [106] |
| PET | KOH | BET, XPS, HRTEM, FTIR analysis | - | 1.25 | Temperature, 25 °C | [103] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hussin, F.; Aroua, M.K.; Kassim, M.A.; Md. Ali, U.F. Transforming Plastic Waste into Porous Carbon for Capturing Carbon Dioxide: A Review. Energies 2021, 14, 8421. https://doi.org/10.3390/en14248421
Hussin F, Aroua MK, Kassim MA, Md. Ali UF. Transforming Plastic Waste into Porous Carbon for Capturing Carbon Dioxide: A Review. Energies. 2021; 14(24):8421. https://doi.org/10.3390/en14248421
Chicago/Turabian StyleHussin, Farihahusnah, Mohamed Kheireddine Aroua, Mohd Azlan Kassim, and Umi Fazara Md. Ali. 2021. "Transforming Plastic Waste into Porous Carbon for Capturing Carbon Dioxide: A Review" Energies 14, no. 24: 8421. https://doi.org/10.3390/en14248421
APA StyleHussin, F., Aroua, M. K., Kassim, M. A., & Md. Ali, U. F. (2021). Transforming Plastic Waste into Porous Carbon for Capturing Carbon Dioxide: A Review. Energies, 14(24), 8421. https://doi.org/10.3390/en14248421







