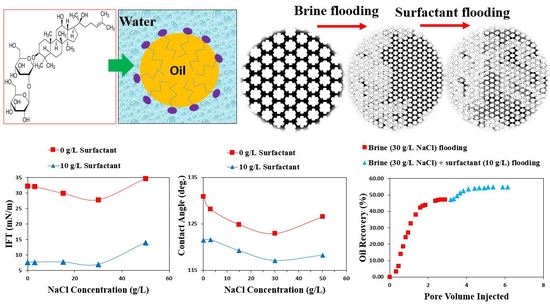
A Systematic Study to Assess Displacement Performance of a Naturally-Derived Surfactant in Flow Porous Systems
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Fourier-Transform Infrared Spectroscopy (FT-IR)
2.2.2. Performance Evaluation of the Surfactant
IFT Measurements
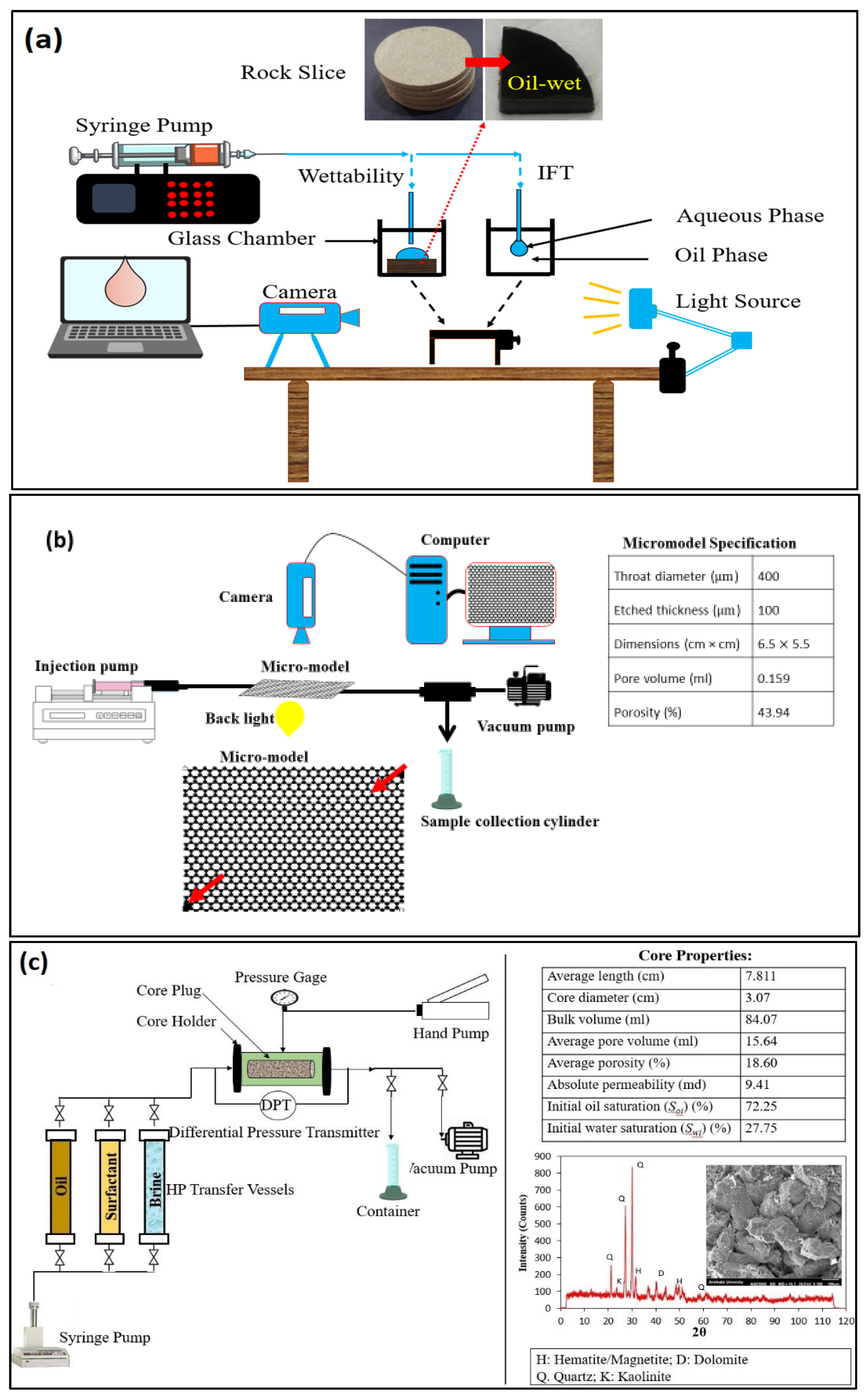
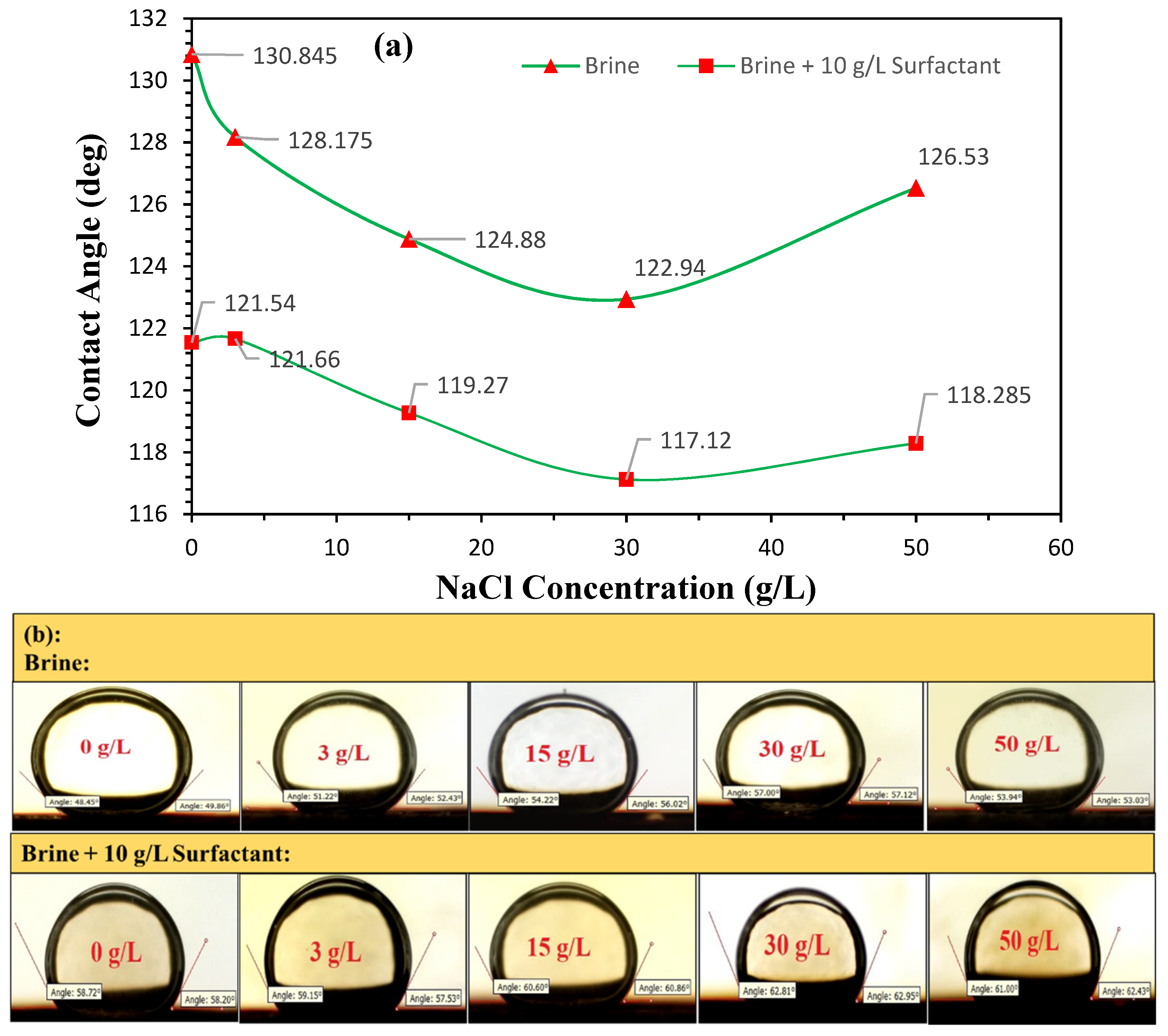
Sessile Drop Technique
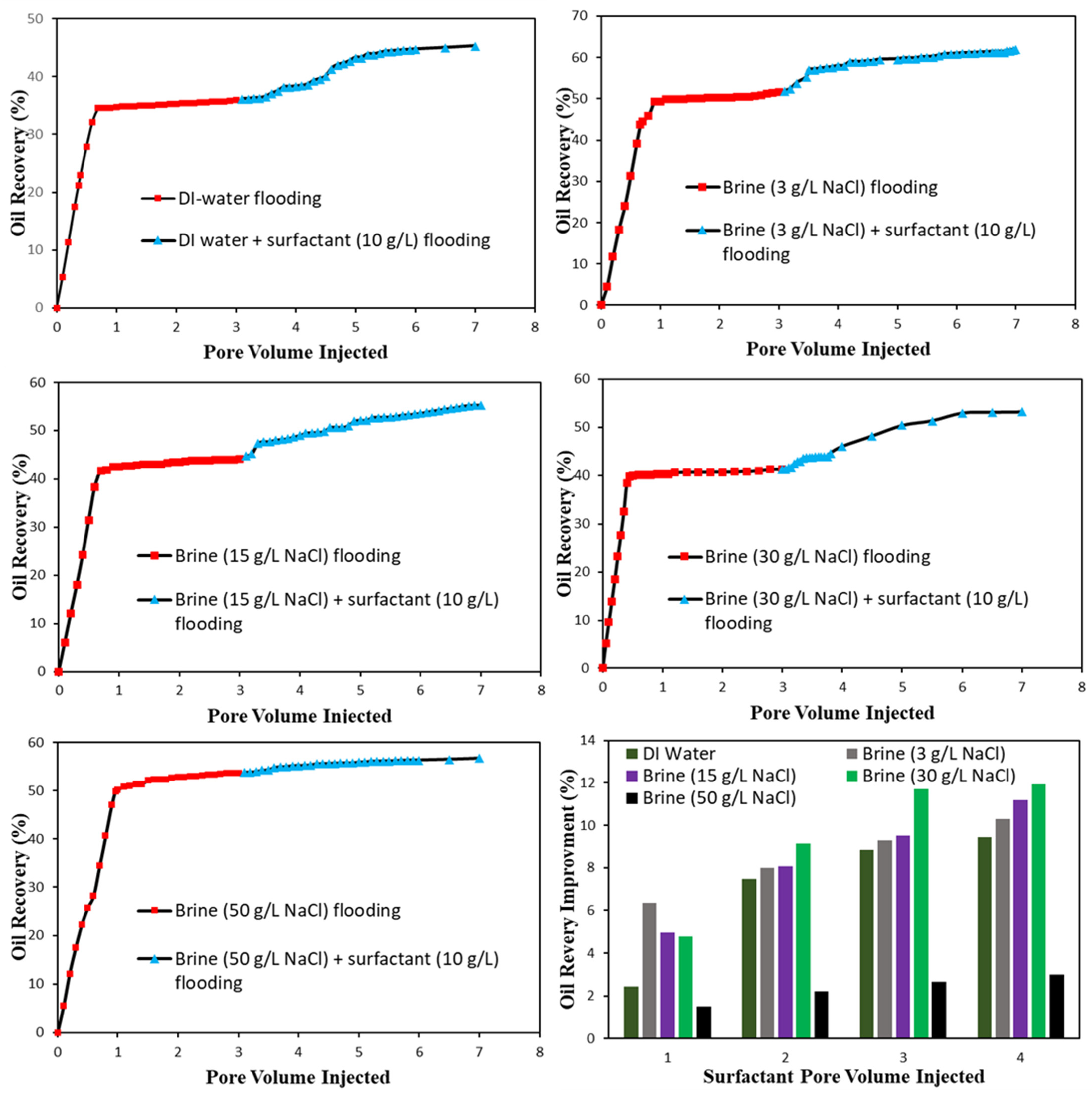
Micromodel Flooding Experiments
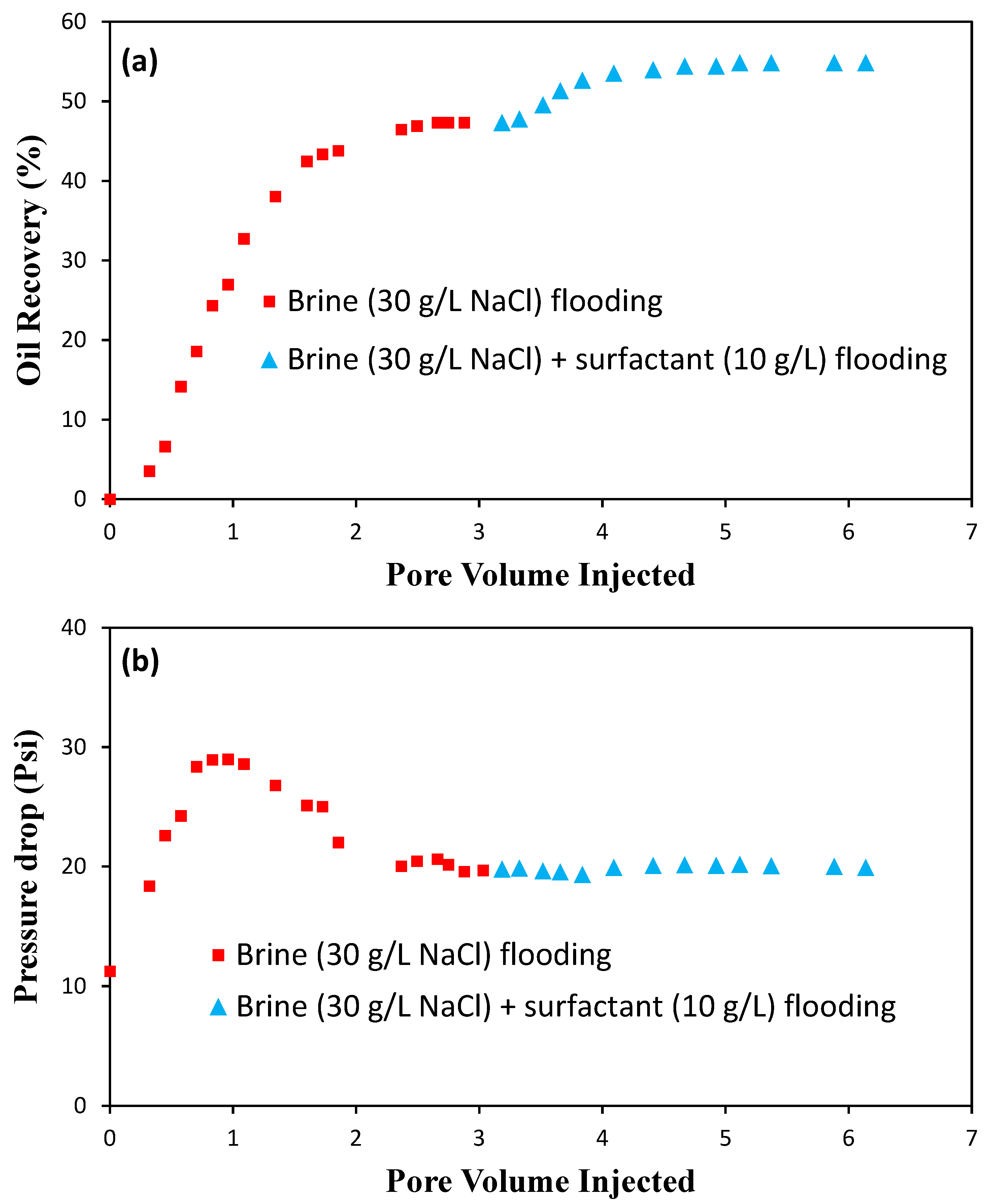
Core-Flood Experiments
- Core cleaning: The core is washed by the Soxhlet extractor using methanol for 48 h to remove the salt contamination. It is then placed in the oven at 80 °C for 24 h and permitted to cool down under ambient conditions. Then, the core dimensions are measured.
- Brine saturation: The core plug is placed in the core holder and vacuumed for about 5 h. In the next step, it is completely saturated with NaCl solution of 30 g/L for about 2 h at a constant injection pressure of 500 psi. In this step, the core bulk volume and the volume of the injected brine are obtained to calculate the effective porosity of the core sample. After achieving saturation, the permeability of the core sample is determined through injecting NaCl solution of 30 g/L into the core at different injection rates, according to Darcy’s law. The core properties are presented in Figure 3c.
- Initial water saturation (Swi) establishment: Swi is maintained by injection of the crude oil into the core with a fixed rate of 0.2 cm3/min until no more brine solution is produced. In this step, the magnitude of Swi can be calculated using the volume of the injected crude oil and core pore volume.
- Thermal aging: The core is taken out from the core holder and then put inside the crude oil under 75 °C for 28 days. It is then removed and located in the core holder to start the flooding process with and without the surfactant.
- Flooding: After placing the core into the core holder, a controlling pressure of 2500 psi is employed. Two flooding tests are carried out using the core sample. For the first flood, the core is subjected to an injection of NaCl brine (30 g/L) at a constant injection rate of 0.2 cm3/min until no crude oil is produced. It should be noted that the magnitude of the pressure drop across the core is carefully recorded to determine the breakthrough time. For the second flood, the aqueous solution of the surfactant (10 g/L) containing 30 g/L NaCl is injected into the core. The volume of produced oil at different pore volumes is recorded versus time, and the oil recovery factor is determined as a percentage of OOIP.
3. Results and Discussion
3.1. FT-IR Analysis
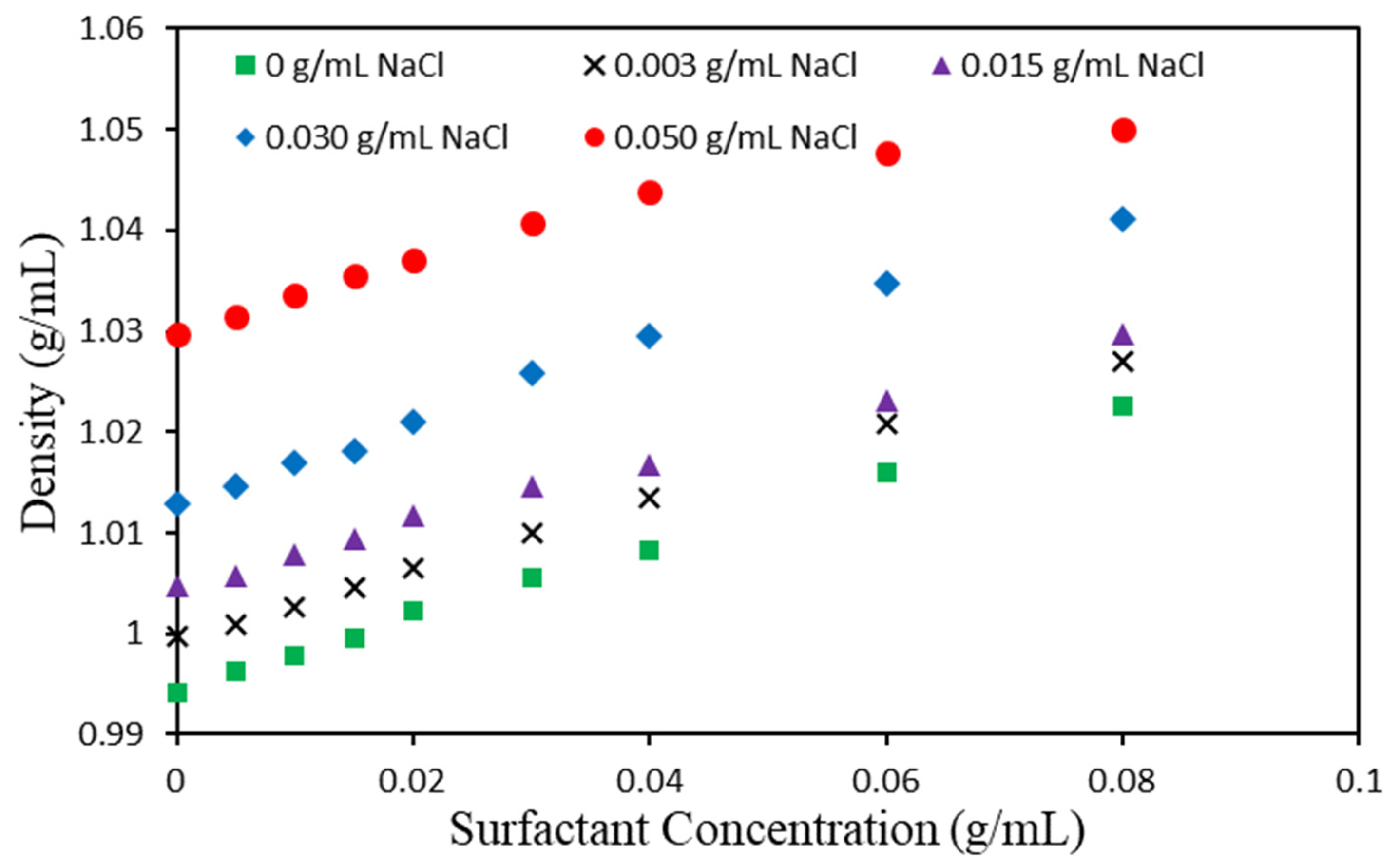
3.2. Determination of Critical Micelle Concentration
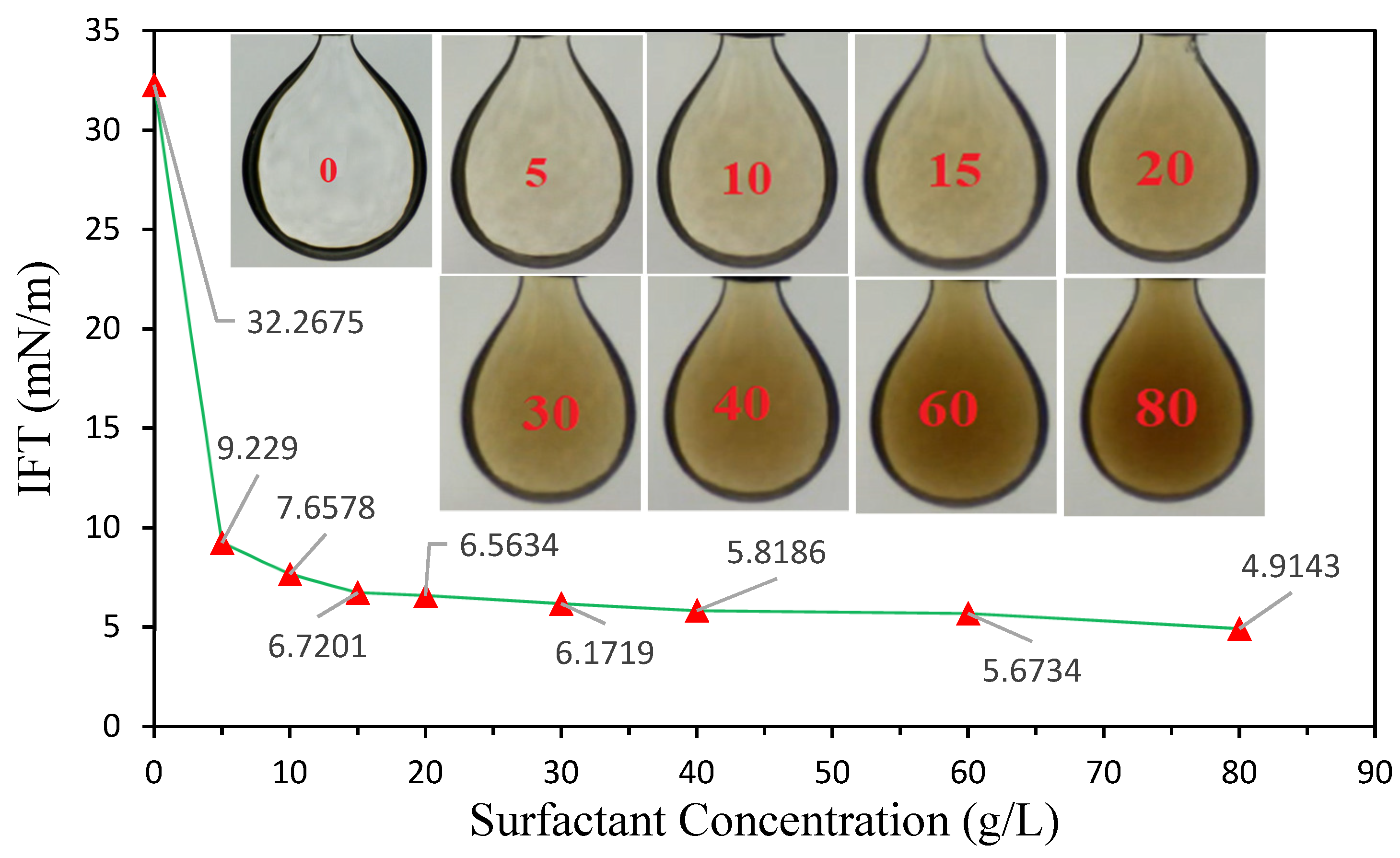
3.3. The Impact of Salinity on IFT
3.4. Contact Angle Measurement
3.5. Displacement Efficiency Analysis
3.5.1. Micromodel Flood
3.5.2. Core Flood
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Nomenclature
| Acronyms | |
| CMC | Critical Micelle Concentration |
| DI | Deionized |
| EOR | Enhanced Oil Recovery |
| FT-IR | Fourier-Transform Infrared Spectroscopy |
| IFT | Interfacial Tension |
| KBr | Potassium Bromide |
| NaCl | Sodium Chloride |
| OOIP | Original Oil in Place |
| PV | Pore Volume |
| SEM | Scanning Electron Microscope |
| XRD | X-Ray Diffraction |
| Variables English Letters | |
| A | empirical constant |
| empirical constant | |
| the activity of the ith component | |
| d | horizontal diameter just at the distance D from the bottom of the drop |
| D | maximum equatorial diameter |
| gravity acceleration | |
| H | shape-dependent factor |
| R | gas constant () |
| S | shape factor |
| Swi | irreducible water saturation |
| T | Temperature (K) |
| Nca | Capillary Number (dimensionless) |
| Greek letters | |
| IFT () | |
| Δρ | density difference between heavy and light phases () |
| alteration in IFT | |
| surface excess concentration of the ith component | |
| μ | Viscosity () |
| Velocity () | |
| θ | Contact angle (°) |
References
- Molnes, S.N.; Torrijos, I.P.; Strand, S.; Paso, K.G.; Syverud, K. Sandstone injectivity and salt stability of cellulose nanocrystals (CNC) dispersions—Premises for use of CNC in enhanced oil recovery. Ind. Crop. Prod. 2016, 93, 152–160. [Google Scholar] [CrossRef]
- Sedaghat, M.H.; Ghazanfari, M.H.; Parvazdavani, M.; Morshedi, S. Experimental Investigation of Microscopic/Macroscopic Efficiency of Polymer Flooding in Fractured Heavy Oil Five-Spot Systems. J. Energy Resour. Technol. 2013, 135, 032901. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, Y.; Zhang, W.; Wang, J.; Soltanian, M.R.; Olabi, A.G. Effectiveness of amino acid salt solutions in capturing CO2: A review. Renew. Sustain. Energy Rev. 2018, 98, 179–188. [Google Scholar] [CrossRef]
- Gassara, F.; Suri, N.; Stanislav, P.; Voordouw, G. Microbially Enhanced Oil Recovery by Sequential Injection of Light Hydrocarbon and Nitrate in Low- And High-Pressure Bioreactors. Environ. Sci. Technol. 2015, 49, 12594–12601. [Google Scholar] [CrossRef]
- Trivedi, J.; Babadagli, T. Efficiency Analysis of Greenhouse Gas Sequestration during Miscible CO2 Injection in Fractured Oil Reservoirs. Environ. Sci. Technol. 2008, 42, 5473–5479. [Google Scholar] [CrossRef] [PubMed]
- Rui, Z.; Wang, X.; Zhang, Z.; Lu, J.; Chen, G.; Zhou, X.; Patil, S. A realistic and integrated model for evaluating oil sands development with Steam Assisted Gravity Drainage technology in Canada. Appl. Energy 2018, 213, 76–91. [Google Scholar] [CrossRef]
- Mirshahghassemi, S.; Lead, J.R. Oil Recovery from Water under Environmentally Relevant Conditions Using Magnetic Nanoparticles. Environ. Sci. Technol. 2015, 49, 11729–11736. [Google Scholar] [CrossRef] [PubMed]
- Ko, K.M.; Chon, B.H.; Jang, S.B.; Jang, H.Y. Surfactant flooding characteristics of dodecyl alkyl sulfate for enhanced oil recovery. J. Ind. Eng. Chem. 2014, 20, 228–233. [Google Scholar] [CrossRef]
- Khojastehmehr, M.; Madani, M.; Daryasafar, A. Screening of enhanced oil recovery techniques for Iranian oil reservoirs using TOPSIS algorithm. Energy Rep. 2019, 5, 529–544. [Google Scholar] [CrossRef]
- Youyi, Z.; Qingfeng, H.; Guoqing, J.; Desheng, M.; Zhe, W. Current development and application of chemical combination flooding technique. Pet. Explor. Dev. 2013, 40, 96–103. [Google Scholar]
- ShamsiJazeyi, H.; Verduzco, R.; Hirasaki, G.J. Reducing adsorption of anionic surfactant for enhanced oil recovery: Part I. Competitive adsorption mechanism. Colloids Surf. A: Physicochem. Eng. Asp. 2014, 453, 162–167. [Google Scholar] [CrossRef]
- Rosestolato, J.C.; Pérez-Gramatges, A.; Lachter, E.R.; Nascimento, R.S. Lipid nanostructures as surfactant carriers for enhanced oil recovery. Fuel 2018, 239, 403–412. [Google Scholar] [CrossRef]
- Hasankhani, G.M.; Madani, M.; Esmaeilzadeh, F.; Mowla, D. Experimental investigation of asphaltene-augmented gel polymer performance for water shut-off and enhancing oil recovery in fractured oil reservoirs. J. Mol. Liq. 2018, 275, 654–666. [Google Scholar] [CrossRef]
- Kamranfar, P.; Jamialahmadi, M. Effect of surfactant micelle shape transition on the microemulsion viscosity and its application in enhanced oil recovery processes. J. Mol. Liq. 2014, 198, 286–291. [Google Scholar] [CrossRef]
- Takassi, M.A.; Zargar, G.; Madani, M.; Zadehnazari, A. The preparation of an amino acid-based surfactant and its potential application as an EOR agent. Pet. Sci. Technol. 2017, 35, 385–391. [Google Scholar] [CrossRef]
- Ding, M.; Wang, Y.; Li, Z.; Zhong, D.; Yuan, F.; Zhu, Y. The role of IFT and emulsification in recovering heavy oil during S/SP flooding. J. Ind. Eng. Chem. 2019, 77, 198–208. [Google Scholar] [CrossRef]
- Heakal, F.E.; Elkholy, A.E. Gemini surfactants as corrosion inhibitors carbon steel. J. Mol. Liq. 2017, 230, 395–417. [Google Scholar] [CrossRef]
- Olajire, A.A. Review of ASP EOR (alkaline surfactant polymer enhanced oil recovery) technology in the petroleum industry: Prospects and challenges. Energy 2014, 77, 963–982. [Google Scholar] [CrossRef]
- Kazempour, M.; Manrique, E.J.; Alvaradov, V.; Zhang, J.; Lantz, M. Role of active clays on alkaline–surfactant–polymer formulation performance in sandstone formations. Fuel 2013, 104, 593–606. [Google Scholar] [CrossRef]
- Madani, M.; Zargar, G.; Takassi, M.A.; Daryasafar, A.; Wood, D.; Zhang, Z. Fundamental investigation of an environmentally-friendly surfactant agent for chemical enhanced oil recovery. Fuel 2018, 238, 186–197. [Google Scholar] [CrossRef]
- Subhash, C.A. Surfactant-Induced Relative Permeability Modifications for Oil Recovery Enhancement. Master Thesis, Louisiana State University, Agricultural and Mechanical College, Baton Rouge, LA, USA, 2002. [Google Scholar]
- Goodarzi, F.; Zendehboudi, S. Effects of Salt and Surfactant on Interfacial Characteristics of Water/Oil Systems: Molecular Dynamic Simulations and Dissipative Particle Dynamics. Ind. Eng. Chem. Res. 2019, 58, 8817–8834. [Google Scholar] [CrossRef] [Green Version]
- Hosseini, H.; Hosseini, H.; Jalili, M.; Apourvari, S.N.; Schaffie, M.; Ranjbar, M. Static adsorption and interfacial tension of Sodium dodecyl sulfate via magnetic field application. J. Pet. Sci. Eng. 2019, 178, 205–215. [Google Scholar] [CrossRef]
- De Freitas, F.A.; Keils, D.; Lachter, E.R.; Maia, C.E.; da Silva, M.I.P.; Nascimento, R.S.V. Synthesis and evaluation of the potential of nonionic surfactants/mesoporous silica systems as nanocarriers for surfactant controlled release in enhanced oil recovery. Fuel 2019, 241, 1184–1194. [Google Scholar] [CrossRef]
- Preux, C.; Malinouskaya, I.; Nguyen, Q.-L.; Flauraud, E.; Ayache, S. Reservoir-Simulation Model with Surfactant Flooding Including Salinity and Thermal Effect, Using Laboratory Experiments. SPE J. 2020, 25, 1761–1770. [Google Scholar] [CrossRef]
- Esene, C.; Onalo, D.; Zendehboudi, S.; James, L.; Aborig, A.; Butt, S. Modeling investigation of low salinity water injection in sandstones and carbonates: Effect of Na+ and SO42−. Fuel 2018, 232, 362–373. [Google Scholar] [CrossRef]
- Jalilian, M.; Tabzar, A.; Ghasemi, V.; Mohammadzadeh, O.; Pourafshary, P.; Rezaei, N.; Zendehboudi, S. An experimental investigation of nanoemulsion enhanced oil recovery: Use of unconsolidated porous systems. Fuel 2019, 251, 754–762. [Google Scholar] [CrossRef]
- Mohammadzadeh, O.; Sedaghat, M.H.; Kord, S.; Zendehboudi, S.; Giesy, J.P. Pore-level visual analysis of heavy oil recovery using chemical-assisted waterflooding process–use of a new chemical agent. Fuel 2019, 239, 202–218. [Google Scholar] [CrossRef]
- Dehdari, B.; Parsaei, R.; Riazi, M.; Rezaei, N.; Zendehboudi, S. New insight into foam stability enhancement mechanism, using polyvinyl alcohol (PVA) and nanoparticles. J. Mol. Liq. 2020, 307, 112755. [Google Scholar] [CrossRef]
- Goodarzi, F.; Kondori, J.; Rezaei, N.; Zendehboudi, S. Meso-and molecular-scale modeling to provide new insights into interfacial and structural properties of hydrocarbon/water/surfactant systems. J. Mol. Liq. 2019, 295, 111357. [Google Scholar] [CrossRef]
- Pordel Shahri, M.; Shadizadeh, S.R.; Jamialahmadi, M. Applicability test of new surfactant produced from Zizyphus spina-christi leaves for enhanced oil recovery in carbonate reservoirs. J. Jpn. Pet. Inst. 2012, 55, 27–32. [Google Scholar] [CrossRef] [Green Version]
- Ahmadi, M.A.; Shadizadeh, S.R. Experimental investigation of adsorption of a new nonionic surfactant on carbonate minerals. Fuel 2013, 104, 462–467. [Google Scholar] [CrossRef]
- Ahmadi, M.A.; Shadizadeh, S.R. Induced effect of adding nano silica on adsorption of a natural surfactant onto sandstone rock: Experimental and theoretical study. J. Pet. Sci. Eng. 2013, 112, 239–247. [Google Scholar] [CrossRef]
- Ahmadi, M.A.; Shadizadeh, S.R. Implementation of a high-performance surfactant for enhanced oil recovery from carbonate reservoirs. J. Pet. Sci. Eng. 2013, 110, 66–73. [Google Scholar] [CrossRef]
- Ahmadi, M.A.; Shadizadeh, S.R. Adsorption of Novel Nonionic Surfactant and Particles Mixture in Carbonates: Enhanced Oil Recovery Implication. Energy Fuels 2012, 26, 4655–4663. [Google Scholar] [CrossRef]
- Ahmadi, M.A.; Shadizadeh, S.R. Spotlight on the New Natural Surfactant Flooding in Carbonate Rock Samples in Low Salinity Condition. Sci. Rep. 2018, 8, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Ahmadi, M.A.; Shadizadeh, S.R.; Chen, Z. Thermodynamic analysis of adsorption of a naturally derived surfactant onto shale sandstone reservoirs. Eur. Phys. J. Plus 2018, 133, 420. [Google Scholar] [CrossRef]
- Moslemizadeh, A.; Dezaki, A.S.; Shadizadeh, S.R. Mechanistic understanding of chemical flooding in swelling porous media using a bio-based nonionic surfactant. J. Mol. Liq. 2017, 229, 76–88. [Google Scholar] [CrossRef]
- Ahmadi, M.A.; Arabsahebi, Y.; Shadizadeh, S.R.; Behbahani, S.S. Preliminary evaluation of mulberry leaf-derived surfactant on interfacial tension in an oil-aqueous system: EOR application. Fuel 2014, 117, 749–755. [Google Scholar] [CrossRef]
- Ravi, S.G.; Shadizadeh, S.R.; Moghaddasi, J. Core Flooding Tests to Investigate the Effects of IFT Reduction and Wettability Alteration on Oil Recovery: Using Mulberry Leaf Extract. Pet. Sci. Technol. 2015, 33, 257–264. [Google Scholar] [CrossRef]
- Moslemizadeh, A.; Dehkordi, A.F.; Barnaji, M.J.; Naseri, M.; Ravi, S.G.; Jahromi, E.K. Novel bio-based surfactant for chemical enhanced oil recovery in montmorillonite rich reservoirs: Adsorption behavior, interaction impact, and oil recovery studies. Chem. Eng. Res. Des. 2016, 109, 18–31. [Google Scholar] [CrossRef]
- Arabloo, M.; Ghazanfari, M.H.; Rashtchian, D. Spotlight on kinetic and equilibrium adsorption of a new surfactant onto sandstone minerals: A comparative study. J. Taiwan Inst. Chem. Eng. 2015, 50, 12–23. [Google Scholar] [CrossRef]
- Barati, A.; Najafi, A.; Daryasafar, A.; Nadali, P.; Moslehi, H. Adsorption of a new nonionic surfactant on carbonate minerals in enhanced oil recovery: Experimental and modeling study. Chem. Eng. Res. Des. 2016, 105, 55–63. [Google Scholar] [CrossRef]
- Barati-Harooni, A.; Najafi-Marghmaleki, A.; Hosseini, S.M.; Moradi, S. Experimental Investigation of Dynamic Adsorption–Desorption of New Nonionic Surfactant on Carbonate Rock: Application to Enhanced Oil Recovery. J. Energy Resour. Technol. 2017, 139, 042202. [Google Scholar] [CrossRef]
- Khayati, H.; Moslemizadeh, A.; Shahbazi, K.; Moraveji, M.K.; Riazi, S.H. An experimental investigation on the use of saponin as a non-ionic surfactant for chemical enhanced oil recovery (EOR) in sandstone and carbonate oil reservoirs: IFT, wettability alteration, and oil recovery. Chem. Eng. Res. Des. 2020, 160, 417–425. [Google Scholar] [CrossRef]
- Ghasemi, M.; Moslemizadeh, A.; Shahbazi, K.; Mohammadzadeh, O.; Zendehboudi, S.; Jafari, S. Primary evaluation of a natural surfactant for inhibiting clay swelling. J. Pet. Sci. Eng. 2019, 178, 878–891. [Google Scholar] [CrossRef]
- Li, Z.; Ji, G.E. Ginseng and obesity. J. Ginseng Res. 2018, 42, 1–8. [Google Scholar] [CrossRef]
- Shin, B.-K.; Kwon, S.W.; Park, J.H. Chemical diversity of ginseng saponins from Panax ginseng. J. Ginseng Res. 2015, 39, 287–298. [Google Scholar] [CrossRef] [Green Version]
- Choi, K.-T. Botanical characteristics, pharmacological effects and medicinal components of Korean Panax ginseng CA Meyer. Acta Pharmacol. Sin. 2008, 29, 1109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Drelich, J.; Fang, C.; White, C. Measurement of interfacial tension in fluid-fluid systems. Encycl. Surf. Colloid Sci. 2002, 3, 3158–3163. [Google Scholar]
- Barati-Harooni, A.; Najafi-Marghmaleki, A.; Tatar, A.; Mohammadi, A.H. Experimental and modeling studies on adsorption of a nonionic surfactant on sandstone minerals in enhanced oil recovery process with surfactant flooding. J. Mol. Liq. 2016, 220, 1022–1032. [Google Scholar] [CrossRef]
- Moeini, F.; Hemmati-Sarapardeh, A.; Ghazanfari, M.-H.; Masihi, M.; Ayatollahi, S. Toward mechanistic understanding of heavy crude oil/brine interfacial tension: The roles of salinity, temperature and pressure. Fluid Phase Equilibria 2014, 375, 191–200. [Google Scholar] [CrossRef]
- Kakati, A.; Sangwai, J. Effect of monovalent and divalent salts on the interfacial tension of pure hydrocarbon-brine systems relevant for low salinity water flooding. J. Pet. Sci. Eng. 2017, 157, 1106–1114. [Google Scholar] [CrossRef]
- Pillai, P.; Kumar, A.; Mandal, A. Mechanistic studies of enhanced oil recovery by imidazolium-based ionic liquids as novel surfactants. J. Ind. Eng. Chem. 2018, 63, 262–274. [Google Scholar] [CrossRef]
- Bera, A.; Mandal, A.; Guha, B.B. Synergistic Effect of Surfactant and Salt Mixture on Interfacial Tension Reduction between Crude Oil and Water in Enhanced Oil Recovery. J. Chem. Eng. Data 2013, 59, 89–96. [Google Scholar] [CrossRef]
- Babu, K.; Pal, N.; Bera, A.; Saxena, V.; Mandal, A. Studies on interfacial tension and contact angle of synthesized surfactant and polymeric from castor oil for enhanced oil recovery. Appl. Surf. Sci. 2015, 353, 1126–1136. [Google Scholar] [CrossRef]
- Pal, N.; Babu, K.; Mandal, A. Surface tension, dynamic light scattering and rheological studies of a new polymeric surfactant for application in enhanced oil recovery. J. Pet. Sci. Eng. 2016, 146, 591–600. [Google Scholar] [CrossRef]
- Babu, K.; Pal, N.; Saxena, V.K.; Mandal, A. Synthesis and characterization of a new polymeric surfactant for chemical enhanced oil recovery. Korean J. Chem. Eng. 2015, 33, 711–719. [Google Scholar] [CrossRef]
- Salager, J.-L.; Forgiarini, A.M.; Márquez, L.; Manchego, L.; Bullón, J. How to Attain an Ultralow Interfacial Tension and a Three-Phase Behavior with a Surfactant Formulation for Enhanced Oil Recovery: A Review. Part 2. Performance Improvement Trends from Winsor’s Premise to Currently Proposed Inter- and Intra-Molecular Mixtures. J. Surfactants Deterg. 2013, 16, 631–663. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gaonkar, A.G. Effects of salt, temperature, and surfactants on the interfacial tension behavior of a vegetable oil/water system. J. Colloid Interface Sci. 1992, 149, 256–260. [Google Scholar] [CrossRef]
- Karnanda, W.; Benzagouta, M.S.; AlQuraishi, A.; Amro, M.M. Effect of temperature, pressure, salinity, and surfactant concentration on IFT for surfactant flooding optimization. Arab. J. Geosci. 2012, 6, 3535–3544. [Google Scholar] [CrossRef]
- Kumar, S.; Mandal, A. Studies on interfacial behavior and wettability change phenomena by ionic and nonionic surfactants in presence of alkalis and salt for enhanced oil recovery. Appl. Surf. Sci. 2016, 372, 42–51. [Google Scholar] [CrossRef]
- Tichelkamp, T.; Teigen, E.; Nourani, M.; Øye, G. Systematic study of the effect of electrolyte composition on interfacial tensions between surfactant solutions and crude oils. Chem. Eng. Sci. 2015, 132, 244–249. [Google Scholar] [CrossRef]
- Eftekhardadkhah, M.; Øye, G. Correlations between Crude Oil Composition and Produced Water Quality: A Multivariate Analysis Approach. Ind. Eng. Chem. Res. 2013, 52, 17315–17321. [Google Scholar] [CrossRef]
- Liu, F.; Wang, M. Review of low salinity waterflooding mechanisms: Wettability alteration and its impact on oil recovery. Fuel 2020, 267, 117112. [Google Scholar] [CrossRef]
- Jarrahian, K.; Seiedi, O.; Sheykhan, M.; Sefti, M.V.; Ayatollahi, S. Wettability alteration of carbonate rocks by surfactants: A mechanistic study. Colloids Surf. A Physicochem. Eng. Aspects 2012, 410, 1–10. [Google Scholar] [CrossRef]
- Zdziennicka, A.; Jańczuk, B. The relationship between the adhesion work, the wettability and composition of the surface layer in the systems polymer/aqueous solution of anionic surfactants and alcohol mixtures. Appl. Surf. Sci. 2010, 257, 1034–1042. [Google Scholar] [CrossRef]
- Bera, A.; Ojha, K.; Kumar, T.; Mandal, A. Mechanistic Study of Wettability Alteration of Quartz Surface Induced by Nonionic Surfactants and Interaction between Crude Oil and Quartz in the Presence of Sodium Chloride Salt. Energy Fuels 2012, 26, 3634–3643. [Google Scholar] [CrossRef]
- Standal, S.; Haavik, J.; Blokhus, A.; Skauge, A. Effect of polar organic components on wettability as studied by adsorption and contact angles. J. Pet. Sci. Eng. 1999, 24, 131–144. [Google Scholar] [CrossRef]
- Saxena, N.; Pal, N.; Dey, S.; Mandal, A. Characterizations of surfactant synthesized from palm oil and its application in enhanced oil recovery. J. Taiwan Inst. Chem. Eng. 2017, 81, 343–355. [Google Scholar] [CrossRef]
- Kestin, J.; Shankland, I.R. Viscosity of aqueous NaCl solutions in the temperature range 25–200 C and in the pressure range 0.1–30 MPa. Int. J. Thermophys. 1984, 5, 241–263. [Google Scholar] [CrossRef]





| Properties | Value | Component | Mole % |
|---|---|---|---|
| Dynamic viscosity (cp) at 30 °C | 13.23 | C2 | 0.06 |
| Dynamic viscosity (cp) at 40 °C | 9.73 | C3 | 0.13 |
| API at 15.6 °C | 43.32 | iC4 | 0.15 |
| Saturates (wt%) | 55.21 | nC4 | 4.69 |
| Aromatics (wt%) | 17.81 | iC5 | 5.83 |
| Resins (wt%) | 3.90 | nC5 | 9.01 |
| Asphaltenes (wt%) | 0.2 | C6 | 10.02 |
| Light fractions (wt%) | 22.88 | C7 | 9.53 |
| - | - | C8 | 8.25 |
| - | - | C9 | 6.73 |
| - | - | C10 | 5.16 |
| - | - | C11 | 9.40 |
| - | - | C12+ | 31.04 |
| Experiments | Quantity | Surfactant (g/L) | NaCL (g/L) | Pressure (atm) | Temperature (°C) |
|---|---|---|---|---|---|
| IFT measurements | 1 | 0 | 0 | 1 | 25 |
| 2 | 5 | ||||
| 3 | 10 | ||||
| 4 | 15 | ||||
| 5 | 20 | ||||
| 6 | 30 | ||||
| 7 | 40 | ||||
| 8 | 60 | ||||
| 9 | 80 | ||||
| 10–13 | 0 | 3, 15, 30, 50 | |||
| 14–17 | 5 | ||||
| 18–21 | 10 | ||||
| 22–25 | 15 | ||||
| 26–29 | 20 | ||||
| 30–33 | 30 | ||||
| 34–37 | 40 | ||||
| 38–41 | 60 | ||||
| 42–45 | 80 | ||||
| Contact angle measurements | 1–5 | 0 | 0, 3, 15, 30, 50 | ||
| 6–10 | 10 | 0, 3, 15, 30, 50 | |||
| Micromodel flooding tests | 1–5 | 10 | 0, 3, 15, 30, 50 | ||
| Core flooding tests | 1 | 10 | 30 | Injection: 2 Overburden:176 | 25 |
| S | A | B0 | B1 | B2 | B3 | B4 |
|---|---|---|---|---|---|---|
| 0.401–0.46 | 2.56651 | 0.18069 | 0.84059 | 0.97553 | 0 | 0.3272 |
| 0.46–0.59 | 2.59725 | 0.13261 | 0.50059 | 0.46898 | 0 | 0.31968 |
| 0.59–0.68 | 2.62435 | 0.05285 | 0.15756 | 0.11714 | 0 | 0.31522 |
| 0.68–0.90 | 2.64267 | 0.05877 | 0.14701 | 0.09155 | 0 | 0.31345 |
| 0.90–1.00 | 2.84636 | 0.2097 | −0.18341 | −1.08315 | −0.69116 | 0.30715 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moslemizadeh, A.; Khayati, H.; Madani, M.; Ghasemi, M.; Shahbazi, K.; Zendehboudi, S.; Hashim Abbas, A. A Systematic Study to Assess Displacement Performance of a Naturally-Derived Surfactant in Flow Porous Systems. Energies 2021, 14, 8310. https://doi.org/10.3390/en14248310
Moslemizadeh A, Khayati H, Madani M, Ghasemi M, Shahbazi K, Zendehboudi S, Hashim Abbas A. A Systematic Study to Assess Displacement Performance of a Naturally-Derived Surfactant in Flow Porous Systems. Energies. 2021; 14(24):8310. https://doi.org/10.3390/en14248310
Chicago/Turabian StyleMoslemizadeh, Aghil, Hossein Khayati, Mohammad Madani, Mehdi Ghasemi, Khalil Shahbazi, Sohrab Zendehboudi, and Azza Hashim Abbas. 2021. "A Systematic Study to Assess Displacement Performance of a Naturally-Derived Surfactant in Flow Porous Systems" Energies 14, no. 24: 8310. https://doi.org/10.3390/en14248310
APA StyleMoslemizadeh, A., Khayati, H., Madani, M., Ghasemi, M., Shahbazi, K., Zendehboudi, S., & Hashim Abbas, A. (2021). A Systematic Study to Assess Displacement Performance of a Naturally-Derived Surfactant in Flow Porous Systems. Energies, 14(24), 8310. https://doi.org/10.3390/en14248310