Recent Progress and Approaches on Transition Metal Chalcogenides for Hydrogen Production
Abstract
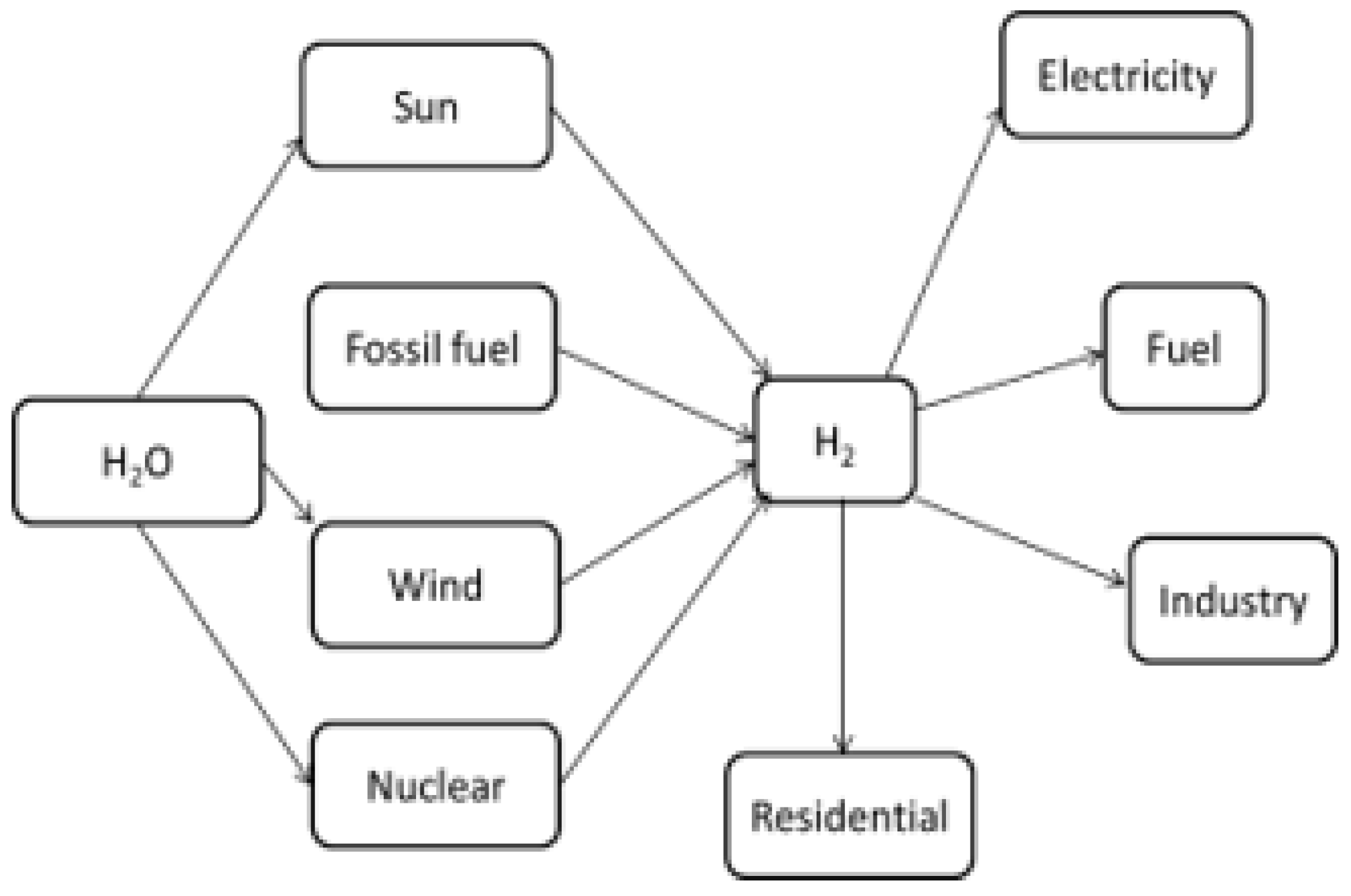
:1. Introduction
2. Transition Metal Chalcogenides (TMCs)
2.1. Synthesis Methods
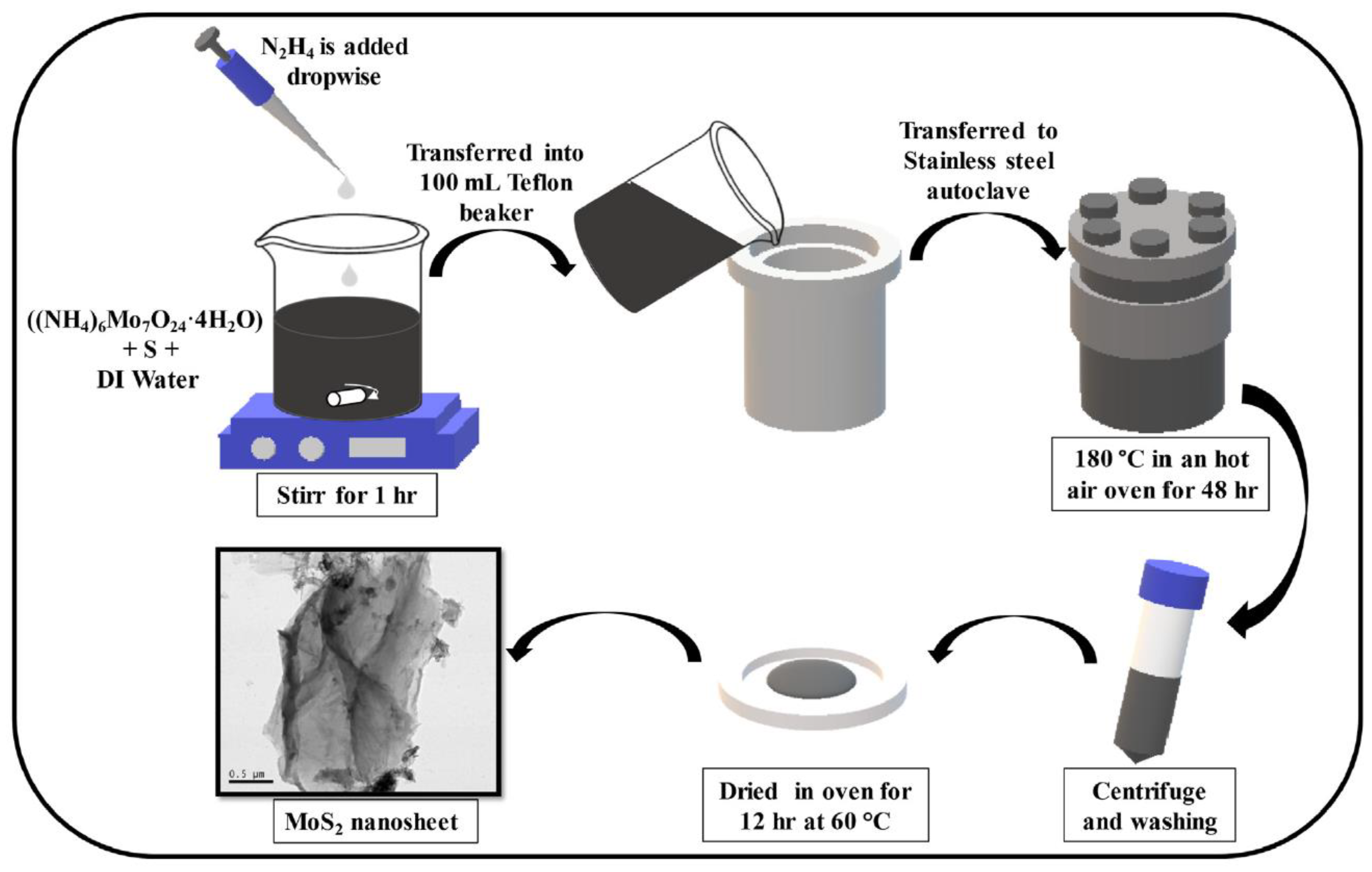
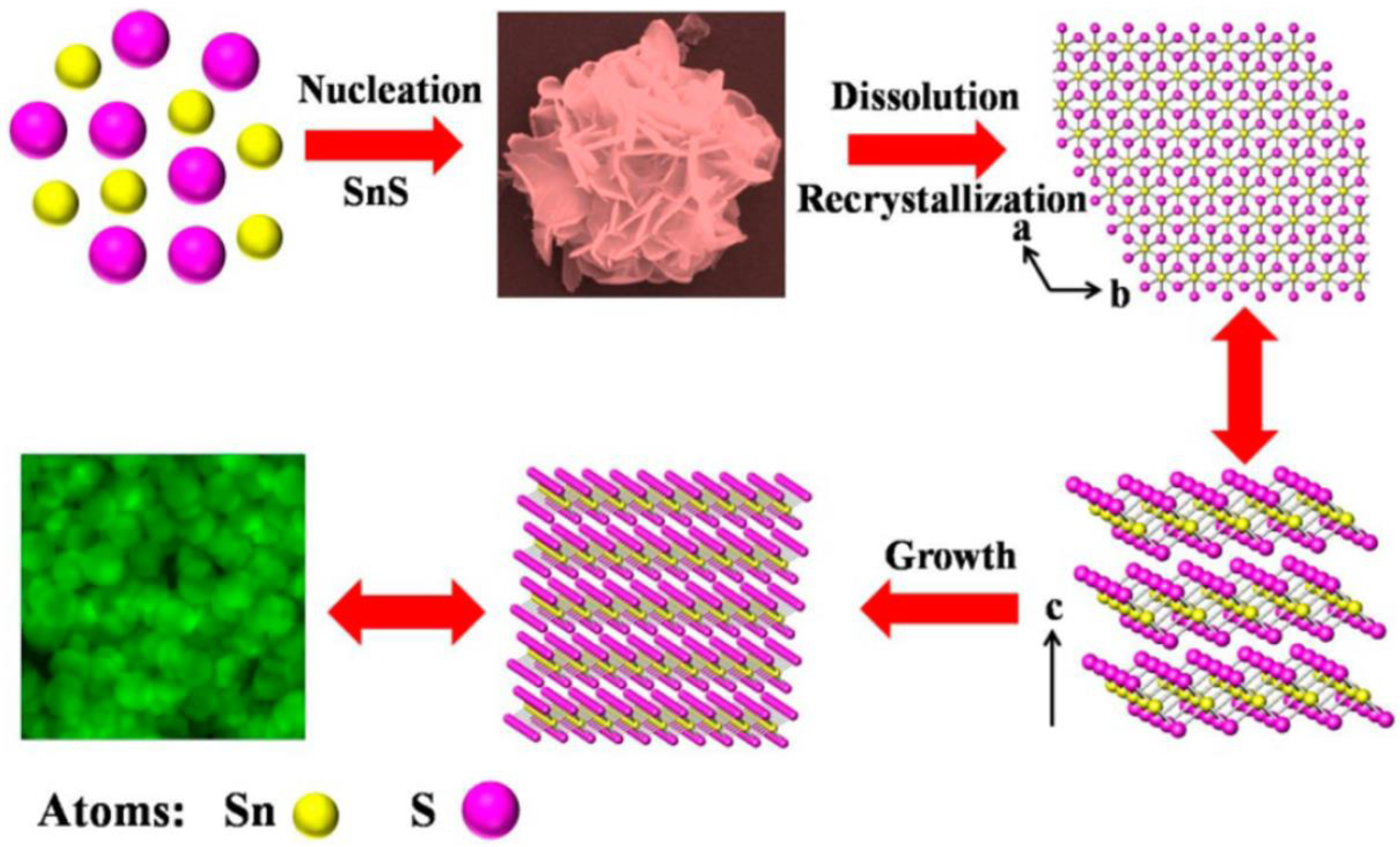
2.1.1. Hydrothermal/Solvothermal Method
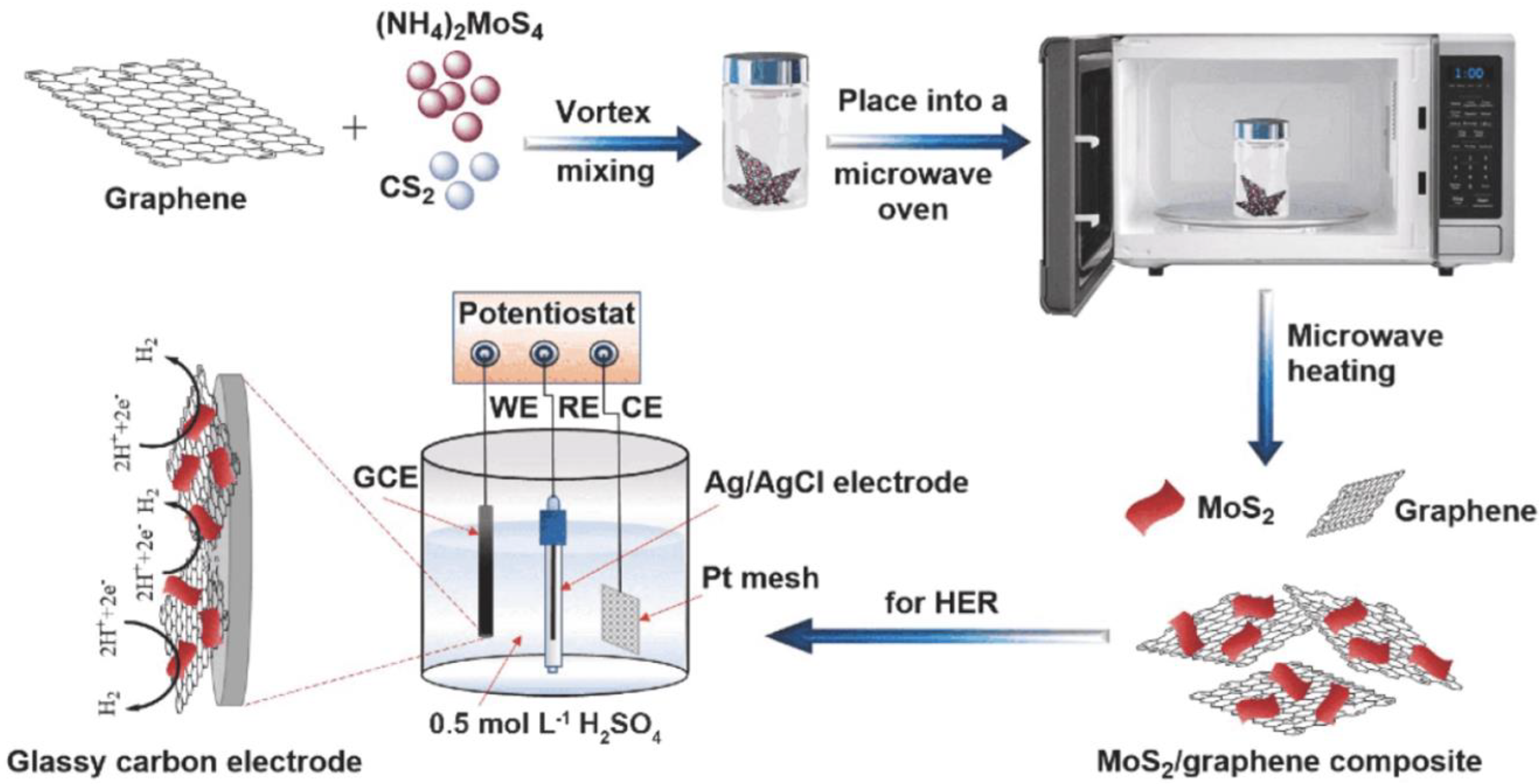
2.1.2. Microwave-Assisted Synthesis
2.1.3. Electrodeposition Method
2.1.4. Photoreduction
2.1.5. Electron Beam Evaporation
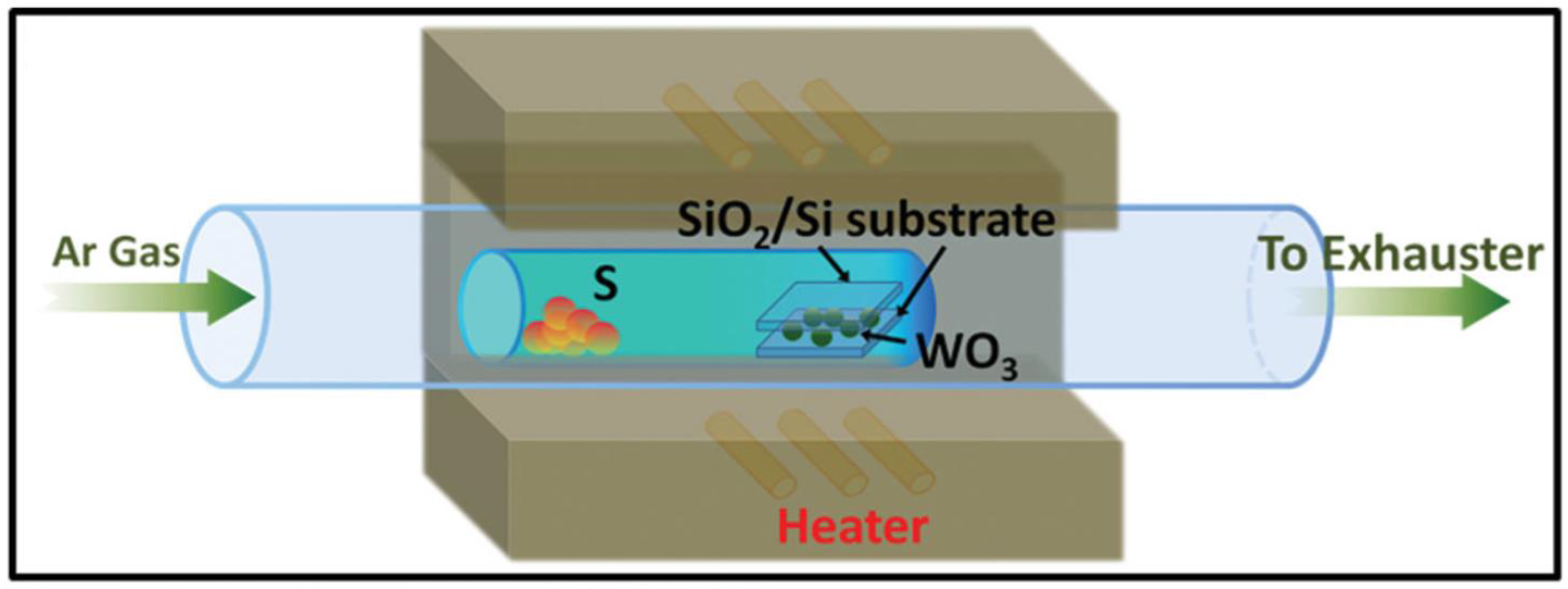
2.1.6. Sulfidation and Selenization
2.1.7. Successive Ionic Layer Adsorption and Reaction (SILAR) Method
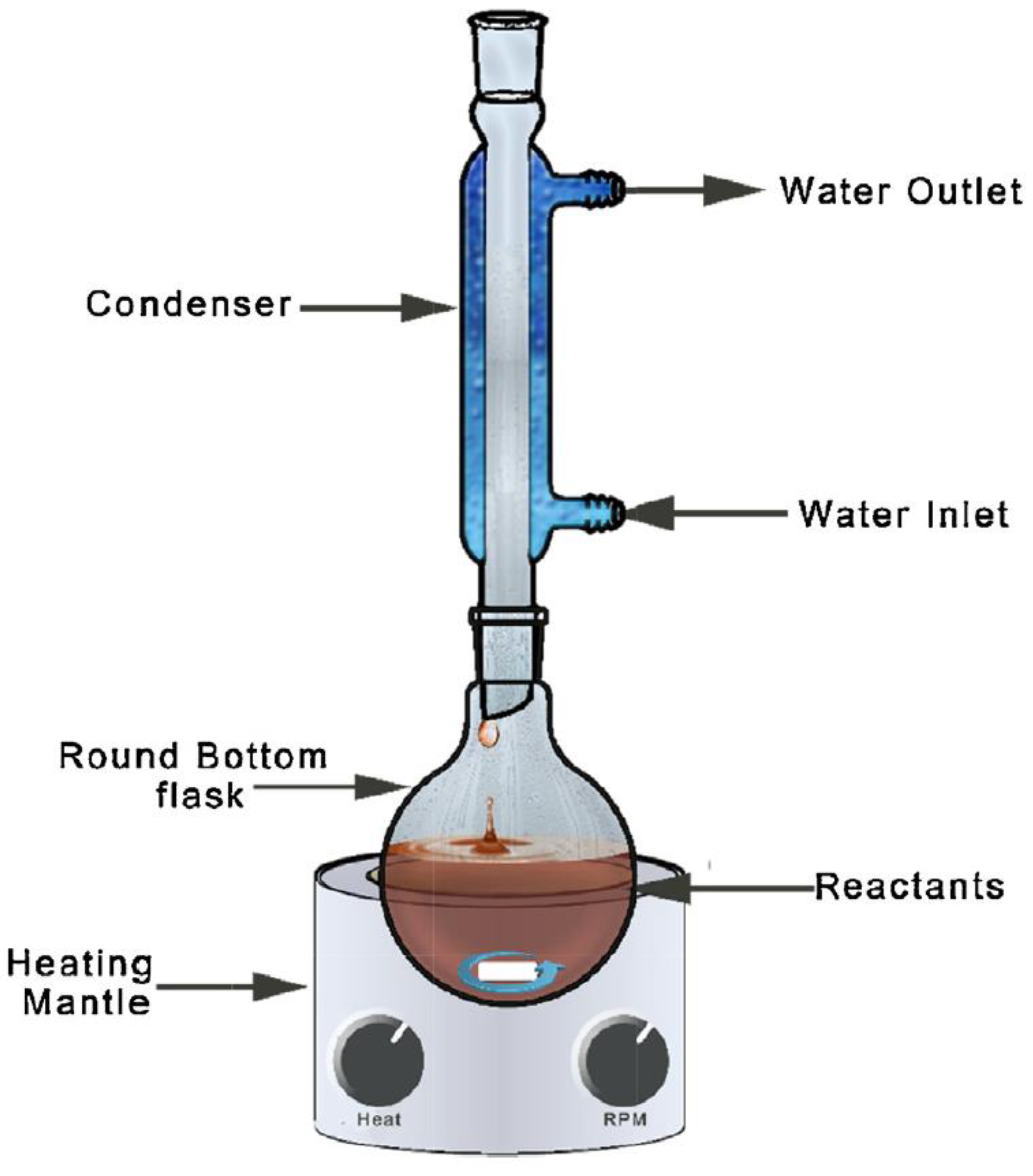
2.1.8. Refluxing Method
2.1.9. Chemical Vapor Deposition (CVD) Method
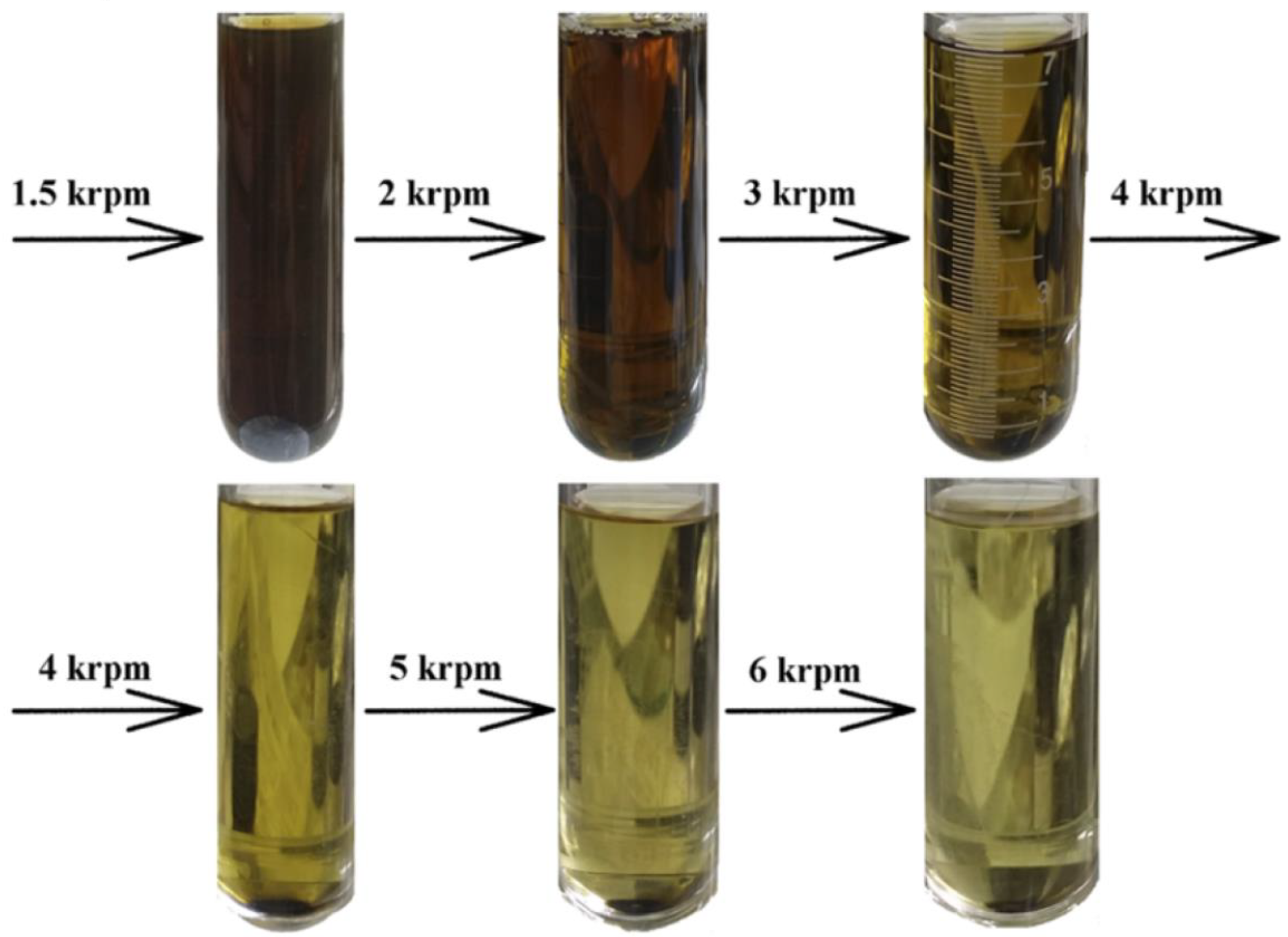
2.1.10. Liquid Cascade Centrifugation (LCC) Method
2.2. Characterization Techniques
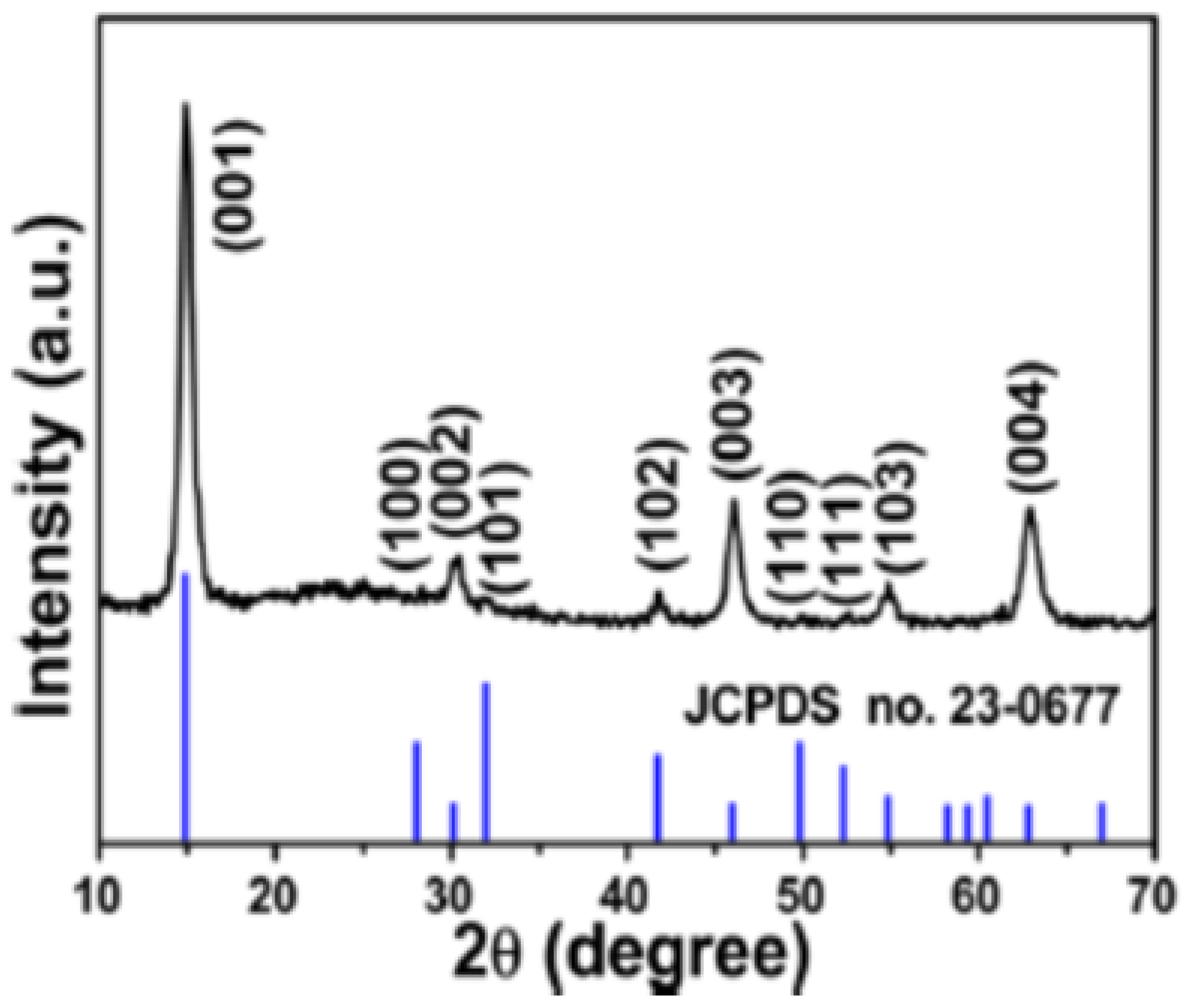
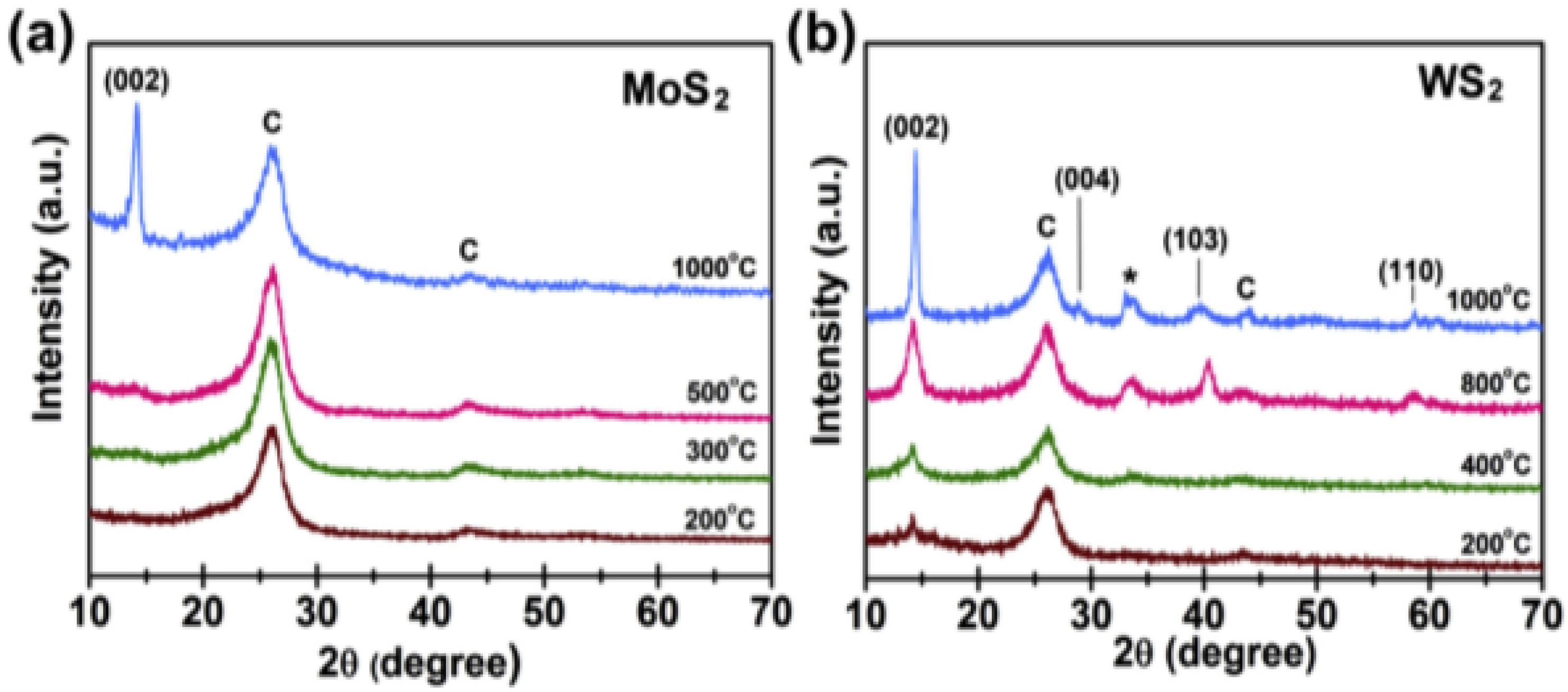
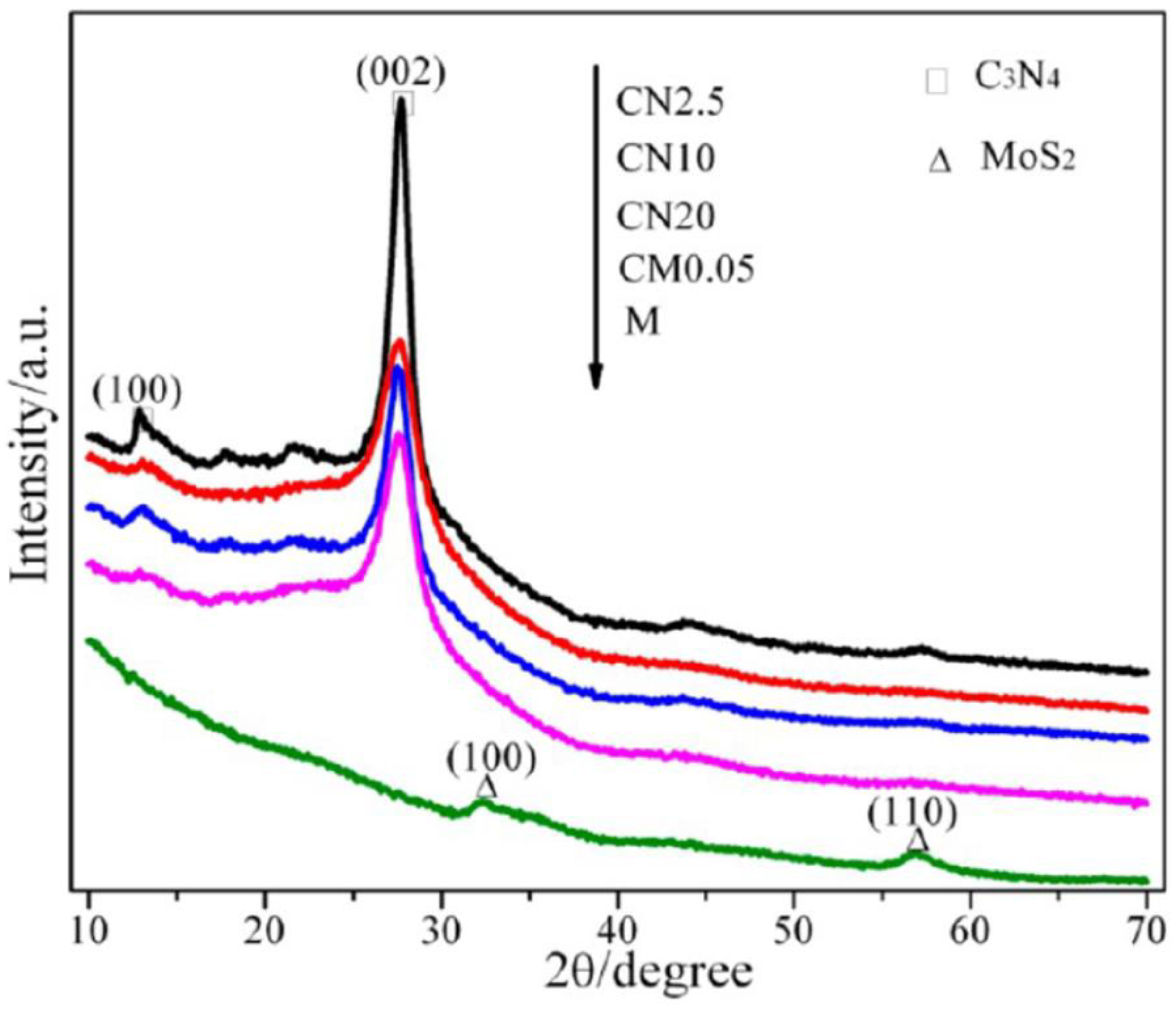
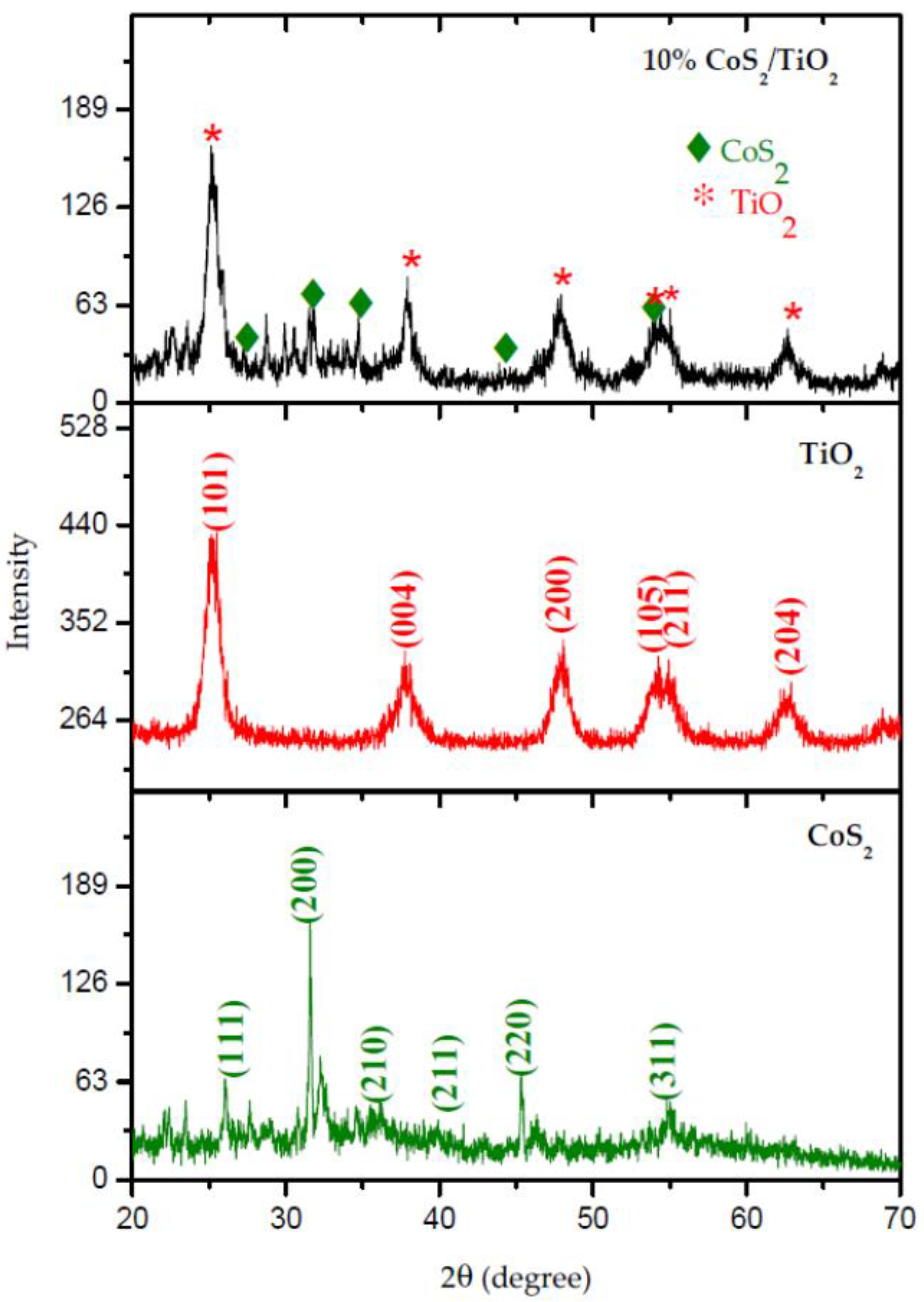
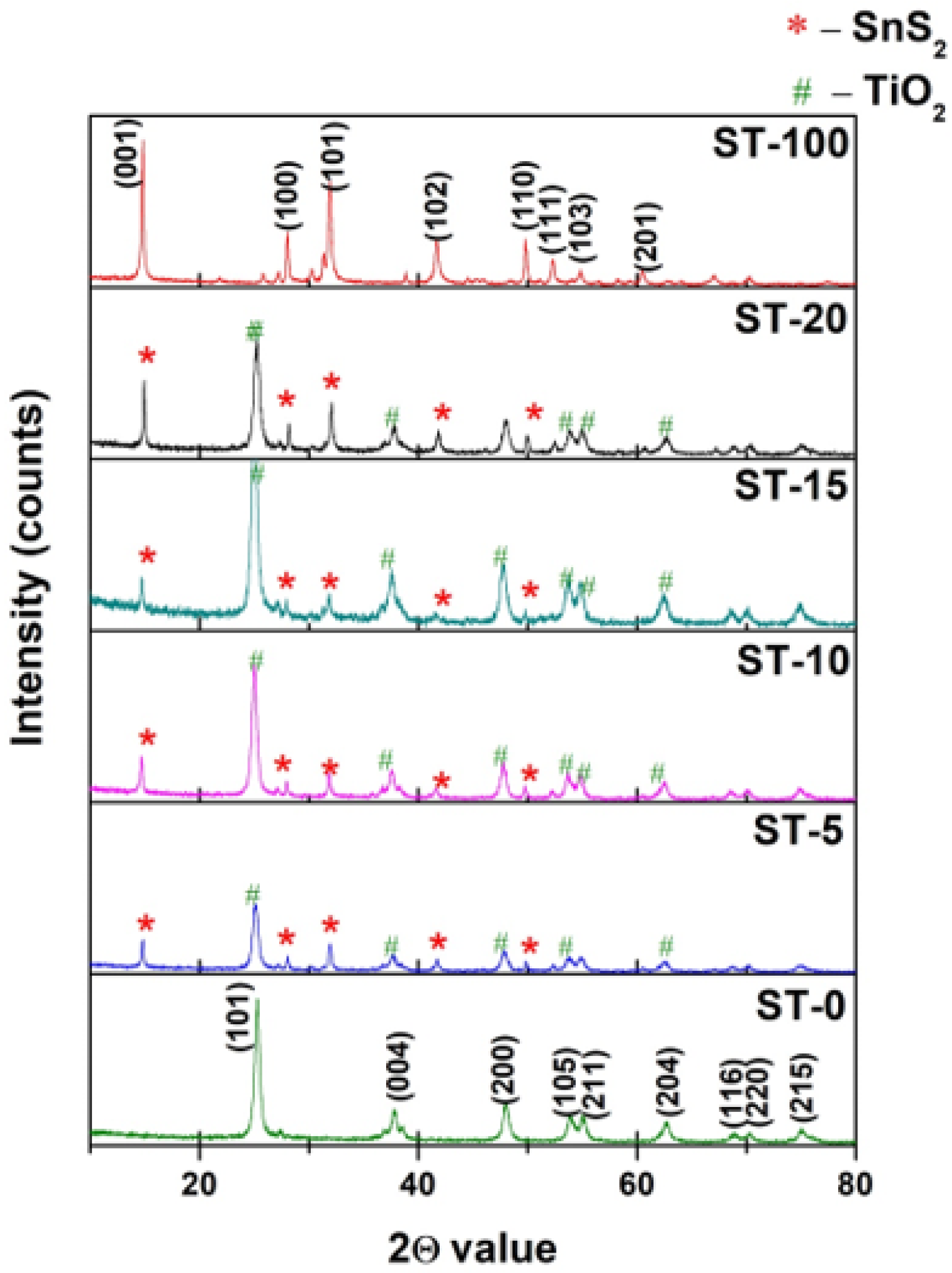
2.2.1. X-ray Diffraction Method (XRD)
2.2.2. Scanning Electron Microscopy (SEM)
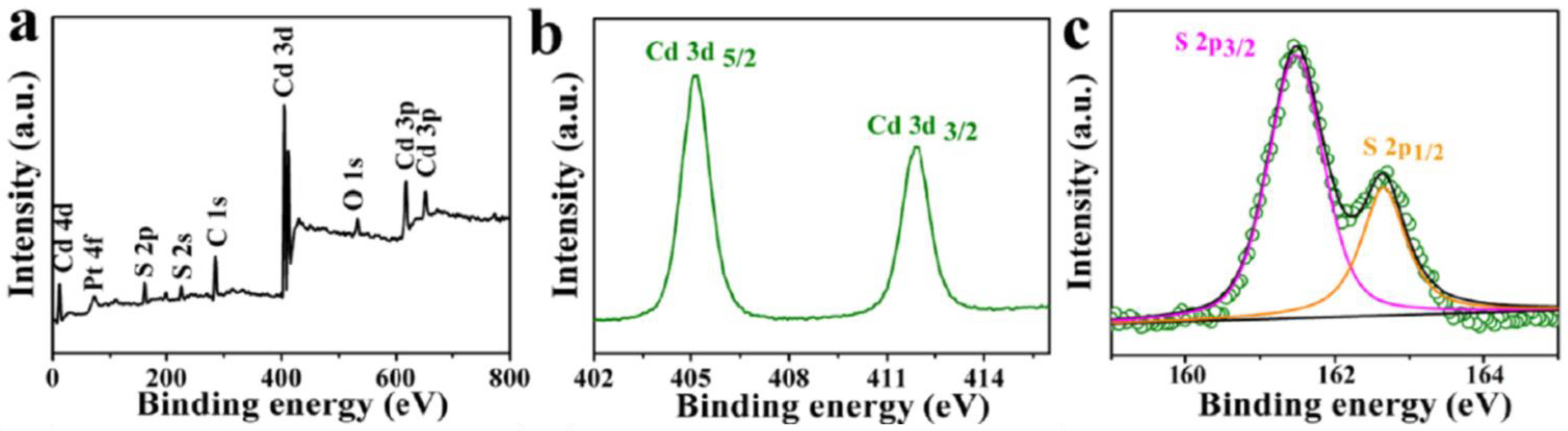
2.2.3. X-ray Photoelectron Spectroscopy (XPS) Studies
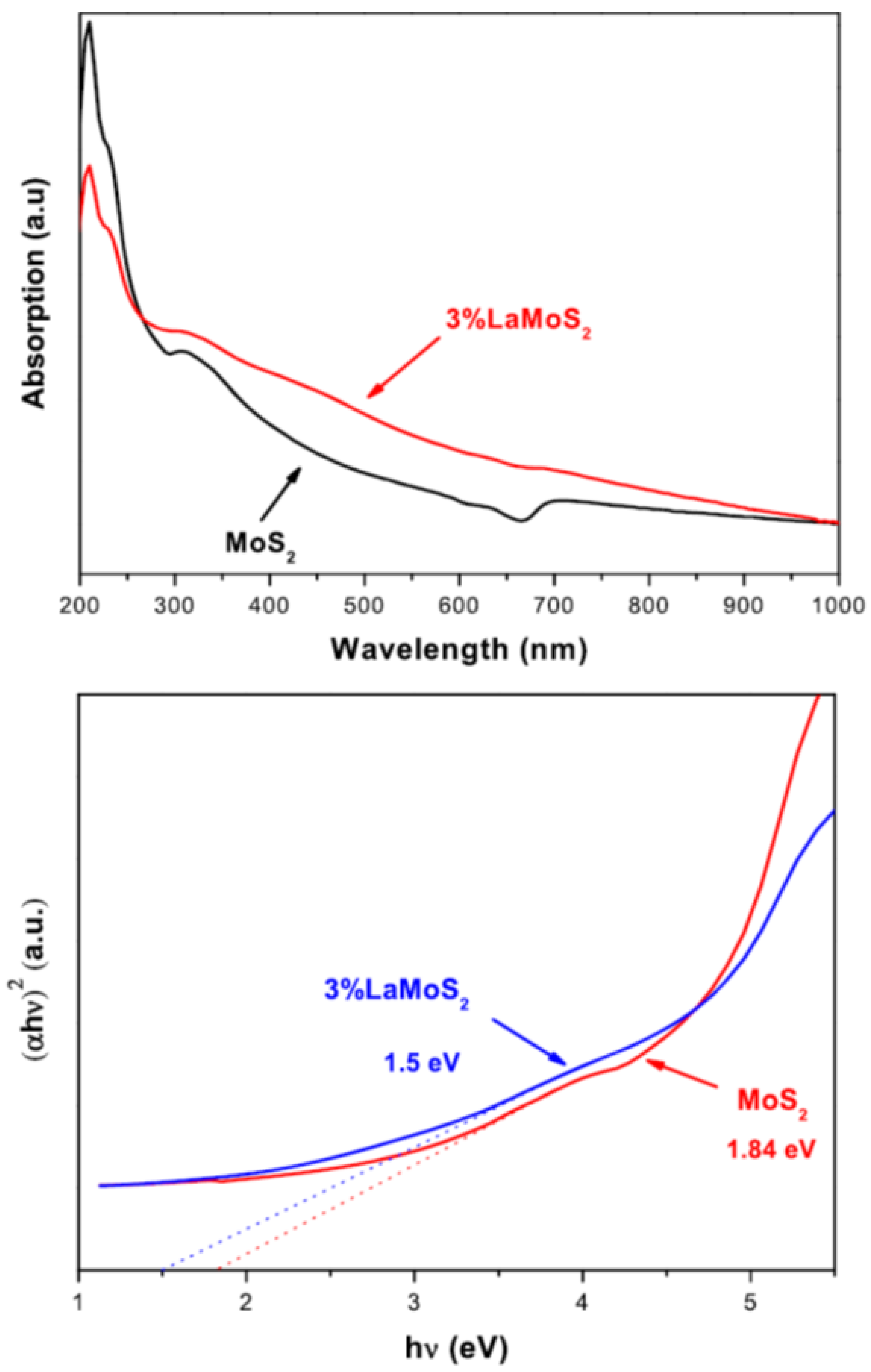
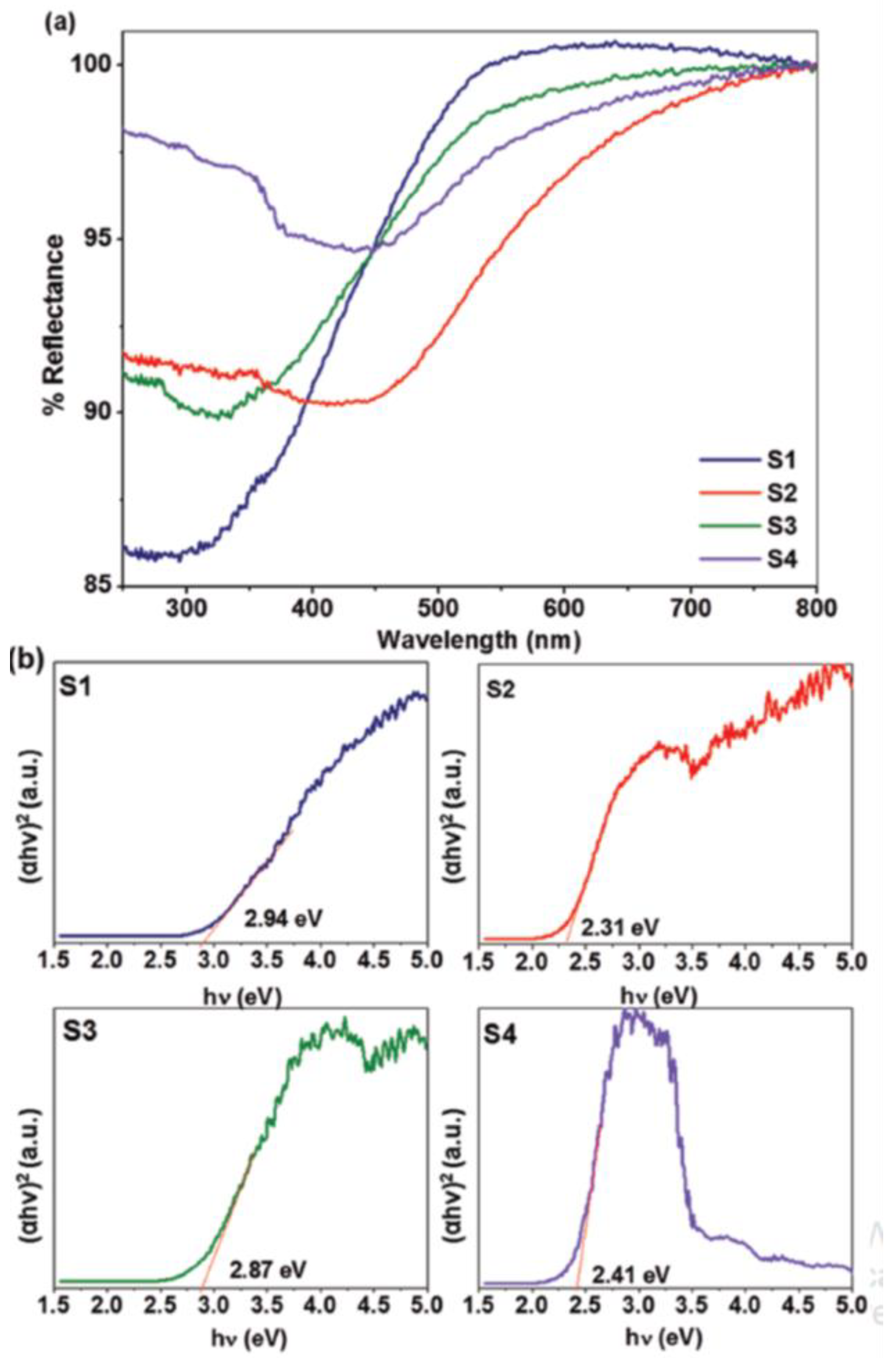
2.2.4. UV–Visible Spectroscopy/Diffuse Reflectance Spectroscopy (DRS)
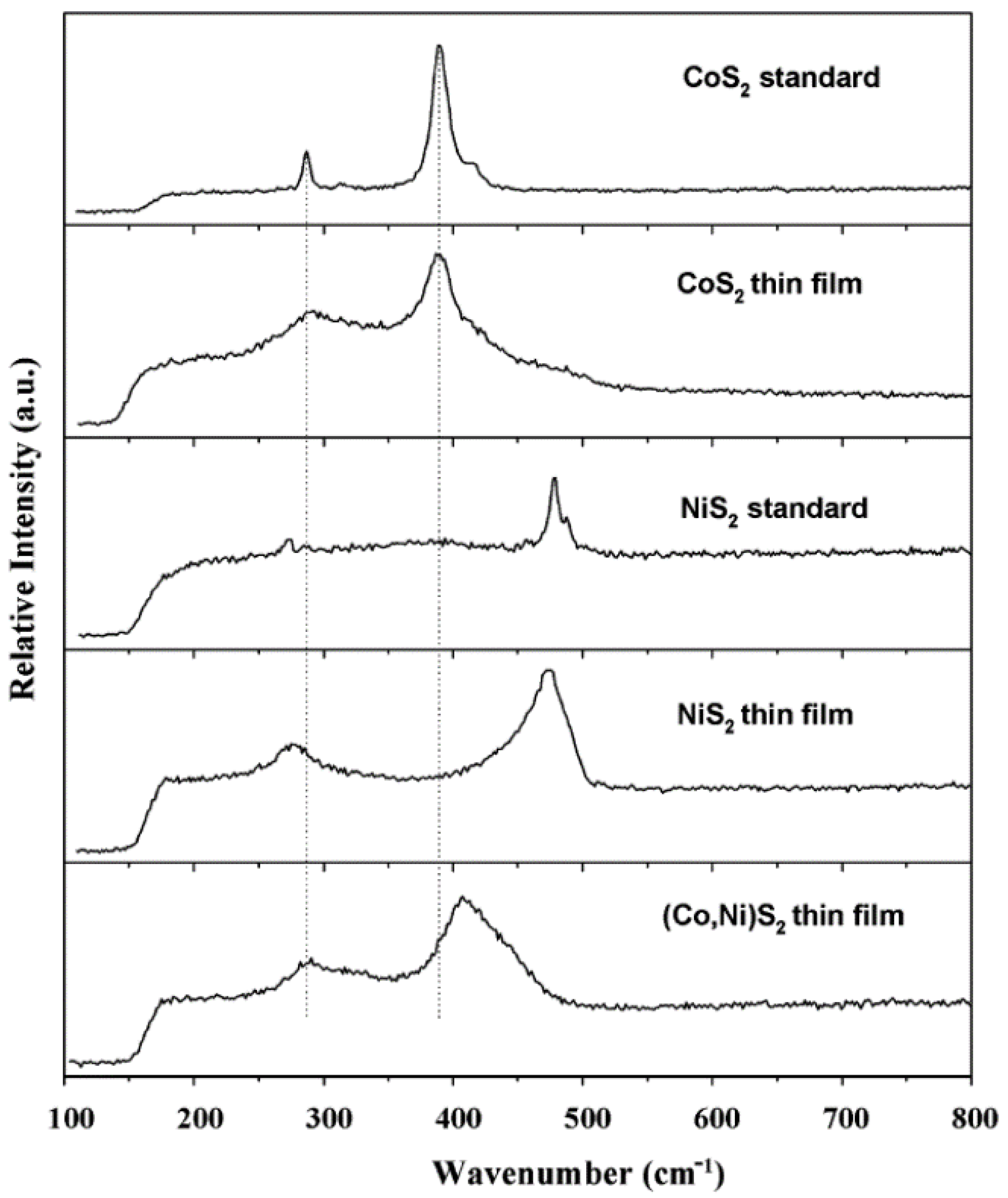
2.2.5. Raman Spectroscopy
3. Hydrogen Evolution Reactions
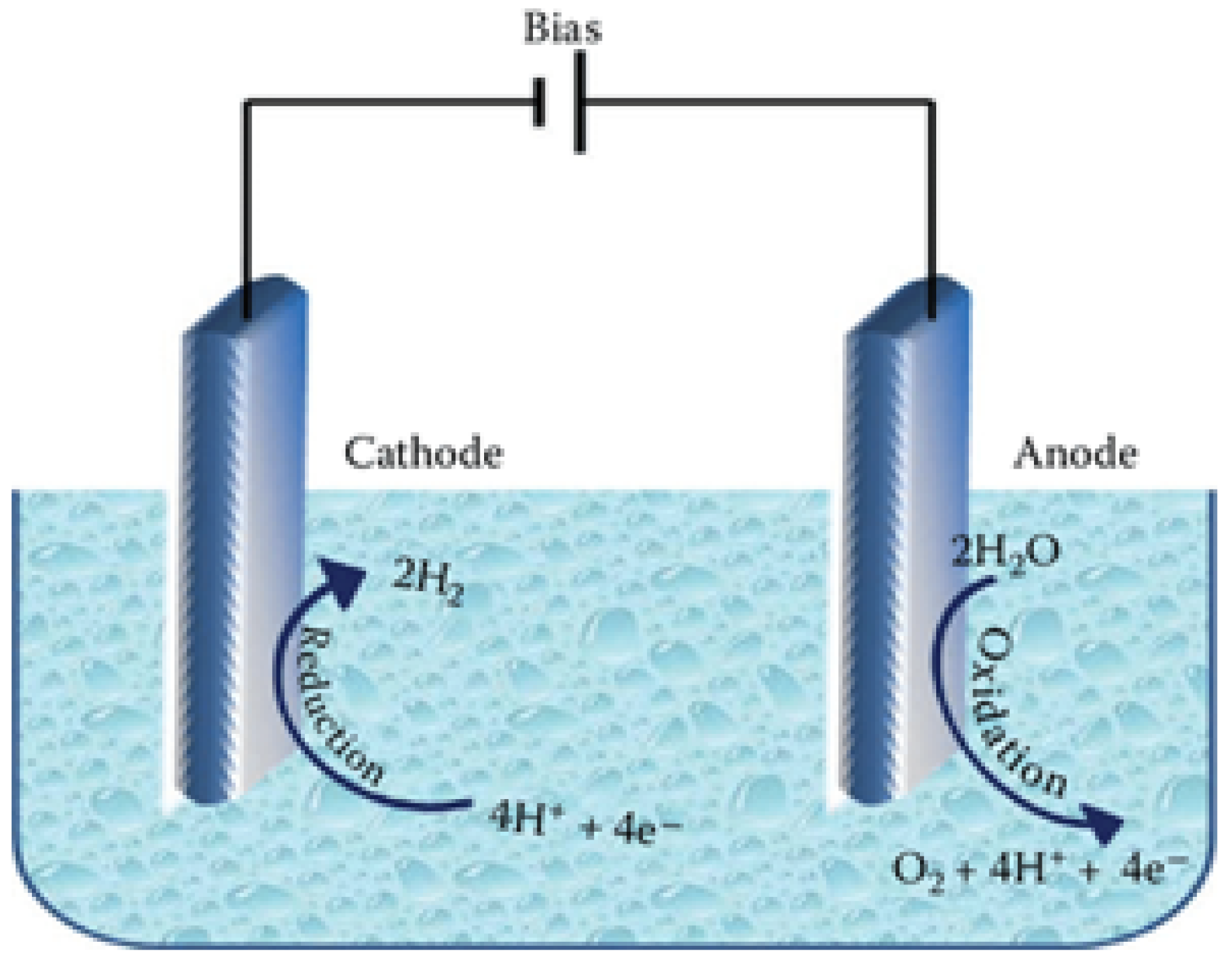
3.1. Photoelectrochemical Hydrogen Evolution
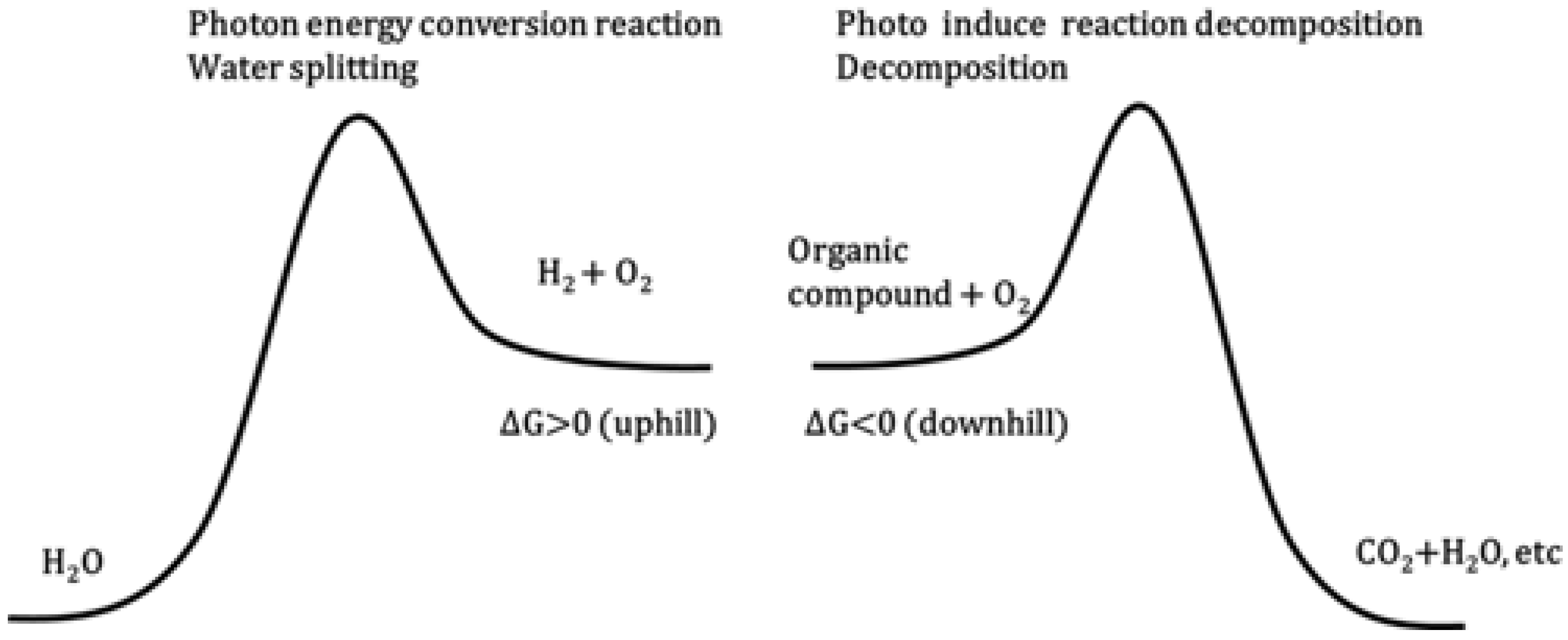
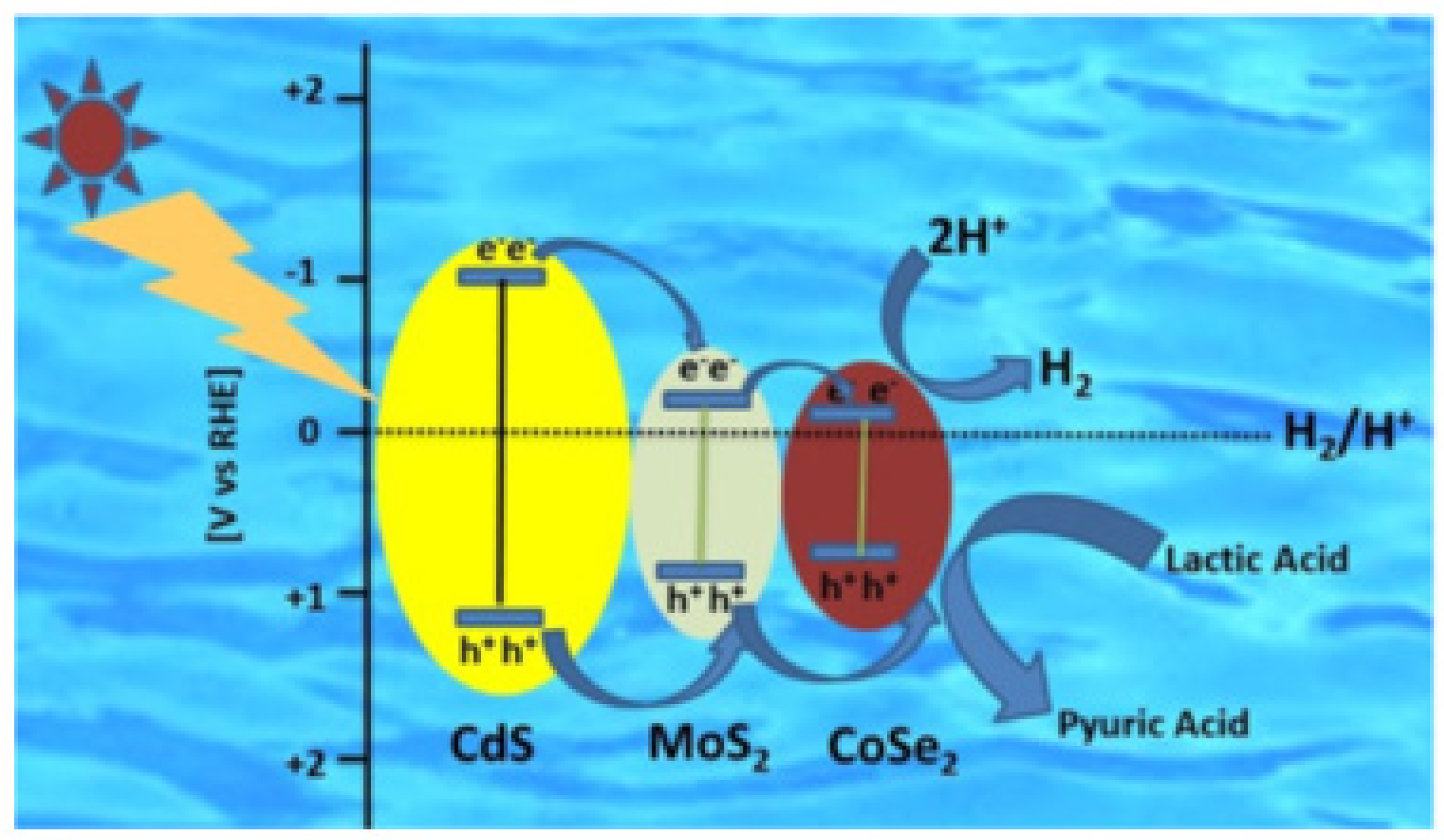
3.2. Photocatalytic Hydrogen Evolution
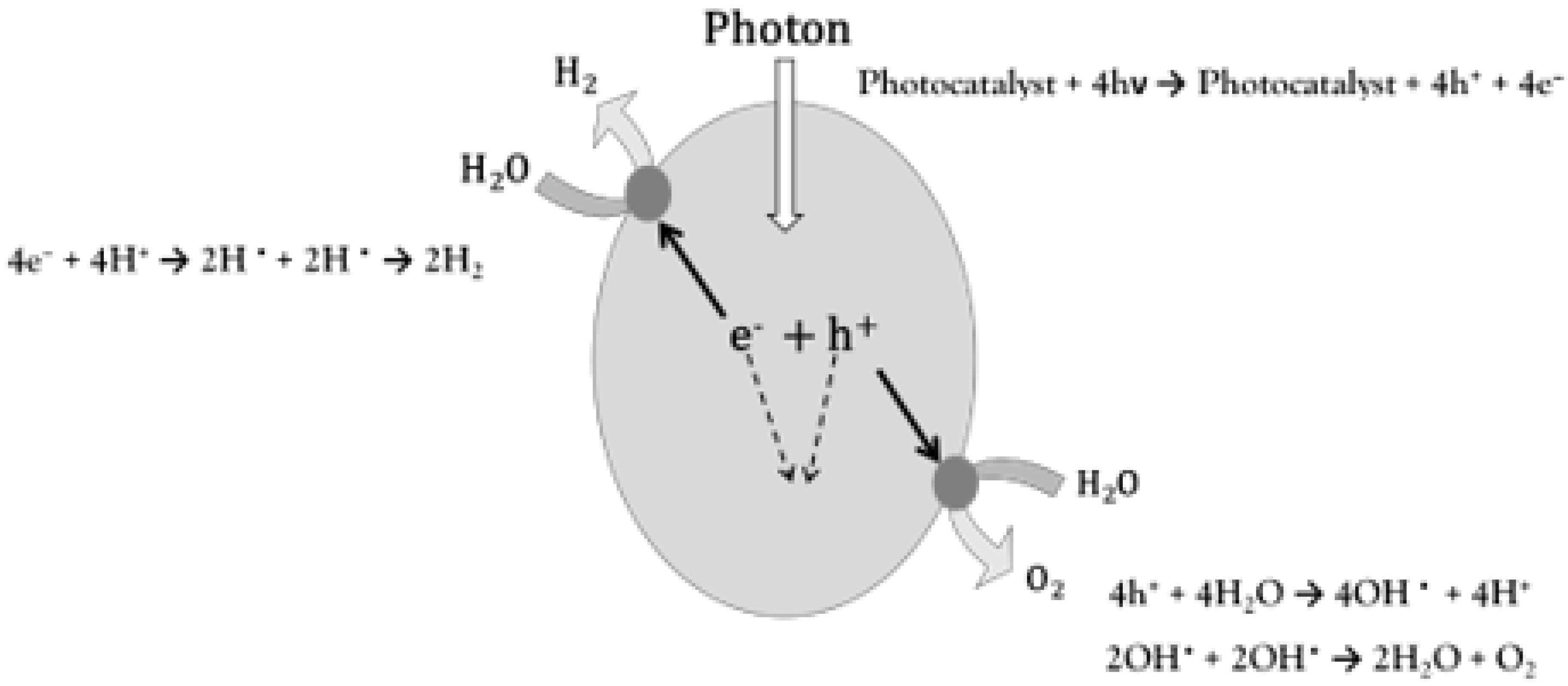
3.2.1. Principle and Mechanism of Photocatalytic Hydrogen Evolution
3.2.2. Measurements of the Rate of Hydrogen Evolution (Quantum Yield)
4. Summary
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Ma, Q.; Hu, C.; Liu, K.; Hung, S.-F.; Ou, D.; Chen, H.M.; Fu, G.; Zheng, N. Identifying the electrocatalytic sites of nickel disulfide in alkaline hydrogen evolution reaction. Nano Energy 2017, 41, 148–153. [Google Scholar] [CrossRef]
- Bai, J.; Meng, T.; Guo, D.; Wang, S.; Mao, B.; Cao, M. Co9S8@MoS2 Core–Shell Heterostructures as Trifunctional Electrocatalysts for Overall Water Splitting and Zn–Air Batteries. ACS Appl. Mater. Interfaces 2018, 10, 1678–1689. [Google Scholar] [CrossRef] [PubMed]
- Naik, S.G.; Rabinal, M.H.K. Molybdenum Disulphide Heterointerfaces as Potential Materials for Solar Cells, Energy Storage, and Hydrogen Evolution. Energy Technol. 2020, 8. [Google Scholar] [CrossRef]
- Feng, K.; Huang, D.; Li, L.; Wang, K.; Li, J.; Harada, T.; Ikeda, S.; Jiang, F. MoSx-CdS/Cu2ZnSnS4-based thin film photocathode for solar hydrogen evolution from water. Appl. Catal. Environ. 2020, 268, 118438. [Google Scholar] [CrossRef]
- Züttel, A. Hydrogen storage methods. Naturwissenschaften 2004, 91, 157–172. [Google Scholar] [CrossRef] [PubMed]
- Züttel, A. Materials for hydrogen storage. Mater. Today 2003, 6, 24–33. [Google Scholar] [CrossRef]
- Paquin, F.; Rivnay, J.; Salleo, A.; Stingelin, N.; Silva, C. Multi-phase semicrystalline microstructures drive exciton dissociation in neat plastic semiconductors. J. Mater. Chem. 2015, 3, 10715–10722. [Google Scholar] [CrossRef]
- Lin, L.; Sherrell, P.; Liu, Y.; Lei, W.; Zhang, S.; Zhang, H.; Wallace, G.G.; Chen, J. Engineered 2D Transition Metal Dichalcogenides—A Vision of Viable Hydrogen Evolution Reaction Catalysis. Adv. Energy Mater. 2020, 10. [Google Scholar] [CrossRef]
- Kudo, A.; Miseki, Y. Heterogeneous photocatalyst materials for water splitting. Chem. Soc. Rev. 2009, 38, 253–278. [Google Scholar] [CrossRef] [PubMed]
- Nikolaidis, P.; Poullikkas, A. A comparative overview of hydrogen production processes. Renew. Sustain. Energy Rev. 2017, 67, 597–611. [Google Scholar] [CrossRef]
- Dincer, I. Green methods for hydrogen production. Int. J. Hydrogen Energy 2012, 37, 1954–1971. [Google Scholar] [CrossRef]
- Ma, L.-C.; Dominguez, B.C.; Kazantzis, N.K.; Ma, Y.H. Integration of membrane technology into hydrogen production plants with CO2 capture: An economic performance assessment study. Int. J. Greenh. Gas Control. 2015, 42, 424–438. [Google Scholar] [CrossRef] [Green Version]
- Luo, P.; Zhang, H.; Liu, L.; Zhang, Y.; Deng, J.; Xu, C.; Hu, N.; Wang, Y. Targeted Synthesis of Unique Nickel Sulfide (NiS, NiS2) Microarchitectures and the Applications for the Enhanced Water Splitting System. ACS Appl. Mater. Interfaces 2017, 9, 2500–2508. [Google Scholar] [CrossRef] [PubMed]
- Leung, D.Y.C.; Fu, X.; Wang, C.; Ni, M.; Leung, M.K.H.; Wang, X.; Fu, X. Hydrogen Production over Titania-Based Photocatalysts. ChemSusChem 2010, 3, 681–694. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.; Jin, Z.; Hou, F. Enhanced solar water-splitting efficiency using core/sheath heterostructure CdS/TiO2 nanotube arrays. Nanotechnology 2007, 18, 495608. [Google Scholar] [CrossRef]
- Kim, S.B.; Hong, S.C. Kinetic study for photocatalytic degradation of volatile organic compounds in air using thin film TiO2 photocatalyst. Appl. Catal. Environ. 2002, 35, 305–315. [Google Scholar] [CrossRef]
- Matthews, R. Photooxidative degradation of coloured organics in water using supported catalysts. TiO2 on sand. Water Res. 1991, 25, 1169–1176. [Google Scholar] [CrossRef]
- Chakrabarti, S.; Dutta, B.K. Photocatalytic degradation of model textile dyes in wastewater using ZnO as semiconductor catalyst. J. Hazard. Mater. 2004, 112, 269–278. [Google Scholar] [CrossRef]
- Mugunthan, E.; Saidutta, M.B.; JagadeeshBabu, P.E. Photocatalytic degradation of diclofenac using TiO2–SnO2 mixed oxide catalysts. Environ. Technol. 2019, 40, 929–941. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.G.; Hwang, D.W.; Kim, J.; Kim, Y.G.; Lee, J.S. Highly donor-doped (110) layered perovskite materials as novel photocatalysts for overall water splitting. Chem. Commun. 1999, 2, 1077–1078. [Google Scholar] [CrossRef] [Green Version]
- Kanazawa, T.; Nozawa, S.; Lu, D.; Maeda, K. Structure and Photocatalytic Activity of PdCrOx Cocatalyst on SrTiO3 for Overall Water Splitting. Catalysts 2019, 9, 59. [Google Scholar] [CrossRef] [Green Version]
- Kong, C.; Min, S.; Lu, G. Dye-Sensitized NiSx Catalyst Decorated on Graphene for Highly Efficient Reduction of Water to Hydrogen under Visible Light Irradiation. ACS Catal. 2014, 4, 2763–2769. [Google Scholar] [CrossRef]
- Zhang, W.; Xiao, X.; Zeng, X.; Li, Y.; Zheng, L.; Wan, C. Enhanced photocatalytic activity of TiO2 nanoparticles using SnS2/RGO hybrid as co-catalyst: DFT study and photocatalytic mechanism. J. Alloys Compd. 2016, 685, 774–783. [Google Scholar] [CrossRef]
- Jafari, T.; Moharreri, E.; Amin, A.S.; Miao, R.; Song, W.; Suib, S.L. Photocatalytic Water Splitting—The Untamed Dream: A Review of Recent Advances. Molecules 2016, 21, 900. [Google Scholar] [CrossRef]
- Li, Y.; Zhou, X.; Xing, Y. In situ thermal-assisted loading of monodispersed Pt nanoclusters on CdS nanoflowers for efficient photocatalytic hydrogen evolution. Appl. Surf. Sci. 2020, 506, 144933. [Google Scholar] [CrossRef]
- Zheng, L.; Zhang, W.; Xiao, X. Preparation of titanium dioxide/tungsten disulfide composite photocatalysts with enhanced photocatalytic activity under visible light. Korean J. Chem. Eng. 2015, 33, 107–113. [Google Scholar] [CrossRef]
- Wang, Q.; Huang, J.; Sun, H.; Ng, Y.H.; Zhang, K.-Q.; Lai, Y. MoS2 Quantum Dots@TiO2 Nanotube Arrays: An Extended-Spectrum-Driven Photocatalyst for Solar Hydrogen Evolution. ChemSusChem 2018, 11, 1708–1721. [Google Scholar] [CrossRef]
- Ali, A.; Oh, W.-C. Photocatalytic Performance of CoS2-Graphene-TiO2 Ternary Composites for Reactive Black B (RBB) Degradation. J. Korean Ceram. Soc. 2017, 54, 308–313. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Wang, H.; Xie, L.; Liang, Y.; Hong, G.; Dai, H. MoS2 Nanoparticles Grown on Graphene: An Advanced Catalyst for the Hydrogen Evolution Reaction. J. Am. Chem. Soc. 2011, 133, 7296–7299. [Google Scholar] [CrossRef] [Green Version]
- He, K.; Xie, J.; Li, M.; Li, X. In situ one-pot fabrication of g-C3N4 nanosheets/NiS cocatalyst heterojunction with intimate interfaces for efficient visible light photocatalytic H2 generation. Appl. Surf. Sci. 2018, 430, 208–217. [Google Scholar] [CrossRef]
- Jing, D.; Guo, L. WS2 sensitized mesoporous TiO2 for efficient photocatalytic hydrogen production from water under visible light irradiation. Catal. Commun. 2007, 8, 795–799. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, X.; Wang, Z.; Lv, X.; Jia, H.; Kong, J.; Yu, M. CdS and SnS2 nanoparticles co-sensitized TiO2 nanotube arrays and the enhanced photocatalytic property. J. Photochem. Photobiol. Chem. 2016, 325, 55–61. [Google Scholar] [CrossRef]
- Ma, D.; Shi, J.-W.; Zou, Y.; Fan, Z.; Ji, X.; Niu, C. Highly Efficient Photocatalyst Based on a CdS Quantum Dots/ZnO Nanosheets 0D/2D Heterojunction for Hydrogen Evolution from Water Splitting. ACS Appl. Mater. Interfaces 2017, 9, 25377–25386. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.-P.; Cao, S.-W.; Yin, L.-S.; Xu, L.; Xue, C. NiS2 Co-catalyst decoration on CdLa2S4 nanocrystals for efficient photocatalytic hydrogen generation under visible light irradiation. Int. J. Hydrogen Energy 2013, 38, 7218–7223. [Google Scholar] [CrossRef]
- Sun, B.; Liu, A.; Li, J.; Wang, J.; Wang, S. Development of novel highly stable synergistic quaternary photocatalyst for the efficient hydrogen evolution reaction. Appl. Surf. Sci. 2020, 510, 145498. [Google Scholar] [CrossRef]
- Makwana, N.M.; Tighe, C.J.; Gruar, R.I.; McMillan, P.F.; Darr, J.A. Pilot plant scale continuous hydrothermal synthesis of nano-titania; effect of size on photocatalytic activity. Mater. Sci. Semicond. Process. 2016, 42, 131–137. [Google Scholar] [CrossRef]
- Huerta-Flores, A.M.; Torres-Martínez, L.M.; Moctezuma, E.; Singh, A.P.; Wickman, B. Green synthesis of earth-abundant metal sulfides (FeS2, CuS, and NiS2) and their use as visible-light active photocatalysts for H2 generation and dye removal. J. Mater. Sci. Mater. Electron. 2018, 29, 11613–11626. [Google Scholar] [CrossRef]
- Yu, J.; Xu, C.; Ma, F.; Hu, S.-P.; Zhang, Y.-W.; Zhen, L. Monodisperse SnS2 Nanosheets for High-Performance Photocatalytic Hydrogen Generation. ACS Appl. Mater. Interfaces 2014, 6, 22370–22377. [Google Scholar] [CrossRef]
- Xu, K.; Wang, F.; Wang, Z.; Zhan, X.; Wang, Q.; Cheng, Z.; Safdar, M.; He, J. Component-Controllable WS2(1–x)Se2x Nanotubes for Efficient Hydrogen Evolution Reaction. ACS Nano 2014, 8, 8468–8476. [Google Scholar] [CrossRef]
- Voiry, D.; Salehi, M.; Silva, R.; Fujita, T.; Chen, M.; Asefa, T.; Shenoy, V.B.; Eda, G.; Chhowalla, M. Conducting MoS2 Nanosheets as Catalysts for Hydrogen Evolution Reaction. Nano Lett. 2013, 13, 6222–6227. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Liu, C.; Grauer, D.C.; Yano, J.; Long, J.R.; Yang, P.; Chang, C.J. Electrodeposited Cobalt-Sulfide Catalyst for Electrochemical and Photoelectrochemical Hydrogen Generation from Water. J. Am. Chem. Soc. 2013, 135, 17699–17702. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.; Wang, H.; Peng, S. Tunable Photodeposition of MoS2 onto a Composite of Reduced Graphene Oxide and CdS for Synergic Photocatalytic Hydrogen Generation. J. Phys. Chem. C 2014, 118, 19842–19848. [Google Scholar] [CrossRef]
- Liu, J.-H.; Huang, G.-F.; Huang, W.-Q.; Miao, H.; Zhou, B.-X. Morphology-controlled SnS2 nanostructures synthesized by refluxing method with high photocatalytic activity. Mater. Lett. 2015, 161, 480–483. [Google Scholar] [CrossRef]
- Wang, Q.; Lei, Y.; Wang, Y.; Liu, Y.; Song, C.; Zeng, J.; Song, Y.; Duan, X.; Wang, D.; Li, Y. Atomic-scale engineering of chemical-vapor-deposition-grown 2D transition metal dichalcogenides for electrocatalysis. Energy Environ. Sci. 2020, 13, 1593–1616. [Google Scholar] [CrossRef]
- Luo, M.; Liu, Y.; Hu, J.; Liu, H.; Li, J. One-Pot Synthesis of CdS and Ni-Doped CdS Hollow Spheres with Enhanced Photocatalytic Activity and Durability. ACS Appl. Mater. Interfaces 2012, 4, 1813–1821. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, L.; Yao, W.; Song, S.; Sun, J.T.; Pan, J.; Ren, X.; Li, C.; Okunishi, E.; Wang, Y.-Q.; et al. Monolayer PtSe2, a New Semiconducting Transition-Metal-Dichalcogenide, Epitaxially Grown by Direct Selenization of Pt. Nano Lett. 2015, 15, 4013–4018. [Google Scholar] [CrossRef] [PubMed]
- Sarwar, S.; Nautiyal, A.; Cook, J.; Yuan, Y.; Li, J.; Uprety, S.; Shahbazian-Yassar, R.; Wang, R.; Park, M.; Bozack, M.J.; et al. Facile microwave approach towards high performance MoS2/graphene nanocomposite for hydrogen evolution reaction. Sci. China Mater. 2020, 63, 62–74. [Google Scholar] [CrossRef] [Green Version]
- Jiao, F.; Yen, H.; Hutchings, G.S.; Yonemoto, B.; Lua, Q.; Kleitz, F. Synthesis, structural characterization, and electrochemical performance of nanocast mesoporous Cu-/Fe-based oxides. J. Mater. Chem. A 2014, 2, 3065–3071. [Google Scholar] [CrossRef]
- Panigrahi, P.K.; Pathak, A. Microwave-assisted synthesis of WS2 nanowires through tetrathiotungstate precursors. Sci. Technol. Adv. Mater. 2008, 9, 045008. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Su, Y.; Chen, P.; Wang, Y. Microwave-assisted solvothermal synthesis of 3D carnation-like SnS2 nanostructures with high visible light photocatalytic activity. J. Mol. Catal. Chem. 2013, 378, 285–292. [Google Scholar] [CrossRef]
- Rao, B.G.; Mukherjee, D.; Reddy, B.M. Novel Approaches for Preparation of Nanoparticles; Elsevier Inc.: Amsterdam, The Netherlands, 2017; ISBN 9780323461481. [Google Scholar]
- Kumar, A. Different Methods Used for the Synthesis of TiO2 Based Nanomaterials: A Review. Am. J. Nano Res. Appl. 2018, 6, 1–10. [Google Scholar] [CrossRef]
- Aliofkhazraei, M. (Ed.) Handbook of Nanoparticles; Springer International Publishing: Cham, Switzerland, 2016. [Google Scholar]
- Pokrovski, G.S.; Roux, J.; Hazemann, J.-L.; Borisova, A.; Gonchar, A.A.; Lemeshko, M.P. In situ X-ray absorption spectroscopy measurement of vapour-brine fractionation of antimony at hydrothermal conditions. Miner. Mag. 2008, 72, 667–681. [Google Scholar] [CrossRef]
- Gautam, A.K.; Faraz, M.; Khare, N. Enhanced thermoelectric properties of MoS2 with the incorporation of reduced graphene oxide (RGO). J. Alloys Compd. 2020, 838, 155673. [Google Scholar] [CrossRef]
- Bricha, M.; Belmamouni, Y.; Essassi, E.M.; Ferreira, J.; El Mabrouk, K. Surfactant-Assisted Hydrothermal Synthesis of Hydroxyapatite Nanopowders. J. Nanosci. Nanotechnol. 2012, 12, 8042–8049. [Google Scholar] [CrossRef]
- Cao, S.; Liu, T.; Zeng, W.; Hussain, S.; Peng, X.; Pan, F. Synthesis and characterization of flower-like WS2 nanospheres via a facile hydrothermal route. J. Mater. Sci. Mater. Electron. 2014, 25, 4300–4305. [Google Scholar] [CrossRef]
- Naskar, B.; Dan, A.; Ghosh, S.; Aswal, V.; Moulik, S.P. Revisiting the self-aggregation behavior of cetyltrimethylammonium bromide in aqueous sodium salt solution with varied anions. J. Mol. Liq. 2012, 170, 1–10. [Google Scholar] [CrossRef]
- Vollath, D.; Szabó, D.V. Synthesis of nanocrystalline MoS2 and WS2 in a microwave plasma. Mater. Lett. 1998, 35, 236–244. [Google Scholar] [CrossRef]
- Shanmugaratnam, S.; Rasalingam, S. Transition Metal Chalcogenide (TMC) Nanocomposites for Environmental Remediation Application over Extended Solar Irradiation. In Nanocatalysts; IntechOpen: London, UK, 2012. [Google Scholar] [CrossRef]
- Park, S.; Park, J.; Selvaraj, R.; Kim, Y. Facile microwave-assisted synthesis of SnS2 nanoparticles for visible-light responsive photocatalyst. J. Ind. Eng. Chem. 2015, 31, 269–275. [Google Scholar] [CrossRef]
- Choubey, S.K.; Tiwary, K.P. Microwave assisted synthesis of CdS nanoparticles for structural and optical characterization. IJIRSET 2014, 3, 10670–10674. [Google Scholar]
- Nethravathi, C.; Rajamathi, J.T.; Rajamathi, M. Microwave-Assisted Synthesis of Porous Aggregates of CuS Nanoparticles for Sunlight Photocatalysis. ACS Omega 2019, 4, 4825–4831. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pang, H.; Wei, C.; Li, X.; Li, G.; Ma, Y.; Li, S.; Chen, J.; Zhang, J. Microwave-assisted synthesis of NiS2 nanostructures for supercapacitors and cocatalytic enhancing photocatalytic H2 production. Sci. Rep. 2014, 4, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Amaresh, S.; Karthikeyan, K.; Jang, I.-C.; Lee, Y.S. Single-step microwave mediated synthesis of the CoS2 anode material for high rate hybrid supercapacitors. J. Mater. Chem. A 2014, 2, 11099–11106. [Google Scholar] [CrossRef]
- Liu, B.; Qu, S.; Kou, Y.; Liu, Z.; Chen, X.; Wu, Y.; Han, X.; Deng, Y.; Hu, W.; Zhong, C. In Situ Electrodeposition of Cobalt Sulfide Nanosheet Arrays on Carbon Cloth as a Highly Efficient Bifunctional Electrocatalyst for Oxygen Evolution and Reduction Reactions. ACS Appl. Mater. Interfaces 2018, 10, 30433–30440. [Google Scholar] [CrossRef] [PubMed]
- Ghahremaninezhad, A.; Asselin, E.; Dixon, D.G. Electrodeposition and Growth Mechanism of Copper Sulfide Nanowires. J. Phys. Chem. C 2011, 115, 9320–9334. [Google Scholar] [CrossRef]
- Mammadov, M.N.; Sh Aliyev, A.; Elrouby, M. Electrodeposition of cadmium sulfide. Int. J. Thin Film Sci. Tec. 2012, 1, 43–53. [Google Scholar]
- Huang, J.; Daryadel, S.; Minary-Jolandan, M. Low-Cost Manufacturing of Metal–Ceramic Composites through Electrodeposition of Metal into Ceramic Scaffold. ACS Appl. Mater. Interfaces 2019, 11, 4364–4372. [Google Scholar] [CrossRef]
- Maharana, H.; Basu, A.; Mondal, K. Structural and tribological correlation of electrodeposited solid lubricating Ni-WSe2 composite coating. Surf. Coatings Technol. 2018, 349, 328–339. [Google Scholar] [CrossRef]
- Safavi, M.S.; Fathi, M.; Charkhesht, V.; Jafarpour, M.; Ahadzadeh, I. Electrodeposition of Co-P Coatings Reinforced by MoS2 + Y2O3 Hybrid Ceramic Nanoparticles for Corrosion-Resistant Applications: Influences of Operational Parameters. Met. Mater. Trans. A 2020, 51, 6740–6758. [Google Scholar] [CrossRef]
- Jayakrishnan, D.S. Electrodeposition: The Versatile Technique for Nanomaterials; Woodhead Publishing Limited: Sawston, UK, 2012. [Google Scholar]
- Aruna, S.; Bindu, C.; Selvi, V.E.; Grips, V.W.; Rajam, K. Synthesis and properties of electrodeposited Ni/ceria nanocomposite coatings. Surf. Coatings Technol. 2006, 200, 6871–6880. [Google Scholar] [CrossRef]
- Shariza, S.; Anand, T.J.S. Effect of deposition time on the structural and optical properties of molybdenum chalcogenides thin films. Chalcogenide Lett. 2011, 8, 529–539. [Google Scholar]
- Chen, Y.-C.; Huang, Y.-S.; Huang, H.; Su, P.-J.; Perng, T.-P.; Chen, L.-J. Photocatalytic enhancement of hydrogen production in water splitting under simulated solar light by band gap engineering and localized surface plasmon resonance of ZnxCd1−xS nanowires decorated by Au nanoparticles. Nano Energy 2020, 67, 104225. [Google Scholar] [CrossRef]
- Sankapal, B.R.; Mane, R.S.; Lokhande, C.D. Successive ionic layer adsorption and reaction (SILAR) method for the deposition of large area (~10 cm2) tin disulfide (SnS2) thin films. Mater. Res. Bull. 2000, 35, 2027–2035. [Google Scholar] [CrossRef]
- Pathan, H.M.; Lokhande, C.D. Deposition of metal chalcogenide thin films by successive ionic layer adsorption and reaction (SILAR) method. Bull. Mater. Sci 2004, 27, 85–111. [Google Scholar]
- Leskell, M. Deposition of manganese-doped successive ionic layer adsorption zinc sulfide thin films by the and reaction (SILAR) method. Thin Solid Film. 1995, 263, 79–84. [Google Scholar]
- Lindroos, S.; Kanniainen, T.; Leskelä, M. Growth of zinc sulfide thin films by the successive ionic layer adsorption and reaction (SILAR) method on polyester substrates. Mater. Res. Bull. 1997, 32, 1631–1636. [Google Scholar] [CrossRef]
- Valkonen, M.P.; Kanniainen, T.; Lindroos, S.; Leskelä, M.; Rauhala, E. Growth of ZnS, CdS and multilayer ZnS/CdS thin films by SILAR technique. Appl. Surf. Sci. 1997, 115, 386–392. [Google Scholar] [CrossRef]
- Aditha, S.K.; Kurdekar, A.D.; Chunduri, L.A.A.; Patnaik, S.; Kamisetti, V. Aqueous based reflux method for green synthesis of nanostructures: Application in CZTS synthesis. MethodsX 2016, 3, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Phuruangrat, A.; Thoonchalong, P.; Thongtem, S.; Thongtem, T. Synthesis of cus with different morphologies by refluxing method: Nanopaticles in clusters and nanoflakes in sponge-like clusters. Chalcogenide Lett. 2012, 9, 421–426. [Google Scholar]
- Wang, L.-C.; Bao, S.-K.; Luo, J.; Wang, Y.-H.; Nie, Y.-C.; Zou, J.-P. Efficient exfoliation of bulk MoS2 to nanosheets by mixed-solvent refluxing method. Int. J. Hydrogen Energy 2016, 41, 10737–10743. [Google Scholar] [CrossRef]
- Phuruangrat, A.; Karthik, K.; Kuntalue, B.; Dumrongrojthanath, P.; Thongtem, S.; Thongtem, T.; Yai, H.; Nadu, T.; Science, M. Refuxing synthesis and characterization of zns nanoparticles and their photocatalytic properties. Chalcogenide Lett. 2019, 16, 387–393. [Google Scholar]
- Dai, Z.; Zang, X.; Yang, J.; Sun, C.; Si, W.; Huang, W.; Dong, X. Template Synthesis of Shape-Tailorable NiS2 Hollow Prisms as High-Performance Supercapacitor Materials. ACS Appl. Mater. Interfaces 2015, 7, 25396–25401. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Yang, X.; Liu, Y.; Huang, B.; Wu, R.; Zhang, Z.; Zhao, B.; Ma, H.; Dang, W.; Wei, Z.; et al. General synthesis of two-dimensional van der Waals heterostructure arrays. Nat. Cell Biol. 2020, 579, 368–374. [Google Scholar] [CrossRef] [PubMed]
- Gong, Y.; Lin, J.; Wang, X.; Shi, G.; Lei, S.; Lin, Z.; Zou, X.; Ye, G.; Vajtai, R.; Yakobson, B.I.; et al. Vertical and in-plane heterostructures from WS2/MoS2 monolayers. Nat. Mater. 2014, 13, 1135–1142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hao, S.; Yang, B.; Gao, Y. Orientation-specific transgranular fracture behavior of CVD-grown monolayer MoS2 single crystal. Appl. Phys. Lett. 2017, 110, 153105. [Google Scholar] [CrossRef]
- Kim, S.; Kwak, J.; Ciobanu, C.V.; Kwon, S. Recent Developments in Controlled Vapor-Phase Growth of 2D Group 6 Transition Metal Dichalcogenides. Adv. Mater. 2019, 31, e1804939. [Google Scholar] [CrossRef]
- Tan, H.; Fan, Y.; Zhou, Y.; Chen, Q.; Xu, W.; Warner, J.H. Ultrathin 2D Photodetectors Utilizing Chemical Vapor Deposition Grown WS2 With Graphene Electrodes. ACS Nano 2016, 10, 7866–7873. [Google Scholar] [CrossRef] [PubMed]
- Chang, R.-J.; Tan, H.; Wang, X.; Porter, B.; Chen, T.; Sheng, Y.; Zhou, Y.; Huang, H.; Bhaskaran, H.; Warner, J.H. High-Performance All 2D-Layered Tin Disulfide: Graphene Photodetecting Transistors with Thickness-Controlled Interface Dynamics. ACS Appl. Mater. Interfaces 2018, 10, 13002–13010. [Google Scholar] [CrossRef] [PubMed]
- Cong, C.; Shang, J.; Wu, X.; Cao, B.; Peimyoo, N.; Qiu, C.; Sun, L.; Yu, T. Synthesis and Optical Properties of Large-Area Single-Crystalline 2D Semiconductor WS2Monolayer from Chemical Vapor Deposition. Adv. Opt. Mater. 2014, 2, 131–136. [Google Scholar] [CrossRef]
- Ye, G.; Gong, Y.; Lei, S.; He, Y.; Li, B.; Zhang, X.; Jin, Z.; Dong, L.; Lou, J.; Vajtai, R.; et al. Synthesis of large-scale atomic-layer SnS2 through chemical vapor deposition. Nano Res. 2017, 10, 2386–2394. [Google Scholar] [CrossRef]
- Dai, C.; Li, B.; Li, J.; Zhao, B.; Wu, R.; Ma, H.; Duan, X. Controllable synthesis of NiS and NiS2 nanoplates by chemical vapor deposition. Nano Res. 2020, 13, 2506–2511. [Google Scholar] [CrossRef]
- Ardahe, M.; Hantehzadeh, M.R.; Ghoranneviss, M. Effect of Growth Temperature on Physical Properties of MoS2 Thin Films Synthesized by CVD. J. Electron. Mater. 2019, 49, 1002–1008. [Google Scholar] [CrossRef]
- Samad, L.; Cabán-Acevedo, M.; Shearer, M.; Park, K.; Hamers, R.; Jin, S. Direct Chemical Vapor Deposition Synthesis of Phase-Pure Iron Pyrite (FeS2) Thin Films. Chem. Mater. 2015, 27, 3108–3114. [Google Scholar] [CrossRef]
- Backes, C.; Szydłowska, B.M.; Harvey, A.; Yuan, S.; Vega-Mayoral, V.; Davies, B.R.; Zhao, P.-L.; Hanlon, D.; Santos, E.J.G.; Katsnelson, M.I.; et al. Production of Highly Monolayer Enriched Dispersions of Liquid-Exfoliated Nanosheets by Liquid Cascade Centrifugation. ACS Nano 2016, 10, 1589–1601. [Google Scholar] [CrossRef]
- Mihai, C.; Sava, F.; Galca, A.C.; Velea, A. Low power non-volatile memory switching in monolayer-rich 2D WS2 and MoS2 devices. AIP Adv. 2020, 10, 025102. [Google Scholar] [CrossRef] [Green Version]
- Chen, T.-Y.; Chang, Y.-H.; Hsu, C.-L.; Wei, K.-H.; Chiang, C.-Y.; Li, L.-J. Comparative study on MoS2 and WS2 for electrocatalytic water splitting. Int. J. Hydrogen Energy 2013, 38, 12302–12309. [Google Scholar] [CrossRef]
- Ma, L.; Chen, W.-X.; Xu, Z.-D.; Xia, J.-B.; Li, X. Carbon nanotubes coated with tubular MoS2layers prepared by hydrothermal reaction. Nanotechnology 2006, 17, 571–574. [Google Scholar] [CrossRef]
- Li, Q.; Zhang, N.; Yang, Y.; Wang, G.; Ng, D.H.L. High Efficiency Photocatalysis for Pollutant Degradation with MoS2/C3N4 Heterostructures. Langmuir 2014, 30, 8965–8972. [Google Scholar] [CrossRef] [PubMed]
- Lukowski, M.A.; Daniel, A.S.; Meng, F.; Forticaux, A.; Li, L.; Jin, S. Enhanced Hydrogen Evolution Catalysis from Chemically Exfoliated Metallic MoS2 Nanosheets. J. Am. Chem. Soc. 2013, 135, 10274–10277. [Google Scholar] [CrossRef]
- Peng, Y.; Meng, Z.; Zhong, C.; Lu, J.; Yu, W.; Jia, Y.; Qian, Y. Hydrothermal synthesis and characterization of single-molecular-layer MoS2 and MoSe2. Chem. Lett. 2001, 30, 772–773. [Google Scholar] [CrossRef]
- Afanasiev, P.; Xia, G.-F.; Berhault, G.; Jouguet, B.; Lacroix, M. Surfactant-Assisted Synthesis of Highly Dispersed Molybdenum Sulfide. Chem. Mater. 1999, 11, 3216–3219. [Google Scholar] [CrossRef]
- Shanmugaratnam, S.; Velauthapillai, D.; Ravirajan, P.; Christy, A.A.; Shivatharsiny, Y. CoS2/TiO2 Nanocomposites for Hydrogen Production under UV Irradiation. Materials 2019, 12, 3882. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shanmugaratnam, S.; Selvaratnam, B.; Baride, A.; Koodali, R.; Ravirajan, P.; Velauthapillai, D.; Shivatharsiny, Y. SnS2/TiO2 Nanocomposites for Hydrogen Production and Photodegradation under Extended Solar Irradiation. Catalysts 2021, 11, 589. [Google Scholar] [CrossRef]
- Cao, J.; Zhou, J.; Zhang, Y.; Wang, Y.; Liu, X. Dominating Role of Aligned MoS2/Ni3S2 Nanoarrays Supported on Three-Dimensional Ni Foam with Hydrophilic Interface for Highly Enhanced Hydrogen Evolution Reaction. ACS Appl. Mater. Interfaces 2018, 10, 1752–1760. [Google Scholar] [CrossRef]
- Gao, B.; Du, X.; Ma, Y.; Li, Y.; Li, Y.; Ding, S.; Song, Z.; Xiao, C. 3D flower-like defected MoS2 magnetron-sputtered on candle soot for enhanced hydrogen evolution reaction. Appl. Catal. Environ. 2020, 263, 117750. [Google Scholar] [CrossRef]
- Nadeem Riaz, K.; Yousaf, N.; Bilal Tahir, M.; Israr, Z.; Iqbal, T. Facile hydrothermal synthesis of 3D flower-like La-MoS2 nanostructure for photocatalytic hydrogen energy production. Int. J. Energy Res. 2019, 43, 491–499. [Google Scholar] [CrossRef] [Green Version]
- Radisavljevic, B.; Radenovic, A.; Brivio, J.; Giacometti, V.; Kis, A. Single-layer MoS2 transistors. Nat. Nanotechnol. 2011, 6, 147–150. [Google Scholar] [CrossRef]
- Gaur, R.; Jeevanandam, P. Synthesis of SnS2 Nanoparticles and Their Application as Photocatalysts for the Reduction of Cr(VI). J. Nanosci. Nanotechnol. 2018, 18, 165–177. [Google Scholar] [CrossRef]
- Zhu, L.; Susac, D.; Teo, M.; Wong, K.; Wong, P.; Parsons, R.; Bizzotto, D.; Mitchell, K.; Campbell, S. Investigation of CoS2-based thin films as model catalysts for the oxygen reduction reaction. J. Catal. 2008, 258, 235–242. [Google Scholar] [CrossRef]
- Wang, J.; Yin, Y. Fermentative hydrogen production using pretreated microalgal biomass as feedstock. Microb. Cell Factories 2018, 17, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Kye, J.; Shin, M.; Lim, B.; Jang, J.-W.; Oh, I.; Hwang, S. Platinum Monolayer Electrocatalyst on Gold Nanostructures on Silicon for Photoelectrochemical Hydrogen Evolution. ACS Nano 2013, 7, 6017–6023. [Google Scholar] [CrossRef]
- Mckone, J.R.; Pieterick, A.P.; Gray, H.B.; Lewis, N.S. Supporting Information: Hydrogen Evolution from Pt /Ru-Coated p-Type WSe2 Photocathodes. J. Am. Chem. Soc. 2013, 135, 223–231. [Google Scholar] [CrossRef] [PubMed]
- Wiensch, J.D.; John, J.; Velazquez, J.M.; Torelli, D.A.; Pieterick, A.P.; McDowell, M.T.; Sun, K.; Zhao, X.; Brunschwig, B.S.; Lewis, N.S. Comparative Study in Acidic and Alkaline Media of the Effects of pH and Crystallinity on the Hydrogen-Evolution Reaction on MoS2 and MoSe2. ACS Energy Lett. 2017, 2, 2234–2238. [Google Scholar] [CrossRef] [Green Version]
- Sun, B.; Wang, H.; Wu, J.; Geng, Y.; Xu, J.; Wang, Y.; Li, Y.; Lin, H.; Wang, L. Designed synthesis of unique ZnS@CdS@Cd0.5Zn0.5S-MoS2 hollow nanospheres for efficient visible-light-driven H2 evolution. CrystEngComm 2020, 22, 2743–2755. [Google Scholar] [CrossRef]
- Komba, N.; Zhang, G.; Pu, Z.; Wu, M.; Rosei, F.; Sun, S. MoS2-supported on free-standing TiO2-nanotubes for efficient hydrogen evolution reaction. Int. J. Hydrogen Energy 2020, 45, 4468–4480. [Google Scholar] [CrossRef]
- Zhao, X.; Ma, X.; Sun, J.; Li, D.; Yang, X. Enhanced Catalytic Activities of Surfactant-Assisted Exfoliated WS2 Nanodots for Hydrogen Evolution. ACS Nano 2016, 10, 2159–2166. [Google Scholar] [CrossRef] [PubMed]
- Mao, Q.; Chen, J.; Chen, H.; Chen, Z.; Chen, J.; Li, Y. Few-layered 1T-MoS2-modified ZnCoS solid-solution hollow dodecahedra for enhanced photocatalytic hydrogen evolution. J. Mater. Chem. A 2019, 7, 8472–8484. [Google Scholar] [CrossRef]
- Oertel, M.; Schmitz, J.; Weirich, W.; Jendryssek-Neumann, D.; Schulten, R. Vapour-liquid Equilibrium Data Collec-tion’ dechema Frankfurt. Izv. Vyss. Uchebn. Zaved. Khim. Tekhnol. 1987, 27, 686–691. [Google Scholar]
- Kumar, S.S.; Himabindu, V. Hydrogen production by PEM water electrolysis—A review. Mater. Sci. Energy Technol. 2019, 2, 442–454. [Google Scholar] [CrossRef]
- Dehghanimadvar, M.; Shirmohammadi, R.; Sadeghzadeh, M.; Aslani, A.; Ghasempour, R. Hydrogen production technologies: Attractiveness and future perspective. Int. J. Energy Res. 2020, 44, 8233–8254. [Google Scholar] [CrossRef]
- FreedomCAR and Fuel Partnership Hydrogen Offers Sustainable Solutions to Our Nation’s Energy and Climate Challenges. Table of Contents Sustainability Urban Air Quality. 2009. Available online: https://www1.eere.energy.gov/hydrogenandfuelcells/pdfs/h2_tech_roadmap.pdf (accessed on 11 October 2021).
- Luo, X.; Wu, T.; Shi, K.; Song, M.; Rao, Y. Biomass Gasification: An Overview of Technological Barriers and Socio-Environmental Impact. Gasif. Low-Grade Feedstock 2018, 3–18. [Google Scholar] [CrossRef] [Green Version]
- Ding, Q.; Song, B.; Xu, P.; Jin, S. Efficient Electrocatalytic and Photoelectrochemical Hydrogen Generation Using MoS2 and Related Compounds. Chem 2016, 1, 699–726. [Google Scholar] [CrossRef] [Green Version]
- Chu, D.; Zhang, C.; Yang, P.; Du, Y.; Lu, C. WS2 as an Effective Noble-Metal Free Cocatalyst Modified TiSi2 for Enhanced Photocatalytic Hydrogen Evolution under Visible Light Irradiation. Catalysts 2016, 6, 136. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Hu, W.; Cao, S.; Piao, L. Recent progress for hydrogen production by photocatalytic natural or simulated seawater splitting. Nano Res. 2020, 13, 2313–2322. [Google Scholar] [CrossRef]
- Liu, J.; Yu, G.; Zhang, R.; Huang, X.; Chen, W. Theoretical predication of the high hydrogen evolution catalytic activity for the cubic and tetragonal SnP systems. Phys. Chem. Chem. Phys. 2019, 21, 5521–5530. [Google Scholar] [CrossRef] [PubMed]
- Som, N.N.; Jha, P.K. Hydrogen evolution reaction of metal di-chalcogenides: ZrS2, ZrSe2 and Janus ZrSSe. Int. J. Hydrogen Energy 2020, 45, 23920–23927. [Google Scholar] [CrossRef]
- Wang, B.; Wang, X.; Yuan, H.; Zhou, T.; Chang, J.; Chen, H. Direct Z-scheme photocatalytic overall water splitting on two dimensional MoSe2/SnS2 heterojunction. Int. J. Hydrogen Energy 2020, 45, 2785–2793. [Google Scholar] [CrossRef]
- Park, H.; Akande, A.; Sanvito, S.; El-Mellouhi, F. The rise of Nb-, Ta-, and Bi-based oxides/chalcogenides for photocatalytic applications. Int. J. Hydrogen Energy 2021, 1–13. [Google Scholar] [CrossRef]
- Ran, N.; Qiu, W.; Song, E.; Wang, Y.; Zhao, X.; Liu, Z.; Liu, J. Bond Electronegativity as Hydrogen Evolution Reaction Catalyst Descriptor for Transition Metal (TM = Mo, W) Dichalcogenides. Chem. Mater. 2020, 32, 1224–1234. [Google Scholar] [CrossRef]
- Faraji, M.; Yousefi, M.; Yousefzadeh, S.; Zirak, M.; Naseri, N.; Jeon, T.H.; Choi, W.; Moshfegh, A.Z. Two-dimensional materials in semiconductor photoelectrocatalytic systems for water splitting. Energy Environ. Sci. 2019, 12, 59–95. [Google Scholar] [CrossRef]
- Huang, D.; Wen, M.; Zhou, C.; Li, Z.; Cheng, M.; Chen, S.; Xue, W.; Lei, L.; Yang, Y.; Xiong, W.; et al. ZnxCd1−xS based materials for photocatalytic hydrogen evolution, pollutants degradation and carbon dioxide reduction. Appl. Catal. Environ. 2020, 267, 118651. [Google Scholar] [CrossRef]
- Kudo, A.; Kato, H.; Tsuji, I. Strategies for the Development of Visible-light-driven Photocatalysts for Water Splitting. Chem. Lett. 2004, 33, 1534–1539. [Google Scholar] [CrossRef]
- Li, K.; Li, Y.; Wang, Y.; Ge, J.; Liu, C.; Xing, W. Enhanced electrocatalytic performance for the hydrogen evolution reaction through surface enrichment of platinum nanoclusters alloying with ruthenium in situ embedded in carbon. Energy Environ. Sci. 2018, 11, 1232–1239. [Google Scholar] [CrossRef]
- Ahn, S.; Yang, J.; Lim, H.; Shin, H.S. Selective synthesis of pure cobalt disulfide on reduced graphene oxide sheets and its high electrocatalytic activity for hydrogen evolution reaction. Nano Converg. 2016, 3, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, C.; Hua, H.; Du, M.; Liu, J.; Wu, X.; Pu, Y.; Li, X. 1T/2H MoS2 nanoflowers decorated amorphous Mo-CoSx skeleton: A ZIF-based composite electrocatalyst for the hydrogen evolution reaction. Appl. Surf. Sci. 2020, 515, 145842. [Google Scholar] [CrossRef]
- Bhat, K.S.; Nagaraja, H. Performance evaluation of molybdenum dichalcogenide (MoX2; X= S, Se, Te) nanostructures for hydrogen evolution reaction. Int. J. Hydrogen Energy 2019, 44, 17878–17886. [Google Scholar] [CrossRef]
- Li, H.; Yang, C.; Wang, X.; Zhang, J.; Xi, J.; Du, G.; Ji, Z. Mixed 3D/2D dimensional TiO2 nanoflowers/MoSe2 nanosheets for enhanced photoelectrochemical hydrogen generation. J. Am. Ceram. Soc. 2020, 103, 1187–1196. [Google Scholar] [CrossRef]
- Sharma, M.D.; Mahala, C.; Basu, M. 2D Thin Sheet Heterostructures of MoS2 on MoSe2 as Efficient Electrocatalyst for Hydrogen Evolution Reaction in Wide pH Range. Inorg. Chem. 2020, 59, 4377–4388. [Google Scholar] [CrossRef]
- Xia, Y.; Wu, W.; Wang, H.; Rao, S.; Zhang, F.; Zou, G. Amorphous RuS2 electrocatalyst with optimized active sites for hydrogen evolution. Nanotechnology 2020, 31, 145401. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Mou, H.; Chen, L.; Wang, D.; Song, C. Design and in-situ synthesis of unique catalyst via embedding graphene oxide shell membrane in NiS2 for efficient hydrogen evolution. Appl. Surf. Sci. 2020, 510, 145483. [Google Scholar] [CrossRef]
- Manjunathaae, C.; Patil, R.S.; Sudeep, M.; Srinivasa, N.; Kumar, R.C.; Aan, M.S.; Ashoka, S. Rational design and synthesis of hetero-nanostructured electrospun PU@PANI@FeS2: A surface tailored hybrid catalyst for H2 production via electrochemical splitting of water. Surf. Interfaces 2020, 18, 100445. [Google Scholar] [CrossRef]
- Zeng, X.; Jiao, Q.; Li, N.; Wang, J. Facile synthesis of CuxS coated electrodes for the efficient hydrogen evolution reaction. Appl. Surf. Sci. 2020, 513, 145785. [Google Scholar] [CrossRef]
- Li, Z.; Liu, P.; Yang, G. Efficient All-2D Amorphous Cobalt Sulfide Nanosheets/Multilayered Molybdenum Disulfide Hybrid Heterojunction Catalyst for Electrochemical Hydrogen Evolution. Glob. Chall. 2020, 4, 1900066. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, X.; Dai, J.; Li, L.; Zhao, D.; Wu, Z.; Tang, Z.; Ma, L.-J.; Chen, S. Hierarchical carbon microflowers supported defect-rich Co3S4 nanoparticles: An efficient electrocatalyst for water splitting. Carbon 2020, 160, 133–144. [Google Scholar] [CrossRef]
- Aslan, E.; Sarilmaz, A.; Yanalak, G.; Chang, C.S.; Cinar, I.; Ozel, F.; Patir, I.H. Facile preparation of amorphous NiWSex and CoWSex nanoparticles for the electrocatalytic hydrogen evolution reaction in alkaline condition. J. Electroanal. Chem. 2019, 856, 113674. [Google Scholar] [CrossRef]
- Li, G.; Chen, Z.; Li, Y.; Zhang, D.; Yang, W.; Liu, Y.; Cao, L. Engineering Substrate Interaction to Improve Hydrogen Evolution Catalysis of Monolayer MoS2 Films beyond Pt. ACS Nano 2020, 14, 1707–1714. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, V.-T.; Le, P.A.; Hsu, Y.-C.; Wei, K.-H. Plasma-Induced Exfoliation Provides Onion-Like Graphene-Surrounded MoS2 Nanosheets for a Highly Efficient Hydrogen Evolution Reaction. ACS Appl. Mater. Interfaces 2020, 12, 11533–11542. [Google Scholar] [CrossRef] [PubMed]
- Shi, Q.; Zhou, Y.; Sun, W.; Wang, H.; Li, S.; Zhang, X.; Wan, J. Hydrogen Evolution and Wastewater Treatment of Hydrangeal-like Catalyst Decroatedby the NiS Nanosheet and PdNanoparticle. ChemistrySelect 2020, 5, 1041–1046. [Google Scholar] [CrossRef]
- Zhao, A.; Zhang, L.; Xu, G.; Zhang, X.; Zhang, S.; Xiao, Y. Hollow ZnxCo1−xSe2 microcubes derived from Metal–Organic framework as efficient bifunctional electrocatalysts for hydrogen evolution and oxygen evolution reactions. Int. J. Hydrogen Energy 2020, 45, 2607–2616. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhang, J.; Ren, H.; Pan, Y.; Yan, Y.; Sun, F.; Wang, X.; Wang, S.; Zhang, J. Mo doping induced metallic CoSe for enhanced electrocatalytic hydrogen evolution. Appl. Catal. B Environ. 2020, 268, 118467. [Google Scholar] [CrossRef]
- Liang, Y.; Yang, Y.; Xu, K.; Yu, T.; Yao, S.; Peng, Q.; Yuan, C. Crystal plane dependent electrocatalytic performance of NiS2 nanocrystals for hydrogen evolution reaction. J. Catal. 2020, 381, 63–69. [Google Scholar] [CrossRef]
- Friedman, F.; Hastie, T.; John, T.; Weatherwax, R.L.; Epstein, D.; Wasserman, L.; Abu-El-Haija, S.; Do, C.B.; Batzoglou, S.; Resknik, C.; et al. Solution-processed GaSe nanoflake-based films for photoelectrochemical water splitting and photoelectrochemical-type. J. Chem. Inf. Model. 2013, 67, 1689–1699. [Google Scholar] [CrossRef]
- Ma, F.; Liang, Y.; Zhou, P.; Tong, F.; Wang, Z.; Wang, P.; Liu, Y.; Dai, Y.; Zheng, Z.; Huang, B. One-step synthesis of Co-doped 1T-MoS2 nanosheets with efficient and stable HER activity in alkaline solutions. Mater. Chem. Phys. 2020, 244, 122642. [Google Scholar] [CrossRef]
- Song, W.; Wang, K.; Jin, G.; Wang, Z.; Li, C.; Yang, X.; Chen, C. Two-Step Hydrothermal Synthesis of CoSe/MoSe 2 as Hydrogen Evolution Electrocatalysts in Acid and Alkaline Electrolytes. ChemElectroChem 2019, 6, 4842–4847. [Google Scholar] [CrossRef]
- Yang, J.-X.; Yu, W.-B.; Li, C.-F.; Dong, W.-D.; Jiang, L.-Q.; Zhou, N.; Zhuang, Z.-P.; Liu, J.; Hu, Z.-Y.; Zhao, H.; et al. PtO nanodots promoting Ti3C2 MXene in-situ converted Ti3C2/TiO2 composites for photocatalytic hydrogen production. Chem. Eng. J. 2021, 420, 129695. [Google Scholar] [CrossRef]
- Guayaquil-Sosa, J.; Rosales, B.S.; Valadés-Pelayo, P.; De Lasa, H. Photocatalytic hydrogen production using mesoporous TiO2 doped with Pt. Appl. Catal. Environ. 2017, 211, 337–348. [Google Scholar] [CrossRef]
- Zhao, H.; Li, C.-F.; Hu, Z.-Y.; Liu, J.; Li, Y.; Hu, J.; Van Tendeloo, G.; Chen, L.-H.; Su, B.-L. Size effect of bifunctional gold in hierarchical titanium oxide-gold-cadmium sulfide with slow photon effect for unprecedented visible-light hydrogen production. J. Colloid Interface Sci. 2021, 604, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Yerga, R.M.N.; Alvarez-Galvan, M.C.; Del Valle, F.; De La Mano, J.A.V.; Fierro, J.L.G. Water Splitting on Semiconductor Catalysts under Visible-Light Irradiation. ChemSusChem 2009, 2, 471–485. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Huang, J.; Sun, H.; Zhang, K.-Q.; Lai, Y. Uniform carbon dots@TiO2 nanotube arrays with full spectrum wavelength light activation for efficient dye degradation and overall water splitting. Nanoscale 2017, 9, 16046–16058. [Google Scholar] [CrossRef]
- Onsuratoom, S.; Chavadej, S.; Sreethawong, T. Hydrogen production from water splitting under UV light irradiation over Ag-loaded mesoporous-assembled TiO2–ZrO2 mixed oxide nanocrystal photocatalysts. Int. J. Hydrogen Energy 2011, 36, 5246–5261. [Google Scholar] [CrossRef]
- Chen, J.; Wu, X.-J.; Yin, L.; Li, B.; Hong, X.; Fan, Z.; Chen, B.; Xue, C.; Zhang, H. One-pot Synthesis of CdS Nanocrystals Hybridized with Single-Layer Transition-Metal Dichalcogenide Nanosheets for Efficient Photocatalytic Hydrogen Evolution. Angew. Chem. Int. Ed. 2015, 54, 1210–1214. [Google Scholar] [CrossRef]
- Zong, X.; Han, J.; Ma, G.; Yan, H.; Wu, G.; Li, C. Photocatalytic H2 Evolution on CdS Loaded with WS2 as Cocatalyst under Visible Light Irradiation. J. Phys. Chem. C 2011, 115, 12202–12208. [Google Scholar] [CrossRef]
- Ran, J.; Zhang, J.; Yu, J.; Jaroniec, M.; Qiao, S.Z. Earth-abundant cocatalysts for semiconductor-based photocatalytic water splitting. Chem. Soc. Rev. 2014, 43, 7787–7812. [Google Scholar] [CrossRef] [PubMed]
- Yan, C.; Xue, X.; Zhang, W.; Li, X.; Liu, J.; Yang, S.; Hu, Y.; Chen, R.; Yan, Y.; Zhu, G.; et al. Well-designed Te/SnS2/Ag artificial nanoleaves for enabling and enhancing visible-light driven overall splitting of pure water. Nano Energy 2017, 39, 539–545. [Google Scholar] [CrossRef]
- Liu, E.; Chen, J.; Ma, Y.; Feng, J.; Jia, J.; Fan, J.; Hu, X. Fabrication of 2D SnS2/g-C3N4 heterojunction with enhanced H2 evolution during photocatalytic water splitting. J. Colloid Interface Sci. 2018, 524, 313–324. [Google Scholar] [CrossRef] [PubMed]
- Ke, X.; Dai, K.; Zhu, G.; Zhang, J.; Liang, C. In situ photochemical synthesis noble-metal-free NiS on CdS-diethylenetriamine nanosheets for boosting photocatalytic H2 production activity. Appl. Surf. Sci. 2019, 481, 669–677. [Google Scholar] [CrossRef]
- Khan, Z.; Chetia, T.R.; Vardhaman, A.K.; Barpuzary, D.; Sastri, C.V.; Qureshi, M. Visible light assisted photocatalytic hydrogen generation and organic dye degradation by CdS–metal oxide hybrids in presence of graphene oxide. RSC Adv. 2012, 2, 12122–12128. [Google Scholar] [CrossRef]
- Chang, K.; Mei, Z.; Wang, T.; Kang, Q.; Ouyang, S.; Ye, J. MoS2/Graphene Cocatalyst for Efficient Photocatalytic H2 Evolution under Visible Light Irradiation. ACS Nano 2014, 8, 7078–7087. [Google Scholar] [CrossRef]
- Jiang, D.; Zhu, L.; Irfan, R.M.; Zhang, L.; Du, P. Integrating noble-metal-free NiS cocatalyst with a semiconductor heterojunction composite for efficient photocatalytic H 2 production in water under visible light. Chin. J. Catal. 2017, 38, 2102–2109. [Google Scholar] [CrossRef]
- Xin, X.; Song, Y.; Guo, S.; Zhang, Y.; Wang, B.; Yu, J.; Li, X. In-situ growth of high-content 1T phase MoS2 confined in the CuS nanoframe for efficient photocatalytic hydrogen evolution. Appl. Catal. Environ. 2020, 269, 118773. [Google Scholar] [CrossRef]
- Nagappagari, L.R.; Samanta, S.; Sharma, N.; Battula, V.R.; Kailasam, K. Synergistic effect of a noble metal free Ni(OH)2 co-catalyst and a ternary ZnIn2S4/g-C3N4 heterojunction for enhanced visible light photocatalytic hydrogen evolution. Sustain. Energy Fuels 2020, 4, 750–759. [Google Scholar] [CrossRef]
- Guo, X.; Guo, P.; Wang, C.; Chen, Y.; Guo, L. Few-layer WSe2 nanosheets as an efficient cocatalyst for improved photocatalytic hydrogen evolution over Zn0.1Cd0.9S nanorods. Chem. Eng. J. 2020, 383, 123183. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, D.; Xi, X.; Shen, Y.; Chang, S.; Zhu, J.; Chen, Y.; Jiang, L.; Zhang, J. Noble metal-free bimetallic NiCo decorated Zn0.5Cd0.5S solid solution for enhanced photocatalytic H2 evolution under visible light. Int. J. Hydrogen Energy 2020, 45, 8300–8309. [Google Scholar] [CrossRef]
- Jia, J.; Bai, X.; Zhang, Q.; Hu, X.; Liu, E.; Fan, J. Porous honeycomb-like NiSe2/red phosphorus heteroarchitectures for photocatalytic hydrogen production. Nanoscale 2020, 12, 5636–5651. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Chen, J.; Shao, L.; Xia, X.; Liu, Y.; Wang, L. Oriented facet heterojunctions on CdS nanowires with high photoactivity and photostability for water splitting. Appl. Catal. B Environ. 2020, 268, 118744. [Google Scholar] [CrossRef]
- Han, J.; Liu, Y.; Wang, Y.; Zhao, R.; Wang, L. Construction of ternary CdxMo1−xSe quantum dots for enhanced photocatalytic hydrogen production. J. Mater. Sci. 2019, 55, 1117–1125. [Google Scholar] [CrossRef]
- Beall, C.; Piipari, K.; Al-qassab, H.; Smith, M.A. MoS2/CdIn2S4 flower-like heterojunctions with enhanced photocatalytic degradation and H2 evolution activity. Biochem. J. 2010, 53, 1–14. [Google Scholar]
- Mangiri, R.; Reddy, D.A.; Subramanyam, K.; Kumar, K.S.; Sudharani, A.; Poornaprakash, B.; Vijayalakshmi, R. Decorating MoS2 and CoSe2 nanostructures on 1D-CdS nanorods for boosting photocatalytic hydrogen evolution rate. J. Mol. Liq. 2019, 289, 2–11. [Google Scholar] [CrossRef]
- Wang, B.; Ding, Y.; Deng, Z.; Li, Z. Rational design of ternary NiS/CQDs/ZnIn2S4 nanocomposites as efficient noble-metal-free photocatalyst for hydrogen evolution under visible light. Chin. J. Catal. 2019, 40, 335–342. [Google Scholar] [CrossRef]
- Chen, Z.; Gong, H.; Liu, Q.; Song, M.; Huang, C. NiSe2 Nanoparticles Grown in Situ on CdS Nanorods for Enhanced Photocatalytic Hydrogen Evolution. ACS Sustain. Chem. Eng. 2019, 7, 16720–16728. [Google Scholar] [CrossRef]
- Yan, A.; Shi, X.; Huang, F.; Fujitsuka, M.; Majima, T. Efficient photocatalytic H2 evolution using NiS/ZnIn2S4 heterostructures with enhanced charge separation and interfacial charge transfer. Appl. Catal. Environ. 2019, 250, 163–170. [Google Scholar] [CrossRef]
- Rangappa, A.P.; Kumar, D.P.; Gopannagari, M.; Reddy, D.A.; Hong, Y.; Kim, Y.; Kim, T.K. Highly efficient hydrogen generation in water using 1D CdS nanorods integrated with 2D SnS2 nanosheets under solar light irradiation. Appl. Surf. Sci. 2020, 508, 144803. [Google Scholar] [CrossRef]
- Ranjith, K.S.; Kumar, D.R.; Huh, Y.S.; Shanmugam, R.K.; Uyar, T.; Kumar, R.T.R. Promotional Effect of Cu2S–ZnS Nanograins as a Shell Layer on ZnO Nanorod Arrays for Boosting Visible Light Photocatalytic H2 Evolution. J. Phys. Chem. C 2020, 124, 3610–3620. [Google Scholar] [CrossRef]
- Puentes-Prado, E.; Garcia, C.; Oliva, J.; Galindo, R.; Bernal-Alvarado, J.; Torres, L.D.; Gomez-Solis, C. Enhancing the solar photocatalytic hydrogen generation of ZnS films by UV radiation treatment. Int. J. Hydrogen Energy 2020, 45, 12308–12317. [Google Scholar] [CrossRef]
- Li, X.; Song, S.; Gao, Y.; Ge, L.; Song, W.; Ma, T.; Liu, J. Identification of the Charge Transfer Channel in Cobalt Encapsulated Hollow Nitrogen-Doped Carbon Matrix@CdS Heterostructure for Photocatalytic Hydrogen Evolution. Small 2021, 17, 2101315. [Google Scholar] [CrossRef]
- Zhang, Z.; Kang, Y.; Yin, L.-C.; Niu, P.; Zhen, C.; Chen, R.; Kang, X.; Wu, F.; Liu, G. Constructing CdSe QDs modified porous g-C3N4 heterostructures for visible light photocatalytic hydrogen production. J. Mater. Sci. Technol. 2021, 95, 167–171. [Google Scholar] [CrossRef]
- Wong, K.-L.; Bünzli, J.-C.G.; Tanner, P.A. Quantum yield and brightness. J. Lumin. 2020, 224, 117256. [Google Scholar] [CrossRef]
- Bouas-Laurent, H.; Dürr, H. Organic photochromism (IUPAC Technical Report) Republication or reproduction of this report or its storage and/or dissemination by electronic means is permitted without the need for formal IUPAC permission on condition that an acknowledgment, with full re. Photochromism 2003, 73, 639–665. [Google Scholar] [CrossRef]
- Meng, X.; Zhang, C.; Dong, C.; Sun, W.; Ji, D.; Ding, Y. Carbon quantum dots assisted strategy to synthesize Co@NC for boosting photocatalytic hydrogen evolution performance of CdS. Chem. Eng. J. 2020, 389, 124432. [Google Scholar] [CrossRef]
- Yuan, Y.; Lu, H.; Ji, Z.; Zhong, J.; Ding, M.; Chen, D.; Li, Y.; Tu, W.; Cao, D.; Yu, Z.; et al. Enhanced visible-light-induced hydrogen evolution from water in a noble-metal-free system catalyzed by ZnTCPP-MoS2/TiO2 assembly. Chem. Eng. J. 2015, 275, 8–16. [Google Scholar] [CrossRef]

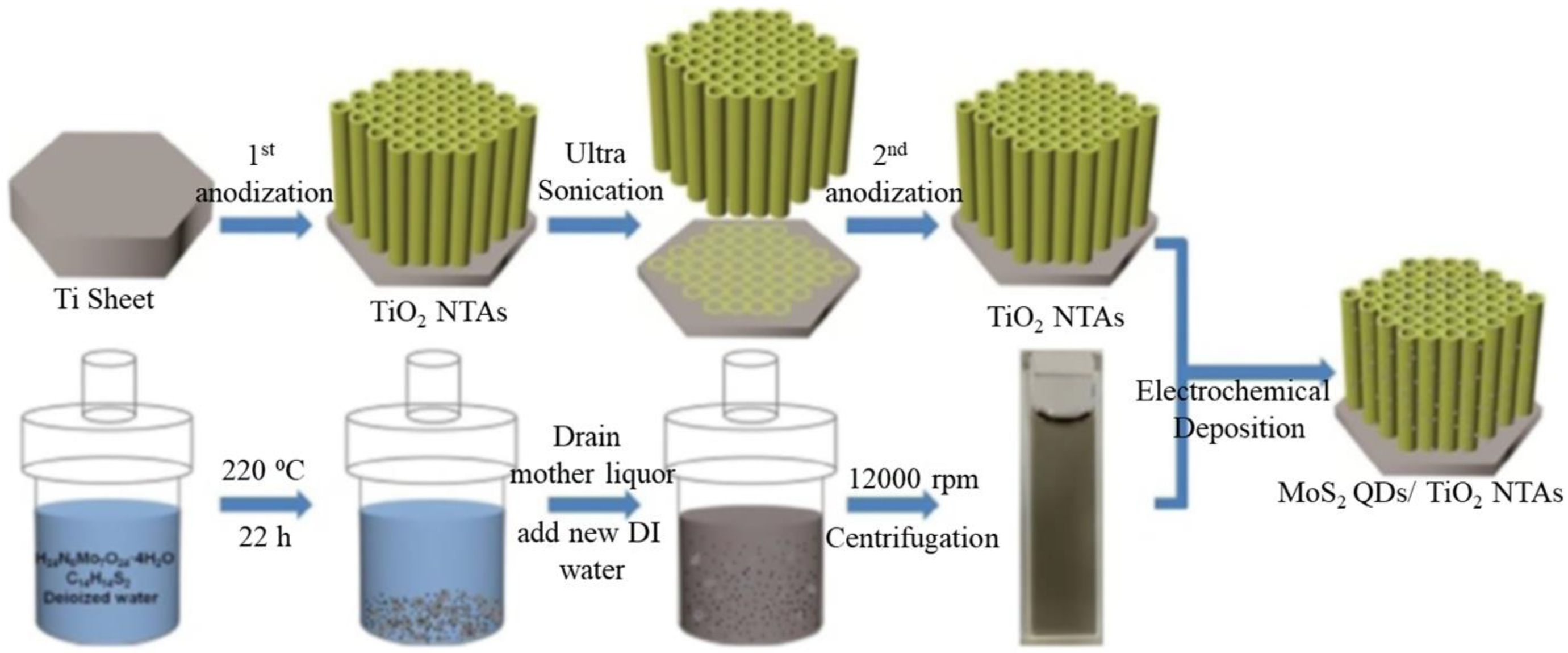
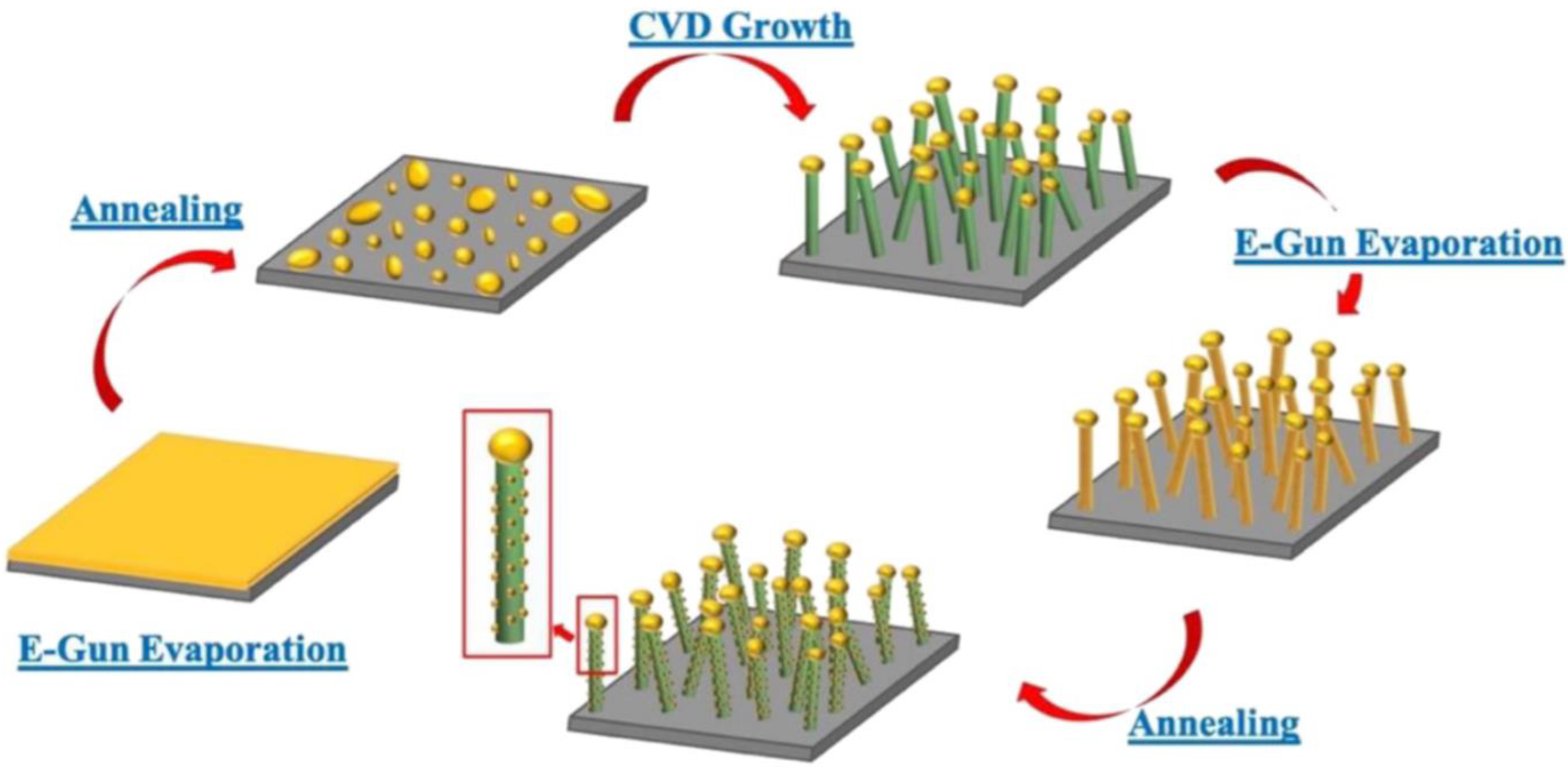
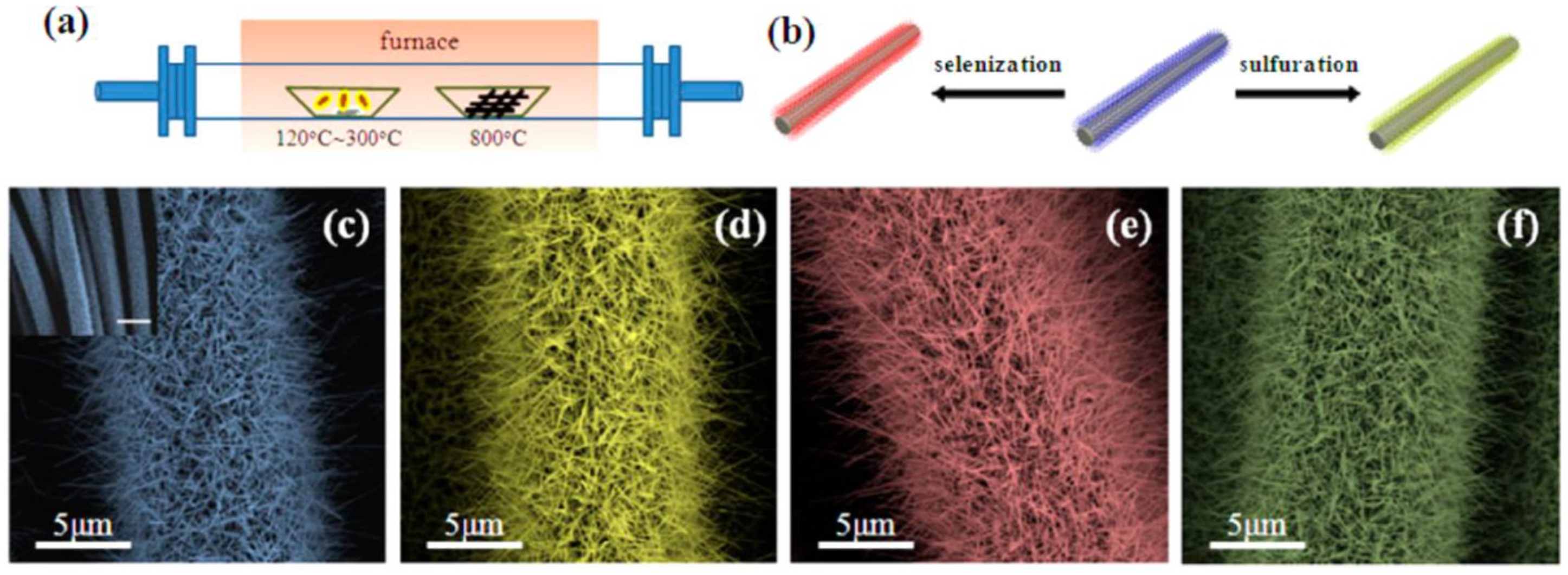
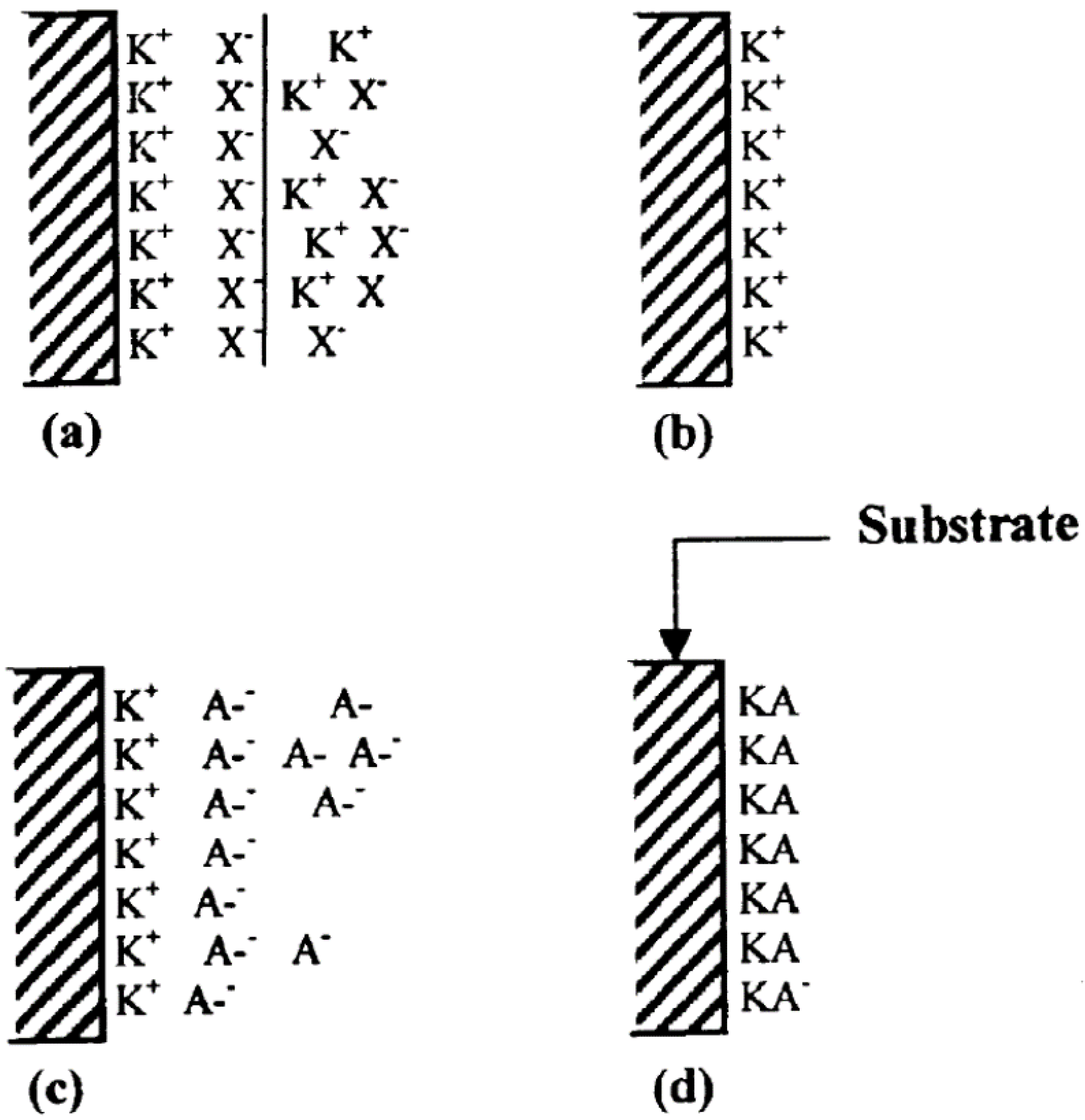
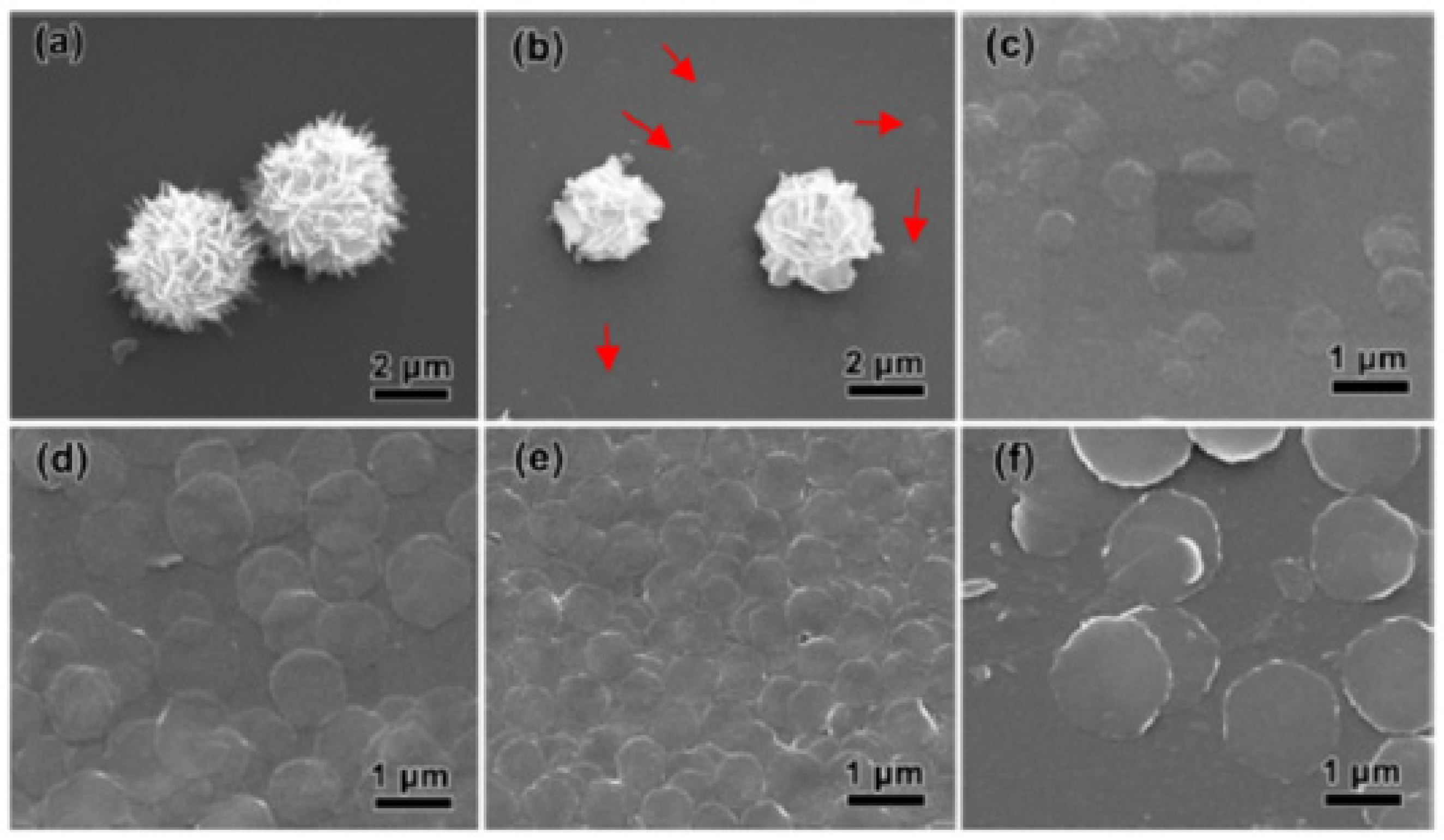
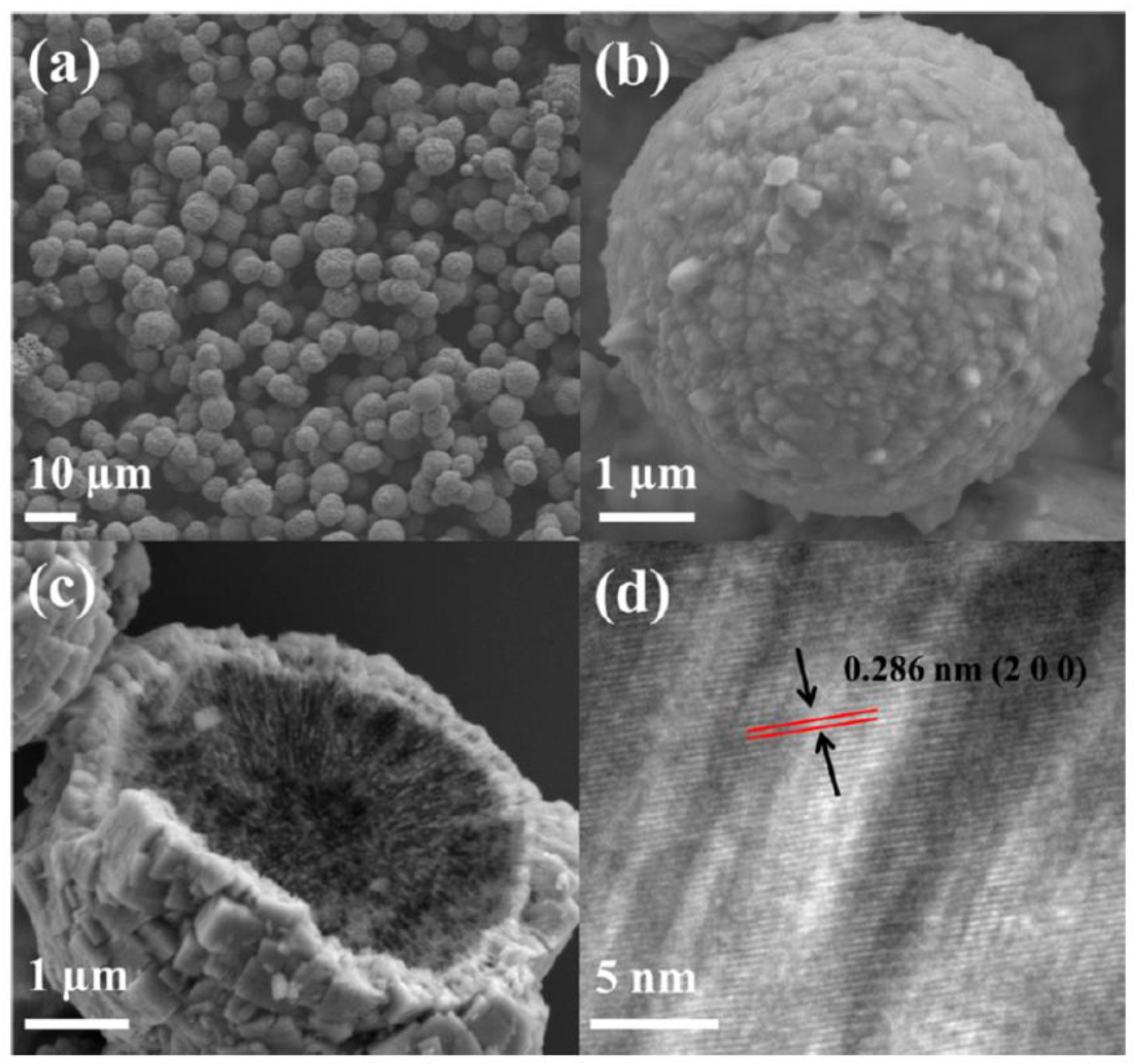

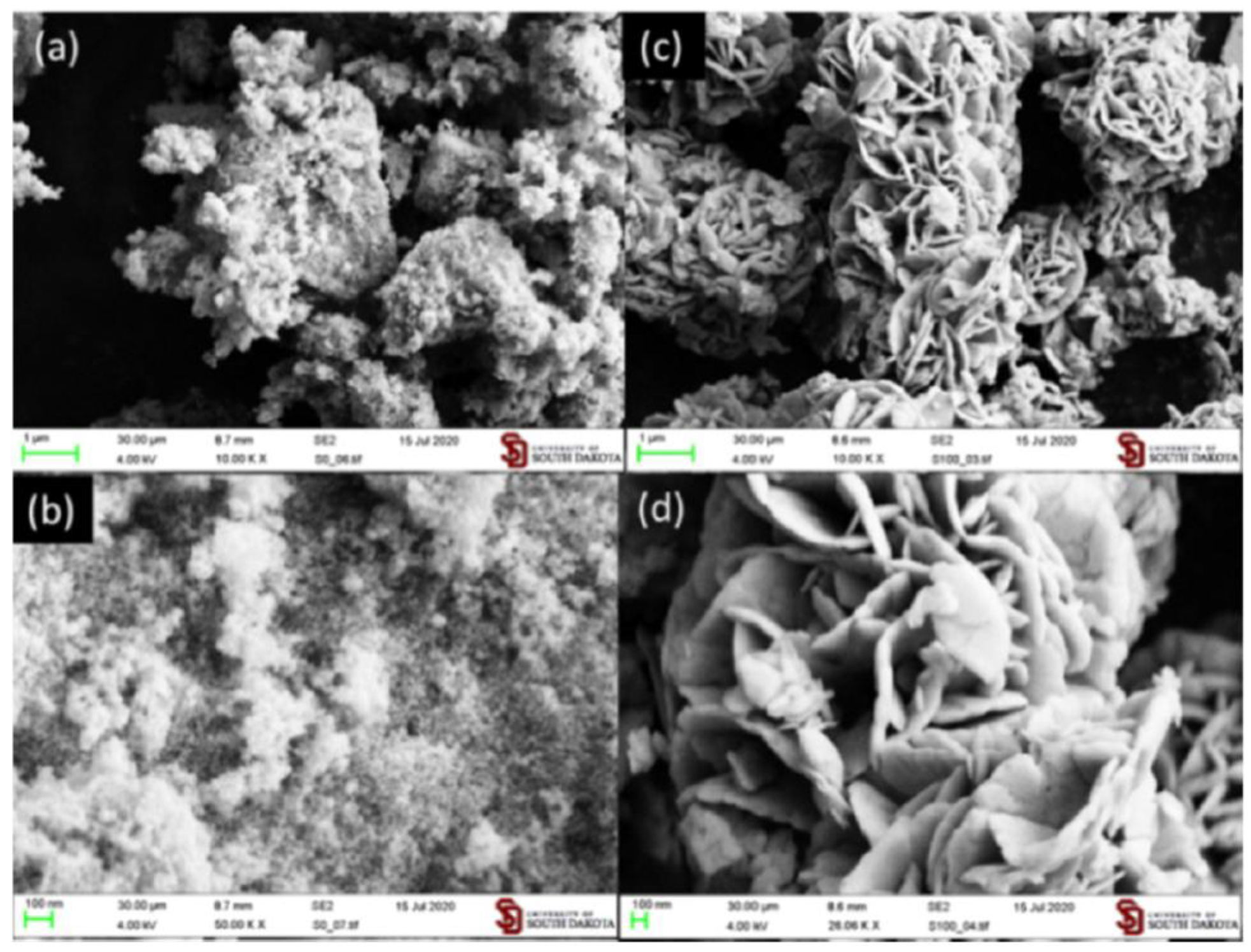

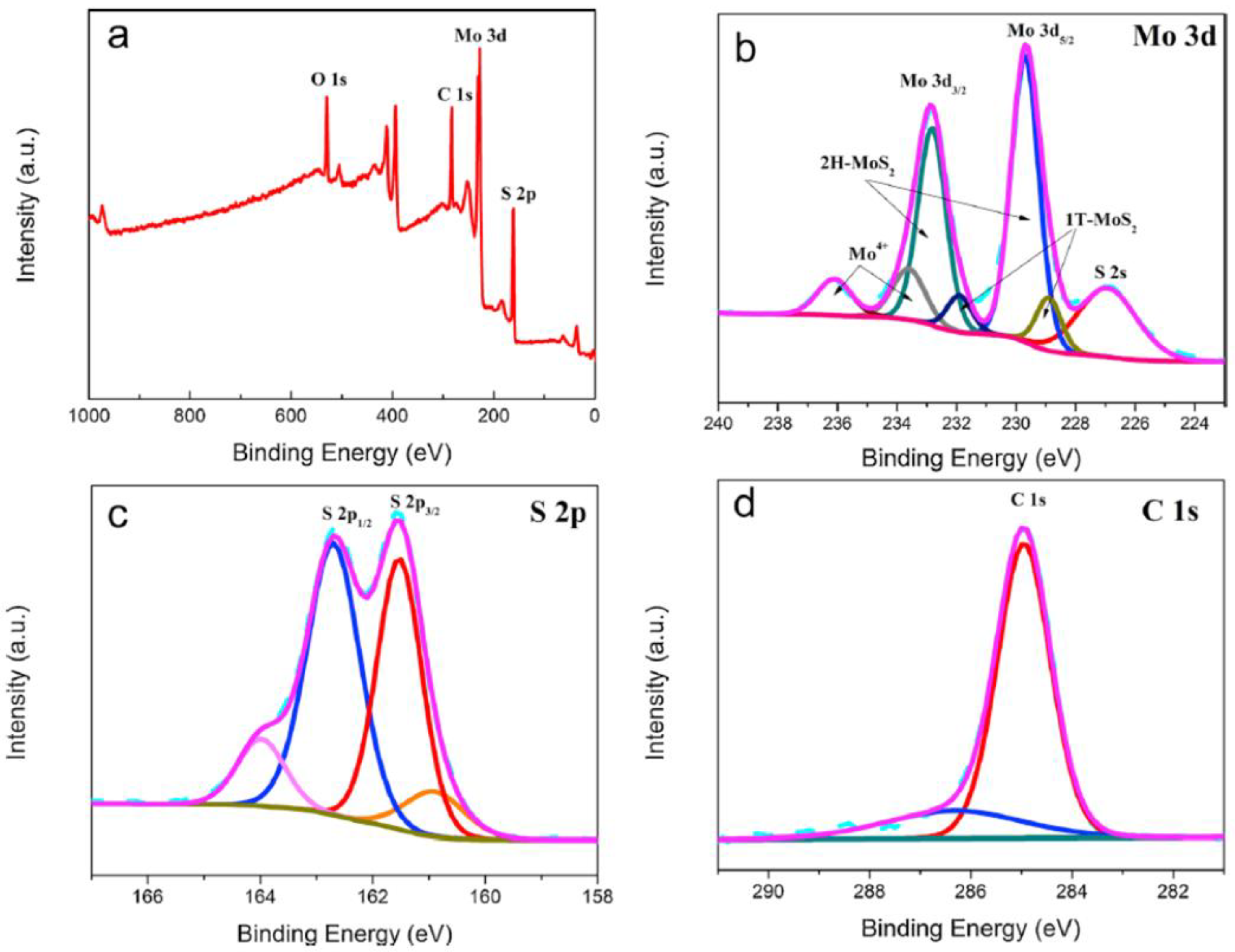
| Synthesis Methods | Advantages/Disadvantages |
|---|---|
| Hydrothermal/Solvothermal Method | Produce nanomaterials with various morphologies and thicknesses at high pressure and low temperature; can synthesize high quality large crystals; cost of equipment is high |
| Microwave-assisted Synthesis | Required less time/rapid process; size can be controlled |
| Electrodeposition Method | Rapid and single-step process; used to produce homogeneous and high-purity crystalline materials at the cathode of the electrochemical system during the coating process |
| Photoreduction | Require higher photon energy; can synthesize the materials with large surface area and many active sites |
| Electron Beam Evaporation | 0D, 1D, and 2D materials can be prepared; used for depositing materials with high melting point; as electrons can be focalized, it is possible to obtain a very localized heating on the material to evaporate with a high density of evaporation power |
| Sulfidation and Selenization | Solution-phase conversion; facile and selectable synthesis method |
| Successive Ionic Layer Adsorption and Reaction (SILAR) Method | Simple, cost effective, and rapid technique for the deposition of binary semiconducting thin films |
| Refluxing Method | Large scale synthesis method; facile and cost effective |
| Chemical Vapor Deposition (CVD) Method | Gas-phase aerosol process for producing high-purity nanoparticles; mainly used for large scale thin-film production |
| Liquid cascade centrifugation (LCC) method | Used to achieve highly efficient nanosheet with selected size and thickness; most efficient, scalable, and versatile method based on a set of iterative centrifugation cascades |
| Hydrogen Production Methods | Description | Advantages | Disadvantages | Reference |
|---|---|---|---|---|
| Steam natural gas reforming | Direct production of hydrogen gas by conversion of feed hydrocarbons at high temperature |
|
| [121,122,123] |
| Coal gasification | Partial oxidation of coal with air. However, it generates the CO2 through traditional combustion |
|
| [123,124] |
| Biomass gasification | Burning of biomass using limited supply of air generates combustible gases, such as CO, CO2, H2 CH4, H2O and N2 with contaminants like small char particles, ash and tars |
|
| [122,125] |
| Thermolysis | Using high temperature from concentrated solar energy and chemical reactions to generate the H2 and O2 from water. |
|
| [122,123] |
| Electrolysis | Usage of electricity to split water into hydrogen and oxygen |
|
| [114,122] |
| Photoelectrochemical | Hydrogen is produced from water in the presence of sunlight and specialized semiconductors immersed in water-based electrolytes |
|
| [10,114,126] |
| Photocatalytic | A simple method to produce hydrogen from water in the presence of catalyst and sunlight |
|
| [10,127,128] |
| Material | Current Density | Over Potential | Photocurrent | Other Condition(s) | References |
|---|---|---|---|---|---|
| MoS2 | 10 mAcm−2 | 173 mV | 109.81 mVdec−1 | Nafion resin | [140] |
| MoSe2 | 10 mAcm−2 | 208 mV | 65.92 mVdec−1 | Nafion resin | [140] |
| MoTe2 | 10 mAcm−2 | 283 mV | 102.06 mVdec−1 | Nafion resin | [140] |
| MoS2/candle soot/Ni foam | 10 mAcm−2 | 56 mV | NA | 1.0 M KOH | [108] |
| 3D/2D TiO2/MoSe2 | 1.40 mAcm−2 | NA | NA | 1.0 M NaOH | [141] |
| 2D MoS2/MoSe2 | 10 mA/cm−2 147 μmol of H2 in ∼50 min. | NA | NA | Wide pH conditions | [142] |
| Amorphous-RuS2 | 10 mAcm−2 | 141 mV | 65.6 mVdec−1 | 0.5 M H2SO4 | [143] |
| MoS2/TiO2 | 10 mAcm−2 | 170 mV | 70 mVdec−1 | 0.5 M H2SO4 | [118] |
| GO/NiS2 | 10 mAcm−2 | 57 mV-HER 294 mV-OER | NA | 1.0 M KOH | [144] |
| PU@PANI@FeS2 | 10 mAcm−2 50 mAcm−2 | 266 mV 372 mV | NA | 0.5M H2SO4 | [145] |
| CuxS | NA | –90 mV | 100 mVdec−1 | 0.5 M H2SO4 | [146] |
| CoS/MoS2 | NA | −147 mV | 126 mVdec−1 | [147] | |
| Carbon/Co3S4 | 10 mVcm−2 | 250 mV and 140 mV for OER and HER | NA | 1.0 M KOH | [148] |
| NiWSex and CoWSex | 1 mAcm−2 | NiWSex (231 mV) CoWSex (281 mV) and WSex (404 mV) | NA | 1.0 M KOH | [149] |
| MoS2/Pt | 0.642 mAcm−2 | NA | 52 mVdec−1 to 32 mVdec−1 | 0.5 M H2S O4 | [150] |
| G/MoS2 | 10 mAcm−2 | 118 mV | 73 mVedec−1 | 0.5 M H2SO4 | [151] |
| NiS/Pd | 10 mAcm−2 | 100 mV | 50 mVdec−1 | NA | [152] |
| ZnxCo1−xSe2 | 10 mAcm−2 | 196 mV-HER 308 mV-OER | NA | HER—acid media OER—alkaline media | [153] |
| Mo/CoSe | 100 mAcm−2 | 186.1 mV | 58.7 mV dec−1 | 0.5 M H2SO4 | [154] |
| MoS2/graphene | 1000 mAcm−2 | 250 mV | 43.3 mVdec−1 | 0.5 M H2SO4 | [47] |
| NiS2 | 10 mA/cm−2 | 302 mV | NA | Nafion ethanol solution | [155] |
| GaSe | −9.3 µAcm−2 83.4 µAcm−2 | +1.23 V vs. RHE | NA | 0.5 M H2SO4 | [156] |
| 1T-MoS2 | 10 mAcm−2 | 240 mV | 68 mVdec−1 | 1 M KOH | [157] |
| CoSe/MoSe2 | 10 mAcm−2 | 192 and 115 mV in acidic and alkaline | NA | Nafion ethanol solution | [158] |
| Materials | Amount of Hydrogen Evolved | Light Source | Co-Catalyst | Scavenging Agents/Other Chemicals Used | Reference |
|---|---|---|---|---|---|
| SnS2 | 1.06 mmol h−1 g−1 | UV–Visible | NA | Na2S and Na2SO3 | [38] |
| Te/SnS2/Ag | 332.4 and 166.2 µmol h−1 for H2 and O2 | UV–Visible | NA | - | [168] |
| SnS2/g-C3N4 | 972.6 µmol h−1g−1 | Visible | Pt | Triethanolamine | [169] |
| CdS/WS2 | 0.42 mmol h−1 | Visible | NA | Lactic acid | [166] |
| NiS /CdS DETA | 230 µmolh−1 | Visible | NA | - | [170] |
| CdS/ZnO/GO, CdS/Al2O3/GO | 22.12 mmol h−1g−1 | 500 W Phoenix tungsten halogen lamp | Pt | Na2SO3 and Na2S | [171] |
| MoS2/RGO/CdS | 99 μmol h−1 | Visible | NA | Lactic acid | [42] |
| MoS2/G | 1.80 mmol h−1 | Visible | NA | Na2S and Na2S2O3 | [172] |
| Eosin Y/NiSx/G | 0.34 mmol h−1 | Visible | NA | Triethanolamine | [22] |
| NiS/CdS/ZnS | 574 μmol h−1 | Visible | NA | Na2S/Na2S2O3 | [173] |
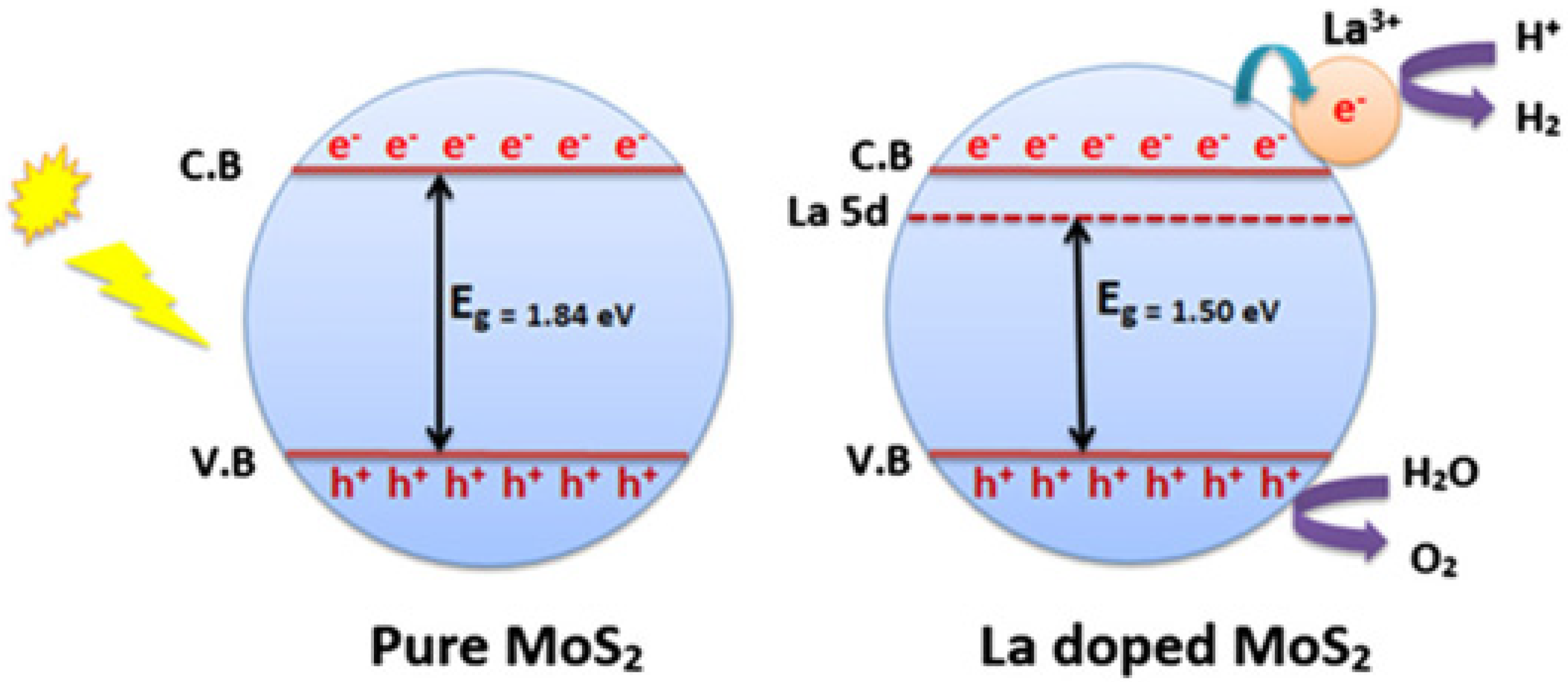
| MoS2 | 110 µmol h−1 | Visible | La3+ | Methanol | [109] |
| CuS–MoS2–1T | 9648.7 μmol g −1h−1 | UV–Visible | NA | 0.3 M Na2SO3 | [174] |
| WS2/TiO2 | 596.4 µmolg−1 | Visible | Pt | 1 wt. % of WS2/TiO2 | [31] |
| ZnIn2S4/g-C3N4 | 450 μmol g−1h−1 | Visible | NA | Triethanolamine | [175] |
| WSe2/ Zn0.1Cd0.9S | 147.32 mmol g−1h−1 | Visible | NA | Lactic acid | [176] |
| NiCo/Zn0.5Cd0.5S | 34.7 mmol g−1h−1 | UV–Visible | NA | Na2S and Na2SO3 | [177] |
| NiSe2/RP | 1968.8μmol g−1h−1 | UV–Visible | NA | Na2S and Na2SO3 | [178] |
| CdS | 3072 μmol g−1h−1 | UV–Visible | NA | Na2S and Na2SO3 | [179] |
| ZnS/CdS/Cd0.5Zn0.5S/MoS2 | 50.65 mmol g−1h−1 | Visible | NA | Lactic acid, Na2S, and Na2SO3 | [117] |
| CdxMo1−xSe | 911.1 mol in 7 h | Visible | NA | Na2S and Na2SO3 | [180] |
| MoS2/CdIn2S4 | 1868.19 μmol g−1h−1 | UV–Visible | NA | Na2S and Na2SO3 | [181] |
| MoS2/CoSe2/1D-CdS | 191.5 mmol g−1h−1 | UV–Visible | NA | Lactic acid | [182] |
| NiS/CQDs/ZnIn2S4 | 28.2 μmol h−1 | Visible | NA | Trimethylamine | [183] |
| NiSe2/CdS | 167.1 mmol g−1h−1 | Visible | NA | [184] | |
| NiS/ZnIn2S4 | 5.0 μmol h−1 | Visible | NA | Methanol and lactic acid | [185] |
| 1T-MoS2-ZnCoS | 15.47 mmol h−1 g−1 | UV–Visible | NA | Trimethylamine, Eosin Y, and acetonitrile | [120] |
| CdS/SnS2 | 20.2 mmol h−1 g−1 | UV–Visible | NA | Lactic acid | [186] |
| ZnO−ZnS−Cu2S | 436 μmol h−1 g−1 | Visible | NA | Na2S and Na2SO3 | [187] |
| ZnS films | 5202.4 μmol h−1 g−1 | UV | NA | Na2SO3 | [188] |
| CoS2/TiO2 | 2.55 mmol g−1 | UV | NA | Methanol | [105] |
| SnS2/TiO2 | 195.0 μmol g−1 | Visible | NA | Methanol | [106] |
| (NC@Co-NCT)/(CdS) | 3.8 mmol h−1 g−1 | Visible | NA | lactic acid | [189] |
| CdSe QDs/g-C3N4 | 192.3 μmol h−1 | Visible | H2PtCl6 | Triethanolamine, Na2S, and Na2SO3 | [190] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shanmugaratnam, S.; Yogenthiran, E.; Koodali, R.; Ravirajan, P.; Velauthapillai, D.; Shivatharsiny, Y. Recent Progress and Approaches on Transition Metal Chalcogenides for Hydrogen Production. Energies 2021, 14, 8265. https://doi.org/10.3390/en14248265
Shanmugaratnam S, Yogenthiran E, Koodali R, Ravirajan P, Velauthapillai D, Shivatharsiny Y. Recent Progress and Approaches on Transition Metal Chalcogenides for Hydrogen Production. Energies. 2021; 14(24):8265. https://doi.org/10.3390/en14248265
Chicago/Turabian StyleShanmugaratnam, Sivagowri, Elilan Yogenthiran, Ranjit Koodali, Punniamoorthy Ravirajan, Dhayalan Velauthapillai, and Yohi Shivatharsiny. 2021. "Recent Progress and Approaches on Transition Metal Chalcogenides for Hydrogen Production" Energies 14, no. 24: 8265. https://doi.org/10.3390/en14248265
APA StyleShanmugaratnam, S., Yogenthiran, E., Koodali, R., Ravirajan, P., Velauthapillai, D., & Shivatharsiny, Y. (2021). Recent Progress and Approaches on Transition Metal Chalcogenides for Hydrogen Production. Energies, 14(24), 8265. https://doi.org/10.3390/en14248265










