Influence of Carbonate Minerals on Heavy Oil Oxidation Behavior and Kinetics by TG-FTIR
Abstract
:1. Introduction
- ·
- Acceleration of crude oil oxidation by kaolinite’s catalytic and surface area effect [10];
- ·
- Shifting of oxidation reactions into lower temperature and reduction in activity energy by increased the specific surface area effect of clays [11];
- ·
- ·
- Promotion in the LTO of linear alkanes by illite [14];
- ·
- ·
- Promotion of fuel formation by carbonate rock [19];
- ·
- Reduced activation energy but not affected reaction model by limestone (major calcite + minor dolomite) [20];
- ·
- Promotion of LTO with higher oxygen consumption and carbon oxides release by montmorillonite > illite > chlorite > kaolinite [21];
- ·
- Increased heat release in LTO by montmorillonite [22];
- ·
- Promoting the formation of cribriform structures on asphaltenes surface that aids the combustion of asphaltenes by increasing its surface area by clays (90 wt % kaolinite and 10 wt % illite) [23];
- ·
- Merging FD into HTO, shifting reaction intervals (LTO and HTO) into lower temperatures, and significantly reducing activation energy by 54% quartz and 46% mica [24];
- ·
- Shift of the reaction intervals into lower temperature and lower activation energy by surface area and catalytic effect of kaolinite, bentonite, and illite [25];
- ·
- Clay minerals may be favorable for the combustion of asphaltenes by forming cribriform structures that increase the surface area of asphaltenes [23].
2. Materials and Methods
2.1. Samples Characterization
2.2. Thermogravimetry–Fourier-Transform-Infrared (TG-FT-IR) Coupled Analysis
3. Results and Discussion
3.1. Thermal Behavior of Heavy Oil Characterized by TG-FT-IR
3.2. Influence of Calcite and Dolomite Rocks on the Thermal Behavior of Heavy Oil
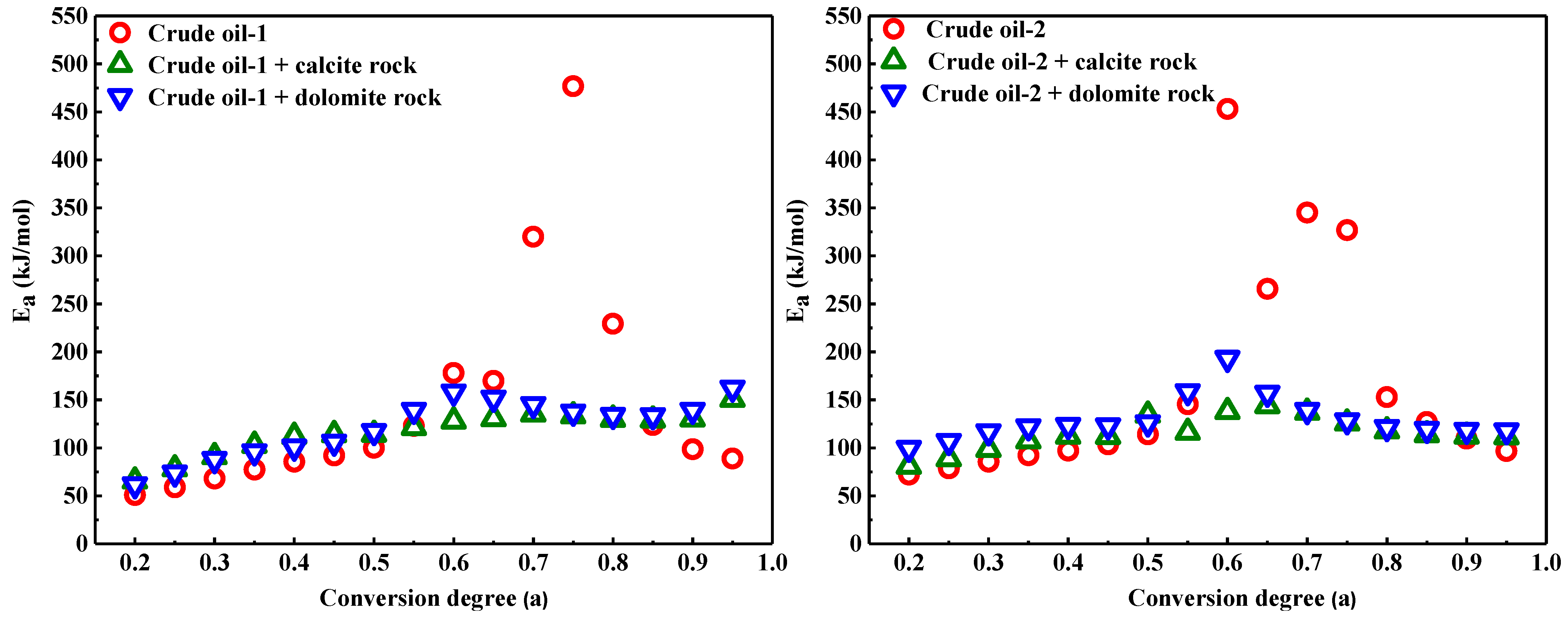
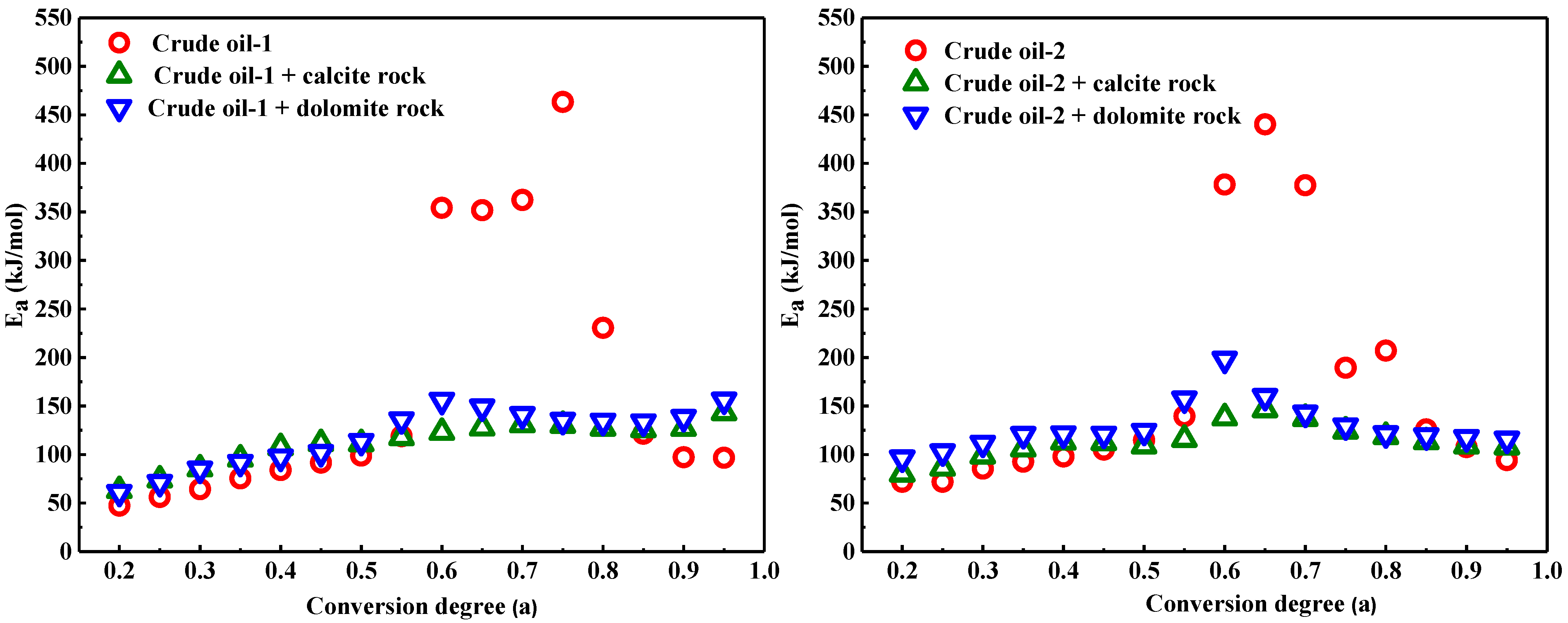
3.3. Influence of Calcite and Dolomite on the Kinetic Analysis of Heavy Oil Oxidation
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Hascakir, B.; Ross, C.M.; Castanier, L.M.; Kovscek, A.R. Fuel formation and conversion during in-situ combustion of crude oil. SPE J. 2013, 18, 1217–1228. [Google Scholar] [CrossRef]
- Yuan, C.; Varfolomeev, M.A.; Emelianov, D.A.; Suwaid, M.A.; Khachatrian, A.A.; Starshinova, V.L.; Vakhitov, I.R.; Al-Muntaser, A.A. Copper stearate as a catalyst for improving the oxidation performance of heavy oil in in-situ combustion process. Appl. Catal. A Gen. 2018, 564, 79–89. [Google Scholar] [CrossRef]
- Mehrabi-Kalajahi, S.; Varfolomeev, M.A.; Yuan, C.; Zinnatullin, A.L.; Rodionov, N.O.; Vagizov, F.G.; Osin, Y.N.; Yakimova, L.S. Improving heavy oil oxidation performance by oil-dispersed CoFe2O4 nanoparticles in In-situ combustion process for enhanced oil recovery. Fuel 2021, 285, 119216. [Google Scholar] [CrossRef]
- Sarathi, P.S. In-Situ Combustion Handbook—Principles and Practices; United States Department of Energy, National Petroleum Technology Office: Tulsa, OK, USA, 1999. [Google Scholar]
- Pope, C.; Ismail, N.B.; Hascakir, B. Impact of carbonates on reaction kinetics of a bitumen combustion. In Proceedings of the Society of Petroleum Engineers—SPE Canada Heavy Oil Conference 2020, Virtual, 29 September–2 October 2020. [Google Scholar]
- Yuan, C.; Emelianov, D.A.; Varfolomeev, M.A. Oxidation Behavior and Kinetics of Light, Medium, and Heavy Crude Oils Characterized by Thermogravimetry Coupled with Fourier Transform Infrared Spectroscopy. Energy Fuels 2018, 32, 5571–5580. [Google Scholar] [CrossRef]
- Yuan, C.; Varfolomeev, M.A.; Emelianov, D.A.; Eskin, A.A.; Nagrimanov, R.N.; Kok, M.V.; Afanasiev, I.S.; Fedorchenko, G.D.; Kopylova, E.V. Oxidation Behavior of Light Crude Oil and Its SARA Fractions Characterized by TG and DSC Techniques: Differences and Connections. Energy Fuels 2018, 32, 801–808. [Google Scholar] [CrossRef]
- Yuan, C.; Emelianov, D.A.; Varfolomeev, M.A.; Pu, W.; Ushakova, A.S. Oxidation Behavior and Kinetics of Eight C20-C54 n -Alkanes by High Pressure Differential Scanning Calorimetry (HP-DSC). Energy Fuels 2018, 32, 7933–7942. [Google Scholar] [CrossRef]
- Yuan, C.; Emelianov, D.A.; Varfolomeev, M.A.; Abaas, M. Combustion behavior of aromatics and their interaction with n-alkane in in-situ combustion enhanced oil recovery process: Thermochemistry. J. Ind. Eng. Chem. 2019, 76, 467–475. [Google Scholar] [CrossRef]
- Vossoughi, S.; Willhite, G.; El Shoubary, Y.; Bartlett, G. Study of the clay effect on crude oil combustion by thermogravimetry and differential scanning calorimetry. J. Therm. Anal. 1983, 27, 17–36. [Google Scholar] [CrossRef]
- Drici, O.; Vossoughi, S. Study of the Surface Area Effect on Crude Oil Combustion By Thermal Analysis Techniques. JPT J. Pet. Technol. 1985, 37, 731–735. [Google Scholar] [CrossRef]
- Kök, M.V. Influence of reservoir rock composition on the combustion kinetics of crude oil. J. Therm. Anal. Calorim. 2009, 97, 397–401. [Google Scholar] [CrossRef]
- Zheng, R.; Pan, J.; Chen, L.; Tang, J.; Liu, D.; Song, Q.; Chen, L.; Yao, Q. Catalytic Effects of Montmorillonite on Coke Formation during Thermal Conversion of Heavy Oil. Energy Fuels 2018, 32, 6737–6745. [Google Scholar] [CrossRef]
- Faure, P.; Landais, P. Evidence for clay minerals catalytic effects during low-temperature air oxidation of n-alkanes. Fuel 2000, 79, 1751–1756. [Google Scholar] [CrossRef]
- Kök, M.V. Effect of clay on crude oil combustion by thermal analysis techniques. J. Therm. Anal. Calorim. 2006, 84, 361–366. [Google Scholar] [CrossRef]
- Kok, M.V.; Gundogar, A.S. Effect of different clay concentrations on crude oil combustion kinetics by thermogravimetry. J. Therm. Anal. Calorim. 2010, 99, 779–783. [Google Scholar] [CrossRef]
- Kok, M.V. Clay concentration and heating rate effect on crude oil combustion by thermogravimetry. Fuel Process. Technol. 2012, 96, 134–139. [Google Scholar] [CrossRef]
- Varfolomeev, M.A.; Nurgaliev, D.K.; Kok, M.V. Calorimetric study approach for crude oil combustion in the presence of clay as catalyst. Pet. Sci. Technol. 2016, 34, 1624–1630. [Google Scholar] [CrossRef]
- Yuan, C.D.; Pu, W.F.; Jin, F.Y.; Zhang, J.J.; Zhao, Q.N.; Li, D.; Li, Y.B.; Chen, Y.F. Characterizing the Fuel Deposition Process of Crude Oil Oxidation in Air Injection. Energy Fuels 2015, 29, 7622–7629. [Google Scholar] [CrossRef]
- Karimian, M.; Schaffie, M.; Fazaelipoor, M.H. Estimation of the kinetic triplet for in-situ combustion of crude oil in the presence of limestone matrix. Fuel 2017, 209, 203–210. [Google Scholar] [CrossRef]
- Zheng, R.; Pan, J.; Cai, G.; Liang, J.; Liu, D.; Song, Q.; Yao, Q. Effects of clay minerals on the low-temperature oxidation of heavy oil. Fuel 2019, 254, 115597. [Google Scholar] [CrossRef]
- Yu, X.; Qu, Z.; Kan, C.; Zhao, X. Effect of Different Clay Minerals on Heavy Oil Oxidation during Ignition Process. Energy Fuels 2017, 31, 12839–12847. [Google Scholar] [CrossRef]
- Ismail, N.B.; Hascakir, B. Impact of asphaltenes and clay interaction on in-situ combustion performance. Fuel 2020, 268, 117358. [Google Scholar] [CrossRef]
- Ariskina, K.A.; Yuan, C.; Abaas, M.; Emelianov, D.A.; Rodionov, N.; Varfolomeev, M.A. Catalytic effect of clay rocks as natural catalysts on the combustion of heavy oil. Appl. Clay Sci. 2020, 193, 105662. [Google Scholar] [CrossRef]
- Kök, M.V.; Varfolomeev, M.A.; Nurgaliev, D.K. TGA and DSC investigation of different clay mineral effects on the combustion behavior and kinetics of crude oil from Kazan region, Russia. J. Pet. Sci. Eng. 2021, 200, 108364. [Google Scholar] [CrossRef]
- Pu, W.; Zhao, S.; Pan, J.; Wang, R.; Chen, L.; Kan, N.; Wang, L. Comparative analysis of quartz sand and detritus effects on thermal behavior and kinetics of heavy crude oil. Thermochim. Acta 2018, 667, 153–159. [Google Scholar] [CrossRef]
- Verşan Kök, M.; Varfolomeev, M.A.; Nurgaliev, D.K. Thermal characterization of crude oils in the presence of limestone matrix by TGA-DTG-FTIR. J. Pet. Sci. Eng. 2017, 154, 495–501. [Google Scholar] [CrossRef]
- Varfolomeev, M.A.; Nurgaliev, D.K.; Kok, M.V. Thermal, kinetics, and oxidation mechanism studies of light crude oils in limestone and sandstone matrix using TG-DTG-DTA: Effect of heating rate and mesh size. Pet. Sci. Technol. 2016, 34, 1647–1653. [Google Scholar] [CrossRef]
- Guowei, Q.; Yaling, W.; Xu, D.; Wenlong, Q.; Mei, W.; Yanzhao, Z. Influence of clay minerals on oxidation reaction of light crude oil. Pet. Sci. Technol. 2019, 37, 566–572. [Google Scholar] [CrossRef]
- Yuan, C.; Emelianov, D.A.; Varfolomeev, M.A.; Rodionov, N.O.; Suwaid, M.A.; Vakhitov, I.R. Mechanistic and kinetic insight into catalytic oxidation process of heavy oil in in-situ combustion process using copper (II) stearate as oil soluble catalyst. Fuel 2021, 284. [Google Scholar] [CrossRef]
- Abaas, M.; Yuan, C.; Emelianov, D.A.; Varfolomeev, M.A.; Ariskina, K.A. Effect of calcite on crude oil combustion characterized by high-pressure differential scanning calorimetry (HP-DSC). Pet. Sci. Technol. 2019, 37, 1216–1221. [Google Scholar] [CrossRef]
- Vyazovkin, S.; Chrissafis, K.; Di Lorenzo, M.L.; Koga, N.; Pijolat, M.; Roduit, B.; Sbirrazzuoli, N.; Suñol, J.J. ICTAC Kinetics Committee recommendations for collecting experimental thermal analysis data for kinetic computations. Thermochim. Acta 2014, 590, 1–23. [Google Scholar] [CrossRef]
- Freitag, N.P. Chemical-reaction mechanisms that govern oxidation rates during in-situ combustion and high-pressure air injection. SPE Reserv. Eval. Eng. 2016, 19, 645–654. [Google Scholar] [CrossRef]
- Zhao, S.; Pu, W.; Sun, B.; Gu, F.; Wang, L. Comparative evaluation on the thermal behaviors and kinetics of combustion of heavy crude oil and its SARA fractions. Fuel 2019, 239, 117–125. [Google Scholar] [CrossRef]
- Amanam, U.U.; Koh Yoo, K.H.; Castanier, L.; Kovscek, A.R. Investigation of the Effects of Select Metal Nanoparticles on Heavy Oil Combustion in Porous Media. Energy Fuels 2020, 34, 130–141. [Google Scholar] [CrossRef]
- Pu, W.F.; Yuan, C.D.; Jin, F.Y.; Wang, L.; Qian, Z.; Li, Y.B.; Li, D.; Chen, Y.F. Low-temperature oxidation and characterization of heavy oil via thermal analysis. Energy Fuels 2015, 29, 1151–1159. [Google Scholar] [CrossRef]
- Niu, B.; Ren, S.; Liu, Y.; Wang, D.; Tang, L.; Chen, B. Low-temperature oxidation of oil components in an air injection process for improved oil recovery. Energy Fuels 2011, 25, 4299–4304. [Google Scholar] [CrossRef]
- Zhao, S.; Pu, W.; Varfolomeev, M.A.; Yuan, C.; Pan, J.; Wang, R.; Chen, L.; Kan, N. Low-temperature oxidation of light and heavy oils via thermal analysis: Kinetic analysis and temperature zone division. J. Pet. Sci. Eng. 2018, 168, 246–255. [Google Scholar] [CrossRef]
- Murugan, P.; Mani, T.; Mahinpey, N.; Asghari, K. The low temperature oxidation of Fosterton asphaltenes and its combustion kinetics. Fuel Process. Technol. 2011, 92, 1056–1061. [Google Scholar] [CrossRef]
- Yuan, C.; Emelianov, D.A.; Varfolomeev, M.A.; Abaas, M. Comparison of oxidation behavior of linear and branched alkanes. Fuel Process. Technol. 2019, 188, 203–211. [Google Scholar] [CrossRef]
- Vyazovkin, S. Isoconversional Kinetics of Thermally Stimulated Processes; Springer: Berlin/Heidelberg, Germany, 2015; ISBN 9783319141756. [Google Scholar]
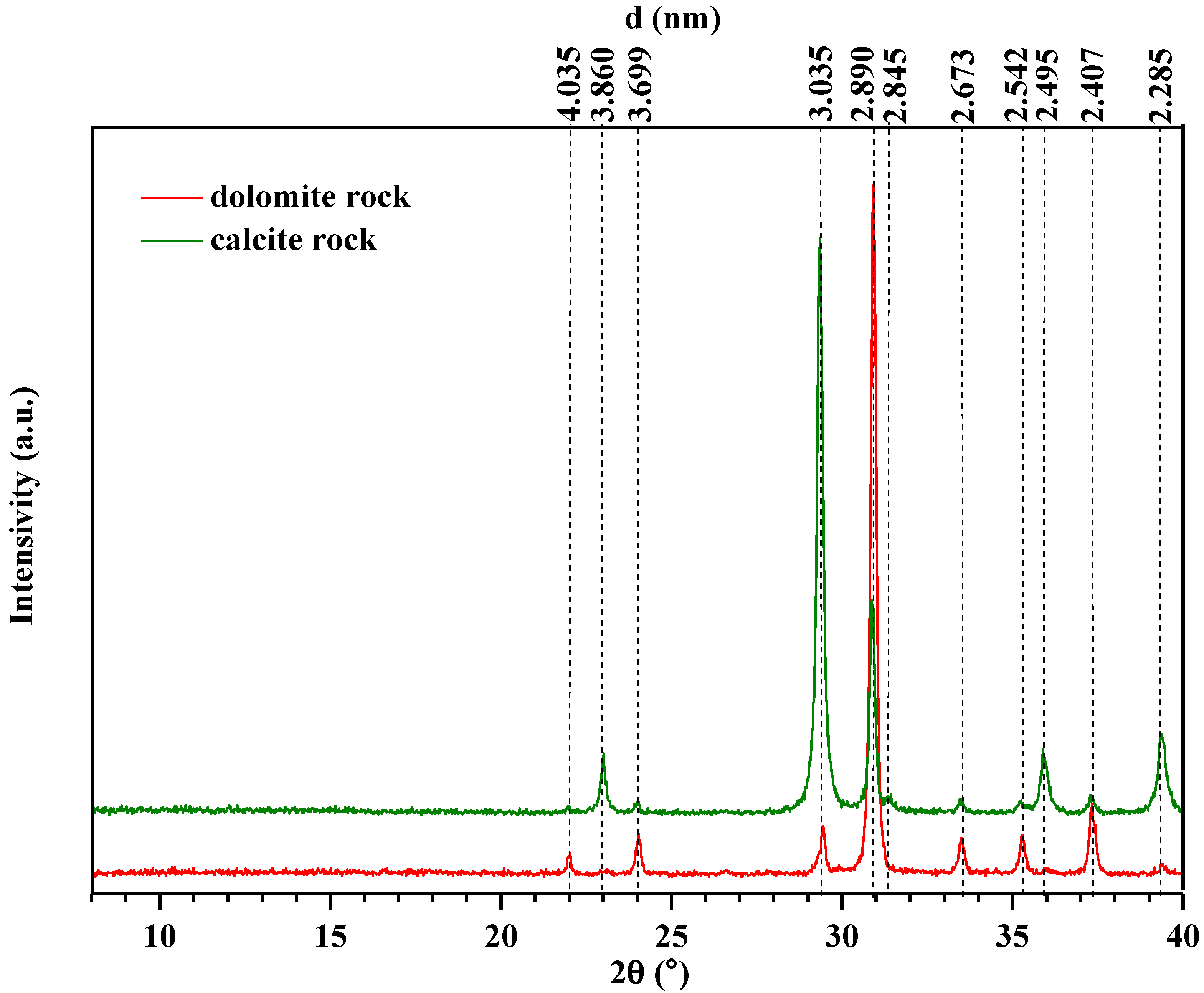

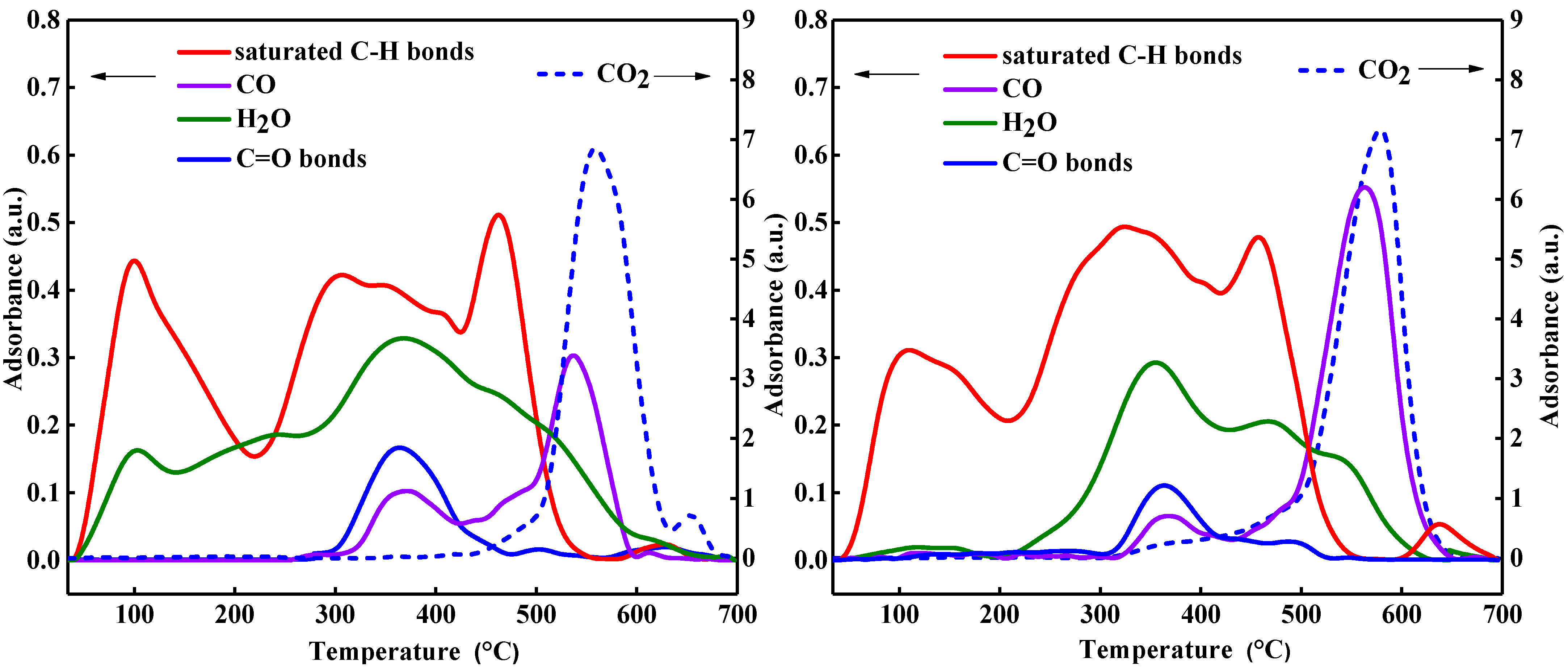
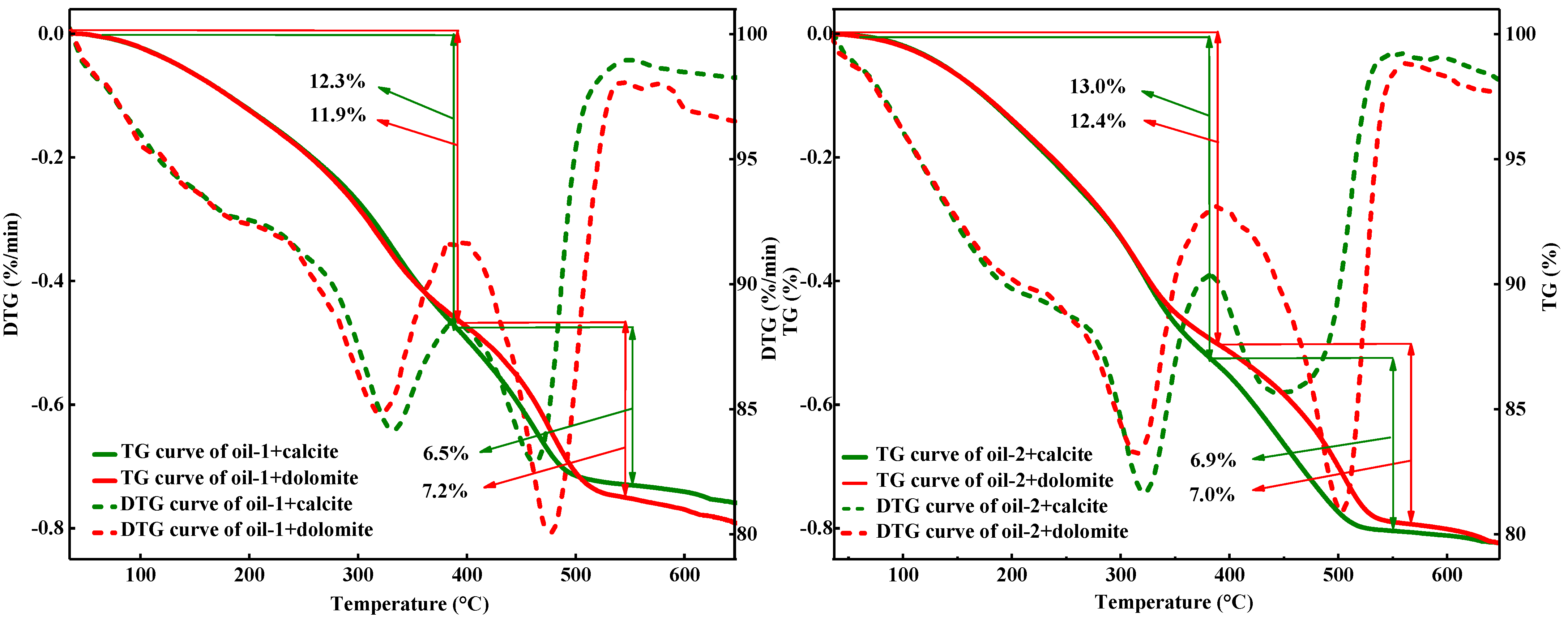

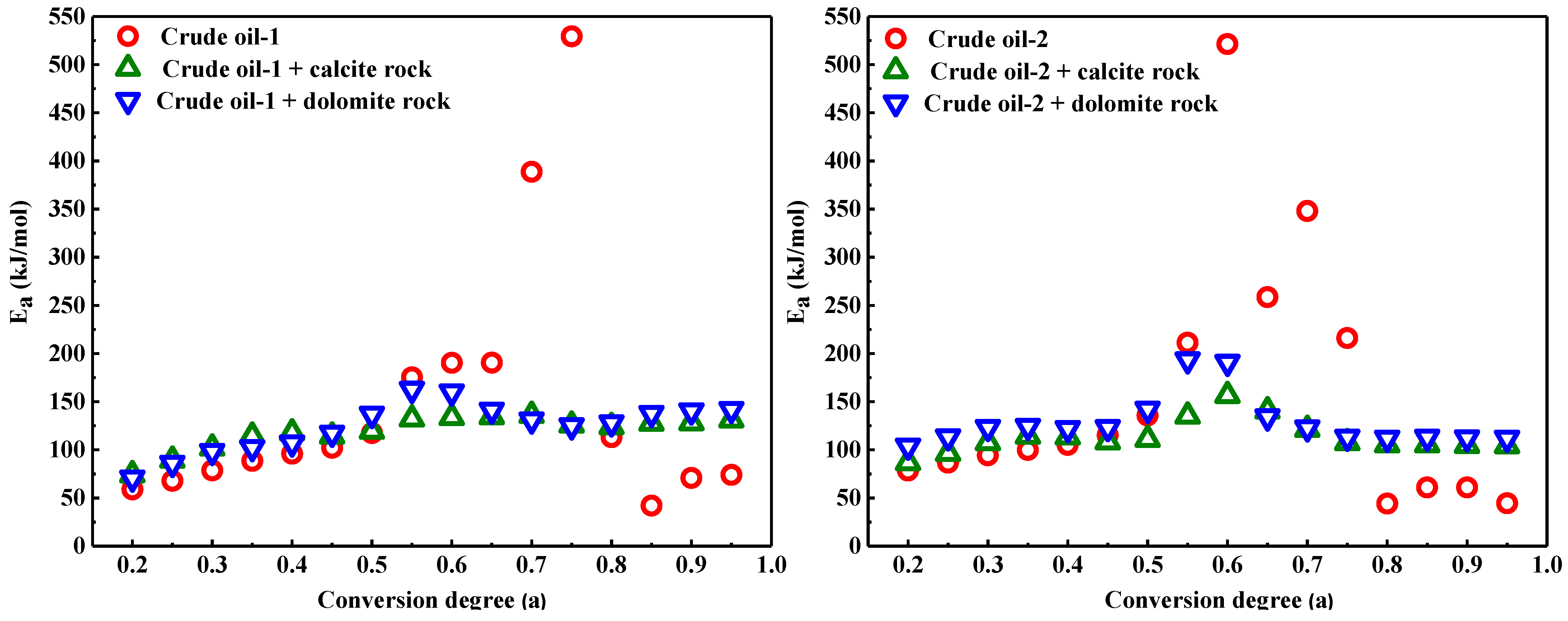
| Property | Oil-1 | Oil-2 |
|---|---|---|
| API gravity (°) | 19.70 | 14.10 |
| Viscosity (MPa·s) | 1438.50 | 2073.00 |
| Saturates (%) | 30.74 | 28.79 |
| Aromatics (%) | 40.22 | 44.32 |
| Resins (%) | 20.47 | 20.98 |
| Asphaltenes (%) | 8.57 | 5.91 |
| Rock Type | Mineral Content, % | |||
|---|---|---|---|---|
| Calcite | Dolomite | Albite | Quartz | |
| Calcite rock | 75.0 | 25.0 | - | - |
| Dolomite rock | 6.0 | 91.5 | 2.2 | 0.3 |
| Heating Rate (°C/min) | LTO | FD | HTO | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Temp. Range (°C) | Peak Temp. (°C) | Mass Loss (%) | Temp. Range (°C) | Peak Temp. (°C) | Mass Loss (%) | Temp. Range (°C) | Peak Temp. (°C) | Mass Loss (%) | |
| Crude oil-1 | |||||||||
| 4 | <367.5 | 318.7 | 55.4 | 367.5–469.1 | 418.1 | 18.3 | 482.6–597.1 | 522.7 | 24.8 |
| 6 | <393.8 | 333.1 | 57.7 | 393.8–486.6 | 453.4 | 18.6 | 490.3–627.4 | 527.7 | 21.7 |
| 8 | <405.5 | 346.8 | 59.2 | 405.5–492.5 | 455.8 | 17.7 | 503.6–644.0 | 536.5 | 21.3 |
| 10 | <426.1 | 351.0 | 64.0 | 426.1–508.2 | 460.1 | 14.9 | 515.6–652.8 | 556.9 | 18.1 |
| Crude oil-2 | |||||||||
| 4 | <366.4 | 314.1 | 56.2 | 366.4–472.6 | 429.5 | 17.8 | 472.6–593.5 | 534.4 | 25.5 |
| 6 | <381.8 | 330.4 | 57.7 | 381.8–486.2 | 448.5 | 19.0 | 486.2–624.4 | 545.0 | 23.0 |
| 8 | <392.4 | 339.7 | 59.2 | 392.4–500.7 | 452.4 | 18.9 | 500.7–642.9 | 562.2 | 21.6 |
| 10 | <428.2 | 347.6 | 65.4 | 428.2–510.4 | 457.7 | 13.0 | 510.4–666.9 | 574.4 | 21.2 |
| Heating Rate (°C/min) | LTO-FD | FD-HTO | ||||
|---|---|---|---|---|---|---|
| Temp. Range (°C) | Peak Temp. (°C) | Mass Loss (%) | Temp. Range (°C) | Peak Temp. (°C) | Mass Loss (%) | |
| Crude oil-1 with calcite rock | ||||||
| 4 | <372.3 | 308.0 | 12.6 | 372.3–524.1 | 428.3 | 6.1 |
| 6 | <381.3 | 319.1 | 12.7 | 381.3–534.8 | 440.6 | 6.1 |
| 8 | <383.0 | 323.2 | 12.3 | 383.0–540.6 | 447.9 | 6.4 |
| 10 | <391.2 | 332.0 | 12.3 | 391.2–572.3 | 463.0 | 6.5 |
| Crude oil-1 with dolomite rock | ||||||
| 4 | <367.3 | 295.0 | 11.7 | 367.3–526.6 | 445.2 | 7.2 |
| 6 | <378.8 | 307.4 | 11.8 | 378.8–538.9 | 460.7 | 7.2 |
| 8 | <384.6 | 312.9 | 11.9 | 384.6–552.1 | 468.4 | 7.3 |
| 10 | <397.0 | 320.7 | 11.9 | 397.0–567.8 | 476.0 | 7.3 |
| Crude oil-2 with calcite rock | ||||||
| 4 | <360.7 | 289.9 | 13.0 | 360.7–526.3 | 404.3 | 6.9 |
| 6 | <369.0 | 309.4 | 12.9 | 369.0–532.9 | 425.0 | 6.9 |
| 8 | <376.4 | 316.2 | 13.1 | 376.4–545.3 | 430.4 | 6.8 |
| 10 | <383.0 | 320.7 | 13.0 | 383.0–572.8 | 440.1 | 6.9 |
| Crude oil-2 with dolomite rock | ||||||
| 4 | <361.1 | 293.9 | 12.0 | 361.1–522.5 | 470.7 | 7.3 |
| 6 | <375.1 | 303.7 | 12.2 | 375.1–548.9 | 487.0 | 7.2 |
| 8 | <385.9 | 311.1 | 12.4 | 385.9–554.7 | 496.0 | 7.1 |
| 10 | <394.9 | 316.0 | 12.4 | 394.9–582.6 | 505.3 | 7.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ariskina, K.A.; Ding, Z.; Abaas, M.; Yuan, C.; Emelianov, D.A.; Chen, Q.; Varfolomeev, M.A. Influence of Carbonate Minerals on Heavy Oil Oxidation Behavior and Kinetics by TG-FTIR. Energies 2021, 14, 8136. https://doi.org/10.3390/en14238136
Ariskina KA, Ding Z, Abaas M, Yuan C, Emelianov DA, Chen Q, Varfolomeev MA. Influence of Carbonate Minerals on Heavy Oil Oxidation Behavior and Kinetics by TG-FTIR. Energies. 2021; 14(23):8136. https://doi.org/10.3390/en14238136
Chicago/Turabian StyleAriskina, Kristina A., Zhenfeng Ding, Mustafa Abaas, Chengdong Yuan, Dmitrii A. Emelianov, Qing Chen, and Mikhail A. Varfolomeev. 2021. "Influence of Carbonate Minerals on Heavy Oil Oxidation Behavior and Kinetics by TG-FTIR" Energies 14, no. 23: 8136. https://doi.org/10.3390/en14238136
APA StyleAriskina, K. A., Ding, Z., Abaas, M., Yuan, C., Emelianov, D. A., Chen, Q., & Varfolomeev, M. A. (2021). Influence of Carbonate Minerals on Heavy Oil Oxidation Behavior and Kinetics by TG-FTIR. Energies, 14(23), 8136. https://doi.org/10.3390/en14238136








