Tin Oxide Modified Titanium Dioxide as Electron Transport Layer in Formamidinium-Rich Perovskite Solar Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Processing of ETL Thin Films
2.3. Fabrication of PSCs
2.4. Characterization of Materials
3. Results and Discussion
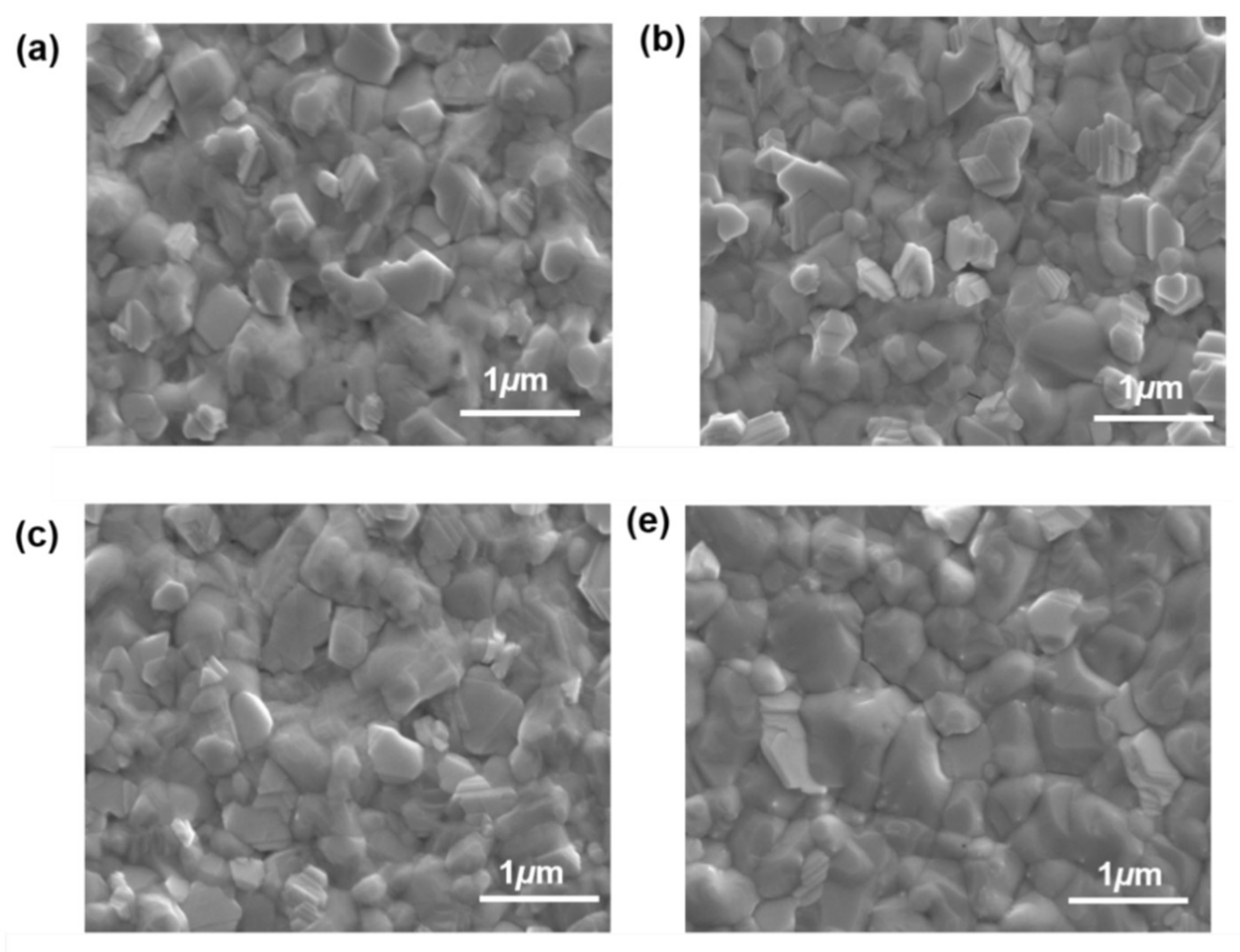
3.1. Structure and Morphology of the ETL and Perovskite Thin Films
3.2. Optoelectronic Properties of the Thin Films
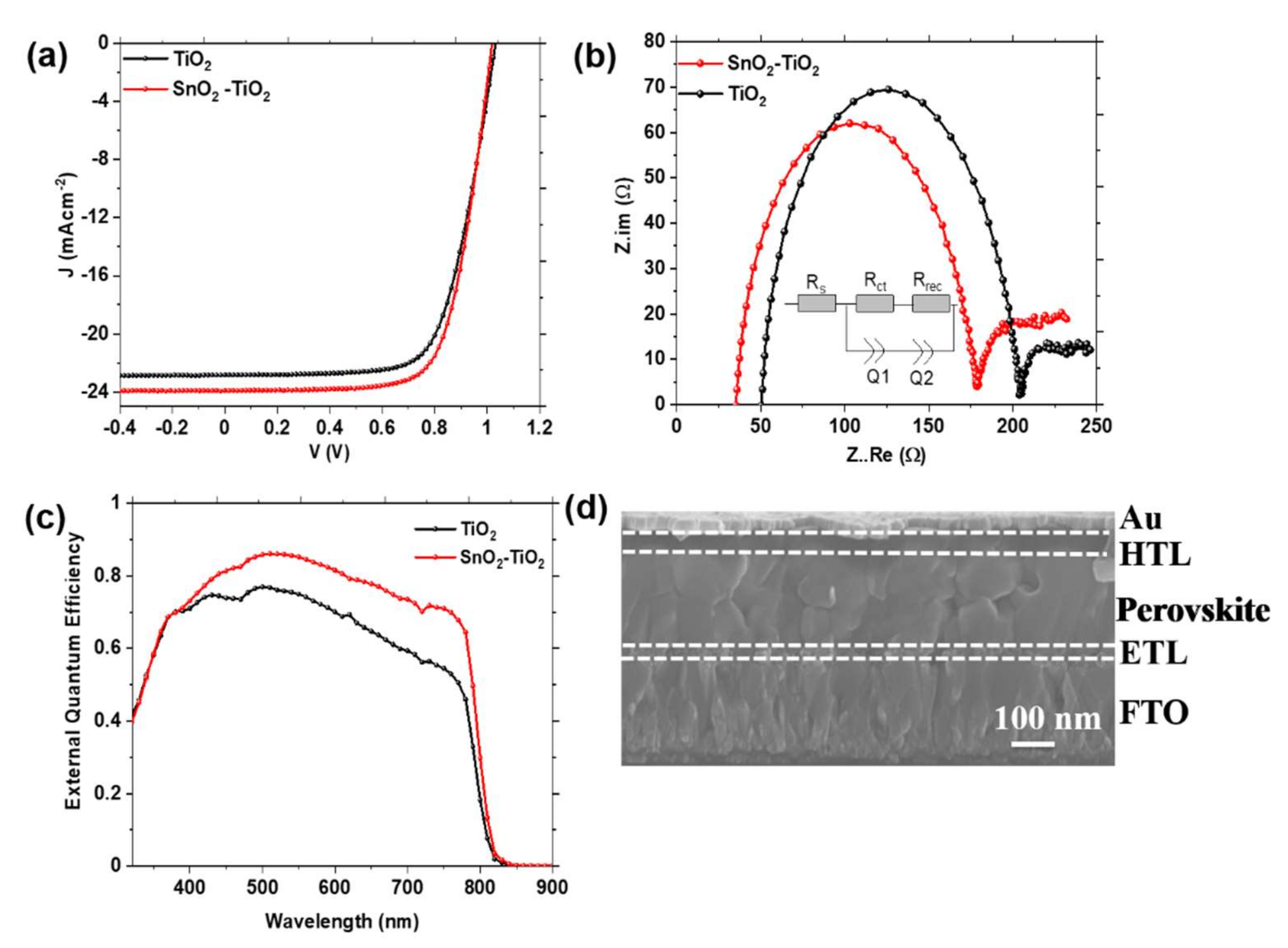
3.3. Performance Characteristics of PSCs
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Even, J.; Boyer-Richard, S.; Carignano, M.; Pedesseau, L.; Jancu, J.-M.; Katan, C. Theoretical insights into hybrid perovskites for photovoltaic applications. Proc. SPIE 2016, 9742, 97421A. [Google Scholar] [CrossRef] [Green Version]
- Koech, R.K.; Kigozi, M.; Bello, A.; Onwualu, P.A.; Soboyejo, W.O. Recent advances in solar energy harvesting materials with particular emphasis on photovoltaic materials. In Proceedings of the IEEE PES/IAS PowerAfrica Conference: Power Economics and Energy Innovation in Africa, PowerAfrica 2019, Abuja, Nigeria, 20–23 August 2019; pp. 627–632. [Google Scholar] [CrossRef]
- Mali, S.S.; Hong, C.K. P-i-n/n-i-p type planar hybrid structure of highly efficient perovskite solar cells towards improved air stability: Synthetic strategies and the role of p-type hole transport layer (HTL) and n-type electron transport layer (ETL) metal oxides. Nanoscale 2016, 8, 10528–10540. [Google Scholar] [CrossRef]
- You, J.; Meng, L.; Hong, Z.; Li, G.; Yang, Y. Inverted planar structure of perovskite solar cells. In Organic-Inorganic Halide Perovskite Photovoltaics: From Fundamentals to Device Architectures; Springer: Cham, Switzerland, 2016. [Google Scholar]
- Thakur, U.; Kisslinger, R.; Shankar, K. One-Dimensional Electron Transport Layers for Perovskite Solar Cells. Nanomaterials 2017, 7, 95. [Google Scholar] [CrossRef] [PubMed]
- Yue, L.; Yan, B.; Attridge, M.; Wang, Z. Light absorption in perovskite solar cell: Fundamentals and plasmonic enhancement of infrared band absorption. Sol. Energy 2016, 124, 143–152. [Google Scholar] [CrossRef]
- Shi, J.; Li, Y.; Li, Y.; Li, D.; Luo, Y.; Wu, H.; Meng, Q. From Ultrafast to Ultraslow: Charge-Carrier Dynamics of Perovskite Solar Cells. Joule 2018, 2, 879–901. [Google Scholar] [CrossRef] [Green Version]
- Hsiao, Y.C.; Wu, T.; Li, M.; Liu, Q.; Qin, W.; Hu, B. Fundamental physics behind high-efficiency organo-metal halide perovskite solar cells. J. Mater. Chem. A 2015, 3, 15372–15385. [Google Scholar] [CrossRef]
- Giorgi, G.; Yamashita, K. Organic-inorganic halide perovskites: An ambipolar class of materials with enhanced photovoltaic performances. J. Mater. Chem. A 2015, 3, 8981–8991. [Google Scholar] [CrossRef]
- Zhang, F.; Yang, B.; Li, Y.; Deng, W.; He, R. Extra long electron-hole diffusion lengths in CH 3 NH 3 PbI 3-: X Cl x perovskite single crystals. J. Mater. Chem. C 2017, 5, 8431–8435. [Google Scholar] [CrossRef]
- Tailor, N.K.; Abdi-Jalebi, M.; Gupta, V.; Lu, H.; Dar, M.I.; Li, G.; Satapathi, S. Recent progress in morphology optimization in perovskite solar cells. J. Mater. Chem. A 2020, 8, 21356–21386. [Google Scholar] [CrossRef]
- Le Corre, V.M.; Stolterfoht, M.; Perdigo, L.; Feuerstein, M.; Wolff, C.; Bolink, H.J.; Neher, D.; Koster, L.J.A. Charge Transport Layers Limiting the Efficiency of Perovskite Solar Cells: How To Optimize Conductivity, Doping, and Thickness. ACS Appl. Energy Mater. 2019, 29, 40. [Google Scholar] [CrossRef] [Green Version]
- Wu, N.; Wu, Y.; Walter, D.; Shen, H.; Duong, T.; Grant, D.; Barugkin, C.; Fu, X.; Peng, J.; White, T.; et al. Identifying the Cause of Voltage and Fill Factor Losses in Perovskite Solar Cells by Using Luminescence Measurements. Energy Technol. 2017, 5, 1827–1835. [Google Scholar] [CrossRef] [Green Version]
- Grill, I.; Aygüler, M.F.; Bein, T.; Docampo, P.; Hartmann, N.F.; Handloser, M.; Hartschuh, A. Charge Transport Limitations in Perovskite Solar Cells: The Effect of Charge Extraction Layers. ACS Appl. Mater. Interfaces 2017, 9, 37655–37661. [Google Scholar] [CrossRef] [Green Version]
- Wolff, C.M.; Caprioglio, P.; Stolterfoht, M.; Neher, D. Nonradiative Recombination in Perovskite Solar Cells: The Role of Interfaces. Adv. Mater. 2019, 31, 1902762. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stolterfoht, M.; Caprioglio, P.; Wolff, C.M.; Márquez, J.A.; Nordmann, J.; Zhang, S.; Rothhardt, D.; Hörmann, U.; Amir, Y.; Redinger, A.; et al. The impact of energy alignment and interfacial recombination on the internal and external open-circuit voltage of perovskite solar cells. Energy Environ. Sci. 2019, 12, 2778–2788. [Google Scholar] [CrossRef] [Green Version]
- Courtier, N.E.; Cave, J.M.; Foster, J.M.; Walker, A.B.; Richardson, G. How transport layer properties affect perovskite solar cell performance: Insights from a coupled charge transport/ion migration model. Energy Environ. Sci. 2019, 12, 396–409. [Google Scholar] [CrossRef] [Green Version]
- Ren, X.; Wang, Z.S.; Choy, W.C.H. Device Physics of the Carrier Transporting Layer in Planar Perovskite Solar Cells. Adv. Opt. Mater. 2019, 7, 1900407. [Google Scholar] [CrossRef]
- Mahmood, K.; Sarwar, S.; Mehran, M.T. Current status of electron transport layers in perovskite solar cells: Materials and properties. RSC Adv. 2017, 7, 17044–17062. [Google Scholar] [CrossRef] [Green Version]
- Yu, M.; Guo, Y.; Yuan, S.; Zhao, J.S.; Qin, Y.; Ai, X.C. The influence of the electron transport layer on charge dynamics and trap-state properties in planar perovskite solar cells. RSC Adv. 2020, 10, 12347–12353. [Google Scholar] [CrossRef]
- Nimens, W.J.; Ogle, J.; Caruso, A.; Jonely, M.; Simon, C.; Smilgies, D.; Noriega, R.; Scarpulla, M.; Whittaker-Brooks, L. Morphology and Optoelectronic Variations Underlying the Nature of the Electron Transport Layer in Perovskite Solar Cells. ACS Appl. Energy Mater. 2018, 1, 602–615. [Google Scholar] [CrossRef]
- Shin, S.S.; Lee, S.J.; Seok, S.I. Metal Oxide Charge Transport Layers for Efficient and Stable Perovskite Solar Cells. Adv. Funct. Mater. 2019, 29, 1900455. [Google Scholar] [CrossRef]
- Zhou, Y.; Li, X.; Lin, H. To Be Higher and Stronger—Metal Oxide Electron Transport Materials for Perovskite Solar Cells. Small 2020, 16, 1902579. [Google Scholar] [CrossRef] [PubMed]
- Zheng, S.; Wang, G.; Liu, T.; Lou, L.; Xiao, S.; Yang, S. Materials and structures for the electron transport layer of efficient and stable perovskite solar cells. Sci. China Chem. 2019, 62, 800–809. [Google Scholar] [CrossRef]
- Chen, X.; Mao, S.S. Titanium dioxide nanomaterials: Synthesis, properties, modifications and applications. Chem. Rev. 2007, 107, 2891–2959. [Google Scholar] [CrossRef]
- Chen, X. Titanium dioxide nanomaterials and their energy applications. Cuihua Xuebao/Chin. J. Catal. 2009, 30, 839–851. [Google Scholar] [CrossRef]
- Pan, X.; Yang, M.Q.; Fu, X.; Zhang, N.; Xu, Y.J. Defective TiO2 with oxygen vacancies: Synthesis, properties and photocatalytic applications. Nanoscale 2013, 5, 3601–3614. [Google Scholar] [CrossRef]
- Yu, J.C.; Hong, J.A.; Jung, E.D.; Kim, D.B.; Baek, S.M.; Lee, S.; Cho, S.; Park, S.S.; Choi, K.J.; Song, M.H. Highly efficient and stable inverted perovskite solar cell employing PEDOT:GO composite layer as a hole transport layer. Sci. Rep. 2018, 8, 1070. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nwankwo, U.; Hong, J.A.; Jung, E.D.; Kim, D.B.; Baek, S.M.; Lee, S.; Cho, S.; Park, S.S.; Choi, K.J.; Song, M.H. Effects of alkali and transition metal-doped TiO2 hole blocking layers on the perovskite solar cells obtained by a two-step sequential deposition method in air and under vacuum. RSC Adv. 2020, 10, 13139–13148. [Google Scholar] [CrossRef] [Green Version]
- Belchi, R.; Habert, A.; Foy, E.; Gheno, A.; Vedraine, S.; Antony, R.; Ratier, B.; Bouclé, J.; Herlin-Boime, N. One-Step Synthesis of TiO2/Graphene Nanocomposites by Laser Pyrolysis with Well-Controlled Properties and Application in Perovskite Solar Cells. ACS Omega 2019, 4, 11906–11913. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saleem, A.; Ullah, N.; Khursheed, K.; Iqbal, T.; Shah, S.A.; Asjad, M.; Sarwar, N.; Saleem, M.; Arshad, M. Graphene Oxide–TiO2 Nanocomposite Films for Electron Transport Applications. J. Electron. Mater. 2018, 47, 3749–3756. [Google Scholar] [CrossRef]
- Li, W.; Elzatahry, A.; Aldhayan, D.; Zhao, D. Core-shell structured titanium dioxide nanomaterials for solar energy utilization. Chem. Soc. Rev. 2018, 47, 8203–8237. [Google Scholar] [CrossRef] [PubMed]
- Apostolopoulou, A.; Sygkridou, D.; Rapsomanikis, A.; Kalarakis, A.N.; Stathatos, E. Enhanced performance of mesostructured perovskite solar cells in ambient conditions with a composite TiO2–In2O3 electron transport layer. Sol. Energy Mater. Sol. Cells 2017, 166, 100–107. [Google Scholar] [CrossRef]
- Akurati, K.K.; Vital, A.; Hany, R.; Bommer, B.; Graule, T.; Winterer, M. One-step flame synthesis of SnO2/TiO2 composite nanoparticles for photocatalytic applications. Int. J. Photoenergy 2005, 7, 153–161. [Google Scholar] [CrossRef] [Green Version]
- Zhen, C.; Wu, T.; Chen, R.; Wang, L.; Liu, G.; Cheng, H.M. Strategies for Modifying TiO 2 Based Electron Transport Layers to Boost Perovskite Solar Cells. ACS Sustain. Chem. Eng. 2019, 7, 4586–4618. [Google Scholar] [CrossRef]
- Larin, A.; Womble, P.C.; Dobrokhotov, V. Hybrid SnO2/TiO2 nanocomposites for selective detection of ultra-low hydrogen sulfide concentrations in complex backgrounds. Sensors 2016, 16, 1373. [Google Scholar] [CrossRef] [PubMed]
- Desai, U.V.; Xu, C.; Wu, J.; Gao, D. Hybrid TiO2-SnO2 nanotube arrays for dye-sensitized solar cells. J. Phys. Chem. C 2013, 117, 3232–3239. [Google Scholar] [CrossRef]
- Duan, Y.; Fu, N.; Liu, Q.; Fang, Y.; Zhou, X.; Zhang, J.; Lin, Y. Sn-doped TiO2 photoanode for dye-sensitized solar cells. J. Phys. Chem. C 2012, 116, 8888–8893. [Google Scholar] [CrossRef]
- Dou, M.; Persson, C. Comparative study of rutile and anatase SnO2 and TiO2: Band-edge structures, dielectric functions, and polaron effects. J. Appl. Phys. 2013, 113, 083703. [Google Scholar] [CrossRef]
- Guo, H.; Zhang, H.; Yang, J.; Chen, H.; Li, Y.; Wang, L.; Niu, X. TiO2/SnO2 Nanocomposites as Electron Transporting Layer for Efficiency Enhancement in Planar CH3NH3PbI3-Based Perovskite Solar Cells. ACS Appl. Energy Mater. 2018, 1, 6936–6944. [Google Scholar] [CrossRef]
- Li, N.; Yan, J.; Ai, Y.; Jiang, E.; Lin, L.; Shou, C.; Yan, B.; Sheng, J.; Ye, J. A low-temperature TiO2/SnO2 electron transport layer for high-performance planar perovskite solar cells. Sci. China Mater. 2020, 63, 207–215. [Google Scholar] [CrossRef] [Green Version]
- Liu, Z.; Sun, B.; Liu, X.; Han, J.; Ye, H.; Tu, Y.; Chen, C.; Shi, T.; Tang, Z.; Liao, G. 15% efficient carbon based planar-heterojunction perovskite solar cells using a TiO2/SnO2 bilayer as the electron transport layer. J. Mater. Chem. A 2018, 6, 7409–7419. [Google Scholar] [CrossRef]
- Song, S.; Kang, G.; Pyeon, L.; Lim, C.; Lee, G.Y.; Park, T.; Choi, J. Systematically Optimized Bilayered Electron Transport Layer for Highly Efficient Planar Perovskite Solar Cells (η = 21.1%). ACS Energy Lett. 2017, 2, 2667–2673. [Google Scholar] [CrossRef]
- Tavakoli, M.M.; Yadav, P.; Tavakoli, R.; Kong, J. Surface Engineering of TiO2 ETL for Highly Efficient and Hysteresis-Less Planar Perovskite Solar Cell (21.4%) with Enhanced Open-Circuit Voltage and Stability. Adv. Energy Mater. 2018, 8, 1800794. [Google Scholar] [CrossRef]
- Martínez-Denegri, G.; Colodrero, S.; Kramarenko, M.; Martorell, J. All-Nanoparticle SnO2/TiO2 Electron-Transporting Layers Processed at Low Temperature for Efficient Thin-Film Perovskite Solar Cells. ACS Appl. Energy Mater. 2018, 1, 5548–5556. [Google Scholar] [CrossRef]
- Wan, F.; Qiu, X.; Chen, H.; Liu, Y.; Xie, H.; Shi, J.; Huang, H.; Yuan, Y.; Gao, Y.; Zhou, C. Accelerated electron extraction and improved UV stability of TiO2 based perovskite solar cells by SnO2 based surface passivation. Org. Electron. 2018, 59, 184–189. [Google Scholar] [CrossRef]
- Liu, N.; Chen, M.; Yang, H.; Ran, M.; Zhang, C.; Luo, X.; Lu, H.; Yang, Y. TiO2/Mg-SnO2 nanoparticle composite compact layer for enhancing the performance of perovskite solar cells. Opt. Mater. Express 2020, 10, 157–169. [Google Scholar] [CrossRef]
- Mohammadbeigi, A.; Mozaffari, S.; Ghorashi, S.M.B. Yolk-shell SnO2@TiO2 nanospheres as electron transport layer in mesoscopic perovskite solar cell. J. Sol-Gel Sci. Technol. 2020, 94, 731–742. [Google Scholar] [CrossRef]
- Oyewole, D.O.; Koech, R.K.; Ichwani, R.; Ahmed, R.; Tamayo, J.H.; Adeniji, S.A.; Cromwell, J.; Ulloa, E.C.; Oyewole, O.K.; Agyei-Tuffour, B.; et al. Annealing effects on interdiffusion in layered FA-rich perovskite solar cells Annealing effects on interdiffusion in layered FA-rich perovskite solar cells AIP Advances. AIP Adv. 2021, 11, 65327. [Google Scholar] [CrossRef]
- Mohamad Noh, M.F.; Teh, C.H.; Daik, R.; Lim, E.L.; Yap, C.C.; Ibrahim, M.A.; Ahmad Ludin, N.; Bin Mohd Yusoff, A.R.; Jang, J.; Mat Teridi, M.A. The architecture of the electron transport layer for a perovskite solar cell. J. Mater. Chem. C 2018, 6, 682–712. [Google Scholar] [CrossRef]
- Ke, W.; Zhao, D.; Cimaroli, A.J.; Grice, C.R.; Qin, P.; Liu, Q.; Xiong, L.; Yan, Y.; Fang, G. Effects of annealing temperature of tin oxide electron selective layers on the performance of perovskite solar cells. J. Mater. Chem. A 2015, 3, 24163–24168. [Google Scholar] [CrossRef]
- Tauc, J.; Menth, A. States in the gap. J. Non. Cryst. Solids 1972, 8, 569–585. [Google Scholar] [CrossRef]
- Makuła, P.; Pacia, M.; Macyk, W. How To Correctly Determine the Band Gap Energy of Modified Semiconductor Photocatalysts Based on UV-Vis Spectra. J. Phys. Chem. Lett. 2018, 6814–6817. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fassl, P.; Zhao, D.; Cimaroli, A.J.; Grice, C.R.; Qin, P.; Liu, Q.; Xiong, L.; Yan, Y.; Fang, G. Effect of density of surface defects on photoluminescence properties in MAPbI3 perovskite films. J. Mater. Chem. C 2019, 7, 5285–5292. [Google Scholar] [CrossRef]
- Handa, T.; Tex, D.M.; Shimazaki, A.; Wakamiya, A.; Kanemitsu, Y. Charge Injection Mechanism at Heterointerfaces in CH3NH3PbI3 Perovskite Solar Cells Revealed by Simultaneous Time-Resolved Photoluminescence and Photocurrent Measurements. J. Phys. Chem. Lett. 2017, 8, 954–960. [Google Scholar] [CrossRef] [PubMed]
- Jain, A.; Kumar, P.; Jain, S.C.; Kumar, V.; Kaur, R.; Mehra, R.M. Trap filled limit voltage (VTFL) and V2 law in space charge limited currents. J. Appl. Phys. 2007, 102, 094505. [Google Scholar] [CrossRef]
- Jin, H.; Debroye, E.; Keshavarz, M.; Scheblykin, I.G.; Roeffaers, M.B.J.; Hofkens, J.; Steele, J.A. It’s a trap! on the nature of localised states and charge trapping in lead halide perovskites. Mater. Horiz. 2020, 7, 397–410. [Google Scholar] [CrossRef] [Green Version]
- Liang, P.W.; Liao, C.Y.; Chueh, C.C.; Zuo, F.; Williams, S.T.; Xin, X.K.; Lin, J.; Jen, A.K.Y. Additive enhanced crystallization of solution-processed perovskite for highly efficient planar-heterojunction solar cells. Adv. Mater. 2014, 26, 3748–3754. [Google Scholar] [CrossRef]
- Zhang, J.; Juárez-Pérez, E.J.; Mora-Seró, I.; Viana, B.; Pauporté, T. Fast and low temperature growth of electron transport layers for efficient perovskite solar cells. J. Mater. Chem. A 2015, 3, 4909–4915. [Google Scholar] [CrossRef]
- Ranjan, R.; Prakash, A.; Singh, A.; Singh, A.; Garg, A.; Gupta, R.K. Effect of tantalum doping in a TiO2 compact layer on the performance of planar spiro-OMeTAD free perovskite solar cells. J. Mater. Chem. A 2018, 6, 1037–1047. [Google Scholar] [CrossRef]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koech, R.K.; Ichwani, R.; Oyewole, D.; Kigozi, M.; Amune, D.; Sanni, D.M.; Adeniji, S.; Oyewole, K.; Bello, A.; Ntsoenzok, E.; et al. Tin Oxide Modified Titanium Dioxide as Electron Transport Layer in Formamidinium-Rich Perovskite Solar Cells. Energies 2021, 14, 7870. https://doi.org/10.3390/en14237870
Koech RK, Ichwani R, Oyewole D, Kigozi M, Amune D, Sanni DM, Adeniji S, Oyewole K, Bello A, Ntsoenzok E, et al. Tin Oxide Modified Titanium Dioxide as Electron Transport Layer in Formamidinium-Rich Perovskite Solar Cells. Energies. 2021; 14(23):7870. https://doi.org/10.3390/en14237870
Chicago/Turabian StyleKoech, Richard K., Reisya Ichwani, Deborah Oyewole, Moses Kigozi, Daniel Amune, Dahiru M. Sanni, Sharafadeen Adeniji, Kehinde Oyewole, Abdulhakeem Bello, Esidor Ntsoenzok, and et al. 2021. "Tin Oxide Modified Titanium Dioxide as Electron Transport Layer in Formamidinium-Rich Perovskite Solar Cells" Energies 14, no. 23: 7870. https://doi.org/10.3390/en14237870
APA StyleKoech, R. K., Ichwani, R., Oyewole, D., Kigozi, M., Amune, D., Sanni, D. M., Adeniji, S., Oyewole, K., Bello, A., Ntsoenzok, E., & Soboyejo, W. (2021). Tin Oxide Modified Titanium Dioxide as Electron Transport Layer in Formamidinium-Rich Perovskite Solar Cells. Energies, 14(23), 7870. https://doi.org/10.3390/en14237870





