Characterization of MOCVD-Prepared CIS Solar Cells
Abstract
:1. Introduction
2. Materials and Methods
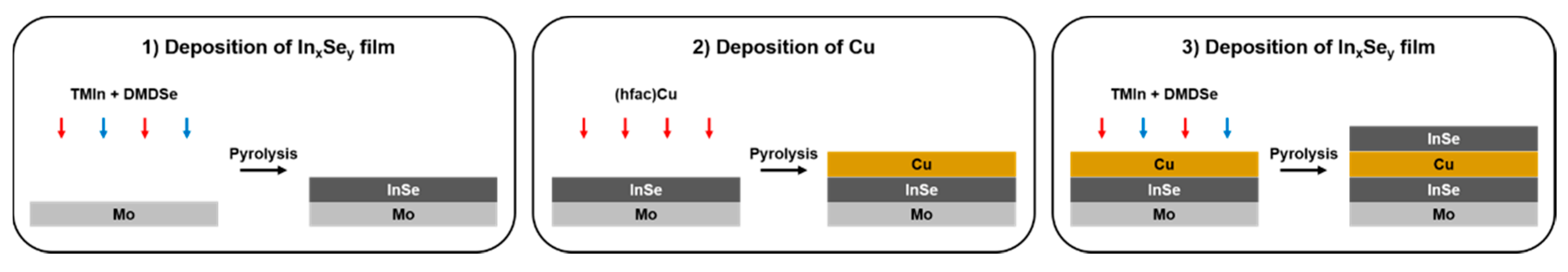
2.1. Fabrication of CIS-Series Solar Cell Samples
2.2. Characterization
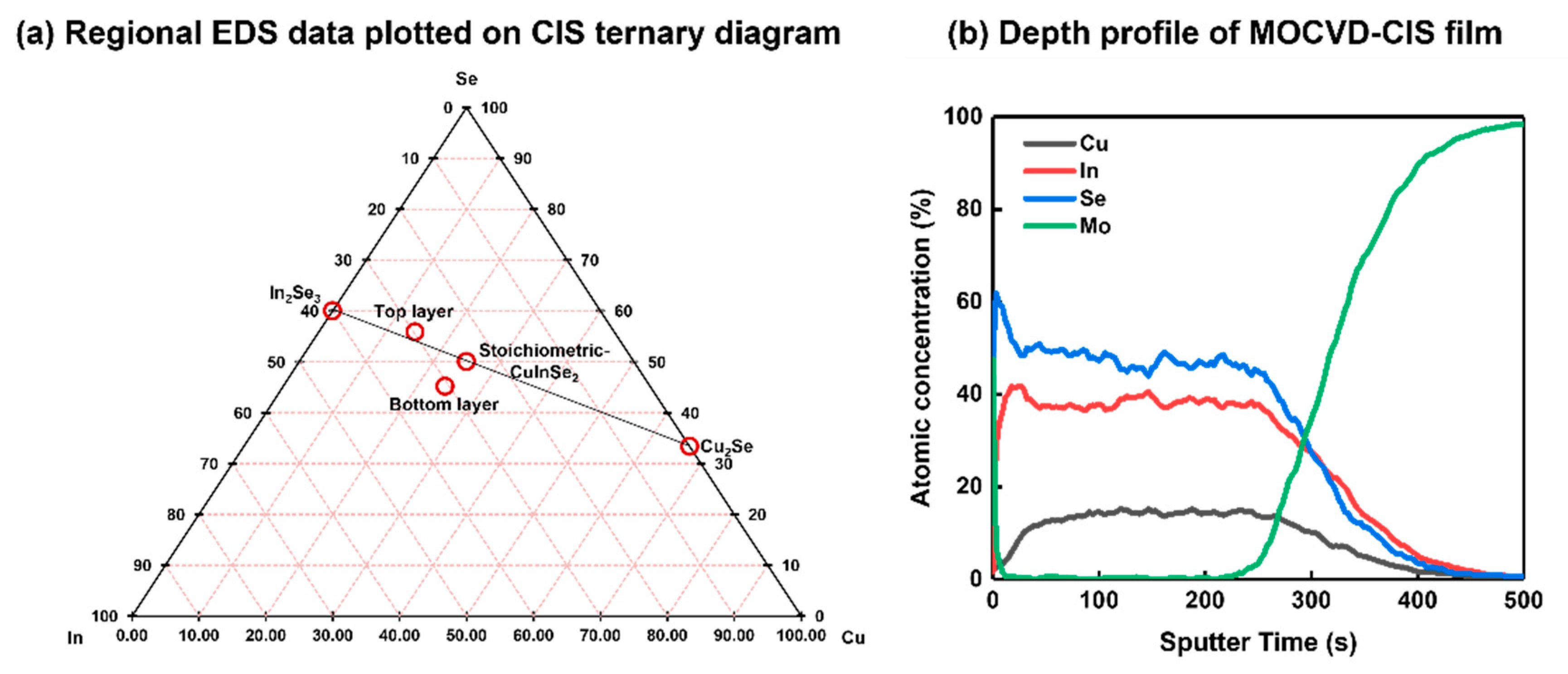
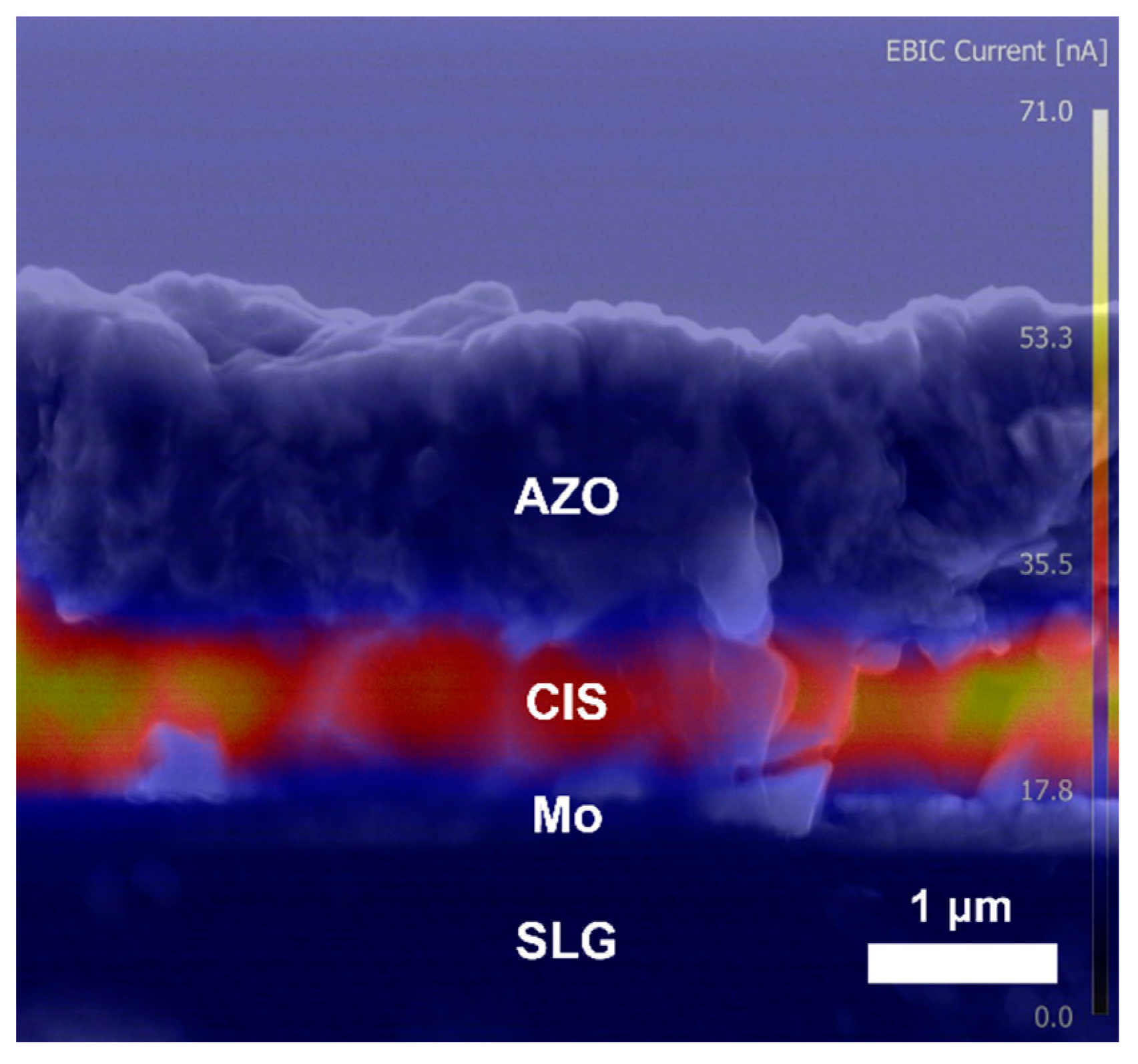
3. Results
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Green, M.; Dunlop, E.; Hohl-Ebinger, J.; Yoshita, M.; Kopidakis, N.; Hao, X. Solar cell efficiency tables (version 57). Prog. Photovolt. Res. Appl. 2021, 29, 3–15. [Google Scholar] [CrossRef]
- Li, R.; Liu, M.; Matta, S.K.; Hiltunen, A.; Deng, Z.; Wang, C.; Dai, Z.; Russo, S.P.; Vivo, P.; Zhang, H. Sulfonated dopant-free hole-transport material promotes interfacial charge transfer dynamics for highly stable perovskite solar cells. Adv. Sustain. Syst. 2021, 2100244. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, M.; Yang, W.; Judin, L.; Hukka, T.I.; Priimagi, A.; Deng, Z.; Vivo, P. Thionation enhances the performance of polymeric dopant-free hole-transporting materials for perovskite solar cells. Adv. Mater. Interfaces 2019, 6, 1901036. [Google Scholar] [CrossRef]
- Mitchell, V.D.; Jones, D.J. Advances toward the effective use of block copolymers as organic photovoltaic active layers. Polym. Chem. 2018, 9, 795–814. [Google Scholar] [CrossRef]
- Zhan, X.; Zhu, D. Conjugated polymers for high-efficiency organic photovoltaics. Polym. Chem. 2010, 1, 409–419. [Google Scholar] [CrossRef]
- Lee, S.H.; Kim, M.K.; Bae, S.; Nam, J.; Park, H.; Lee, S.W.; Jang, Y.J.; Min, B.K.; Yang, J.Y.; Kang, Y.; et al. Absorber delamination-induced shunt defects in alcohol-based solution-processed Cu(In,Ga)(S,Se)2 solar modules. ACS Appl. Energy Mater. 2020, 3, 10384–10392. [Google Scholar] [CrossRef]
- Irvine, S.J.C.; Barrioz, V.; Lamb, D.; Jones, E.W.; Rowlands-Jones, R.L. MOCVD of thin film photovoltaic solar cells—Next-generation production technology? J. Cryst. Growth 2008, 310, 5198–5203. [Google Scholar] [CrossRef]
- Niki, S.; Contreras, M.; Repins, I.; Powalla, M.; Kushiya, K.; Ishizuka, S.; Matsubara, K. CIGS absorbers and processes. Prog. Photovolt. Res. Appl. 2010, 18, 453–466. [Google Scholar] [CrossRef]
- Thompson, A.G. MOCVD technology for semiconductors. Mater. Lett. 1997, 30, 255–263. [Google Scholar] [CrossRef]
- Mufti, N.; Amrillah, T.; Taufiq, A.; Diantoro, M.; Nur, H. Review of CIGS-based solar cells manufacturing by structural engineering. Sol. Energy 2020, 207, 1146–1157. [Google Scholar] [CrossRef]
- Sagnes, B.; Salesse, A.; Artaud, M.C.; Duchemin, S.; Bougnot, J.; Bougnot, G. MOCVD growth of CuInSe2: First results. J. Cryst. Growth 1992, 124, 620–627. [Google Scholar] [CrossRef]
- Artaud, M.C.; Ouchen, F.; Martin, L.; Duchemin, S. CuInSe2 thin films grown by MOCVD: Characterization, first devices. Thin Solid Films 1998, 324, 115–123. [Google Scholar] [CrossRef]
- Choi, I.H. The preparation of a CuInSe2 solar cell by metal organic chemical vapor deposition. Thin Solid Films 2012, 525, 137–140. [Google Scholar] [CrossRef]
- Cho, J.Y.; Jeong, H.C.; Kim, K.S.; Kang, D.H.; Kim, H.K.; Shim, I.W. Preparation of phase-pure InSe thin films by MOCVD using a new single-source precursor [(Me)2In(μ-SeMe)]2. Bull. Korean Chem. Soc. 2003, 24, 645–646. [Google Scholar]
- Choi, I.H.; Lee, D.H. Preparation of CuIn1−xGaxSe2 films by metalorganic chemical vapor deposition using three precursors. Thin Solid Films 2007, 515, 4778–4782. [Google Scholar] [CrossRef]
- Gwak, J.; Lee, M.; Yun, J.H.; Ahn, S.; Cho, A.; Ahn, S.; Humle, J.P.; Shin, K.E.; Yoon, K.H. Selenium flux effect on Cu(In,Ga)Se2 thin films grown by a 3-stage co-evaporation process. Isr. J. Chem. 2015, 55, 1115–1122. [Google Scholar] [CrossRef]
- Luque, A.; Hegedus, S. (Eds.) Handbook of Photovoltaic Science and Engineering; John Wiley & Sons, Ltd.: Chichester, UK, 2011. [Google Scholar]
- Delgado, G.E.; Manfredy, L.; López-Rivera, S.A. Crystal structure of the ternary semiconductor Cu2In14/3 4/3Se8 determined by X-ray powder diffraction data. Powder Diffr. 2018, 33, 237–241. [Google Scholar] [CrossRef]
- Liang, X.; Zhu, H.; Chen, J.; Zhou, D.; Zhang, C.; Guo, Y.; Niu, X.; Li, Z.; Mai, Y. Substrate temperature optimization for Cu(In,Ga)Se2 solar cells on flexible stainless steels. Appl. Surf. Sci. 2016, 368, 464–469. [Google Scholar] [CrossRef]
- Klinkert, T.; Jubault, M.; Donsanti, F.; Lincot, D.; Guillemoles, J.F. Ga gradients in Cu(In,Ga)Se2: Formation, characterization, and consequences. J. Renew. Sustain. Energy 2014, 6, 011403. [Google Scholar] [CrossRef]
- Zhang, W.; Zhu, H.; Zhang, L.; Guo, Y.; Niu, X.; Li, Z.; Chen, J.; Liu, Q.; Mai, Y. Cu(In,Ga)Se2 thin film solar cells grown at low temperatures. Solid State Electron. 2017, 132, 57–63. [Google Scholar] [CrossRef]
- Wei, W.C.J.; Lo, M.H. Processing and properties of (Mo, Cr) oxycarbides from MOCVD. Appl. Organomet. Chem. 1998, 12, 201–220. [Google Scholar] [CrossRef]
- Djajanti, S.D.; Howe, R.F. MOCVD in zeolites using Mo(CO)6 and W(CO)6 as precursors. In Studies in Surface Science and Catalysis; Elsevier: Amsterdam, The Netherlands, 1995; pp. 197–204. [Google Scholar]
- Schaefer, C.M.; Caicedo Roque, J.M.; Sauthier, G.; Bousquet, J.; Hébert, C.; Sperling, J.R.; Pérez-Tomás, A.; Santiso, J.; del Corro, E.; Garrido, J.A. Carbon incorporation in MOCVD of MoS2 thin films grown from an organosulfide precursor. Chem. Mater. 2021, 33, 4474–4487. [Google Scholar] [CrossRef]
- Ishizuka, S.; Yamada, A.; Fons, P.J.; Kamikawa-Shimizu, Y.; Komaki, H.; Shibata, H.; Niki, S. Buried pn junction formation in CuGaSe2 thin-film solar cells. Appl. Phys. Lett. 2014, 104, 031606. [Google Scholar] [CrossRef]
- Schmid, D.; Ruckh, M.; Grunwald, F.; Schock, H.W. Chalcopyrite/defect chalcopyrite heterojunctions on the basis of CuInSe2. J. Appl. Phys. 1993, 73, 2902–2909. [Google Scholar] [CrossRef]
- Zhao, Y.; Yuan, S.; Chang, Q.; Zhou, Z.; Kou, D.; Zhou, W.; Qi, Y.; Wu, S. Controllable formation of ordered vacancy compound for high efficiency solution processed Cu(In,Ga)Se2 solar cells. Adv. Funct. Mater. 2021, 31, 2007928. [Google Scholar] [CrossRef]
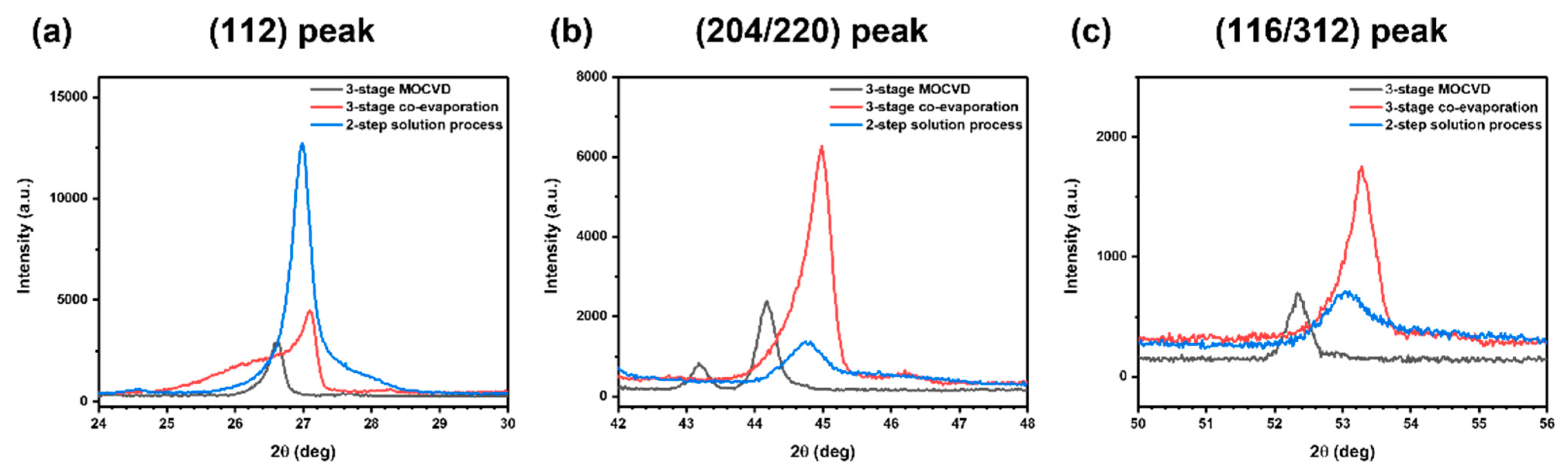

| Crystallographic Planes | Diffraction Peak Positions (2θ) | ||
|---|---|---|---|
| MOCVD-Processed CIS | Co-Evaporation-Processed CIGS | Solution-Processed CIGSSe | |
| (112) | 26.62° | 27.10° | 26.98° |
| (204/220) | 44.18° | 44.98° | 44.74° |
| (116/312) | 52.34° | 53.28° | 53.04° |
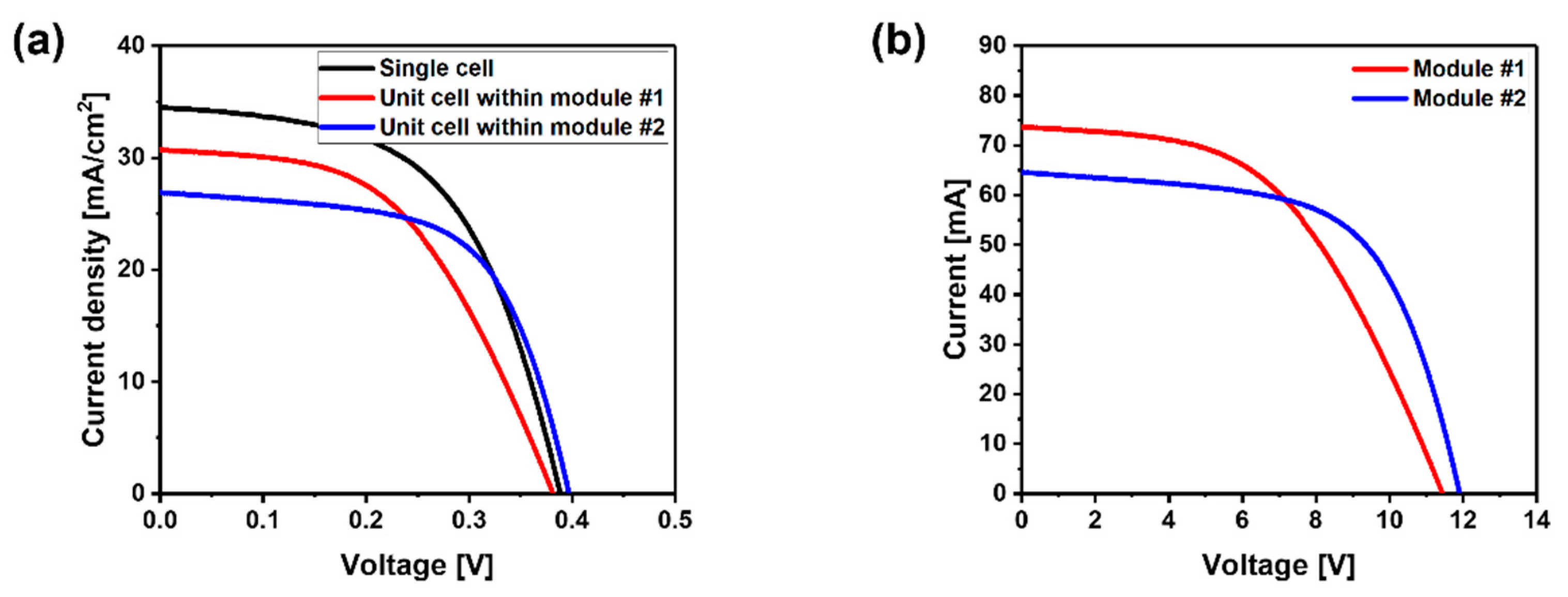
| Sample | Single Cell | Module #1 | Module #2 |
|---|---|---|---|
| VOC [V] | 0.387 | 0.381 | 0.397 |
| JSC [mA/cm2] | 34.5 | 30.7 | 26.9 |
| Fill factor [%] | 55.4 | 50.3 | 61.6 |
| Series resistance [Ω cm2] | 2.542 | 4.226 | 2.353 |
| Series resistance [Ω cm2] | 175.1 | 192.1 | 148.3 |
| Efficiency [%] | 7.39 | 5.88 | 6.57 |
| Area | 0.5 × 0.5 cm | 8 × 9 cm (with 30-unit cells) | 8 × 9 cm (with 30-unit cells) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, S.H.; Lee, G.H.; Lee, H.-S.; Kim, D.; Kang, Y. Characterization of MOCVD-Prepared CIS Solar Cells. Energies 2021, 14, 7721. https://doi.org/10.3390/en14227721
Lee SH, Lee GH, Lee H-S, Kim D, Kang Y. Characterization of MOCVD-Prepared CIS Solar Cells. Energies. 2021; 14(22):7721. https://doi.org/10.3390/en14227721
Chicago/Turabian StyleLee, Seung Hoon, Gyu Hyun Lee, Hae-Seok Lee, Donghwan Kim, and Yoonmook Kang. 2021. "Characterization of MOCVD-Prepared CIS Solar Cells" Energies 14, no. 22: 7721. https://doi.org/10.3390/en14227721
APA StyleLee, S. H., Lee, G. H., Lee, H.-S., Kim, D., & Kang, Y. (2021). Characterization of MOCVD-Prepared CIS Solar Cells. Energies, 14(22), 7721. https://doi.org/10.3390/en14227721








