Experimental and Kinetic Studies on Steam Gasification of a Biomass Char
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Char Samples
2.2. Steam Gasification Tests
2.3. Kinetic Models
3. Results and Discussion
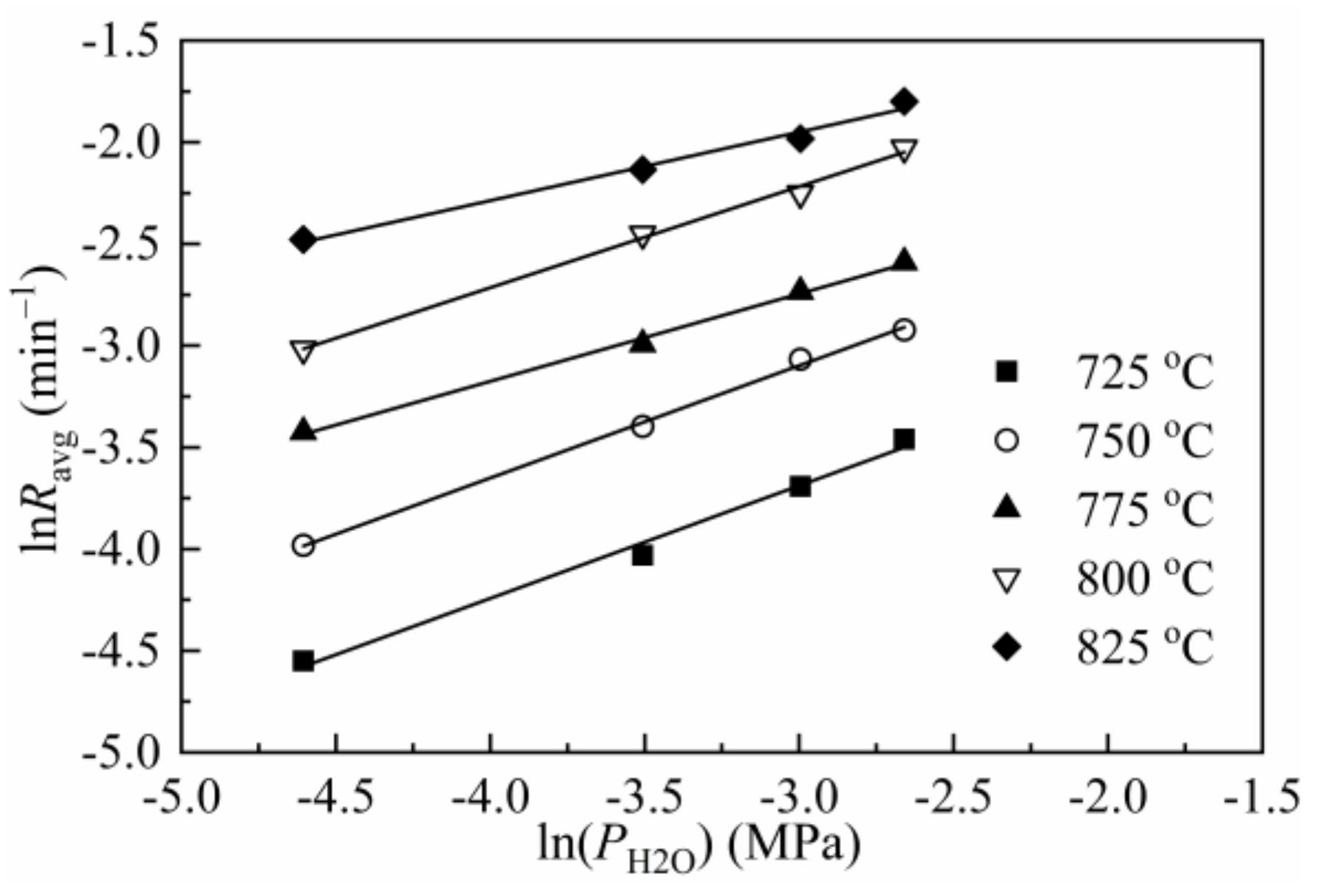
3.1. Effect of Gasification Pressure
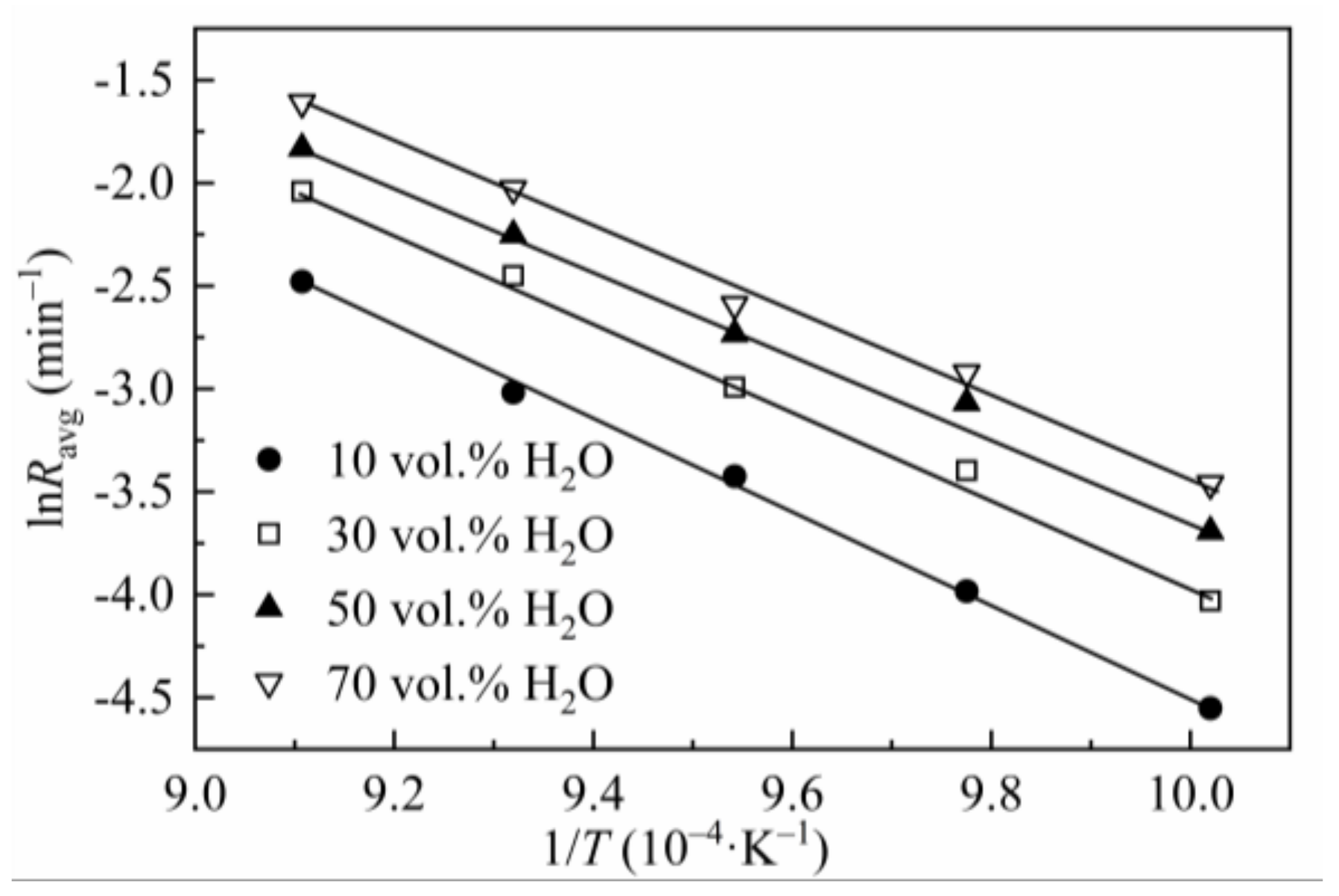
3.2. Effect of Gasification Temperature
3.3. Reaction Order
3.4. Kinetics
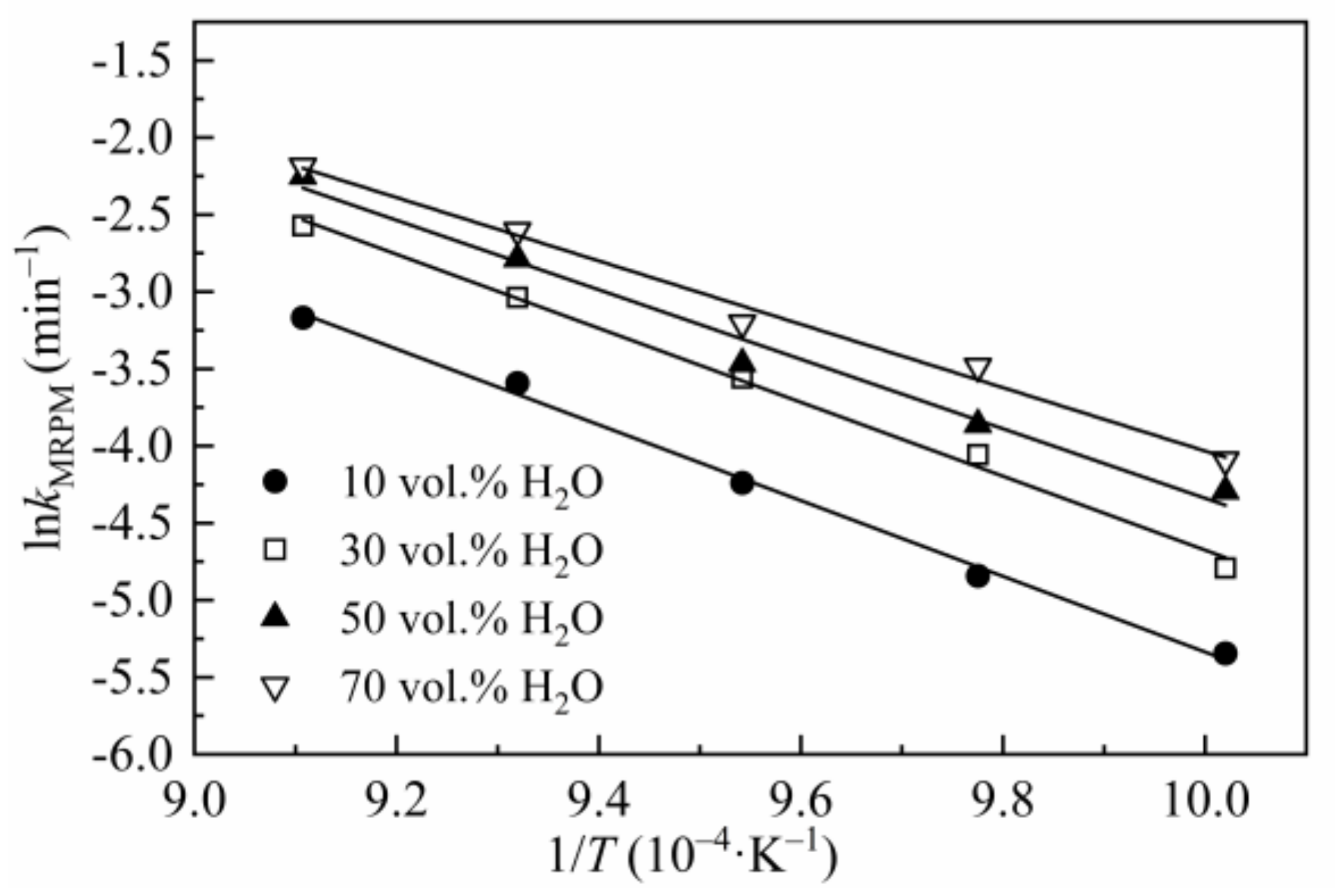
3.4.1. The Application of MRPM to Describe Biomass Char Gasification Behaviors
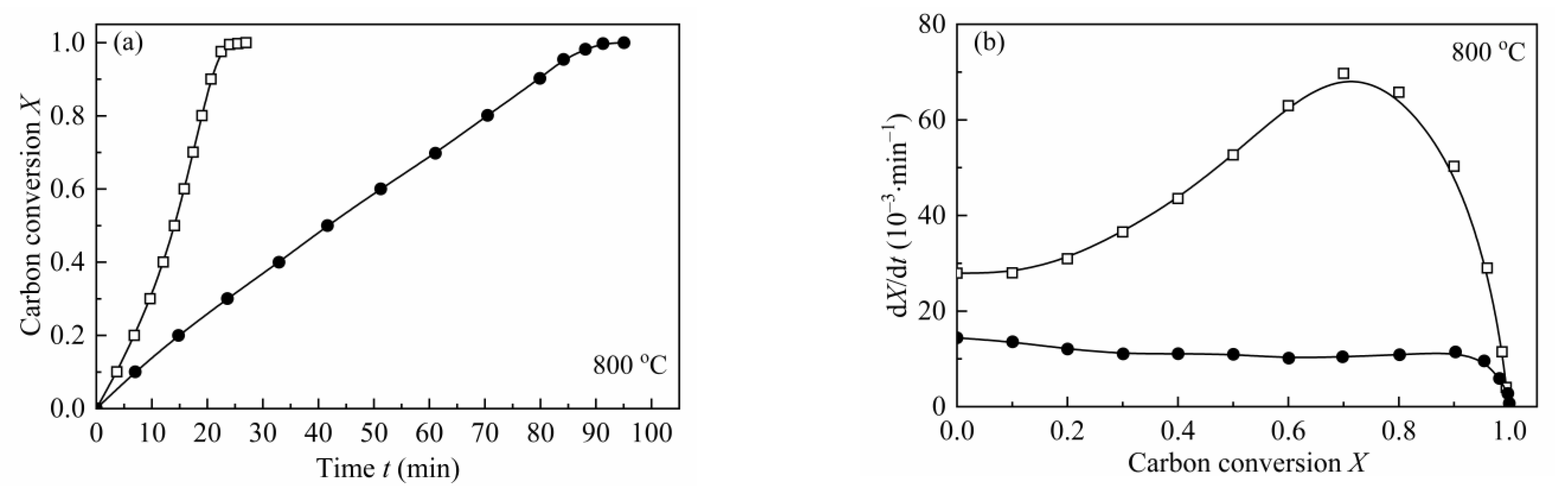
3.4.2. The Effect of Water Leaching on Biomass Char Gasification Behaviors
3.4.3. The Application of the L-H Model to Interpret Biomass Char Gasification
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Di Blasi, C. Combustion and gasification rates of lignocellulosic chars. Prog. Energy Combust. Sci. 2009, 35, 121–140. [Google Scholar] [CrossRef]
- Widjaya, E.R.; Chen, G.; Bowtell, L.; Hills, C. Gasification of non-woody biomass: A literature review. Renew. Sustain. Energy Rev. 2018, 89, 184–193. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Geng, P.; Zheng, Y. Exploration and practice to improve the kinetic analysis of char-CO2 gasification via thermogravimetric analysis. Chem. Eng. J. 2019, 359, 298–304. [Google Scholar] [CrossRef]
- Safarian, S.; Unnþórsson, R.; Richter, C. A review of biomass gasification modelling. Renew. Sustain. Energy Rev. 2019, 110, 378–391. [Google Scholar] [CrossRef]
- Gao, X.; Zhang, Y.; Li, B.; Zhao, Y.; Jiang, B. Determination of the intrinsic reactivities for carbon dioxide gasification of rice husk chars through using random pore model. Bioresour. Technol. 2016, 218, 1073–1081. [Google Scholar] [CrossRef]
- Lin, L.; Strand, M. Investigation of the intrinsic CO2 gasification kinetics of biomass char at medium to high temperatures. Appl. Energy 2013, 109, 220–228. [Google Scholar] [CrossRef]
- Chew, J.J.; Soh, M.; Sunarso, J.; Yong, S.-T.; Doshi, V.; Bhattacharya, S. Isothermal kinetic study of CO2 gasification of torrefied oil palm biomass. Biomass Bioenergy 2020, 134, 105487. [Google Scholar] [CrossRef]
- Strandberg, A.; Holmgren, P.; Wagner, D.R.; Molinder, R.; Wiinikka, H.; Umeki, K.; Broström, M. Effects of Pyrolysis Conditions and Ash Formation on Gasification Rates of Biomass Char. Energy Fuels 2017, 31, 6507–6514. [Google Scholar] [CrossRef]
- Cetin, E.; Moghtaderi, B.; Gupta, R.; Wall, T.F. Biomass gasification kinetics: Influences of pressure and char structure. Combust. Sci. Technol. 2005, 177, 765–791. [Google Scholar] [CrossRef]
- Okumura, Y.; Hanaoka, T.; Sakanishi, K. Effect of pyrolysis conditions on gasification reactivity of woody biomass-derived char. Proc. Combust. Inst. 2009, 32, 2013–2020. [Google Scholar] [CrossRef]
- Septien, S.; Escudero Sanz, F.J.; Salvador, S.; Valin, S. The effect of pyrolysis heating rate on the steam gasification reactivity of char from woodchips. Energy 2018, 142, 68–78. [Google Scholar] [CrossRef] [Green Version]
- Dahou, T.; Defoort, F.; Thiéry, S.; Grateau, M.; Campargue, M.; Bennici, S.; Jeguirim, M.; Dupont, C. The Influence of Char Preparation and Biomass Type on Char Steam Gasification Kinetics. Energies 2018, 11, 2126. [Google Scholar] [CrossRef] [Green Version]
- Xiao, R.; Yang, W. Kinetics characteristics of straw semi-char gasification with carbon dioxide. Bioresour. Technol. 2016, 207, 180–187. [Google Scholar] [CrossRef] [PubMed]
- Feng, D.; Zhao, Y.; Zhang, Y.; Sun, S.; Gao, J. Steam Gasification of Sawdust Biochar Influenced by Chemical Speciation of Alkali and Alkaline Earth Metallic Species. Energies 2018, 11, 205. [Google Scholar] [CrossRef] [Green Version]
- Kirtania, K.; Axelsson, J.; Matsakas, L.; Christakopoulos, P.; Umeki, K.; Furusjö, E. Kinetic study of catalytic gasification of wood char impregnated with different alkali salts. Energy 2017, 118, 1055–1065. [Google Scholar] [CrossRef]
- Sadhwani, N.; Adhikari, S.; Eden, M.R.; Wang, Z.; Baker, R. Southern pines char gasification with CO2—Kinetics and effect of alkali and alkaline earth metals. Fuel Process. Technol. 2016, 150, 64–70. [Google Scholar] [CrossRef] [Green Version]
- Perander, M.; DeMartini, N.; Brink, A.; Kramb, J.; Karlström, O.; Hemming, J.; Moilanen, A.; Konttinen, J.; Hupa, M. Catalytic effect of Ca and K on CO2 gasification of spruce wood char. Fuel 2015, 150, 464–472. [Google Scholar] [CrossRef]
- Lahijani, P.; Zainal, Z.A.; Mohamed, A.R.; Mohammadi, M. CO2 gasification reactivity of biomass char: Catalytic influence of alkali, alkaline earth and transition metal salts. Bioresour. Technol. 2013, 144, 288–295. [Google Scholar] [CrossRef]
- Zhang, Y.; Ashizawa, M.; Kajitani, S.; Miura, K. Proposal of a semi-empirical kinetic model to reconcile with gasification reactivity profiles of biomass chars. Fuel 2008, 87, 475–481. [Google Scholar] [CrossRef]
- Dupont, C.; Nocquet, T.; Da Costa, J.A., Jr.; Verne-Tournon, C. Kinetic modelling of steam gasification of various woody biomass chars: Influence of inorganic elements. Bioresour. Technol. 2011, 102, 9743–9748. [Google Scholar] [CrossRef]
- Mitsuoka, K.; Hayashi, S.; Amano, H.; Kayahara, K.; Sasaoaka, E.; Uddin, M.A. Gasification of woody biomass char with CO2: The catalytic effects of K and Ca species on char gasification reactivity. Fuel Process. Technol. 2011, 92, 26–31. [Google Scholar] [CrossRef]
- Long, J.; Song, H.; Jun, X.; Sheng, S.; Lun-shi, S.; Kai, X.; Yao, Y. Release characteristics of alkali and alkaline earth metallic species during biomass pyrolysis and steam gasification process. Bioresour. Technol. 2012, 116, 278–284. [Google Scholar] [CrossRef]
- Wang, G.; Zhang, J.; Shao, J.; Liu, Z.; Wang, H.; Li, X.; Zhang, P.; Geng, W.; Zhang, G. Experimental and modeling studies on CO2 gasification of biomass chars. Energy 2016, 114, 143–154. [Google Scholar] [CrossRef] [Green Version]
- Ding, L.; Zhang, Y.; Wang, Z.; Huang, J.; Fang, Y. Interaction and its induced inhibiting or synergistic effects during co-gasification of coal char and biomass char. Bioresour. Technol. 2014, 173, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Dahou, T.; Defoort, F.; Jeguirim, M.; Dupont, C. Towards understanding the role of K during biomass steam gasification. Fuel 2020, 282, 118806. [Google Scholar] [CrossRef]
- Wang, Y.; Bell, D.A. Reaction kinetics of Powder River Basin coal gasification in carbon dioxide using a modified drop tube reactor. Fuel 2015, 140, 616–625. [Google Scholar] [CrossRef]
- Jing, X.; Wang, Z.; Yu, Z.; Zhang, Q.; Li, C.; Fang, Y. Experimental and Kinetic Investigations of CO2 Gasification of Fine Chars Separated from a Pilot-Scale Fluidized-Bed Gasifier. Energy Fuels 2013, 27, 2422–2430. [Google Scholar] [CrossRef]
- Kajitani, S.; Hara, S.; Matsuda, H. Gasification rate analysis of coal char with a pressurized drop tube furnace. Fuel 2002, 81, 539–546. [Google Scholar] [CrossRef]
- Peng, F.F.; Lee, I.C.; Yang, R.Y.K. Reactivities of in situ and ex situ coal chars during gasification in steam at 1000–1400 °C. Fuel Process. Technol. 1995, 41, 233–251. [Google Scholar] [CrossRef]
- Arnold, R.A.; Habibi, R.; Kopyscinski, J.; Hill, J.M. Interaction of Potassium and Calcium in the Catalytic Gasification of Biosolids and Switchgrass. Energy Fuels 2017, 31, 6240–6247. [Google Scholar] [CrossRef]
- Zhang, R.; Wang, Q.H.; Luo, Z.Y.; Fang, M.X.; Cen, K.F. Competition and Inhibition Effects during Coal Char Gasification in the Mixture of H2O and CO2. Energy Fuels 2013, 27, 5107–5115. [Google Scholar] [CrossRef]
- Roberts, D.G.; Harris, D.J. A Kinetic Analysis of Coal Char Gasification Reactions at High Pressures. Energy Fuels 2006, 20, 2314–2320. [Google Scholar] [CrossRef]
- Dupont, C.; Jacob, S.; Marrakchy, K.O.; Hognon, C.; Grateau, M.; Labalette, F.; Da Silva Perez, D. How inorganic elements of biomass influence char steam gasification kinetics. Energy 2016, 109, 430–435. [Google Scholar] [CrossRef]
- Song, Y.-C.; Li, Q.-T.; Li, F.-Z.; Wang, L.-S.; Hu, C.-C.; Feng, J.; Li, W.-Y. Pathway of biomass-potassium migration in co-gasification of coal and biomass. Fuel 2019, 239, 365–372. [Google Scholar] [CrossRef]
- Chen, G.; Yu, Q.; Sjöström, K. Reactivity of char from pyrolysis of birch wood. J. Anal. Appl. Pyrolysis 1997, 40–41, 491–499. [Google Scholar] [CrossRef]
- DeGroot, W.F.; Shafizadeh, F. Kinetics of gasification of Douglas Fir and Cottonwood chars by carbon dioxide. Fuel 1984, 63, 210–216. [Google Scholar] [CrossRef]
- Fermoso, J.; Stevanov, C.; Moghtaderi, B.; Arias, B.; Pevida, C.; Plaza, M.G.; Rubiera, F.; Pis, J.J. High-pressure gasification reactivity of biomass chars produced at different temperatures. J. Anal. Appl. Pyrolysis 2009, 85, 287–293. [Google Scholar] [CrossRef]
- Fermoso, J.; Arias, B.; Pevida, C.; Plaza, M.G.; Rubiera, F.; Pis, J.J. Kinetic models comparison for steam gasification of different nature fuel chars. J. Therm. Anal. Calorim. 2008, 91, 779–786. [Google Scholar] [CrossRef] [Green Version]
- Tanner, J.; Bhattacharya, S. Kinetics of CO2 and steam gasification of Victorian brown coal chars. Chem. Eng. J. 2016, 285, 331–340. [Google Scholar] [CrossRef]
- Fermoso, J.; Gil, M.V.; Garcia, S.; Pevida, C.; Pis, J.J.; Rubiera, F. Kinetic Parameters and Reactivity for the Steam Gasification of Coal Chars Obtained under Different Pyrolysis Temperatures and Pressures. Energy Fuels 2011, 25, 3574–3580. [Google Scholar] [CrossRef] [Green Version]
- Seo, D.K.; Lee, S.K.; Kang, M.W.; Hwang, J.; Yu, T.-U. Gasification reactivity of biomass chars with CO2. Biomass Bioenergy 2010, 34, 1946–1953. [Google Scholar] [CrossRef]
- Wang, Y.; Bell, D.A. Competition between H2O and CO2 during the gasification of Powder River Basin coal. Fuel 2017, 187, 94–102. [Google Scholar] [CrossRef]
- Wall, T.F.; Liu, G.-s.; Wu, H.-w.; Roberts, D.G.; Benfell, K.E.; Gupta, S.; Lucas, J.A.; Harris, D.J. The effects of pressure on coal reactions during pulverised coal combustion and gasification. Prog. Energy Combust. Sci. 2002, 28, 405–433. [Google Scholar] [CrossRef]
- Roberts, D.G.; Harris, D.J.; Wall, T.F. Total pressure effects on chemical reaction rates of chars with O2, CO2 and H2O. Fuel 2000, 79, 1997–1998. [Google Scholar] [CrossRef]
- Park, H.Y.; Ahn, D.H. Gasification kinetics of five coal chars with CO2 at elevated pressure. Korean J. Chem. Eng. 2007, 24, 24–30. [Google Scholar] [CrossRef]
- Vyazovkin, S.; Wight, C.A. Model-free and model-fitting approaches to kinetic analysis of isothermal and nonisothermal data. Thermochim. Acta 1999, 340–341, 53–68. [Google Scholar] [CrossRef]
- Vyazovkin, S. Computational aspects of kinetic analysis. Part C. The ICTAC Kinetics Project—The light at the end of the tunnel? Thermochim. Acta 2000, 355, 155–163. [Google Scholar] [CrossRef]
- Lahijani, P.; Zainal, Z.A.; Mohamed, A.R. Catalytic effect of iron species on CO2 gasification reactivity of oil palm shell char. Thermochim. Acta 2012, 546, 24–31. [Google Scholar] [CrossRef]
- Roberts, D.G.; Hodge, E.M.; Harris, D.J.; Stubington, J.F. Kinetics of Char Gasification with CO2 under Regime II Conditions: Effects of Temperature, Reactant, and Total Pressure. Energy Fuels 2010, 24, 5300–5308. [Google Scholar] [CrossRef]
- Yuan, S.; Dai, Z.-H.; Zhou, Z.-J.; Chen, X.-L.; Yu, G.-S.; Wang, F.-C. Rapid co-pyrolysis of rice straw and a bituminous coal in a high-frequency furnace and gasification of the residual char. Bioresour. Technol. 2012, 109, 188–197. [Google Scholar] [CrossRef]
- Zhang, R.; Wang, Q.H.; Luo, Z.Y.; Fang, M.X.; Cen, K.F. Coal Char Gasification in the Mixture of H2O, CO2, H2, and CO under Pressured Conditions. Energy Fuels 2014, 28, 832–839. [Google Scholar] [CrossRef]
- Marquez-Montesinos, F.; Cordero, T.; Rodriguez-Mirasol, J.; Rodriguez, J.J. CO2 and steam gasification of a grapefruit skin char. Fuel 2002, 81, 423–429. [Google Scholar] [CrossRef]
- Lopez, G.; Alvarez, J.; Amutio, M.; Arregi, A.; Bilbao, J.; Olazar, M. Assessment of steam gasification kinetics of the char from lignocellulosic biomass in a conical spouted bed reactor. Energy 2016, 107, 493–501. [Google Scholar] [CrossRef]
- Bhatia, S.K.; Perlmutter, D.D. A random pore model for fluid-solid reactions: I. Isothermal, kinetic control. AIChE J. 1980, 26, 379–386. [Google Scholar] [CrossRef]
- Lahijani, P.; Zainal, Z.A.; Mohamed, A.R.; Mohammadi, M. Co-gasification of tire and biomass for enhancement of tire-char reactivity in CO2 gasification process. Bioresour. Technol. 2013, 138, 124–130. [Google Scholar] [CrossRef]
- Feng, D.; Zhao, Y.; Zhang, Y.; Xu, H.; Zhang, L.; Sun, S. Catalytic mechanism of ion-exchanging alkali and alkaline earth metallic species on biochar reactivity during CO2/H2O gasification. Fuel 2018, 212, 523–532. [Google Scholar] [CrossRef]
- Jiang, L.; Hu, S.; Sun, L.-S.; Su, S.; Xu, K.; He, L.-M.; Xiang, J. Influence of different demineralization treatments on physicochemical structure and thermal degradation of biomass. Bioresour. Technol. 2013, 146, 254–260. [Google Scholar] [CrossRef]
- González-Vázquez, M.P.; García, R.; Gil, M.V.; Pevida, C.; Rubiera, F. Unconventional biomass fuels for steam gasification: Kinetic analysis and effect of ash composition on reactivity. Energy 2018, 155, 426–437. [Google Scholar] [CrossRef]
- Everson, R.C.; Neomagus, H.W.J.P.; Kaitano, R.; Falcon, R.; du Cann, V.M. Properties of high ash coal-char particles derived from inertinite-rich coal: II. Gasification kinetics with carbon dioxide. Fuel 2008, 87, 3403–3408. [Google Scholar] [CrossRef]
- Ahmed, I.I.; Gupta, A.K. Kinetics of woodchips char gasification with steam and carbon dioxide. Appl. Energy 2011, 88, 1613–1619. [Google Scholar] [CrossRef]
- Fermoso, J.; Gil, M.V.; Borrego, A.G.; Pevida, C.; Pis, J.J.; Rubiera, F. Effect of the Pressure and Temperature of Devolatilization on the Morphology and Steam Gasification Reactivity of Coal Chars. Energy Fuels 2010, 24, 5586–5595. [Google Scholar] [CrossRef] [Green Version]



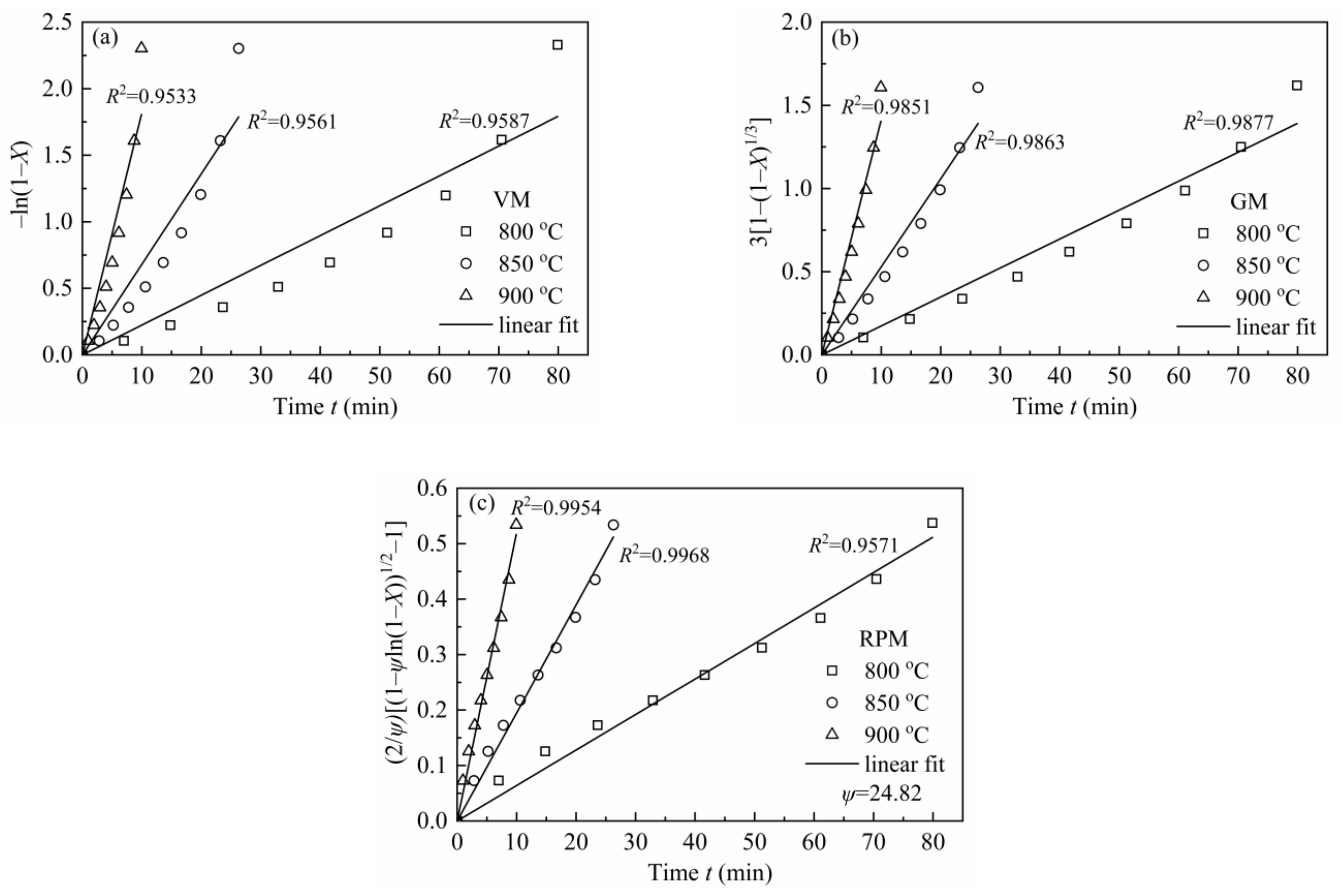
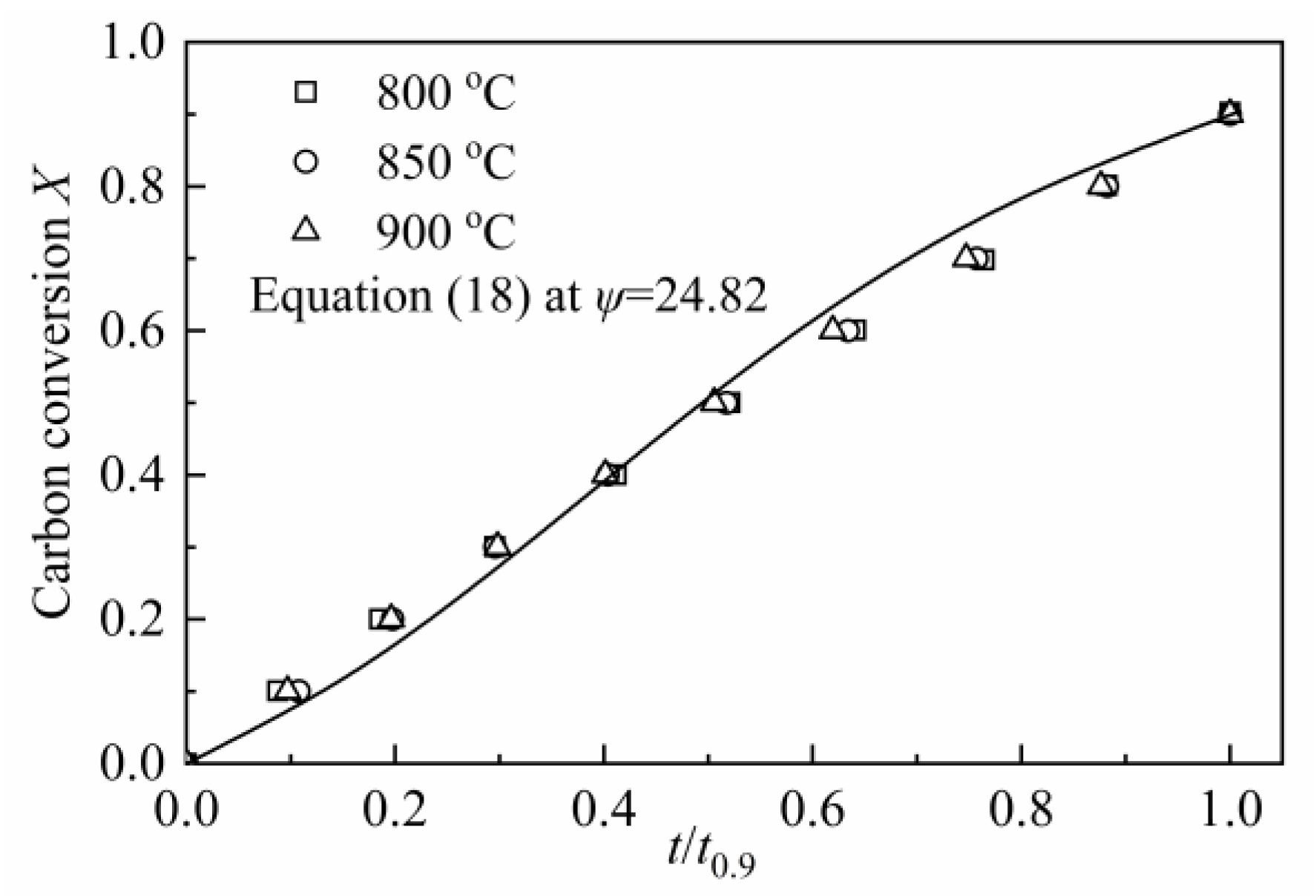
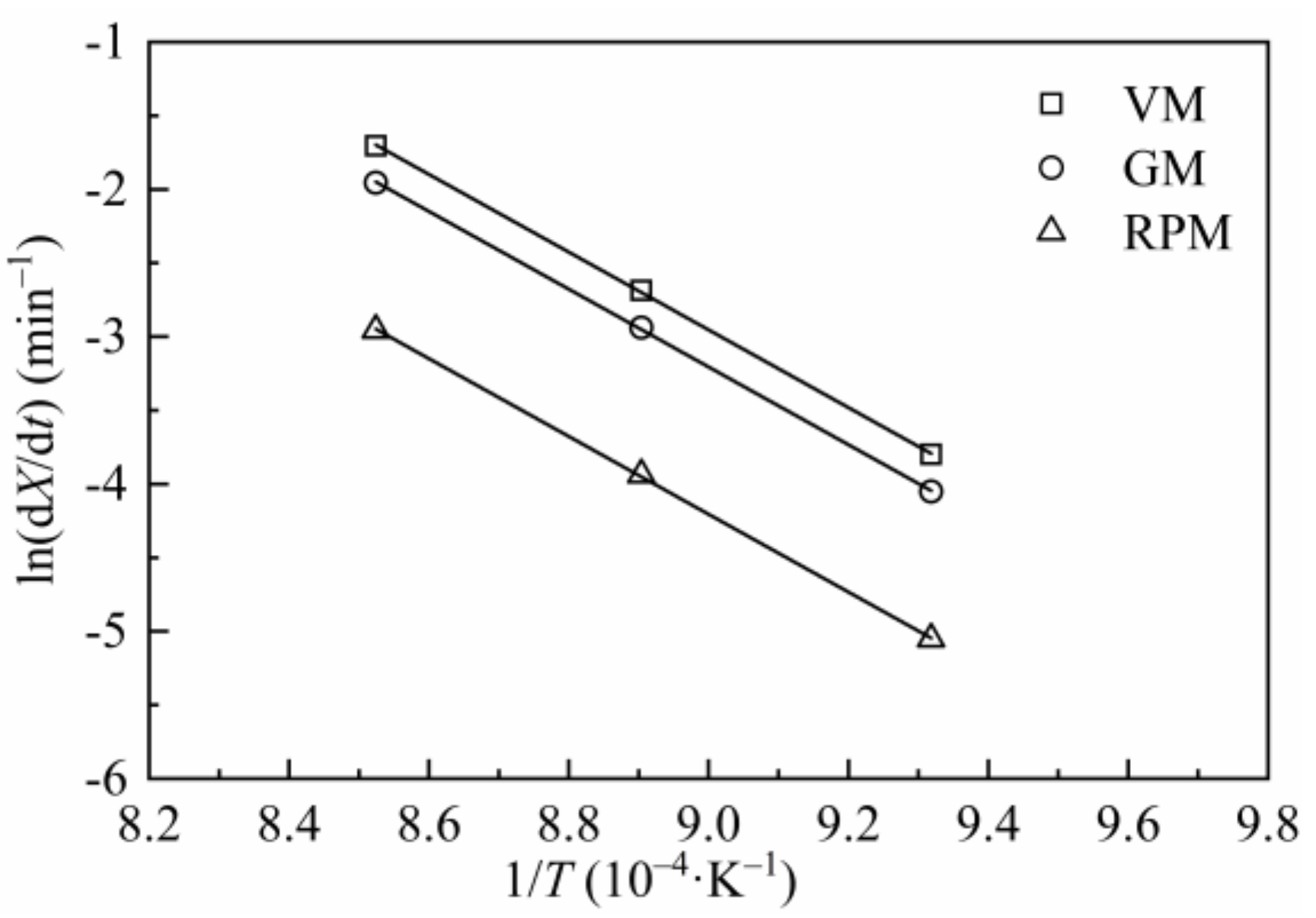
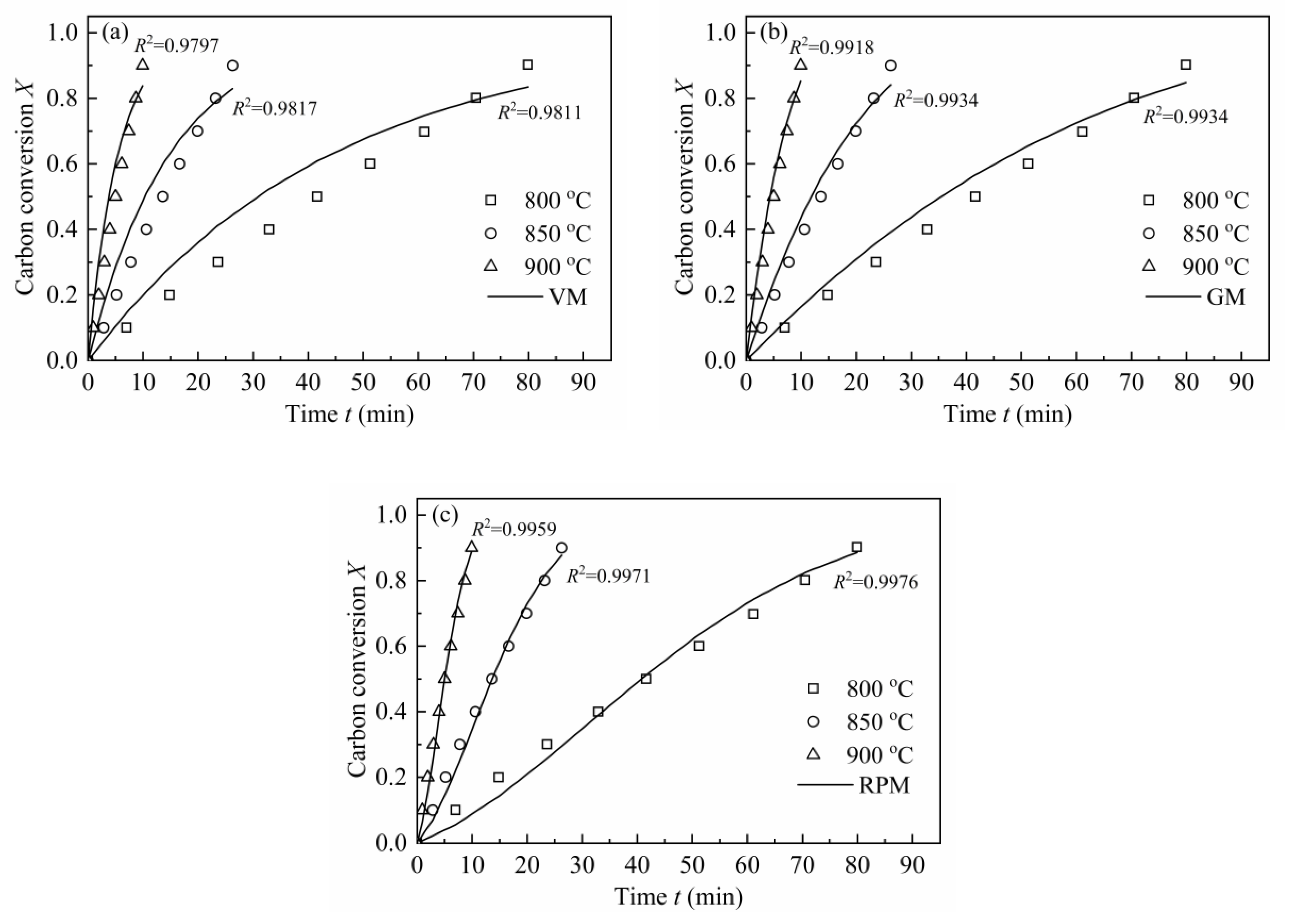

| Reference | Materials | Pyrolysis Conditions [Temperature (T); Atmosphere; Pressure; Heating Rate (HR); Retention Time (t); Reactor; etc.] | Gasification Conditions [Temperature (T); Agents; Pressure; Reactor; etc.] | Key Findings |
|---|---|---|---|---|
| Gao et al. [5] | rice husk | T = 700–900 °C; N2, 1000 mL/min; atmospheric pressure; HR: not available; t = 3 min; micro quartz reactor | T = 850–950 °C; CO2, 100 mL/min; atmospheric pressure; TGA | 1. Reaction order increased as the pyrolysis temperature increased. 2. Pyrolysis temperature had slight effect on the intrinsic activation energy but led to a decrease in the pre-exponential factor. |
| Lin et al. [6] | wood, miscanthus, straw | T = 600–800 °C; N2; atmospheric pressure; HR: not available; t = 3, 5, 15 min; tubular reactor | T = 800–900 °C (TGA), 1100–1300 °C (aerosol reactor); CO2 (33 vol.%) + N2, 150 mL/min; atmospheric pressure; TGA and aerosol reactor | 1. Wood chars pyrolyzed at 800 °C with different retention times had similar reactivity at low temperatures. 2. At higher temperatures, for wood char, the reactivities of short retention time were higher than long retention time. For miscanthus and straw chars made within same retention time, pyrolysis temperature had no clear effect on the reactivity. |
| Chew et al. [7] | oil palm biomass, <0.25 mm | T = 280 °C (t = 120 min), 300 °C (t = 30 min); N2, 150 mL/min; atmospheric pressure; HR = 10 °C/min; vertical fixed-bed reactor | T = 800–900 °C; CO2; atmospheric pressure; TGA | Average reactivity index of three kinds of oil palm biomass reduced due to torrefaction. |
| Strandberg et al. [8] | scots pine, wheat straw, 125–150 μm; 400–425 μm; 600–630 μm | T = 900, 1100 °C; N2, primary gas 380 mL/min, secondary carrier gas 5000 mL/min; atmospheric pressure; HR = 800–1000 °C/s; t: not available; drop tube furnace | T = 700–900 °C; CO2 (20 vol.%) + N2, 100 mL/min; atmospheric pressure; TGA | 1. Pyrolysis temperature had no obvious effect on char reactivity. 2. Char produced from smaller pine wood particles had higher reactivity, while wheat straw char showed less dependence on initial particle size. |
| Cetin et al. [9] | radiata pine, spotted gum, sugarcane bagasse, 180–350 μm and 1–2 mm | 1. T = 950 °C; N2, 2.0 MPa; HR = 500 °C/s; t = 20 s; wire-mesh reactor 2. T = 950 °C; N2; atmospheric pressure; HR = 20 °C/s; t = 5 min; tubular reactor | 1. T = 800–1050 °C; CO2; atmospheric pressure; TGA 2. T = 800–900 °C; CO2; atmospheric pressure; tubular reactor 3. T = 800–900 °C; CO2 (20–100 vol.%) + N2; 0.5–2.0 MPa; high-pressure thermogravimetric analyzer | Global char gasification reactivity decreased with increasing pyrolysis pressure. |
| Okumura et al. [10] | Douglas fir | T = 800 °C; N2, 100 mL/min; 0.1–3.0 MPa; HR = 15–600 °C/s; t: not available; not available | T = 700–1100 °C; CO2; atmospheric pressure; TGA | 1. Increased pyrolysis pressure led to a decrease in char reactivity. 2. High pyrolysis heating rate led to an increase in char reactivity. |
| Septien et al. [11] | beech woodchips | 1. T = 650–950 °C; N2; atmospheric pressure; HR = 100 °C/s; t = 10 min; macro TGA 2. T = 750 °C; N2; atmospheric pressure; HR = 1 °C/s; t = 60 min; screw reactor 3. T = 900 °C; N2; atmospheric pressure; HR = 0.05 °C/s; t = 0 min; batch furnace 4. T = 800 °C; N2, 18.8 L/min; atmospheric pressure; HR = 1000 °C/s; t = 5 s; drop tube reactor | T = 750–950 °C; H2O (15–50 vol.%) + N2, 11.8–14.2 L/min; atmospheric pressure; macro TGA | 1. Char reactivity increased with pyrolysis heating rate. 2. Among the high heating rate chars, the reactivity increased with pyrolysis temperature; among the low heating rating samples, no significant difference was observed. |
| Dahou et al. [12] | rice husk, wheat straw, apple orchard residue, apricot orchard residue, vineyard residue, sunflower seed shells, alfalfa | 1. T = 450 °C; N2, 1000 mL/min; atmospheric pressure; HR = 10 °C/s; t = 60 min; fixed-bed reactor 2. T = 800 °C; N2, 1000 mL/min; atmospheric pressure; HR = 24 °C/s; t = 60 min; induction vertical reactor 3. T = 800 °C; N2, 1000 mL/min; atmospheric pressure; HR = 24 °C/s; t = 30 min; TGA | T = 800 °C; H2O (20 vol.%) + N2, 50 mL/min; atmospheric pressure; TGA | Char prepared in different conditions had the same reactivity during gasification. |
| Xiao et al. [13] | rice straw, 0.180–0.425 mm | T = 300–600 °C; N2, H2, CO2, 350 mL/min; atmospheric pressure; HR = 5 °C/s; t = 90 min; tubular reactor | T = 900–1050 °C; CO2; atmospheric pressure; TGA | 1. Char reactivity increased with pyrolysis temperature and reached a maximum at 400 °C then decreased. 2. Reactivity of char under different pyrolysis atmosphere was in the order of: H2 > N2 > CO2. |
| Reference | Materials | Catalyst Type, Ratio and Loading Methods | Char Preparation Conditions [Temperature (T); Atmosphere; Pressure; Heating Rate (HR); Retention Time (t); Reactor; etc.] | Gasification Conditions [Temperature; Agents; Pressure; Reactor; etc.] | Key Findings |
|---|---|---|---|---|---|
| Feng et al. [14] | Manchurian walnut sawdust, 0.15–0.25 mm | Inherent AAEMs and investigated by chemical fractionation analysis. | T = 800 °C; N2, 5700 mL/min; atmospheric pressure; HR: not available; t = 4.2 s; entrained-flow reactor | T = 800 °C; H2O (15 vol.%) + N2; atmospheric pressure; fluidized bed/fixed-bed reactor | H2O-soluble AAEMs were important in determining the highest reactivity of char. The effect of NH4Ac-soluble AAEMs on char activity was mainly concentrated in the high carbon conversion stage, and that of HCl-soluble AAEMs was reflected in the whole testing stage. |
| Kirtania et al. [15] | pine sawdust | Solutions of K2CO3, Na2CO3, NaOH (0.1 and 1 mol/L), and NaCl (1 mol/L) was impregnated on sawdust (the ratio of solution to biomass was kept at 16 mL/g). | T = 600 °C; N2, 5000 mL/min; atmospheric pressure; HR: not available; t = 4–5 min; macro-TGA | T = 750–900 °C; CO2; atmospheric pressure; macro-TGA | Char reactivity increased with the loading alkali content at low temperatures and up to a certain level. When the temperature increased to 900 °C, no correlation could be observed. |
| Sadhwani et al. [16] | pine wood chips, <0.8 mm | 30 g of char was added to the aqueous solution of metal acetate (K, Na, Ca, Mg) to get a loading target of 0.1 g metal/carbon in the char. | T = 800 °C; N2, 7000 mL/min; atmospheric pressure; HR = 10 °C/min; t = 60 min; tubular reactor | T = 800–945 °C; CO2, 1200 mL/min; atmospheric pressure; stainless steel tubular reactor | The reactivity of the chars was in the order of: K-char > Ca-char > Na-char> Mg-char. |
| Perander et al. [17] | Norwegian spruce, 125–250 μm | 1. Metal nitrate (K, Ca) was loaded on acid washed wood by ion-exchange method (K: 1200–125000 mg/kg wood; Ca: 740–4600 mg/kg wood). 2. K2CO3 was impregnated on acid washed wood (K: 3300–17000 mg/kg wood). 3. CaC2O4·H2O was added to acid-washed wood by ion-exchange method (Ca: 610–3000 mg/kg wood). | In situ pyrolysis | T = 850 °C; CO2; atmospheric pressure; TGA | Char gasification rate increased with the loading content of Ca and K. Organically bound K and K2CO3 showed a similar char reactivity. CaC2O4 addition resulted in lower reactivity. |
| Lahijani et al. [18] | pistachios shell | 3 wt.% of metal nitrates of K, Ca, Mg, Fe and 3–7 wt.% of NaNO3 was impregnated on wood char. | T = 900 °C; N2, 400 mL/min; atmospheric pressure; HR: not available; t = 90 min; vertical tubular reactor | 1. T = 800–1000 °C; CO2, 50 mL/min; atmospheric pressure; TGA 2. T = 800–875 °C; CO2, 500 mL/min; atmospheric pressure; horizontal tubular furnace | The catalytic effect of metal nitrates was in the order of Na > Ca > Fe > K > Mg > raw char, among which 5 wt% NaNO3-loaded char had the highest reactivity. |
| Zhang et al. [19] | 14 biomass samples: Hinoki cypress sawdust, etc. | 100 mg activated carbon was added into 1 mL of an aqueous solution of Ca(COOH)2·H2O, KCOOH, NaCOOH or Mg(COOH)2·4H2O, containing 0.1 mmol metal cation. | T = 900 °C; N2, 200 mL/min; atmospheric pressure; HR = 10 °C/s; t = 90 min; infrared furnace | T = 850 °C; H2O (50 vol.%) + N2, 400 mL/min; atmospheric pressure; TGA | The maximum rate of char at high conversion range was mainly attributed to the catalytic effect of K. |
| Dupont et al. [20] | 21 samples of wood chips: spruce, etc. | This research mainly focused on the inherent AAEMs. | T = 450 °C; N2; atmospheric pressure; HR = a few °C/min; t = 360 min; low heating rate furnace | T = 850 °C; H2O (2–27 vol.%) + N2, 50 mL/min; atmospheric pressure; TGA | Char gasification rate seemed to be correlated with the ratio K/Si, which indicated effect of K and the inhibitor effect of Si on steam gasification of biomass chars. |
| Mitsuoka et al. [21] | Japanese cypress | 1. 2.4 wt.% of Ca(OH)2 was impregnated on cypress chip. 2. 2.4 wt.% of K2CO3 was impregnated on cypress chip char. | T = 900 °C; N2; atmospheric pressure; HR = 9 °C/min; t = 120 min; not available | T = 850–950 °C; CO2 (20–80 vol.%) + N2, 900 mL/min; atmospheric pressure; TGA | K and Ca compounds improved the reactivity of biomass char for CO2 gasification. |
| Sample | Proximate Analysis wad/% | Ultimate Analysis wdaf/% | |||||||
|---|---|---|---|---|---|---|---|---|---|
| M | A | V | FC | C | H | O * | N | S | |
| CS | 11.78 | 4.51 | 71.58 | 12.13 | 50.90 | 5.17 | 43.67 | 0.08 | 0.18 |
| CSC | 2.10 | 10.62 | 2.00 | 85.28 | 97.56 | 0.46 | 1.17 | 0.41 | 0.40 |
| H2O-CSC | 2.00 | 6.91 | 2.10 | 88.99 | 97.21 | 0.46 | 1.58 | 0.38 | 0.37 |
| Sample | Major Inorganic Elements (mmol/g Dry Sample) | ||||||
|---|---|---|---|---|---|---|---|
| Al | Ca | Fe | K | Mg | Na | Si | |
| CSC | 0.04 | 0.24 | 0.03 | 1.32 | 0.27 | 0.05 | 0.60 |
| H2O-CSC | 0.03 | 0.18 | 0.02 | 0.25 | 0.20 | 0.01 | 0.54 |
| Steam Concentration (vol.%) | T (°C) | Ravg (10−3∙min−1) | R2 | Ea |
|---|---|---|---|---|
| (kJ/mol) | ||||
| 10 | 725 | 10.57 | 0.9982 | 186.21 |
| 750 | 18.66 | |||
| 775 | 32.58 | |||
| 800 | 48.94 | |||
| 825 | 83.86 | |||
| 30 | 725 | 17.78 | 0.9967 | 179.63 |
| 750 | 33.50 | |||
| 775 | 50.17 | |||
| 800 | 86.23 | |||
| 825 | 130.00 | |||
| 50 | 725 | 24.92 | 0.9942 | 165.38 |
| 750 | 46.55 | |||
| 775 | 65.00 | |||
| 800 | 105.15 | |||
| 825 | 160.00 | |||
| 70 | 725 | 31.42 | 0.9947 | 167.39 |
| 750 | 53.80 | |||
| 775 | 75.00 | |||
| 800 | 131.50 | |||
| 825 | 200.00 |
| Partial Pressure | 0.1 MPa (10, 30, 50, 70 vol.%) | |
|---|---|---|
| T (°C) | n | R2 |
| 725 | 0.5539 | 0.9911 |
| 750 | 0.5525 | 0.9980 |
| 775 | 0.4299 | 0.9971 |
| 800 | 0.4969 | 0.9961 |
| 825 | 0.4332 | 0.9875 |
| Average | 0.4933 | - |
| Steam Concentration (vol.%) | T (°C) | kMRPM (10−3 min−1) | ψ | c | p | R2 | Ea | A (min−1) |
|---|---|---|---|---|---|---|---|---|
| (kJ/mol) | ||||||||
| 10 | 725 | 4.80 | 3.22 | 2.30 | 2.92 | 0.9951 | 204.26 | 2.25 × 108 |
| 750 | 7.90 | 5.45 | 2.01 | 3.12 | 0.9883 | |||
| 775 | 14.40 | 7.79 | 1.91 | 2.90 | 0.9845 | |||
| 800 | 27.50 | 2.62 | 2.21 | 2.58 | 0.9979 | |||
| 825 | 42.10 | 7.97 | 2.01 | 3.12 | 0.9875 | |||
| 30 | 725 | 8.30 | 7.39 | 1.85 | 3.32 | 0.9941 | 199.42 | 2.43 × 108 |
| 750 | 17.30 | 4.75 | 1.93 | 3.12 | 0.9827 | |||
| 775 | 28.50 | 4.52 | 1.90 | 2.77 | 0.9982 | |||
| 800 | 48.10 | 9.00 | 1.54 | 3.10 | 0.9871 | |||
| 825 | 76.50 | 5.79 | 1.56 | 2.13 | 0.9947 | |||
| 50 | 725 | 13.68 | 4.68 | 1.84 | 3.26 | 0.9915 | 187.38 | 8.01 × 107 |
| 750 | 21.06 | 5.95 | 2.03 | 2.83 | 0.9883 | |||
| 775 | 31.26 | 10.05 | 1.55 | 3.21 | 0.9899 | |||
| 800 | 61.66 | 9.23 | 1.44 | 3.06 | 0.9779 | |||
| 825 | 105.30 | 7.46 | 1.30 | 3.33 | 0.9806 | |||
| 70 | 725 | 16.73 | 10.25 | 1.51 | 3.42 | 0.9892 | 170.87 | 1.49 × 107 |
| 750 | 30.67 | 5.21 | 2.12 | 3.08 | 0.9859 | |||
| 775 | 40.65 | 8.39 | 1.55 | 2.49 | 0.9901 | |||
| 800 | 74.04 | 11.59 | 1.39 | 3.44 | 0.9778 | |||
| 825 | 112.30 | 5.37 | 1.57 | 2.24 | 0.9839 |
| Sample | Time of X = 0.9 (min) | Ravg (10−3∙min−1) | ||||
|---|---|---|---|---|---|---|
| 800 °C | 850 °C | 900 °C | 800 °C | 850 °C | 900 °C | |
| CSC | 20.65 | 8.31 | 4.53 | 48.94 | 115.38 | 228.70 |
| H2O-CSC | 79.90 | 26.26 | 9.92 | 11.30 | 34.49 | 89.88 |
| Parameters | Reaction Model | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| VM | GM | RPM | |||||||
| T(°C) | 800 | 850 | 900 | 800 | 850 | 900 | 800 | 850 | 900 |
| k(10−2·min−1) | 2.24 | 6.81 | 18.2 | 1.74 | 5.30 | 14.15 | 0.64 | 1.95 | 5.21 |
| Ea(kJ/mol) | 219.33 | 219.43 | 219.54 | ||||||
| A(min−1) | 1.07 × 109 | 8.40 × 108 | 3.13 × 108 | ||||||
| ψ | - | - | 24.82 | ||||||
| R2 | 0.9999 | 0.9999 | 0.9999 | ||||||
| Parameter | k1 | k3 | R2 | Ea1 | A1 | R2 | Ea3 | A3 | R2 |
|---|---|---|---|---|---|---|---|---|---|
| T (°C) | (min−1·MPa−1) | (min−1) | (kJ/mol) | (min−1·MPa−1) | (kJ/mol) | (min−1) | |||
| 725 | 1.44 | 0.04 | 0.9716 | 202.08 | 4.17 × 1010 | 0.9904 | 157.84 | 7.17 × 106 | 0.9795 |
| 750 | 2.50 | 0.07 | 0.9894 | ||||||
| 775 | 5.17 | 0.09 | 0.9744 | ||||||
| 800 | 7.01 | 0.16 | 0.9899 | ||||||
| 825 | 13.33 | 0.22 | 0.9687 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, S.; Ding, L.; Ruan, Y.; Bai, B.; Qiu, Z.; Li, Z. Experimental and Kinetic Studies on Steam Gasification of a Biomass Char. Energies 2021, 14, 7229. https://doi.org/10.3390/en14217229
Zhao S, Ding L, Ruan Y, Bai B, Qiu Z, Li Z. Experimental and Kinetic Studies on Steam Gasification of a Biomass Char. Energies. 2021; 14(21):7229. https://doi.org/10.3390/en14217229
Chicago/Turabian StyleZhao, Shengguo, Liang Ding, Yun Ruan, Bin Bai, Zegang Qiu, and Zhiqin Li. 2021. "Experimental and Kinetic Studies on Steam Gasification of a Biomass Char" Energies 14, no. 21: 7229. https://doi.org/10.3390/en14217229
APA StyleZhao, S., Ding, L., Ruan, Y., Bai, B., Qiu, Z., & Li, Z. (2021). Experimental and Kinetic Studies on Steam Gasification of a Biomass Char. Energies, 14(21), 7229. https://doi.org/10.3390/en14217229







