3D Unsteady Simulation of a Scale-Up Methanation Reactor with Interconnected Cooling Unit
Abstract
:1. Introduction
2. Mathematical Models
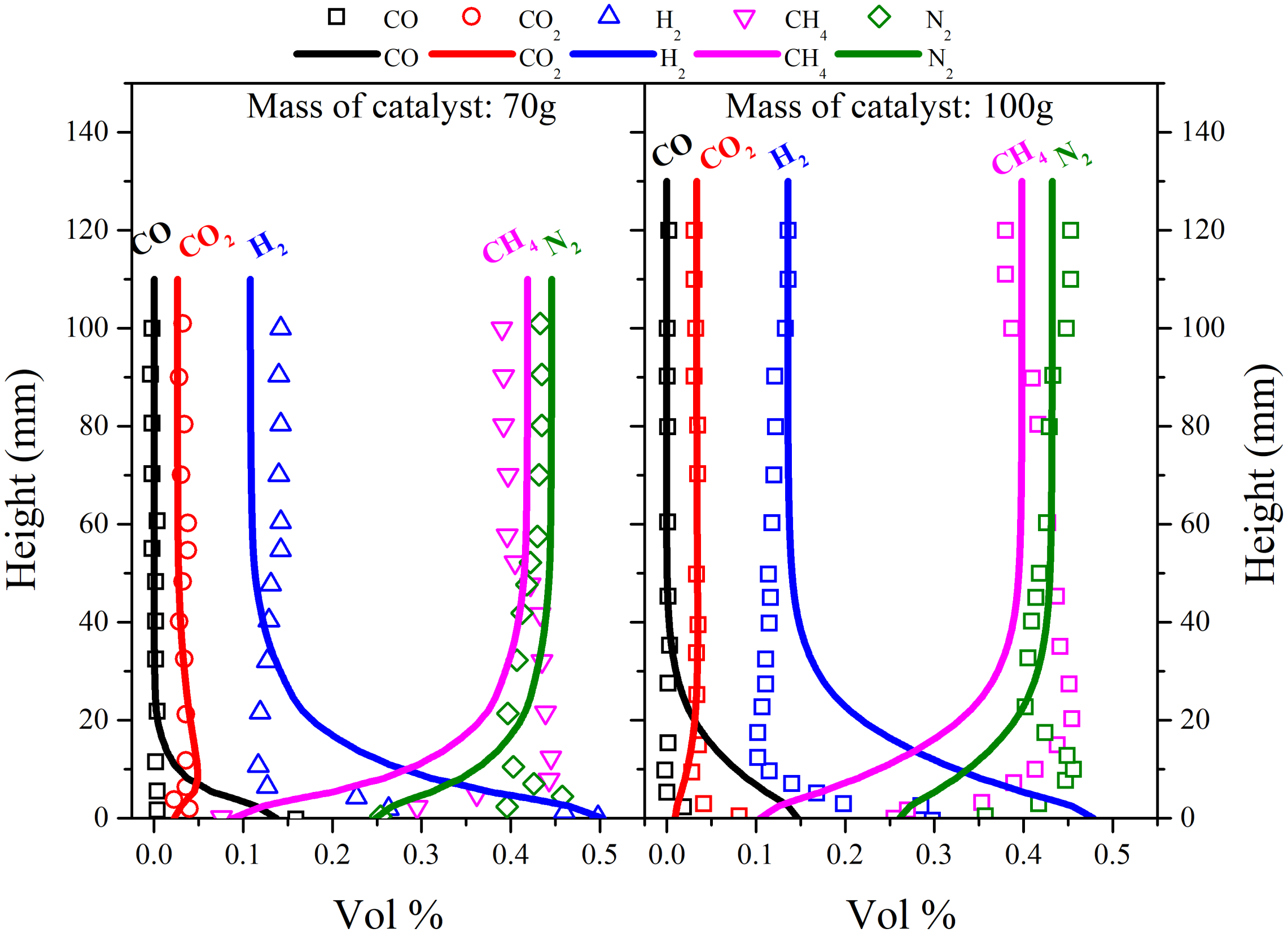
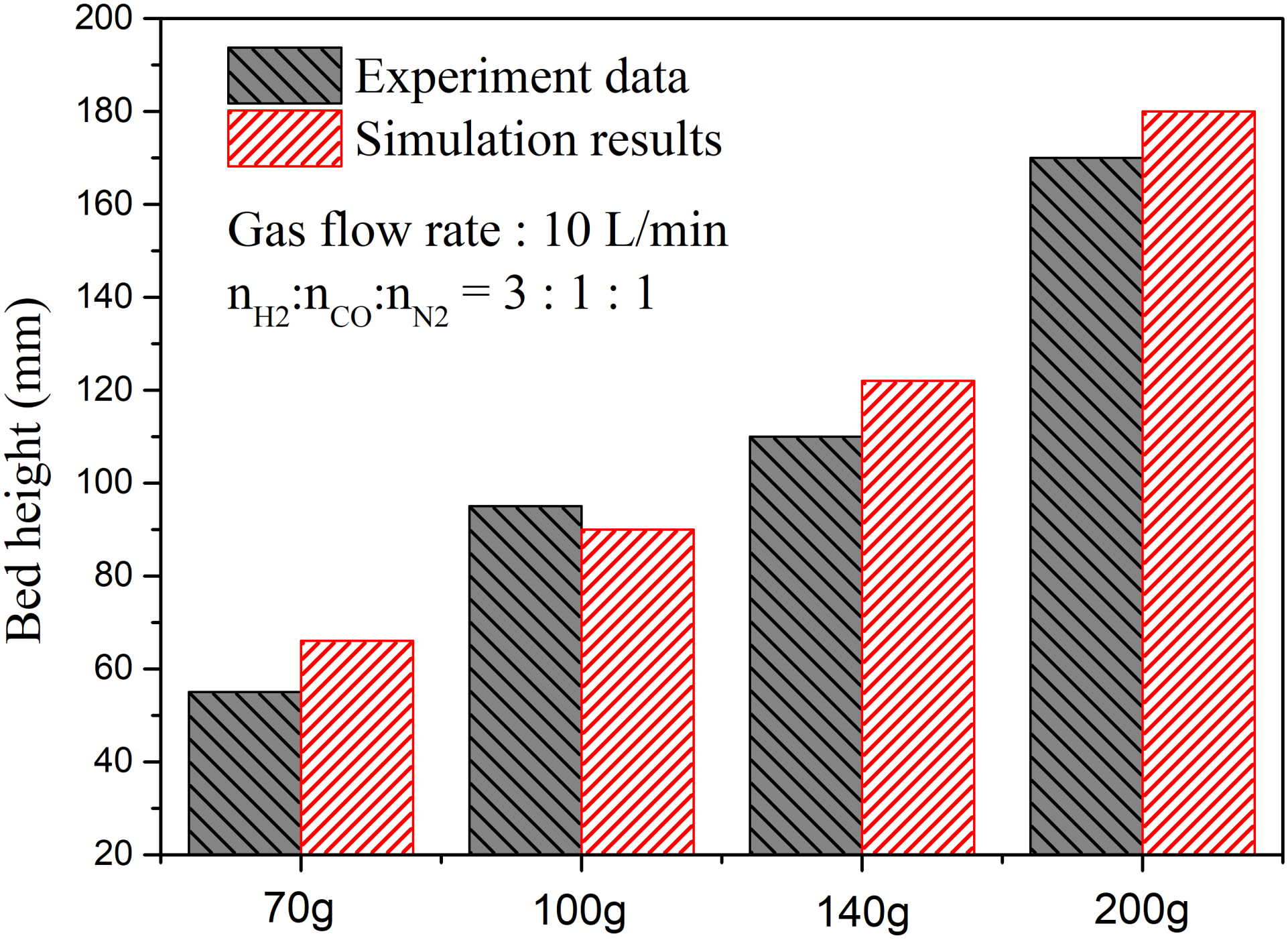
3. Validation of the Model
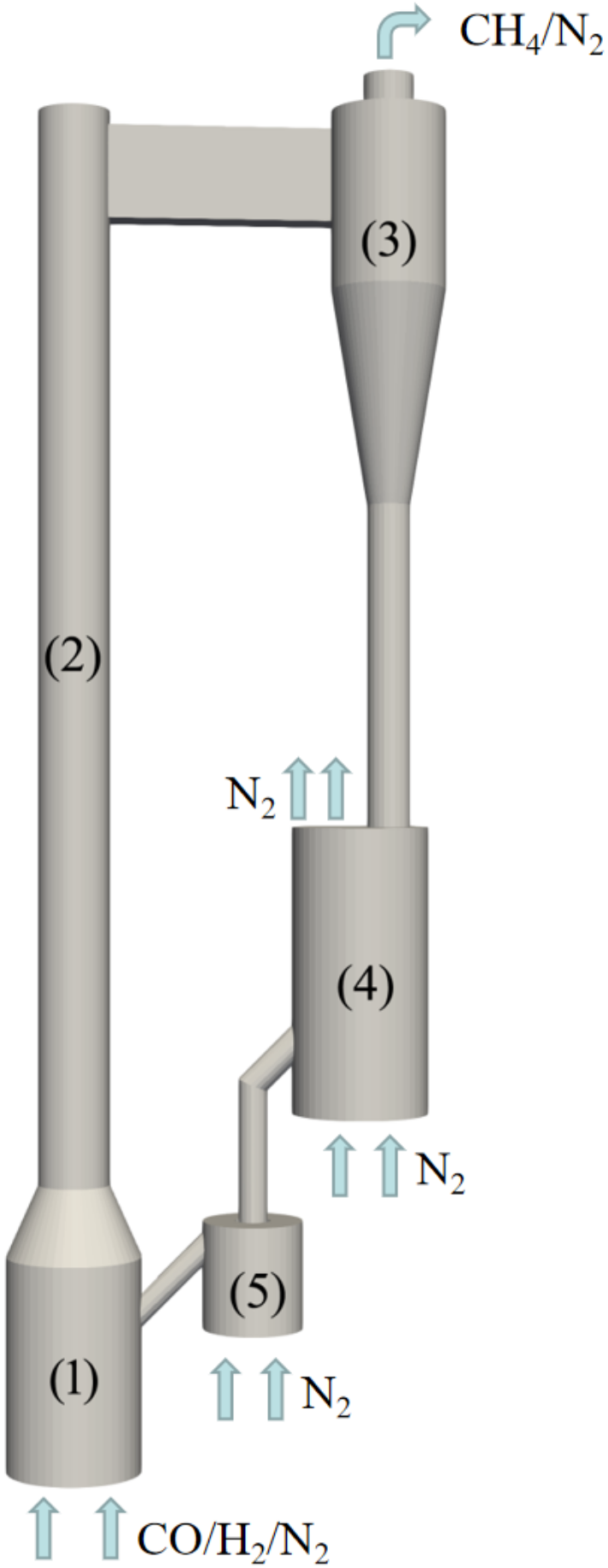
4. Simulation of 3D Circulating Fluidized Bed Reactor
4.1. Design and Setup of Simulations
4.2. Results and Discussion
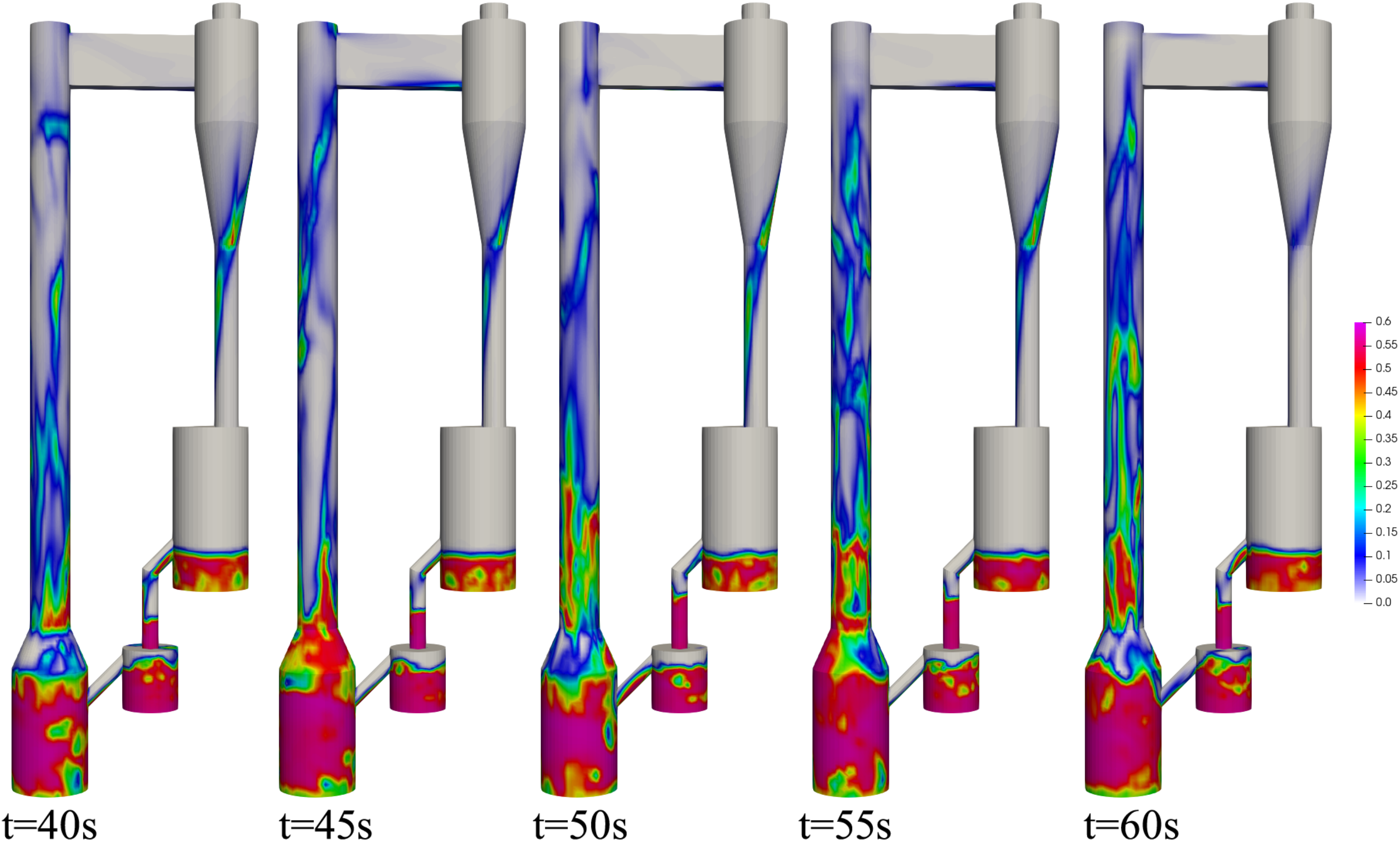
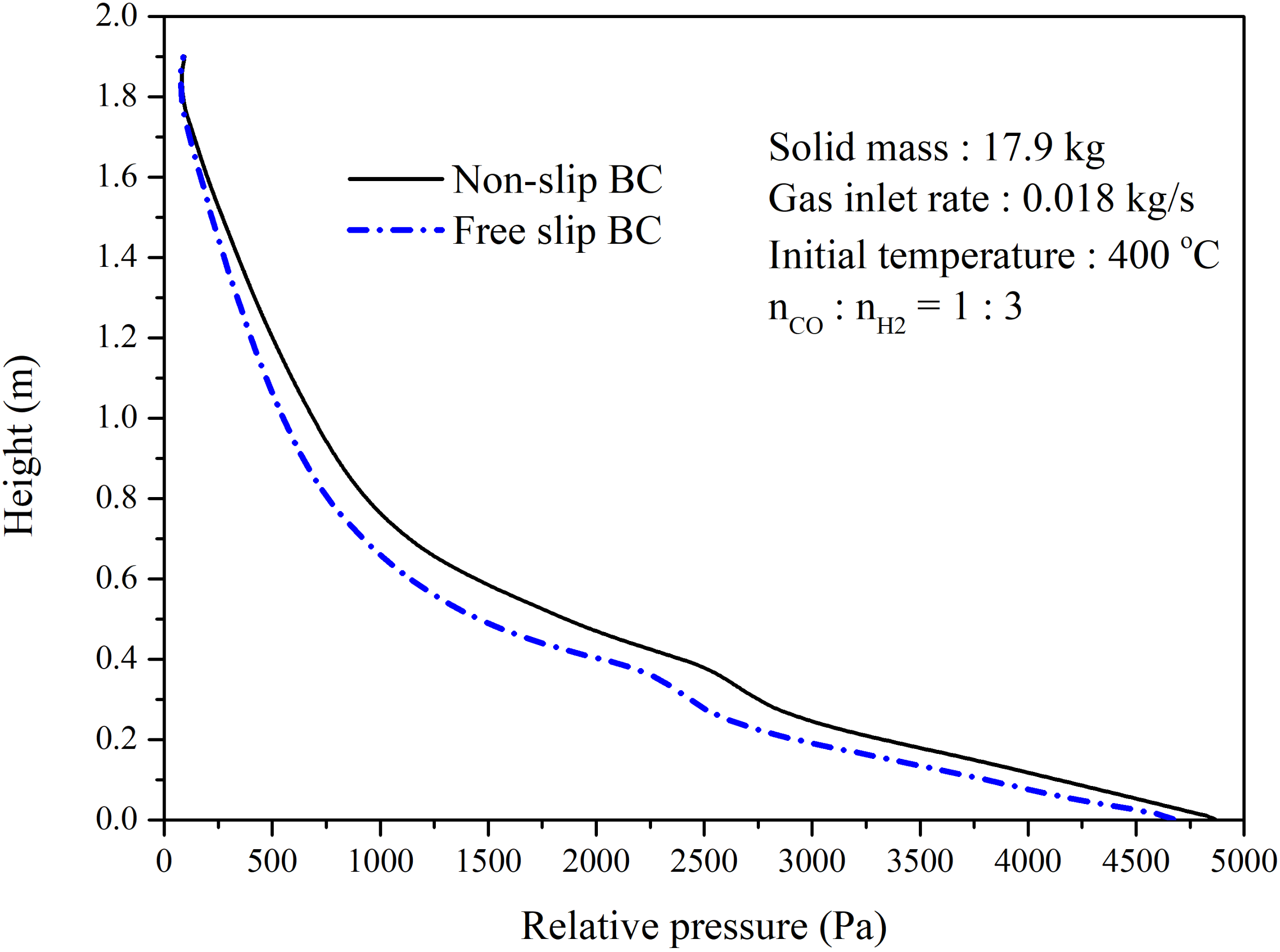
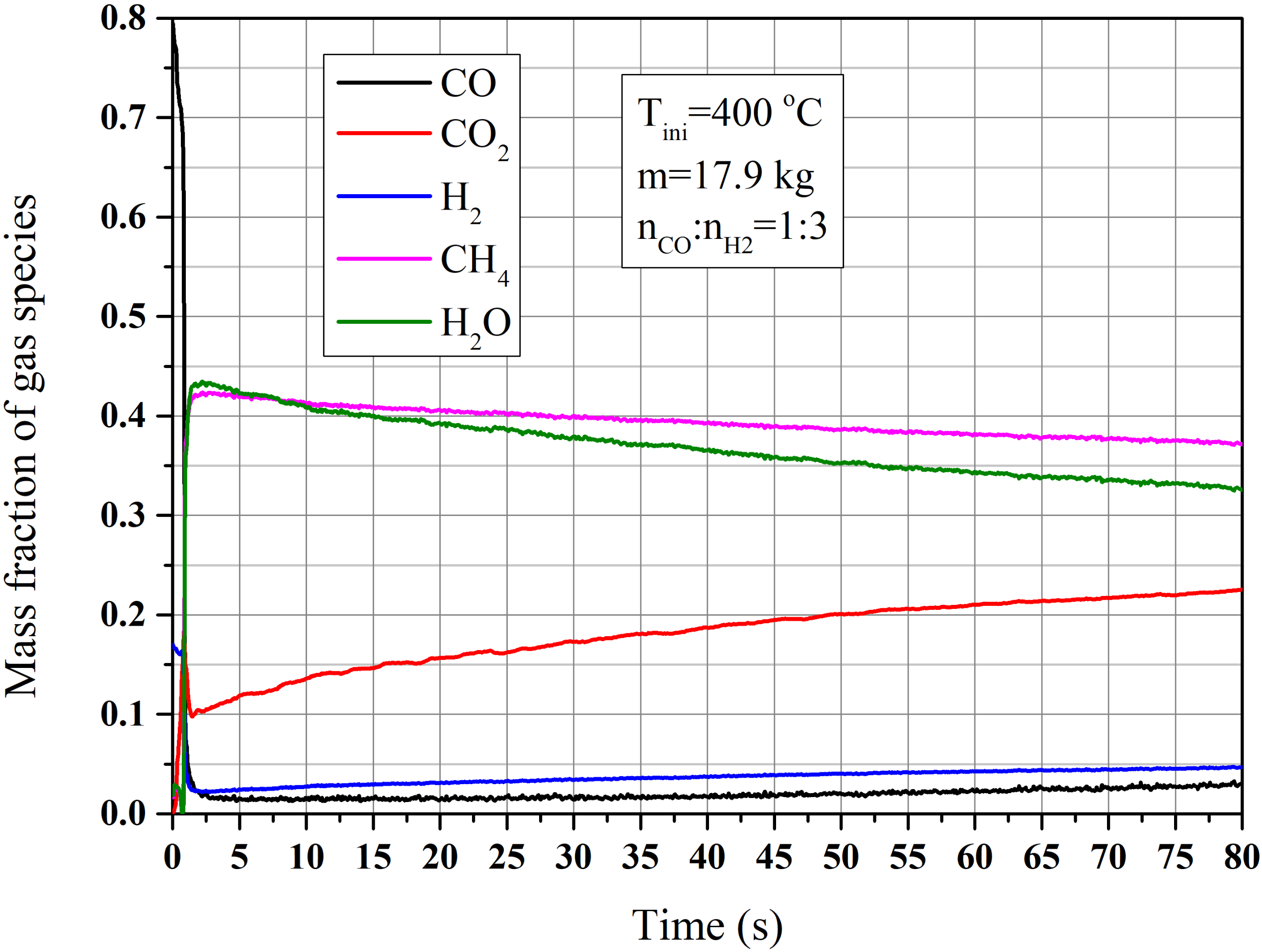
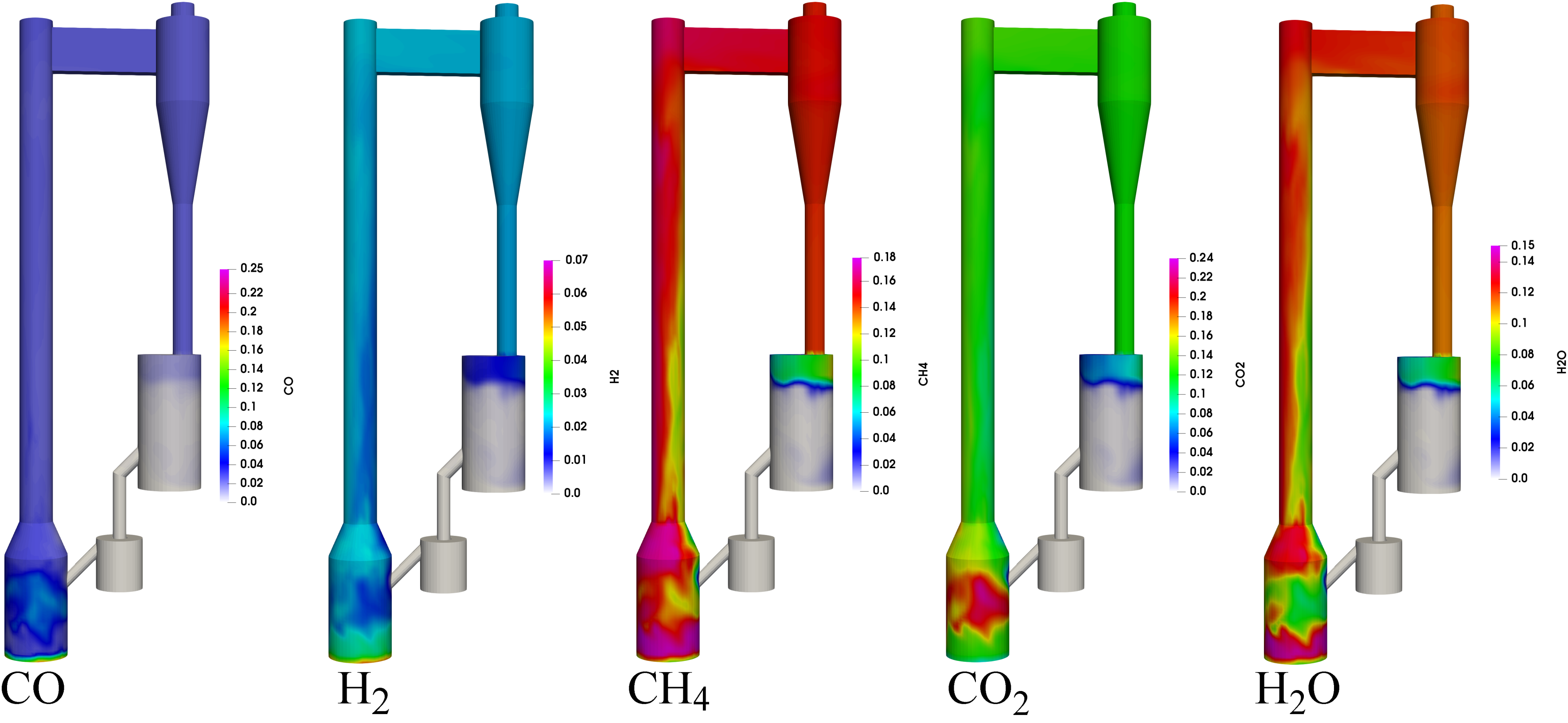
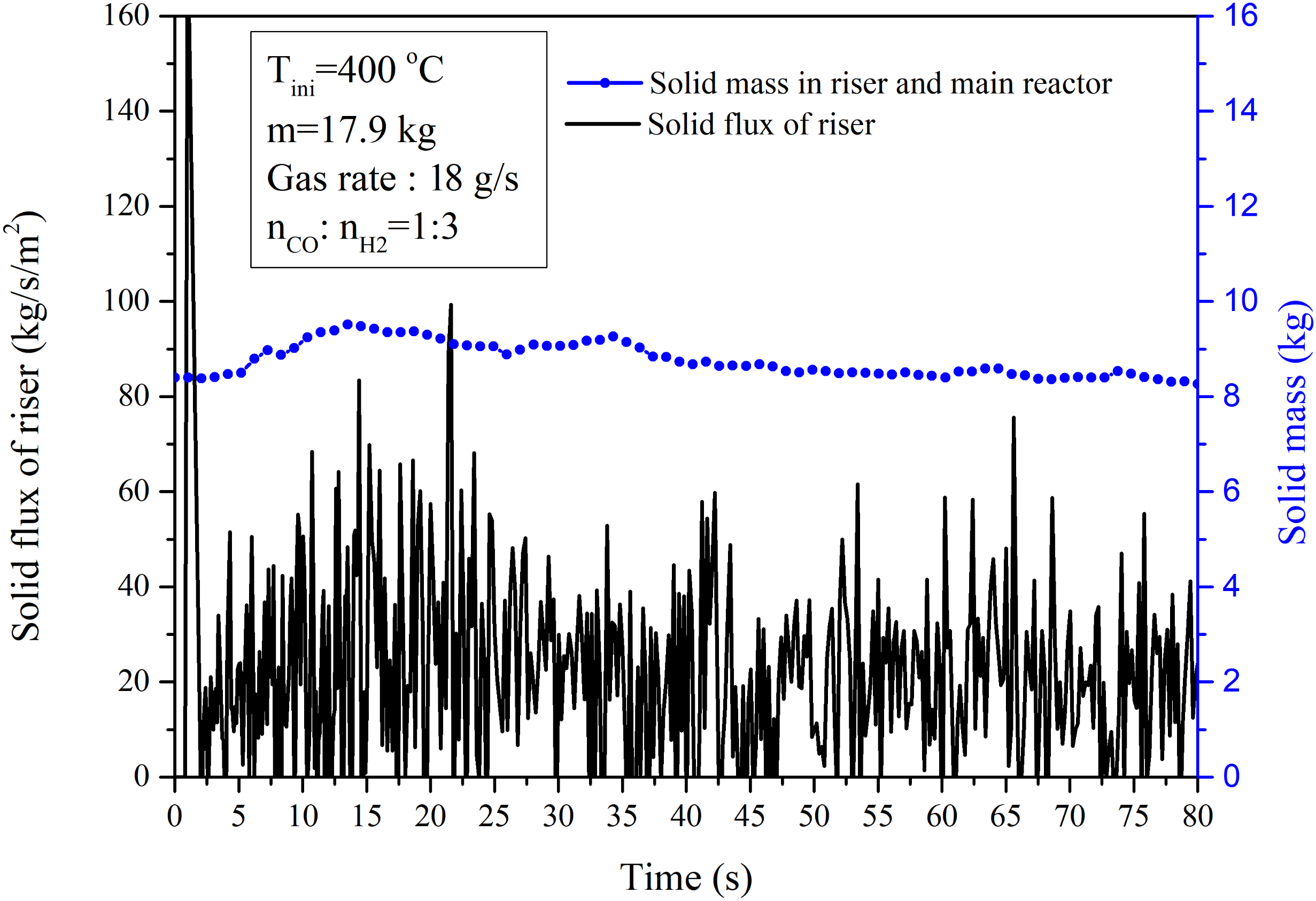
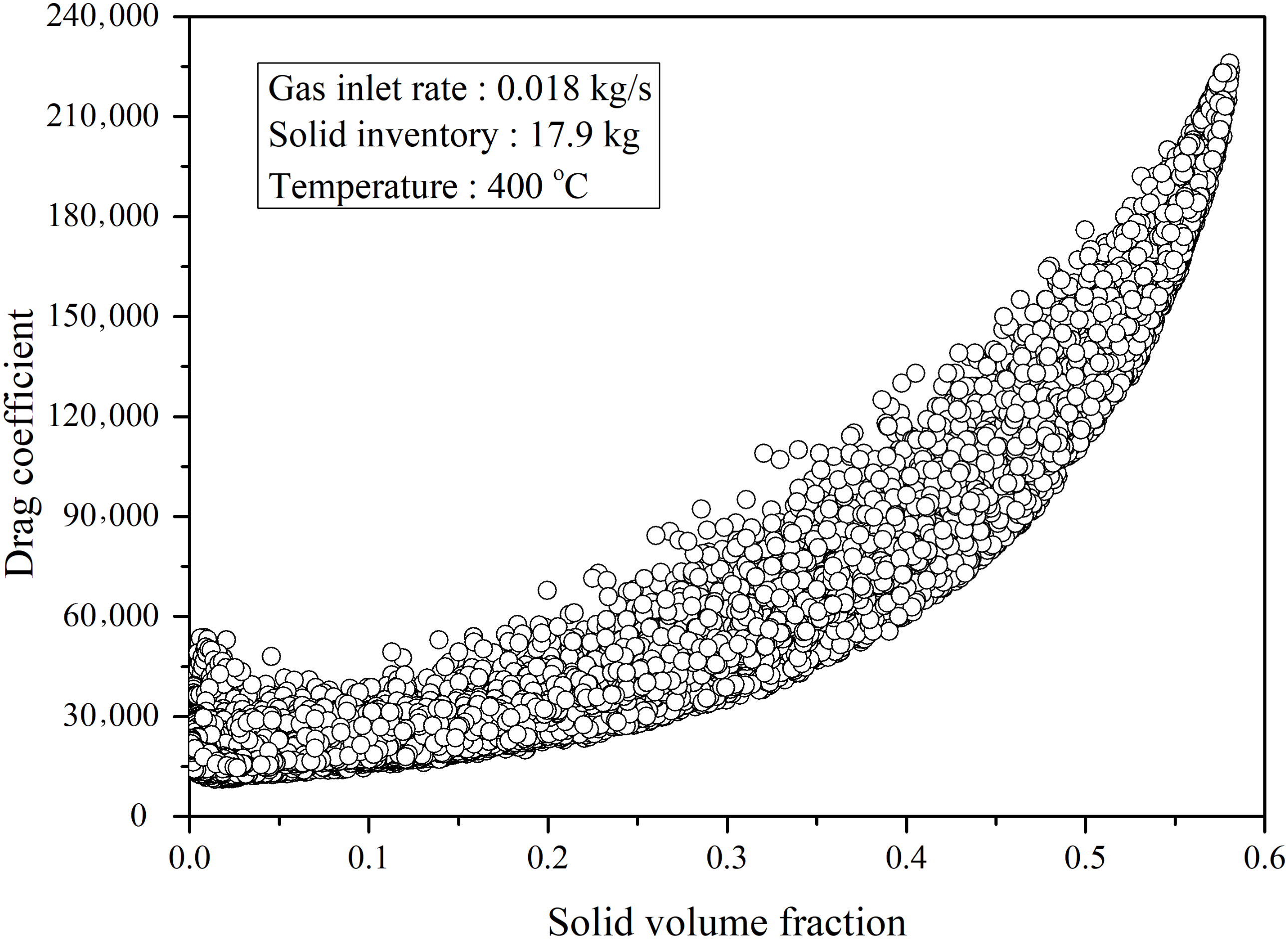
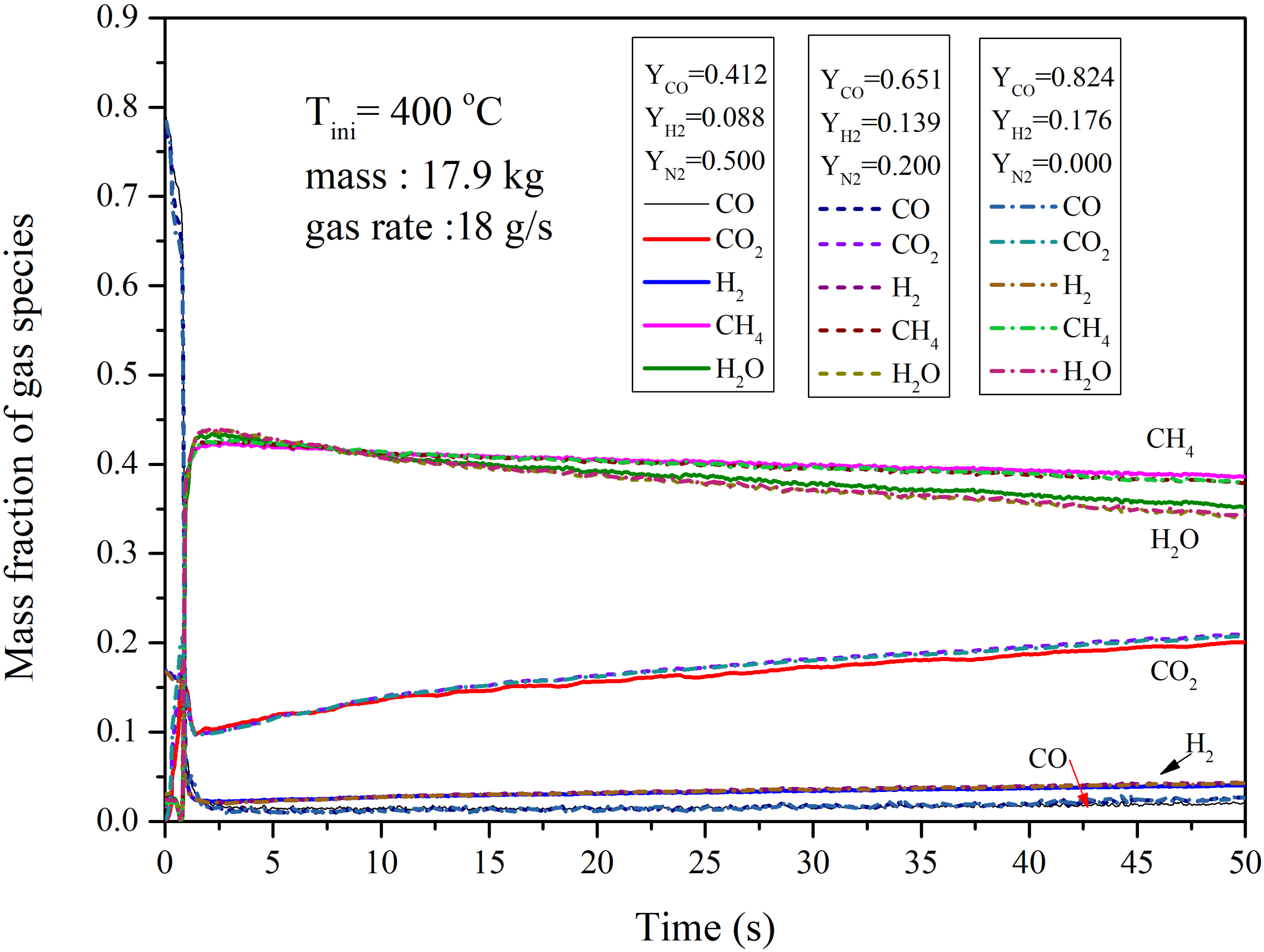
4.2.1. Performance of the Circulating Fluidized Bed Reactor
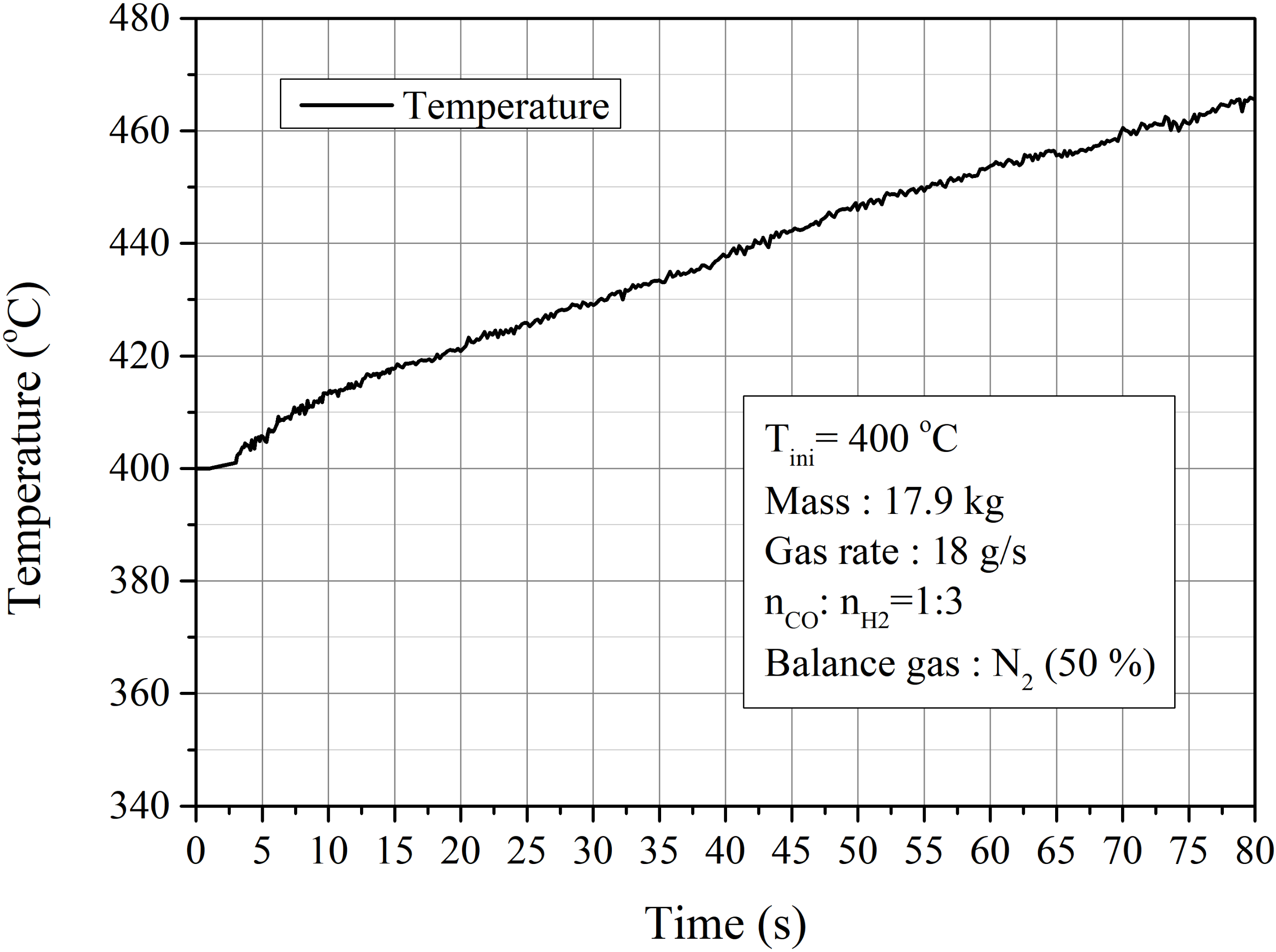
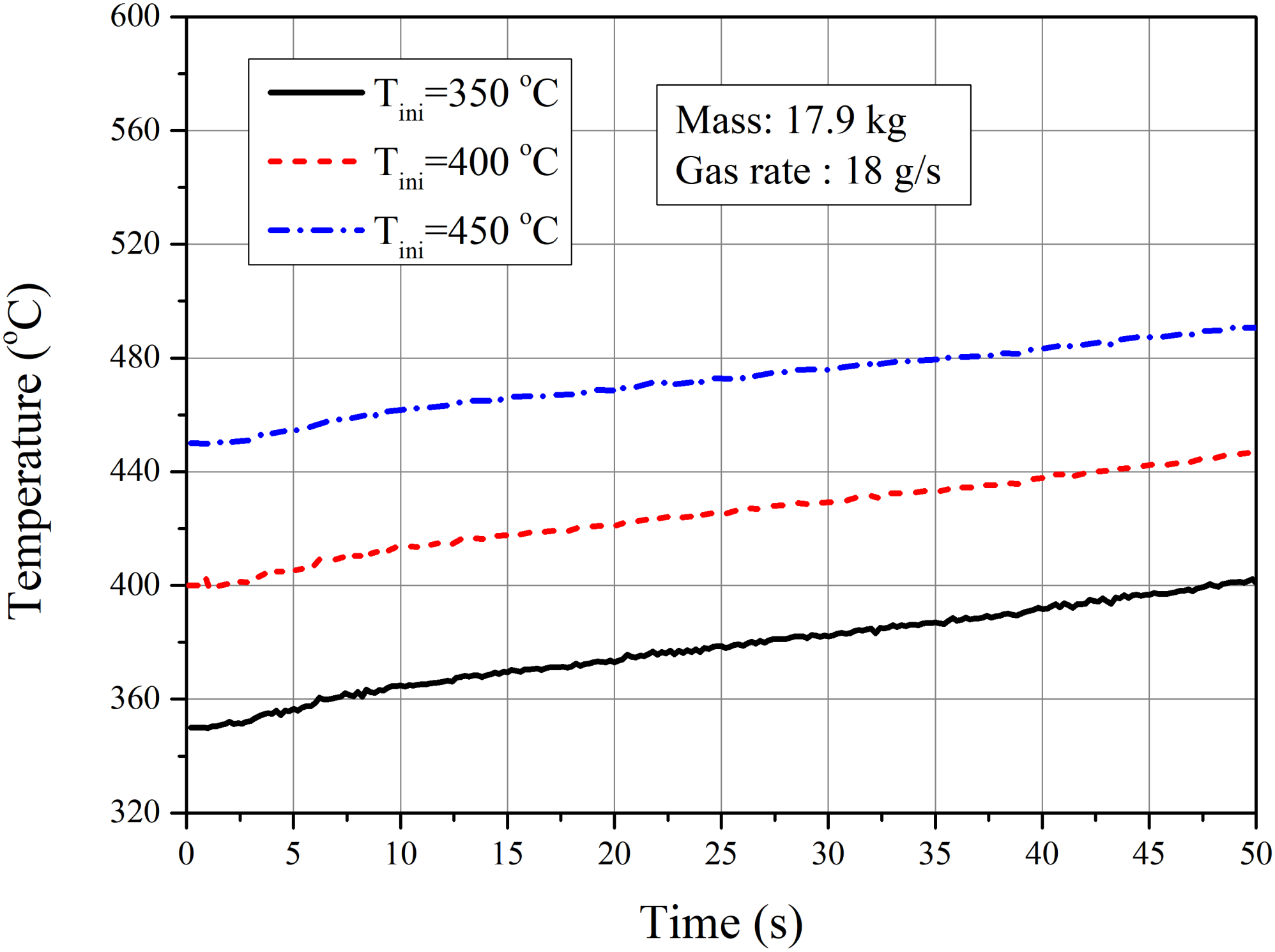
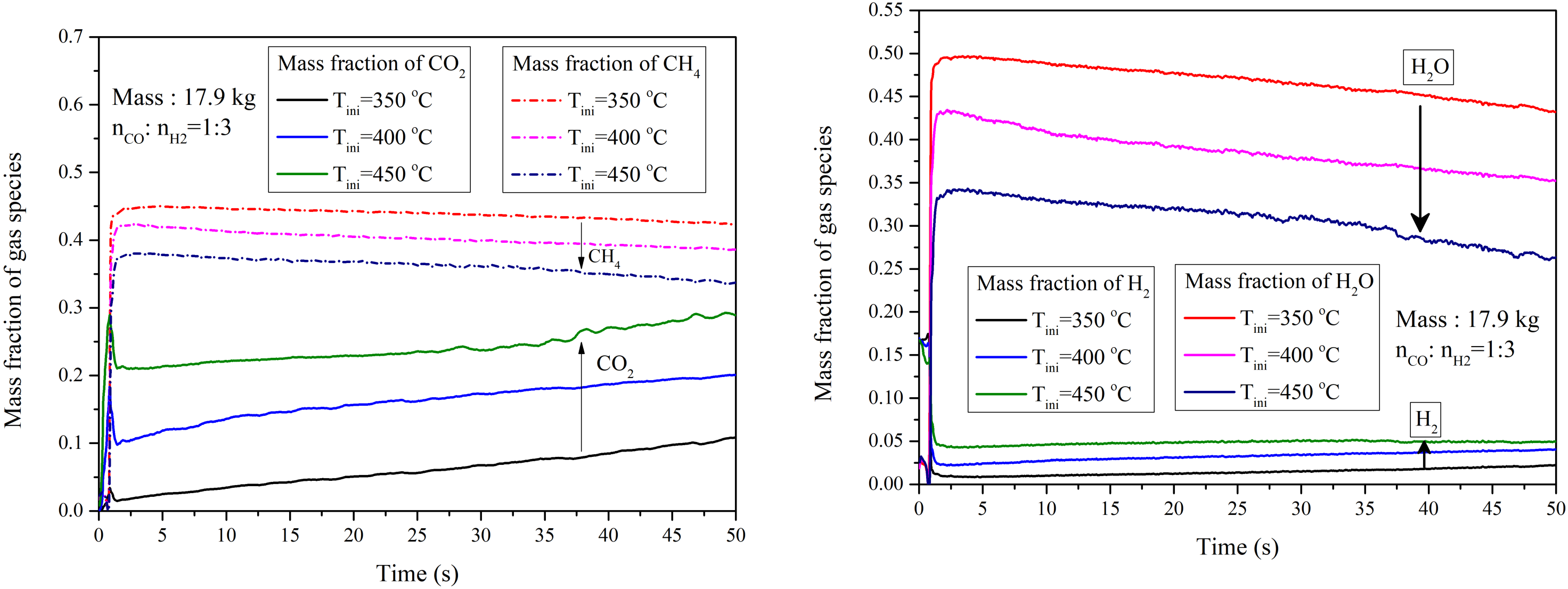
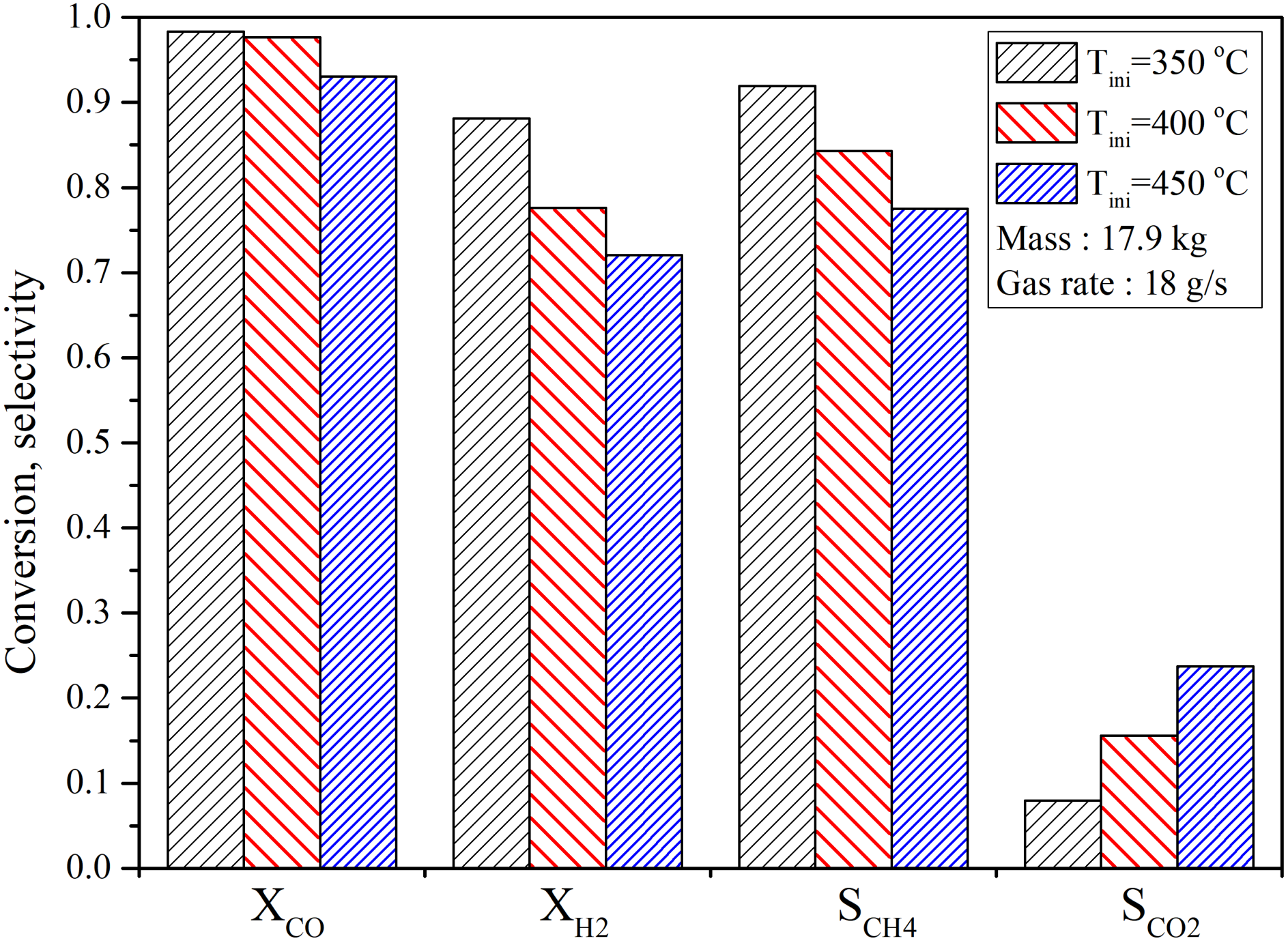
4.2.2. Influence of Initial Temperature
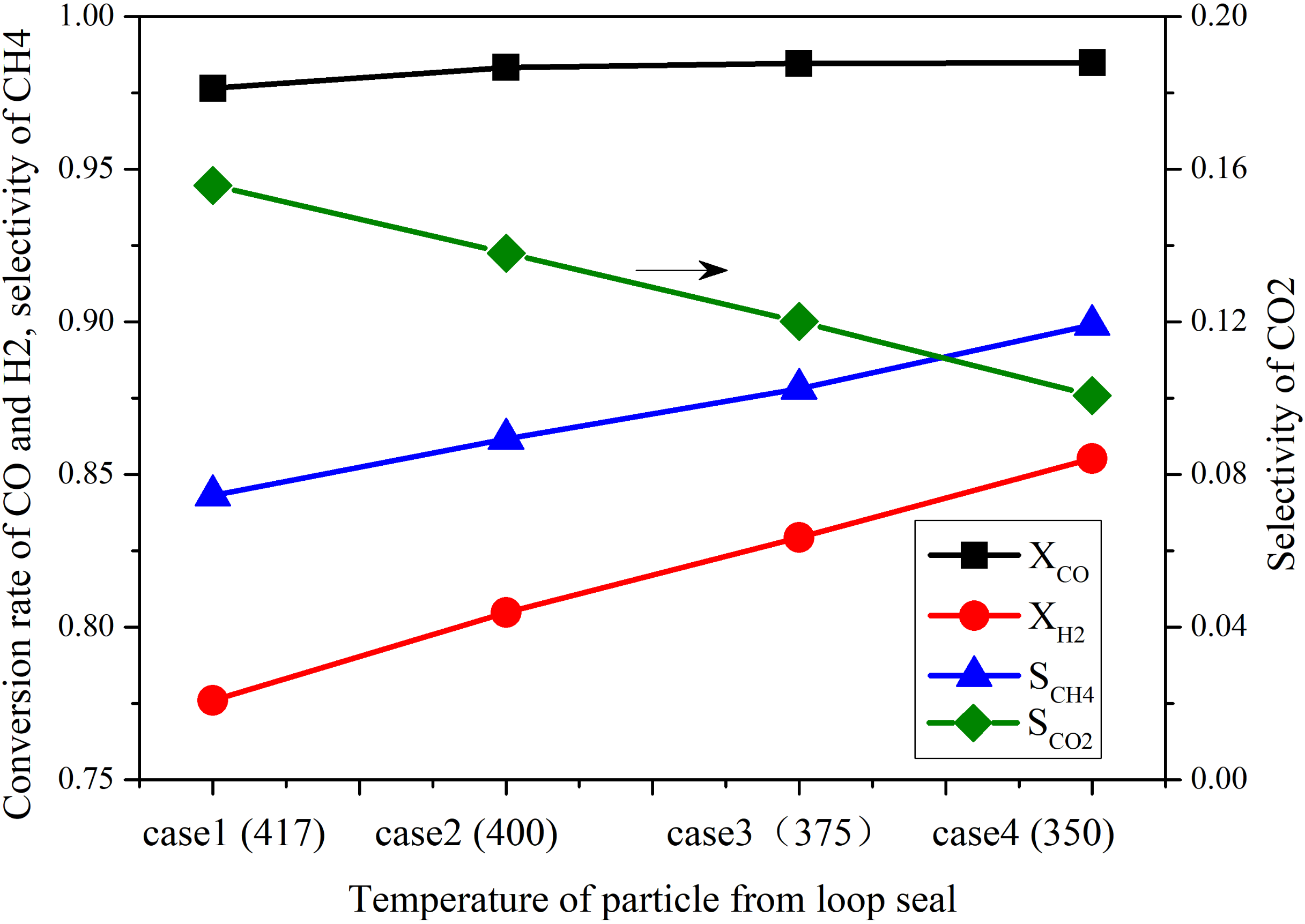
4.2.3. Influence of Cooling Reactor
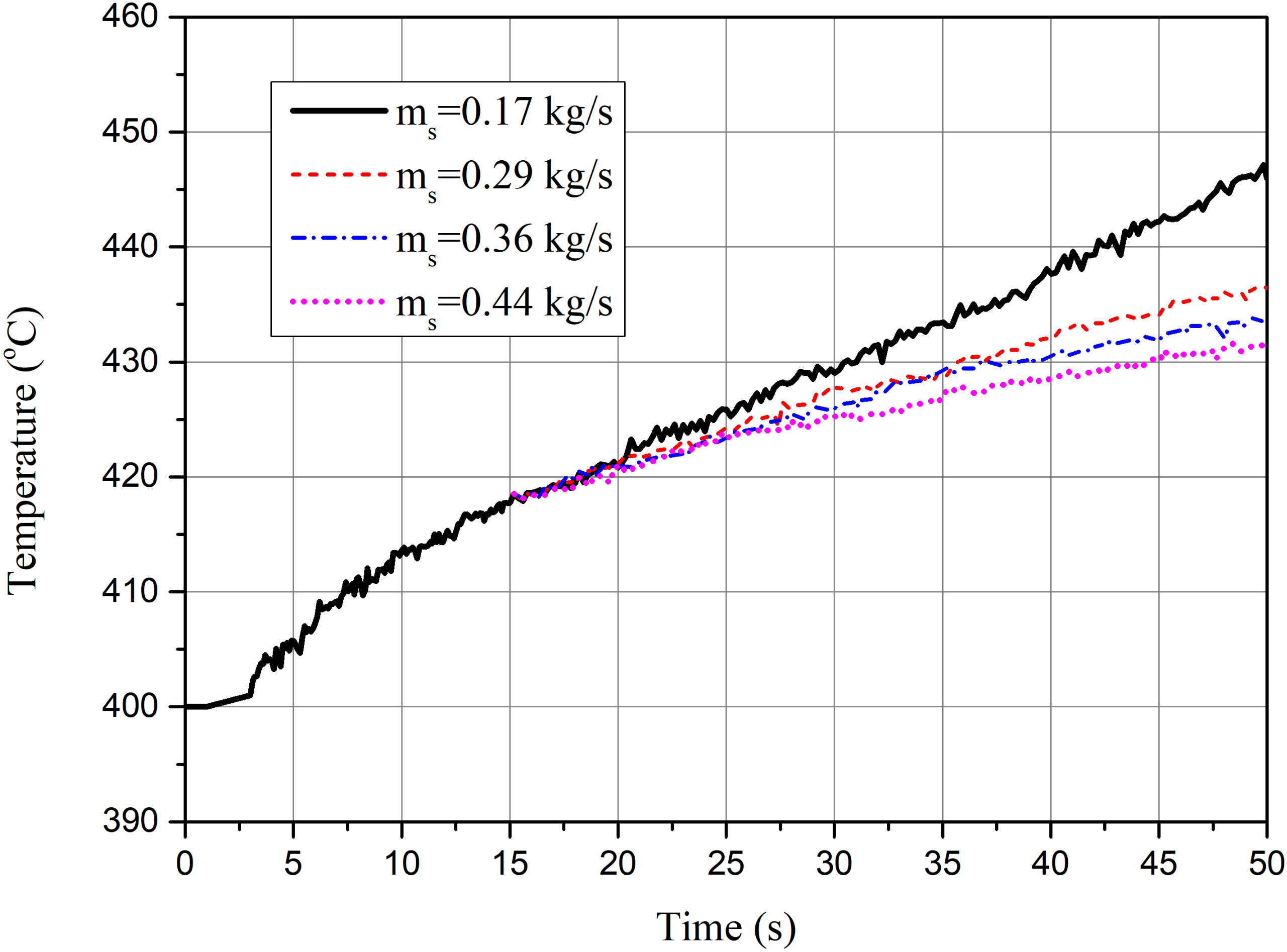
4.2.4. Influence of the Inlet Rate
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Nomenclature
| Drag coefficient | |
| Particle diameter | |
| Particle shear tensor | |
| Energy dissipation rate | |
| Normal restitution coefficient | |
| g | Gravity |
| Radial distribution function | |
| Generation of turbulence kinetic energy | |
| h | Heat transfer coefficient |
| H | Enthalpy |
| I | Interphase momentum transfer |
| Diffusion flux of species | |
| k | Turbulent kinetic energy |
| Conductivity of fluctuating energy | |
| Nusselt number | |
| P | Pressure |
| Prandtl Number | |
| Turbulent-Reynolds stress tensor | |
| Particle Reynolds number | |
| Particle kinetic stress tensor | |
| S | Source term for enthalpy |
| T | Temperature |
| Fluctuating velocity | |
| u | Velocity |
| W | Mole mass |
| Y | Mass fraction |
| Greek letters | |
| Volume fraction | |
| Interphase momentum transfer coefficient | |
| Density | |
| Laminar dynamic viscosity | |
| Frictional viscosity | |
| Viscous stress tensor | |
| Collisional stress tensor | |
| Turbulent kinematic viscosity | |
| Kinetic energy dissipation rate | |
| Stress tensor of gas phase | |
| Stress tensor of gas phase | |
| Dissipation of fluctuation kinetic energy | |
| Particle collision time | |
| Solid bulk viscosity | |
| Thermal conductivity | |
| Production rate of gas species |
References
- Seemann, M.C.; Schildhauer, T.J.; Biollaz, S.M.A. Fluidized Bed Methanation of Wood-Derived Producer Gas for the Production of Synthetic Natural Gas. Ind. Eng. Chem. Res. 2010, 49, 7034–7038. [Google Scholar] [CrossRef]
- Li, X.; Han, Y.; Huang, Y.; Lin, J.; Pan, X.; Zhao, Z.; Zhou, Y.; Wang, H.; Yang, X.; Wang, A.; et al. Hydrogenated TiO2 supported Ru for selective methanation of CO in practical conditions. Appl. Catal. B Environ. 2021, 298, 120597. [Google Scholar] [CrossRef]
- Qin, S.; Li, J.; Long, J.; Yang, X.; Miao, P. Promotion Effect of Cerium on Mo/Al2O3 Catalyst for Methanation. Appl. Catal. A Gen. 2020, 598, 117559. [Google Scholar] [CrossRef]
- Gao, W.; Meng, X.; Jin, D.; Xu, B.; Dai, W.; Zhao, R.; Xin, Z. Polyol-pretreated SBA-16 supported Ni-Fe bimetallic catalyst applied in CO methanation at low temperature. Mol. Catal. 2021, 512, 111769. [Google Scholar] [CrossRef]
- Xiao, Y.S.; Song, Y.H.; Luo, Q.X.; Shi, X.Y.; Li, J.; Hao, Q.Q.; Liu, Z.T.; Liu, Z.W. Kinetics behavior of Co/Ni-ordered mesoporous alumina for the CO methanation. Chem. Eng. Sci. X 2021, 10, 100094. [Google Scholar]
- Mohanty, U.S.; Ali, M.; Azhar, M.R.; Al-Yaseri, A.; Keshavarz, A.; Iglauer, S. Current advances in syngas (CO + H2) production through bi-reforming of methane using various catalysts: A review. Int. J. Hydrogen Energy 2021, 46, 32809–32845. [Google Scholar] [CrossRef]
- Zhang, Q.; Guo, X.; Yao, X.; Cao, Z.; Sha, Y.; Chen, B.; Zhou, H. Modeling, simulation, and systematic analysis of high-temperature adiabatic fixed-bed process of CO methanation with novel catalysts. Appl. Energy 2020, 279, 115822. [Google Scholar] [CrossRef]
- Kopyscinski, J.; Schildhauer, T.J.; Biollaz, S.M. Methanation in a fluidized bed reactor with high initial CO partial pressure: Part II—Modeling and sensitivity study. Chem. Eng. Sci. 2011, 66, 1612–1621. [Google Scholar] [CrossRef]
- Kopyscinski, J.; Schildhauer, T.J.; Biollaz, S.M. Methanation in a fluidized bed reactor with high initial CO partial pressure: Part I—Experimental investigation of hydrodynamics, mass transfer effects, and carbon deposition. Chem. Eng. Sci. 2011, 66, 924–934. [Google Scholar] [CrossRef]
- Kopyscinski, J.; Schildhauer, T.J.; Biollaz, S.M.A. Fluidized-Bed Methanation: Interaction between Kinetics and Mass Transfer. Ind. Eng. Chem. Res. 2011, 50, 2781–2790. [Google Scholar] [CrossRef]
- Li, J.; Yang, B. A Multi-scale Model for the Simulation of Bubbling Fluidized Bed Methanation. In Proceedings of the 25th International Symposium on Chemical Reaction Engineering, Florence, Italy, 20–23 May 2018. [Google Scholar]
- Yu, H.; Zhang, H.; Buahom, P.; Liu, J.; Xia, X.; Park, C.B. Prediction of thermal conductivity of micro/nano porous dielectric materials: Theoretical model and impact factors. Energy 2021, 233, 121140. [Google Scholar] [CrossRef]
- Kopyscinski, J.; Schildhauer, T.J.; Vogel, F.; Biollaz, S.M.; Wokaun, A. Applying spatially resolved concentration and temperature measurements in a catalytic plate reactor for the kinetic study of CO methanation. J. Catal. 2010, 271, 262–279. [Google Scholar] [CrossRef]
- Kopyscinski, J.; Schildhauer, T.J.; Biollaz, S.M.A. Employing Catalyst Fluidization to Enable Carbon Management in the Synthetic Natural Gas Production from Biomass. Chem. Eng. Technol. 2009, 32, 343–347. [Google Scholar] [CrossRef]
- Li, J.; Yang, B. Bubbling fluidized bed methanation study with resolving the mesoscale structure effects. AIChE J. 2019, 65, 16561. [Google Scholar] [CrossRef]
- Liu, Y.; Hinrichsen, O. CFD Simulation of Hydrodynamics and Methanation Reactions in a Fluidized-Bed Reactor for the Production of Synthetic Natural Gas. Ind. Eng. Chem. Res. 2014, 53, 9348–9356. [Google Scholar] [CrossRef]
- Chein, R.Y.; Yu, C.T.; Wang, C.C. Numerical simulation on the effect of operating conditions and syngas compositions for synthetic natural gas production via methanation reaction. Fuel 2016, 185, 394–409. [Google Scholar] [CrossRef]
- Gidaspow, D. Multiphase Flow and Fluidization: Continuum and Kinetic Theory Descriptions; Academic Press: Cambridge, MA, USA, 1994. [Google Scholar]
- Wang, S.; Wang, Q.; Chen, J.; Liu, G.; Lu, H.; Sun, L. Extension of cluster-structure dependent drag model to simulation of riser with Geldart B particles. Adv. Powder Technol. 2016, 27, 57–63. [Google Scholar] [CrossRef]
- Liu, J.; Shen, W.; Cui, D.; Yu, J.; Su, F.; Xu, G. Syngas methanation for substitute natural gas over Ni–Mg/Al2O3 catalyst in fixed and fluidized bed reactors. Catal. Commun. 2013, 38, 35–39. [Google Scholar] [CrossRef]
- Sun, L.; Luo, K.; Fan, J. Production of synthetic natural gas by CO methanation over Ni/Al2O3 catalyst in fluidized bed reactor. Catal. Commun. 2018, 105, 37–42. [Google Scholar] [CrossRef]
- Sun, L.; Luo, K.; Fan, J. Numerical Simulation of CO Methanation for the Production of Synthetic Natural Gas in a Fluidized Bed Reactor. Energy Fuels 2017, 31, 10267–10273. [Google Scholar] [CrossRef]




| Stress tensors | |
| Solid pressure for KTGF | |
| Conductivity of fluctuating energy | |
| Radial distribution function | |
| Dissipation of fluctuation kinetic energy | |
| Particle collision time | |
| Energy dissipation rate | |
| Solid shear viscosity | |
| Solid bulk viscosity | |
| Heat transfer coefficient | |
| Nusselt number | |
| Prandtl Number | |
| Generation of turbulence kinetic energy | |
| Gas phase shear viscosity | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, L.; Luo, K.; Fan, J. 3D Unsteady Simulation of a Scale-Up Methanation Reactor with Interconnected Cooling Unit. Energies 2021, 14, 7095. https://doi.org/10.3390/en14217095
Sun L, Luo K, Fan J. 3D Unsteady Simulation of a Scale-Up Methanation Reactor with Interconnected Cooling Unit. Energies. 2021; 14(21):7095. https://doi.org/10.3390/en14217095
Chicago/Turabian StyleSun, Liyan, Kun Luo, and Jianren Fan. 2021. "3D Unsteady Simulation of a Scale-Up Methanation Reactor with Interconnected Cooling Unit" Energies 14, no. 21: 7095. https://doi.org/10.3390/en14217095
APA StyleSun, L., Luo, K., & Fan, J. (2021). 3D Unsteady Simulation of a Scale-Up Methanation Reactor with Interconnected Cooling Unit. Energies, 14(21), 7095. https://doi.org/10.3390/en14217095






