Physicochemical Variation of the Main Components during Wild Pretreatment Process Based on the Concept of the Whole Utilization of Bamboo
Abstract
:1. Introduction
2. Materials and Methods
2.1. Material
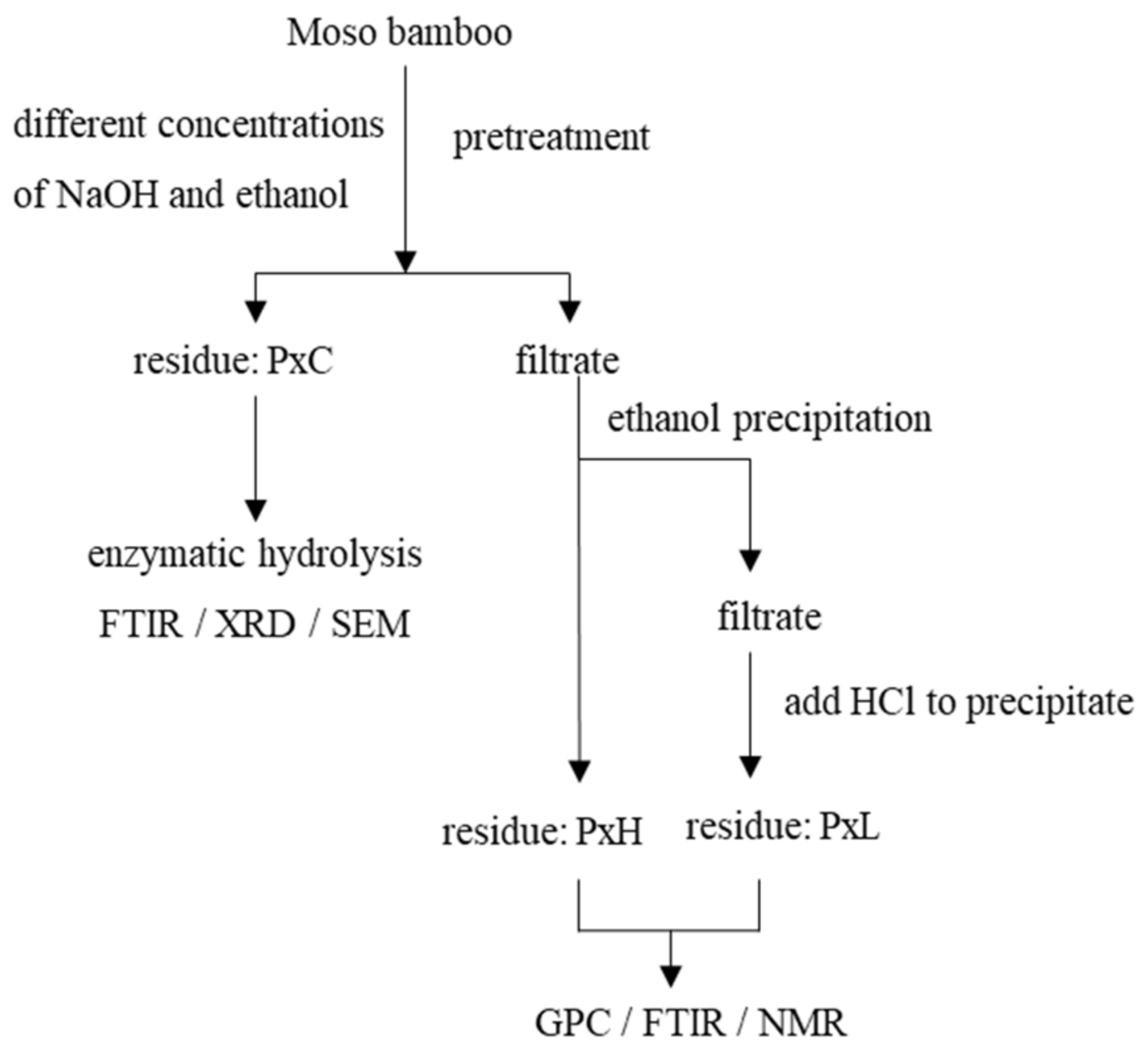
2.2. Fractionation of Bamboo
2.3. Characteristic Analysis
2.3.1. Compositional Analysis
2.3.2. Physicochemical Structure Analysis
2.4. Enzymatic Hydrolysis of the Cellulosic Samples
3. Results and Discussion
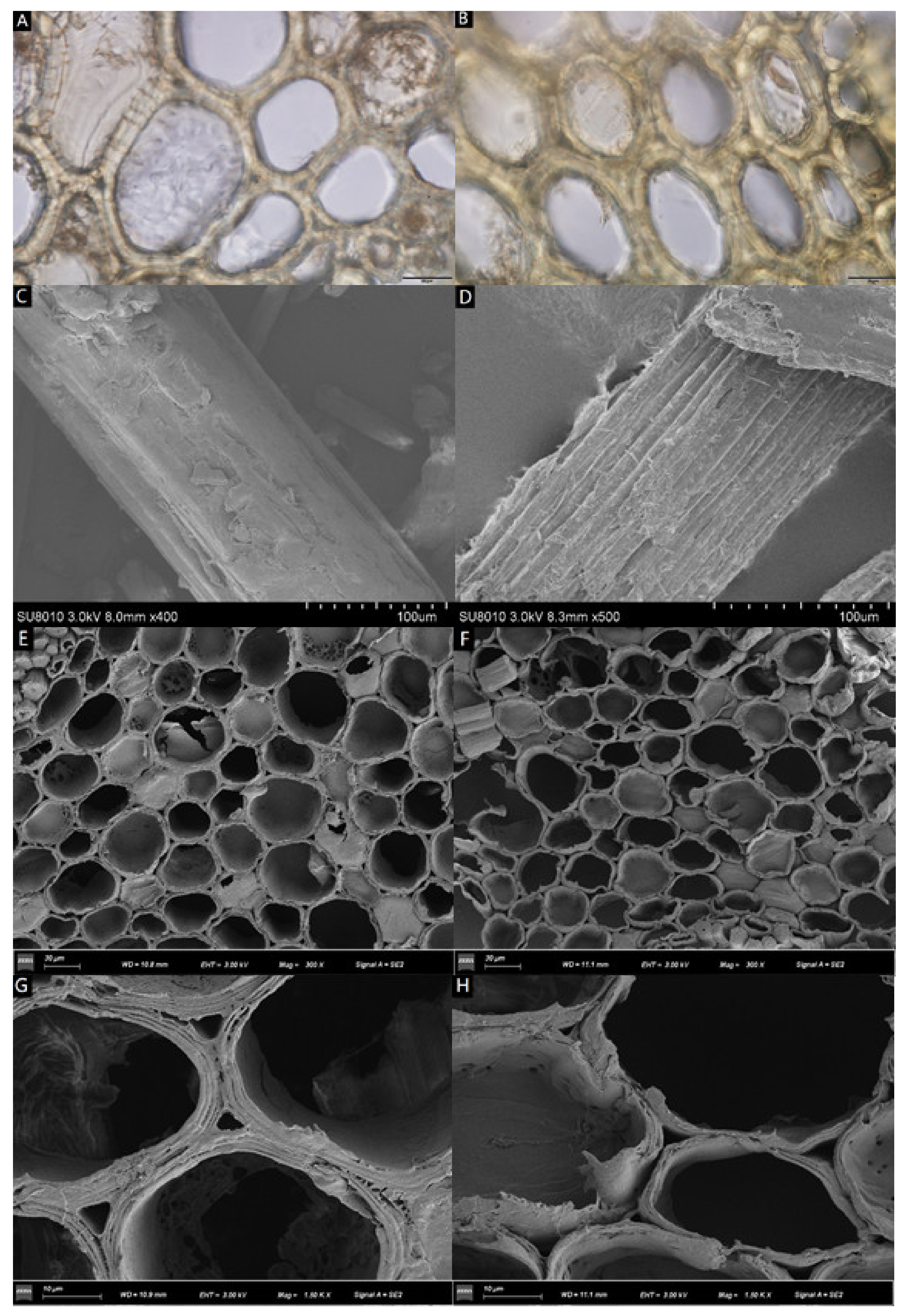
3.1. Fractionation Efficiency and Morphologic Variation of Cell Wall during Pretreatment
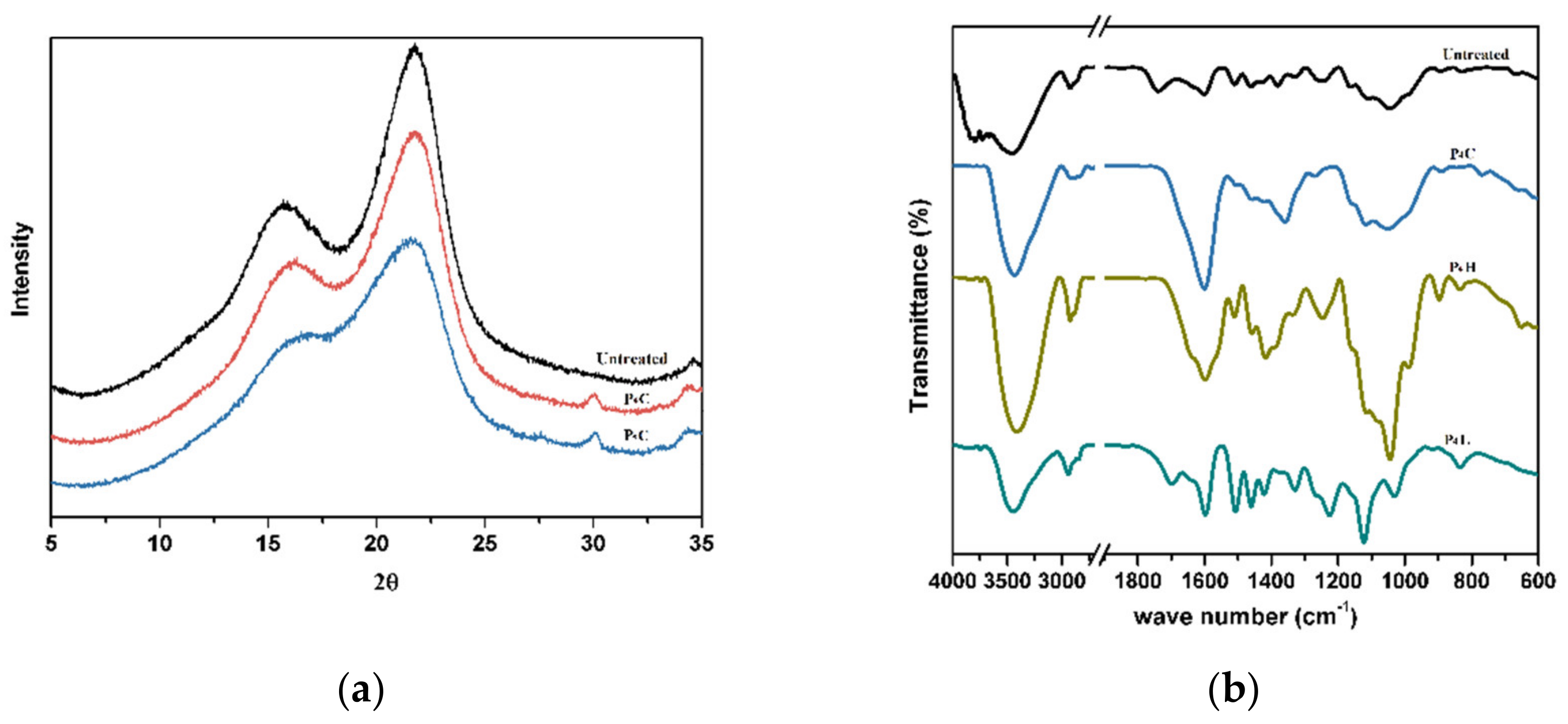
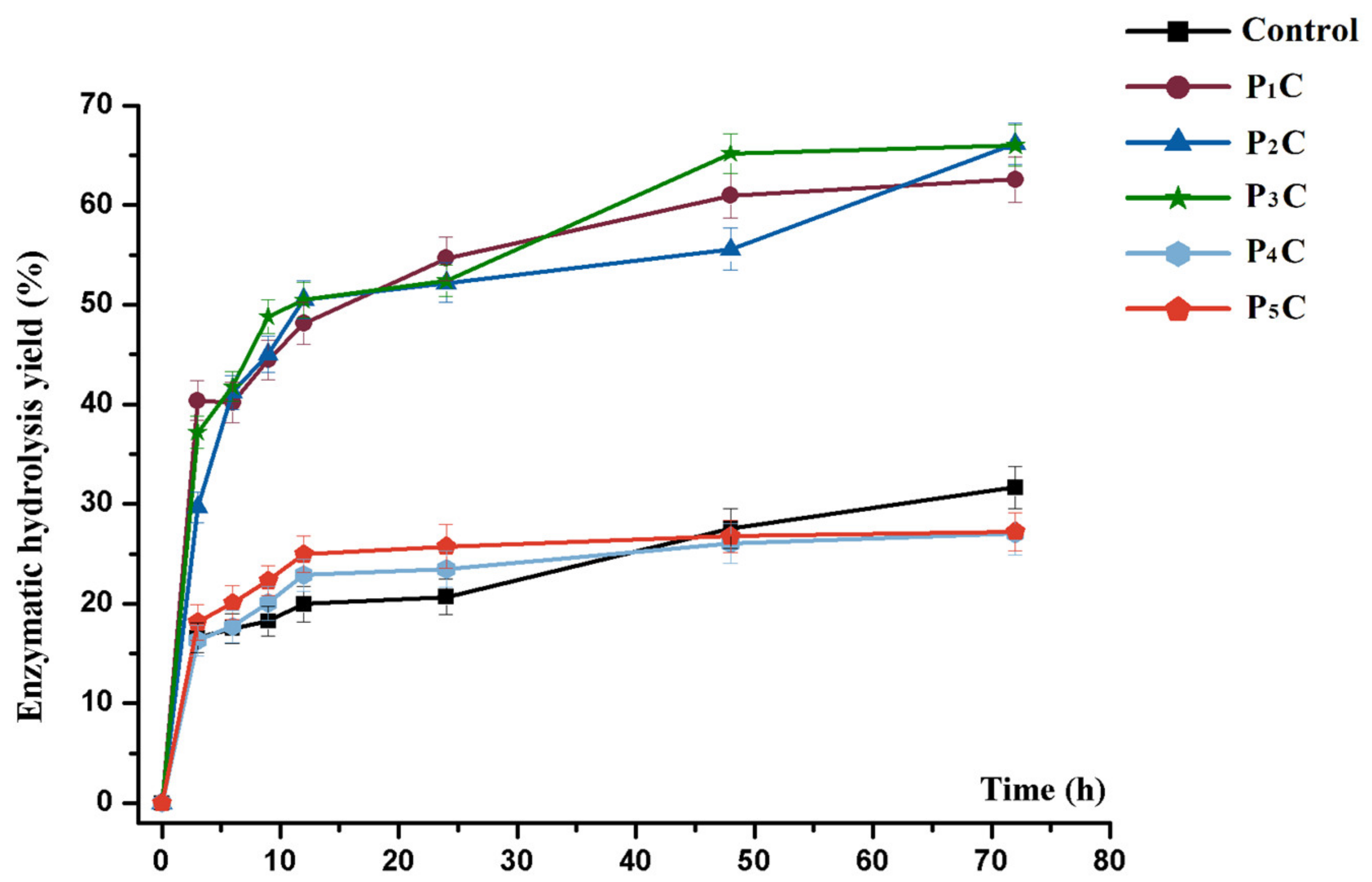
3.2. Physicochemical Analysis and Enzymatic Hydrolysis Efficiency of the Cellulosic Samples
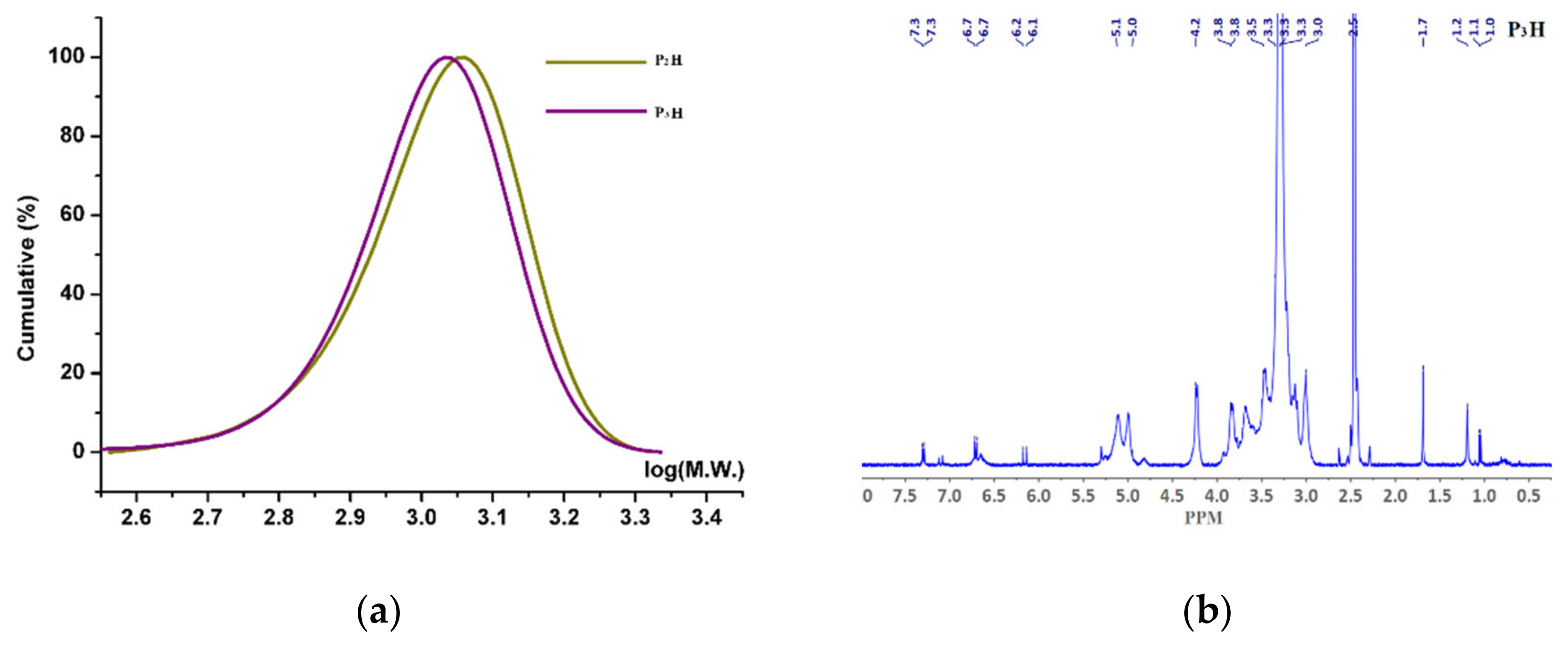
3.3. Physicochemical Analysis of the Hemicellulosic Samples
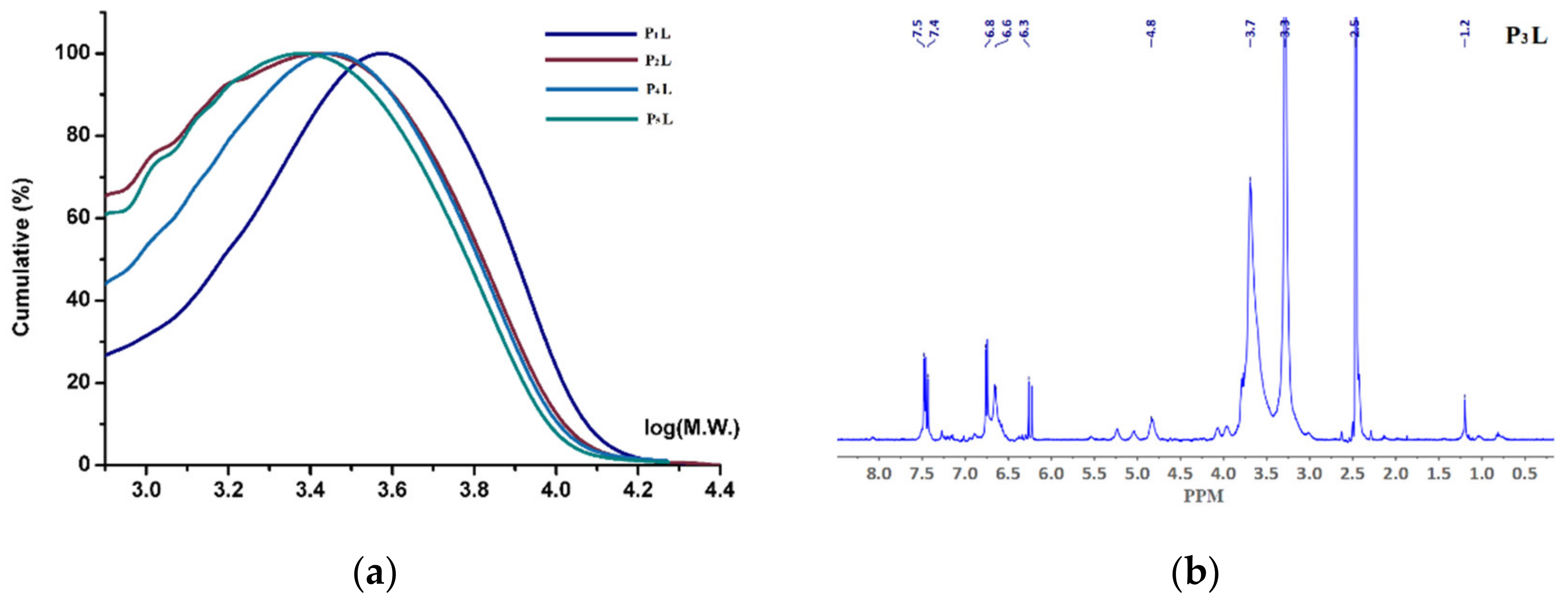
3.4. Physicochemical Analysis of the Lignin Samples
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Saito, K.; Horikawa, Y.; Sugiyama, J.; Watanabe, T.; Kobayashi, Y.; Takabe, K. Effect of thermochemical pretreatment on lignin alteration and cell wall microstructural degradation in Eucalyptus globulus: Comparison of acid, alkali, and water pretreatments. J. Wood Sci. Off. J. Jpn. Wood Res. Soc. 2016, 62, 276–284. [Google Scholar] [CrossRef]
- Bichot, A.; Lerosty, M.; Radoiu, M.; Mechin, V.; Bernet, N.; Delgenes, J.; Garcia-Bernet, D. Decoupling thermal and non-thermal effects of the microwaves for lignocellulosic biomass pretreatment. Energ. Convers. Manag. 2020, 203, 112220–112221. [Google Scholar] [CrossRef]
- Hendriks, A.; Zeeman, G. Pretreatments to enhance the digestibility of lignocellulosic biomass. Bioresour. Technol. 2009, 100, 10–18. [Google Scholar] [CrossRef]
- Pu, Y.; Fan, H.; Huang, F.; Davison, B. Assessing the molecular structure basis for biomass recalcitrance during dilute acid and hydrothermal pretreatments. Biotechnol. Biofuels 2013, 6, 15. [Google Scholar] [CrossRef] [Green Version]
- Zhao, X.; Zhang, L.; Liu, D. Biomass recalcitrance. Part II: Fundamentals of different pre-treatments to increase the enzymatic digestibility of lignocellulose. Biofuel. Bioprod. Biorefin. 2012, 6, 561–579. [Google Scholar] [CrossRef]
- Wang, G. Promoting the development of China’s bamboo industry by the progress of science and technology. State Acad. For. Admin. J. 2011, 10, 8–11. [Google Scholar]
- Fan, B.; Li, Z.; Chen, Y. Analysis on the status quo and development potential of bamboo and rattan resources in China. For. Resour. Manag. 2004, 1, 18–20. [Google Scholar]
- Vorontsova, M.S.; Clark, L.G.; Dransfield, J.; Govaerts, R.; Baker, W.J. World Checklist of Bamboos and Rattans; International Network of Bamboo and Rattan & the Board of Trustees of the Royal Botanic Gardens, Kew Publishing: Richmond, Surrey, UK, 2016; p. 120. [Google Scholar]
- Dam, J.; Elbersen, H.W.; Montaño, C.M.D. Bamboo production for industrial utilization. In Perennial Grasses for Bioenergy and Bioproducts; Elsevier: Amsterdam, The Netherlands, 2018; pp. 175–216. [Google Scholar]
- Dou, Y.; Yu, X.; Fumiyo, I. The current situation and countermeasures of bamboo resource development and utilization of China. Chin. J. Agric. Resour. Reg. Plan. 2011, 32, 65–70. [Google Scholar]
- Liu, Z.; Fei, B.; Jiang, Z.; Liu, X. Combustion characteristics of bamboo-biochars. Bioresour. Technol. 2014, 167C, 94–99. [Google Scholar] [CrossRef] [PubMed]
- Borowski, P.F. Innovation strategy on the example of companies using bamboo. J. Innov. Entrep. 2021, 10, 3. [Google Scholar] [CrossRef] [PubMed]
- Hada, S.; Roat, P.; Chechani, B.; Kumar, S.; Yadav, D.K.; Kumari, N. An Overview on Biomass of Bamboo as a Source of Bioenergy. In Biotechnology for Biofuels: A Sustainable Green Energy Solution; Springer: Singapore, 2020; pp. 241–265. [Google Scholar]
- Zhao, Z.G.; Cheng, K.K.; Zhang, J.A.; Gao, F. Advances in pretreatment technology of lignocellulose renewable biomass. Mod. Chem. Ind. 2006, 26, 39. [Google Scholar]
- Zhu, Z.X.; Nie, J.H.; Yan, Y.J. Chemical pretreatment technology of lignocellulose biomass for fuel ethanol. Chem. Biodivers. 2009, 26, 11–14. [Google Scholar]
- Xu, F.; Zhang, X.; Zhou, X.; Ji, Z.; Ma, J.; Ma, J. An investigation of dissolution mechanism of major components in cell walls of agricultural and forest biomass. J. For. Eng. 2016, 1, 1–9. [Google Scholar]
- Wang, K.; Yang, H.; Guo, S.; Tang, Y.; Jiang, J.; Xu, F.; Sun, R.C. Organosolv fractionation process with various catalysts for improving bioconversion of triploid poplar. Process. Biochem. 2012, 47, 1503–1509. [Google Scholar] [CrossRef]
- Wang, K.; Xu, F.; Sun, R.C. Molecular characteristics of kraft-aq pulping lignin fractionated by sequential organic solvent extraction. Int. J. Mol. Sci. 2010, 11, 2988. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Pillay, B.; Singh, S. Purification and biochemical characteristics of β-D-xylanase from a thermophilic fungus, Thermomyces lanuginosus-SSBP. Biotechnol. Appl. Bioc. 2011, 30, 73–79. [Google Scholar]
- Yamashita, Y.; Shono, M.; Sasaki, C.; Nakamura, Y. Alkaline peroxide pretreatment for efficient enzymatic saccharification of bamboo. Carbohydr. Polym. 2010, 79, 914–920. [Google Scholar] [CrossRef]
- Chi, C.; Liu, M.; Gong, Y.; Li, H.; Wu, Y. Effects of alkaline pretreatment on the enzymatic hydrolysis efficiency of corn straw. J. Shaanxi Univ. Sci. Technol. 2015, 33, 13–18. [Google Scholar]
- Bai, Y.Y.; Xiao, L.P.; Shi, Z.J.; Sun, R.C. Structural variation of bamboo lignin before and after ethanol organosolv pretreatment. Int. J. Mol. Sci. 2013, 14, 21394–21413. [Google Scholar] [CrossRef] [PubMed]
- Jagannathan, P.; Muthukumaran, C.; Tamilarasan, K. A sequential pretreatment of lignocelluloses in bamboo biomass to fermentable sugars by acid/enzymatic hydrolysis. 3 Biotech 2017, 7, 260. [Google Scholar] [CrossRef]
- Chu, L.Q.; Masyuko, R.; Sweedler, J.V.; Bohn, P.W. Base-induced delignification of miscanthus x giganteus studied by three-dimensional confocal raman imaging. Bioresour. Technol. 2010, 101, 4919–4925. [Google Scholar] [CrossRef] [PubMed]
- Zhe, J.; Zhe, L.; Xun, Z.; Ma, J.; Xu, F. Effect of pretreatment on topochemical and ultrastructural changes of lignocellulose plant cell walls: A review. Chin. J. Biotechnol. 2014, 30, 707. [Google Scholar]
- Wang, K.; Xu, F.; Sun, R.C.; Jones, G.L. Influence of incubation time on the physicochemical properties of the isolated hemicelluloses from steam-exploded lespedeza stalks. Ind. Eng. Chem. Res. 2010, 49, 8797–8804. [Google Scholar] [CrossRef]
- Methacanon, P.; Chaikumpollert, O.; Thavorniti, P.; Suchiva, K. Hemicellulosic polymer from Vetiver grass and its physicochemical properties. Carbohydr. Polym. 2003, 54, 335–342. [Google Scholar] [CrossRef]
- Mosier, N.S.; Ladisch, C.M.; Ladisch, M.R. Characterization of acid catalytic domains for cellulose hydrolysis and glucose degradation. Biotechnol. Bioeng. 2002, 79, 610–618. [Google Scholar] [CrossRef]
- Wang, K.; Yang, H.; Xi, Y.; Xu, F.; Sun, R.C. Structural transformation of hemicelluloses and lignin from triploid poplar during acid-pretreatment based biorefinery process. Bioresour. Technol. 2012, 116, 99–106. [Google Scholar] [CrossRef]
- Pandey, K.K. A study of chemical structure of soft and hardwood and wood polymers by FTIR spectroscopy. J. Appl. Polym. Sci. 1999, 71, 1969–1975. [Google Scholar] [CrossRef]
- Ding, D.; Zhou, X.; Ji, Z.; You, T.; Xu, F. How does hemicelluloses removal alter plant cell wall nanoscale architecture and correlate with enzymatic digestibility? Bioenergy Res. 2016, 9, 601–609. [Google Scholar] [CrossRef]
- Xu, F.; Sun, R.C.; Zhai, M.Z.; Sun, J.X.; Jiang, J.X.; Zhao, G.J. Comparative study of three lignin fractions isolated from mild ball-milled Tamarix austromogoliac and Caragana sepium. J. Appl. Polym. Sci. 2008, 108, 1158–1168. [Google Scholar] [CrossRef]
- Popescu, C.M.; Larsson, P.T.; Vasile, C. Carbon-13 CP/MAS solid state NMR and X-ray diffraction spectroscopy studies on lime wood decayed by Chaetomium globosum. Carbohydr. Polym. 2011, 83, 808–812. [Google Scholar] [CrossRef]
- Qin, T.; Huang, L.; Li, G. Functional groups and chemical bonds of lignin of Neosinocalamus affinis and Phyllostachys pubescens. J. Beijing For. Univ. 2010, 32, 161–165. [Google Scholar]
- Egüés, I.; Sanchez, C.; Mondragon, I.; Labidi, J. Effect of alkaline and autohydrolysis processes on the purity of obtained hemicelluloses from corn stalks. Bioresour. Technol. 2012, 103, 239–248. [Google Scholar] [CrossRef] [PubMed]
- Peng, P.; She, D. Isolation, structural characterization, and potential applications of hemicelluloses from bamboo: A review. Carbohydr. Polym. 2014, 112, 701–720. [Google Scholar] [CrossRef]
- Gierlinger, N.; Goswami, L.; Schmidt, M.; Burgert, I.; Coutand, C.; Rogge, T.; Schwanninger, M. In Situ FT-IR microscopic study on enzymatic treatment of poplar wood cross-sections. Macromolecules 2008, 9, 2194–2201. [Google Scholar] [CrossRef]
- Herrera, R.; Erdocia, X.; Llano-Ponte, R.; Labidi, J. Characterization of hydrothermally treated wood in relation to changes on its chemical composition and physical properties. J. Anal. Appl. Pyrolysis 2014, 107, 256–266. [Google Scholar] [CrossRef]
- Xiao, L.P.; Shi, Z.J.; Xu, F.; Sun, R.C. Characterization of lignins isolated with alkaline ethanol from the hydrothermal pretreated tamarix ramosissima. Bioenergy Res. 2013, 6, 519–532. [Google Scholar] [CrossRef]
- Xu, B.; Shang, Y.W.; Zhao, D.; Yang, H. Research on bamboo lignin with supercritical carbon dioxide by FTIR and 1H NMR. J. Beijing Inst. Petro-Chem. Technol. 2014, 22, 4–6. [Google Scholar]
- Han, M. Effects of different extraction on lignin molecular mass and distribution. J. Luoyang Inst. Sci. Technol. (Nat. Sci. Ed.) 2013, 23, 1–4. [Google Scholar]
| Samples | Yield a (%) | Chemical Components b (Relative wt%) | ||||
|---|---|---|---|---|---|---|
| Glc | Xyl | AIL | ASL | |||
| Raw material | -- | 40.7 | 23.5 | 34.1 | 1.7 | |
| Cellulosic Fractions | P1C | 70.8 | 48.6 | 25.0 | 24.2 | 2.2 |
| P2C | 71.7 | 53.4 | 21.1 | 23.5 | 2.0 | |
| P3C | 62.8 | 61.2 | 17.1 | 19.9 | 1.8 | |
| P4C | 69.7 | 50.1 | 19.3 | 28.6 | 2.0 | |
| P5C | 73.4 | 46.0 | 18.3 | 34.0 | 1.8 | |
| Hemicellulosic Fractions | P1H | T c | -- | -- | -- | -- |
| P2H | 0.5 | 0.5 | 62.3 | 34.6 | 2.6 | |
| P3H | 25.6 | 0.7 | 60.5 | 36.2 | 2.6 | |
| P4H | 7.8 | 2.0 | 69.0 | 26.7 | 2.4 | |
| P5H | 8.3 | 0.8 | 65.4 | 31.3 | 2.5 | |
| Lignin Fractions | P1L | 2.6 | 0.6 | T | 96.4 | 0.2 |
| P2L | 6.8 | 1.0 | 0.9 | 95.6 | 0.2 | |
| P3L | 3.6 | 0.7 | T | 94.6 | 0.1 | |
| P4L | 5.8 | 0.4 | 0.2 | 97.0 | 0.3 | |
| P5L | 7.2 | 0.5 | T | 96.6 | 0.3 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, X.; Fan, K.; Wang, K.; Jiang, J.; Peng, X.; Yang, H.; Wang, M. Physicochemical Variation of the Main Components during Wild Pretreatment Process Based on the Concept of the Whole Utilization of Bamboo. Energies 2021, 14, 6857. https://doi.org/10.3390/en14216857
Yu X, Fan K, Wang K, Jiang J, Peng X, Yang H, Wang M. Physicochemical Variation of the Main Components during Wild Pretreatment Process Based on the Concept of the Whole Utilization of Bamboo. Energies. 2021; 14(21):6857. https://doi.org/10.3390/en14216857
Chicago/Turabian StyleYu, Xiaojuan, Kai Fan, Kun Wang, Jianxin Jiang, Xiaopeng Peng, Haiyan Yang, and Meng Wang. 2021. "Physicochemical Variation of the Main Components during Wild Pretreatment Process Based on the Concept of the Whole Utilization of Bamboo" Energies 14, no. 21: 6857. https://doi.org/10.3390/en14216857
APA StyleYu, X., Fan, K., Wang, K., Jiang, J., Peng, X., Yang, H., & Wang, M. (2021). Physicochemical Variation of the Main Components during Wild Pretreatment Process Based on the Concept of the Whole Utilization of Bamboo. Energies, 14(21), 6857. https://doi.org/10.3390/en14216857







