An Experimental and Kinetic Modelling Study on Laminar Premixed Flame Characteristics of Ethanol/Acetone Mixtures
Abstract
:1. Introduction
2. Experimental and Computational Methods
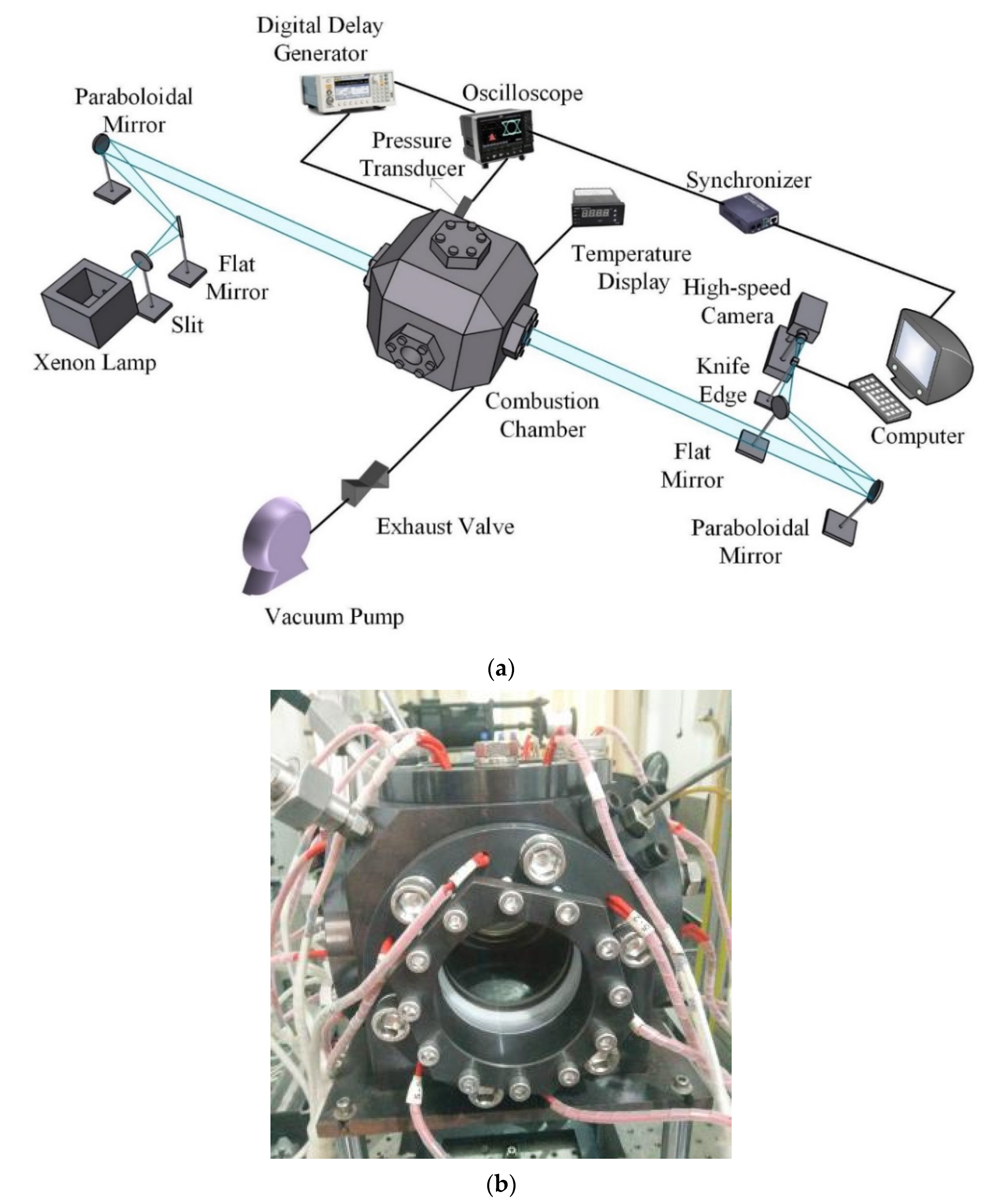
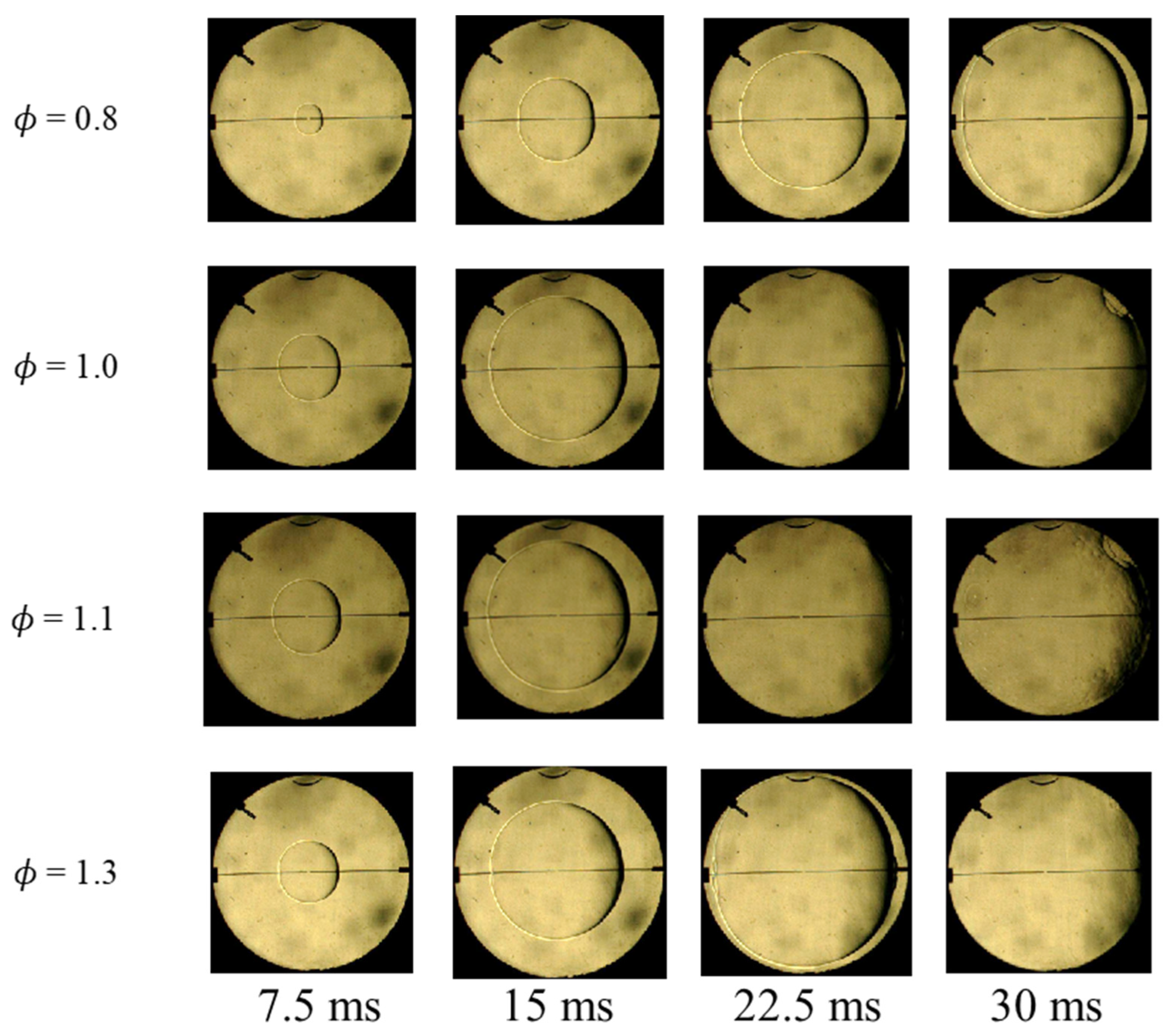
2.1. Experimental Device
2.2. Data Processing
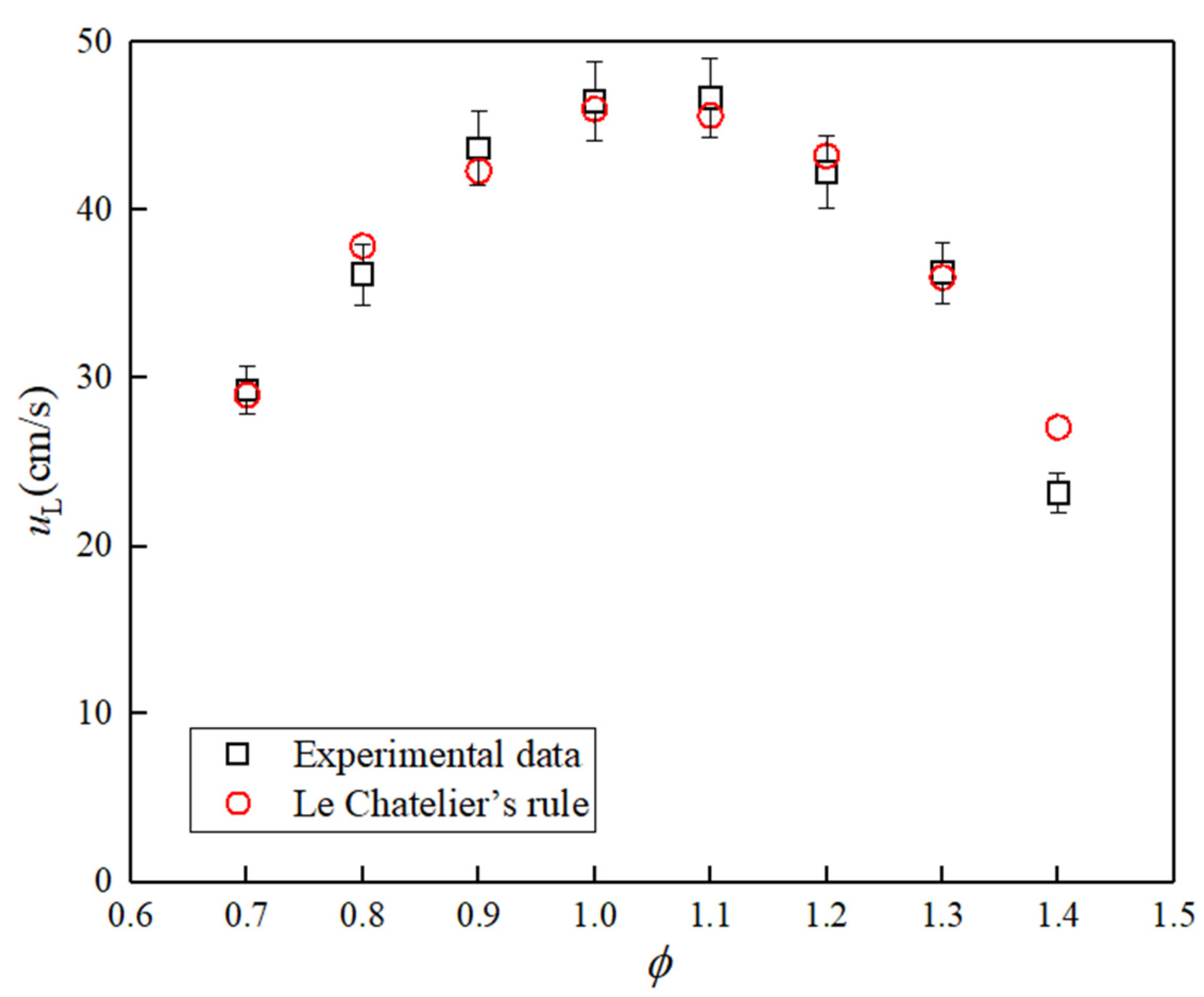
2.3. Uncertainty Assessment
3. Results and Discussion
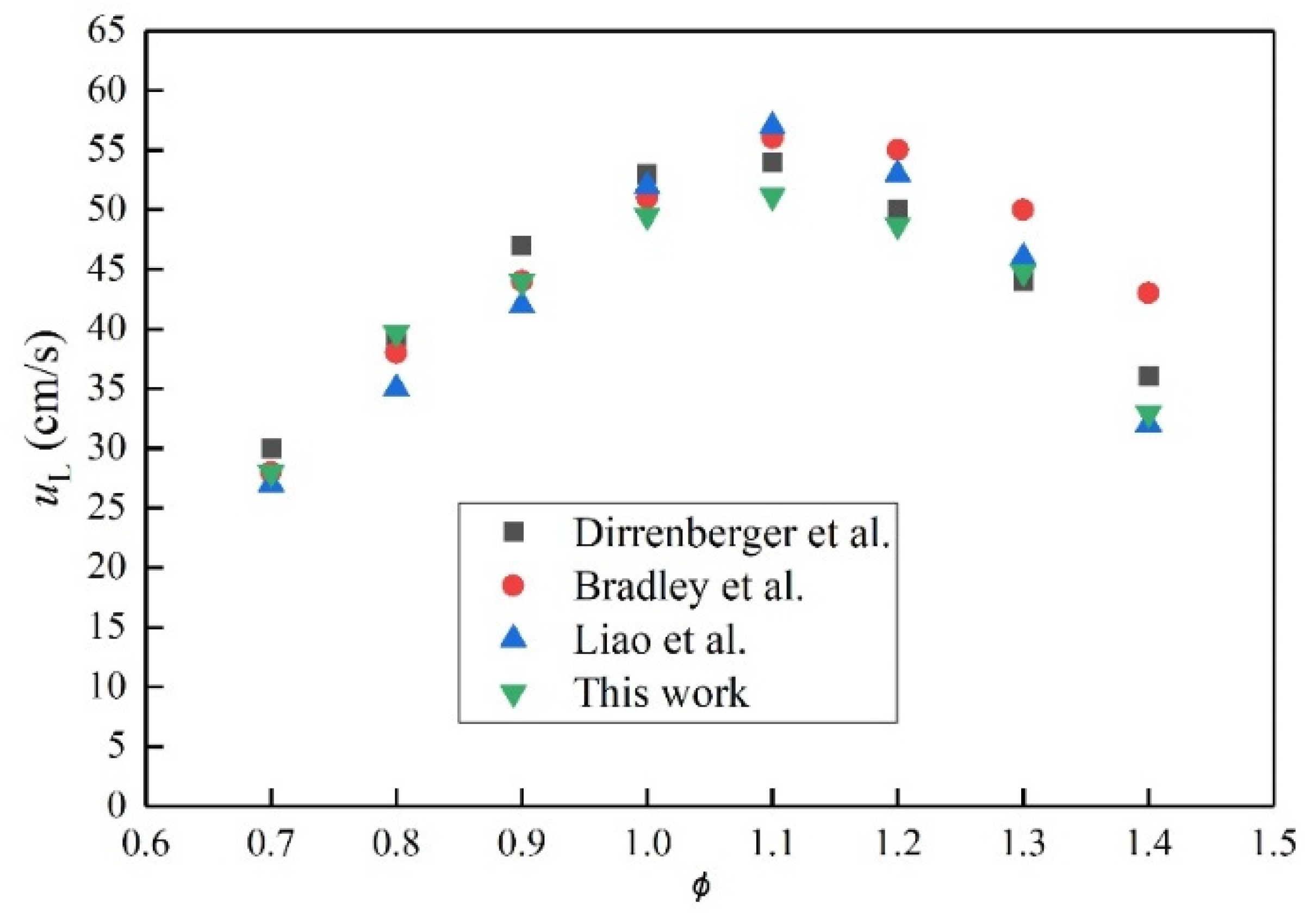
3.1. System Validation
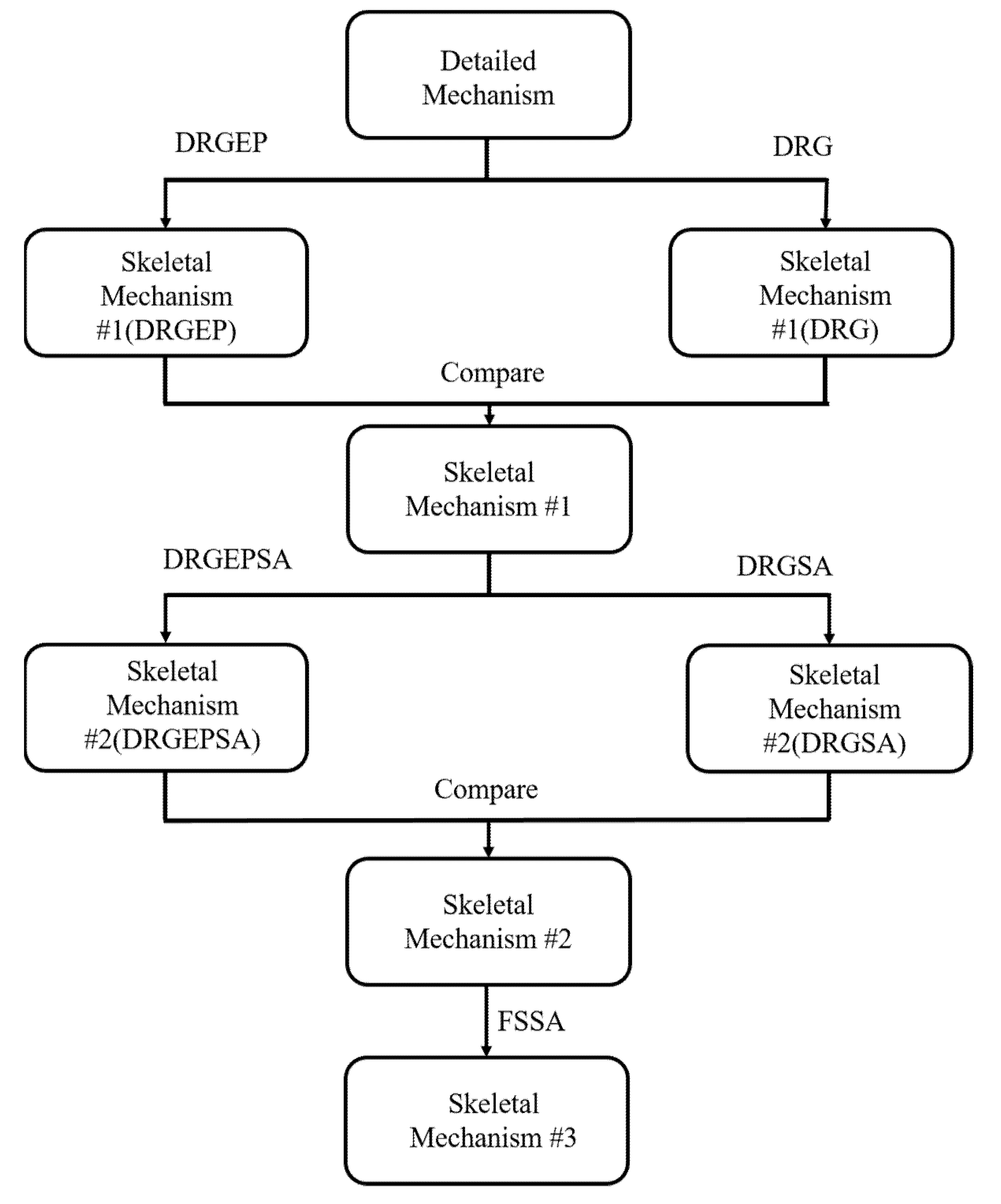
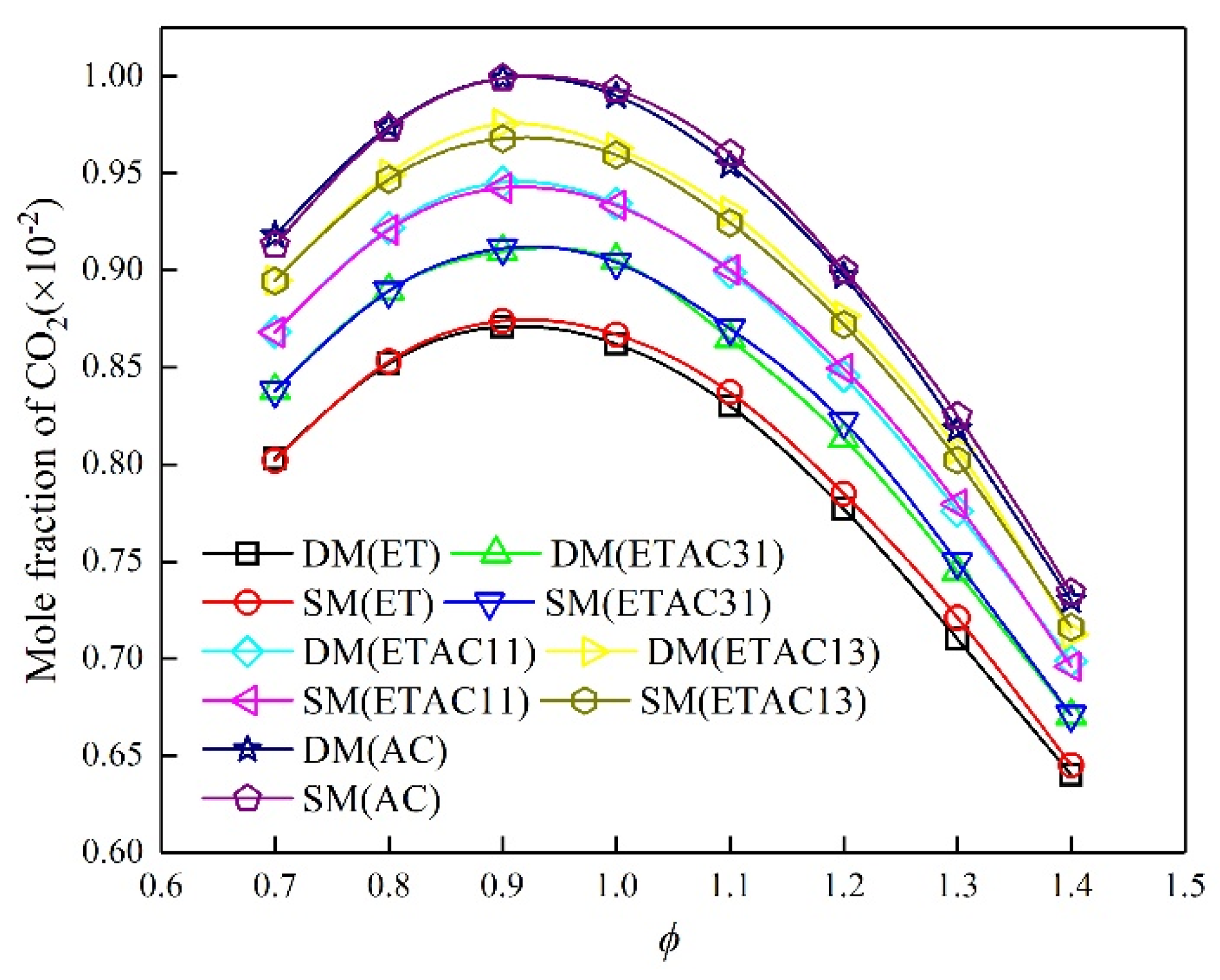
3.2. Mechanism Reduction
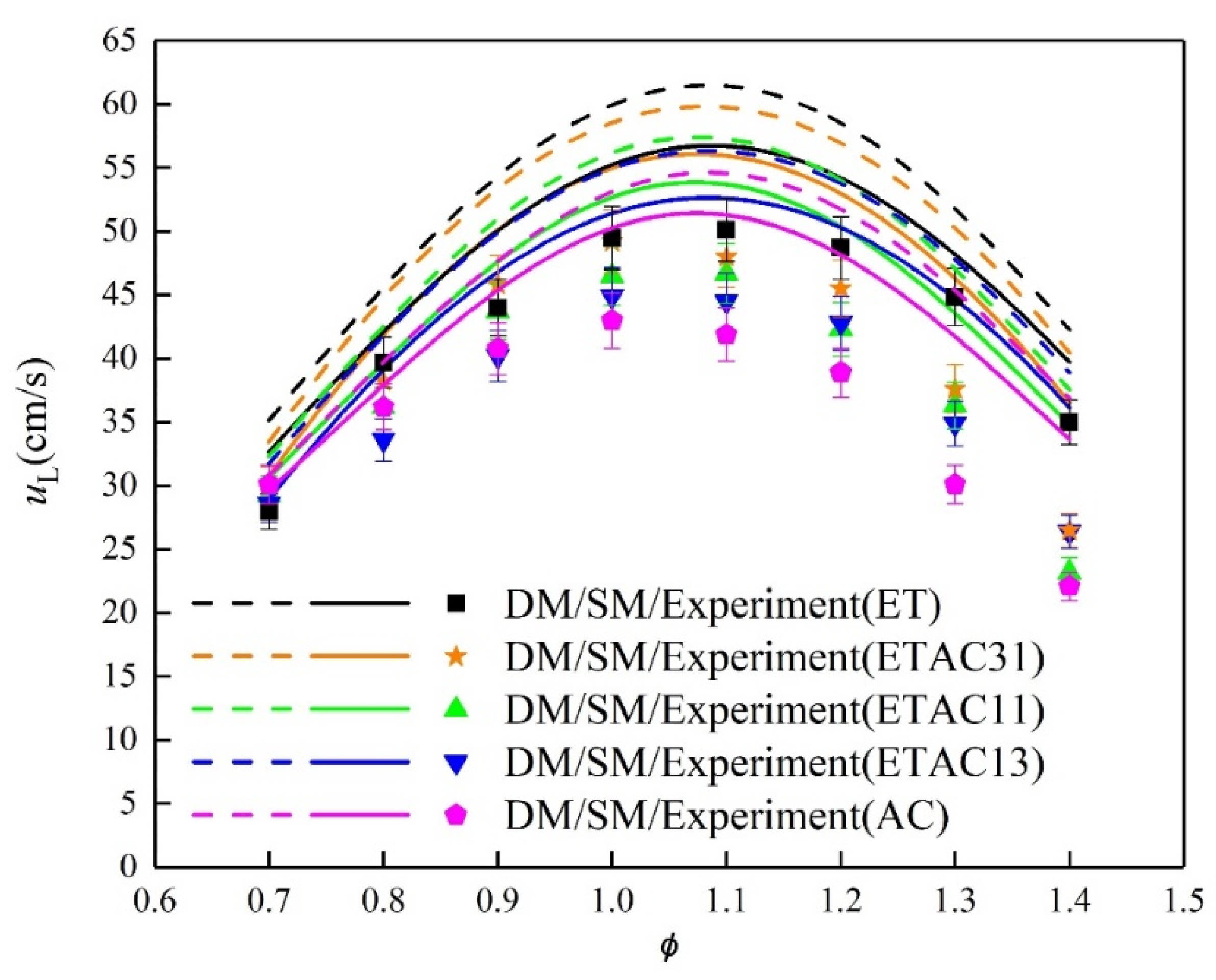
3.3. Laminar Burning Velocity
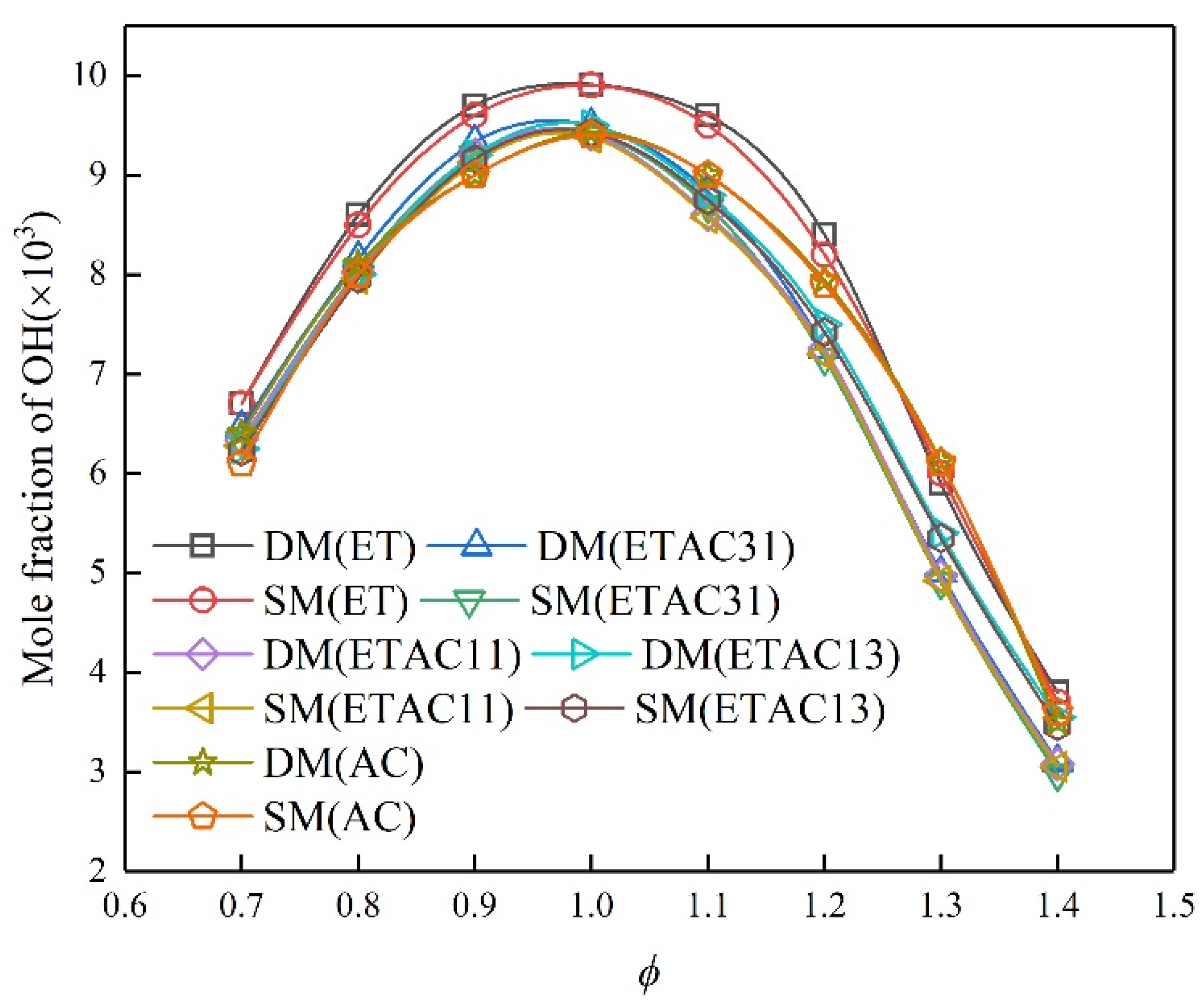
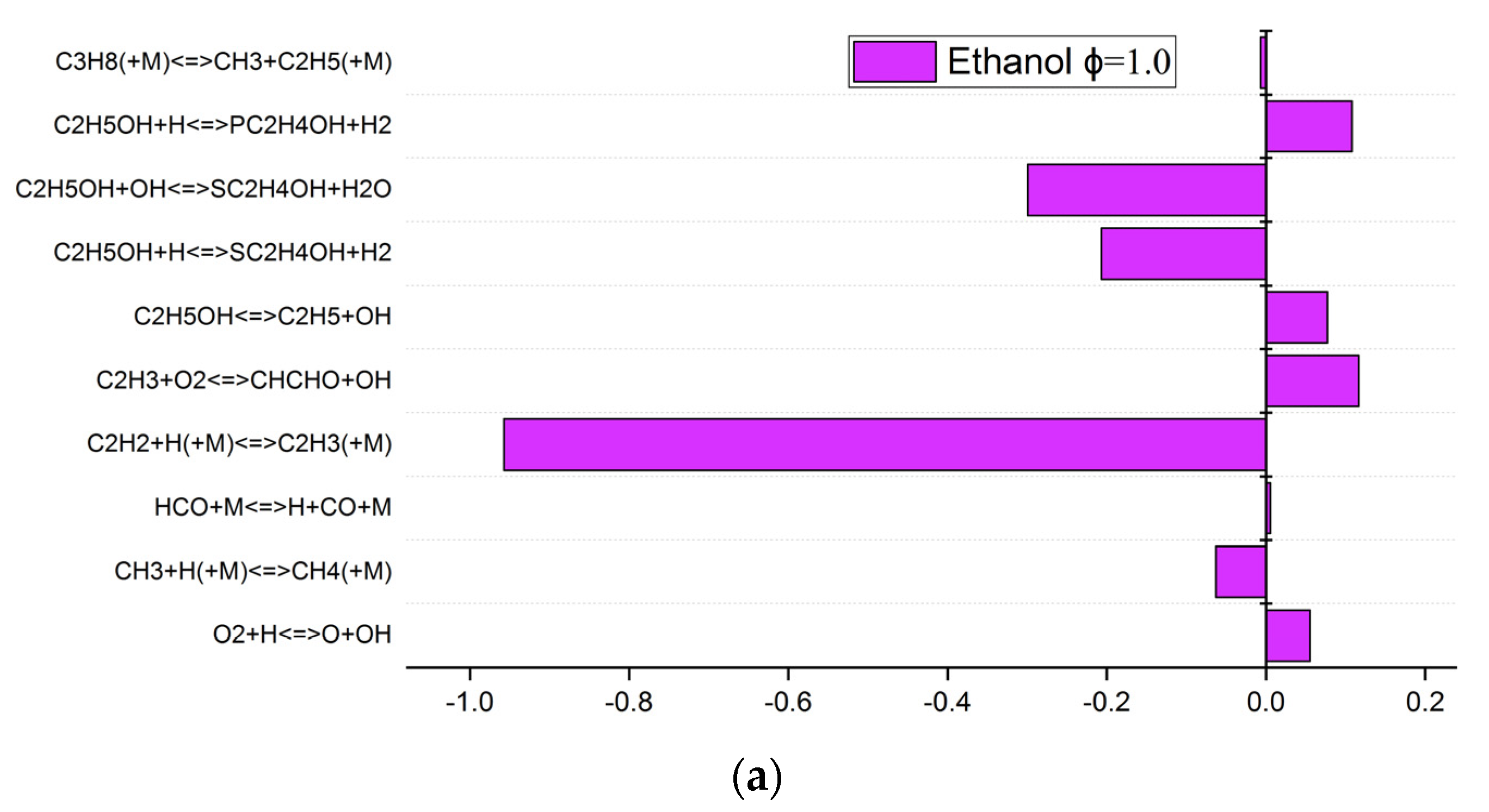
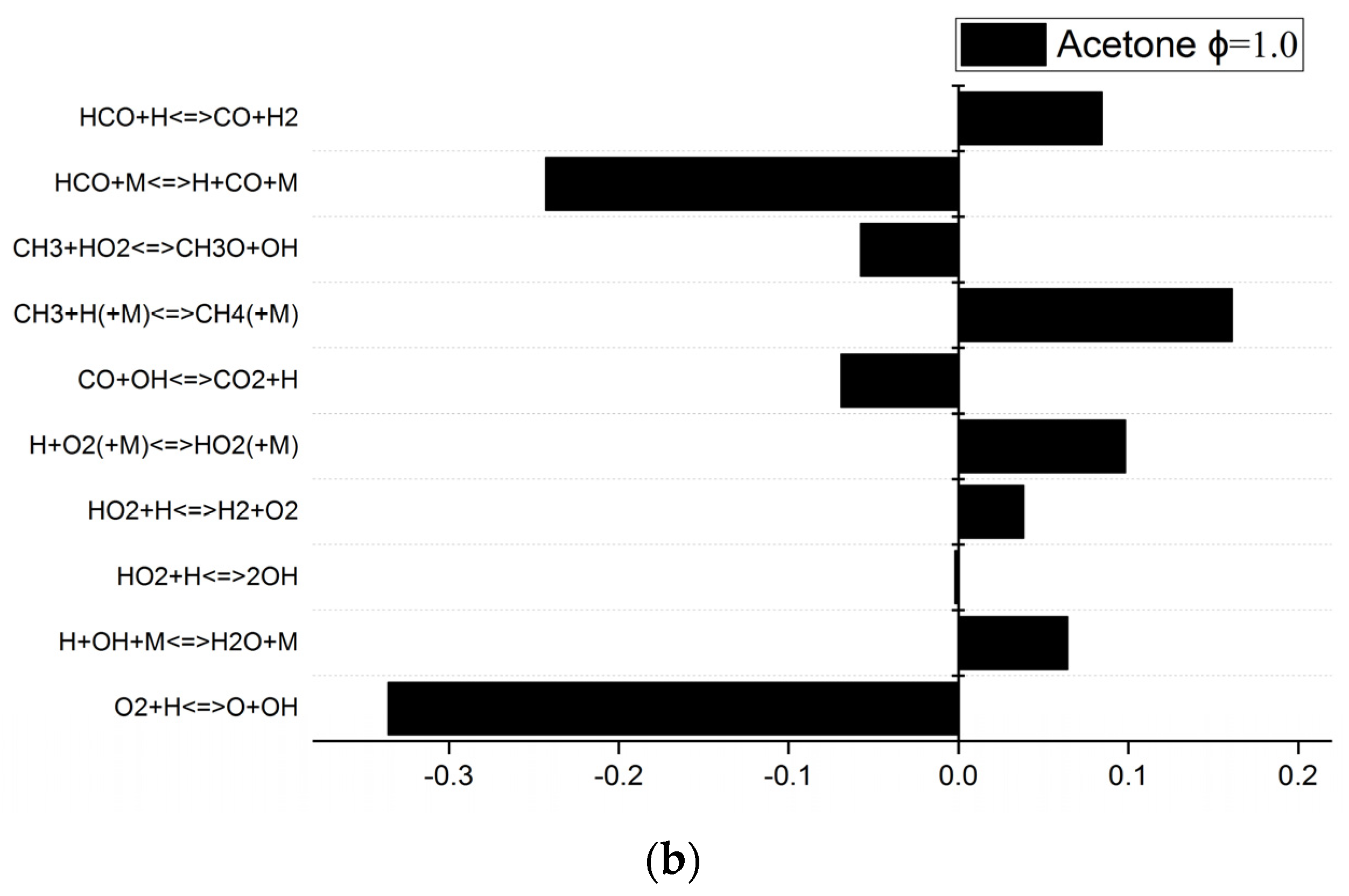
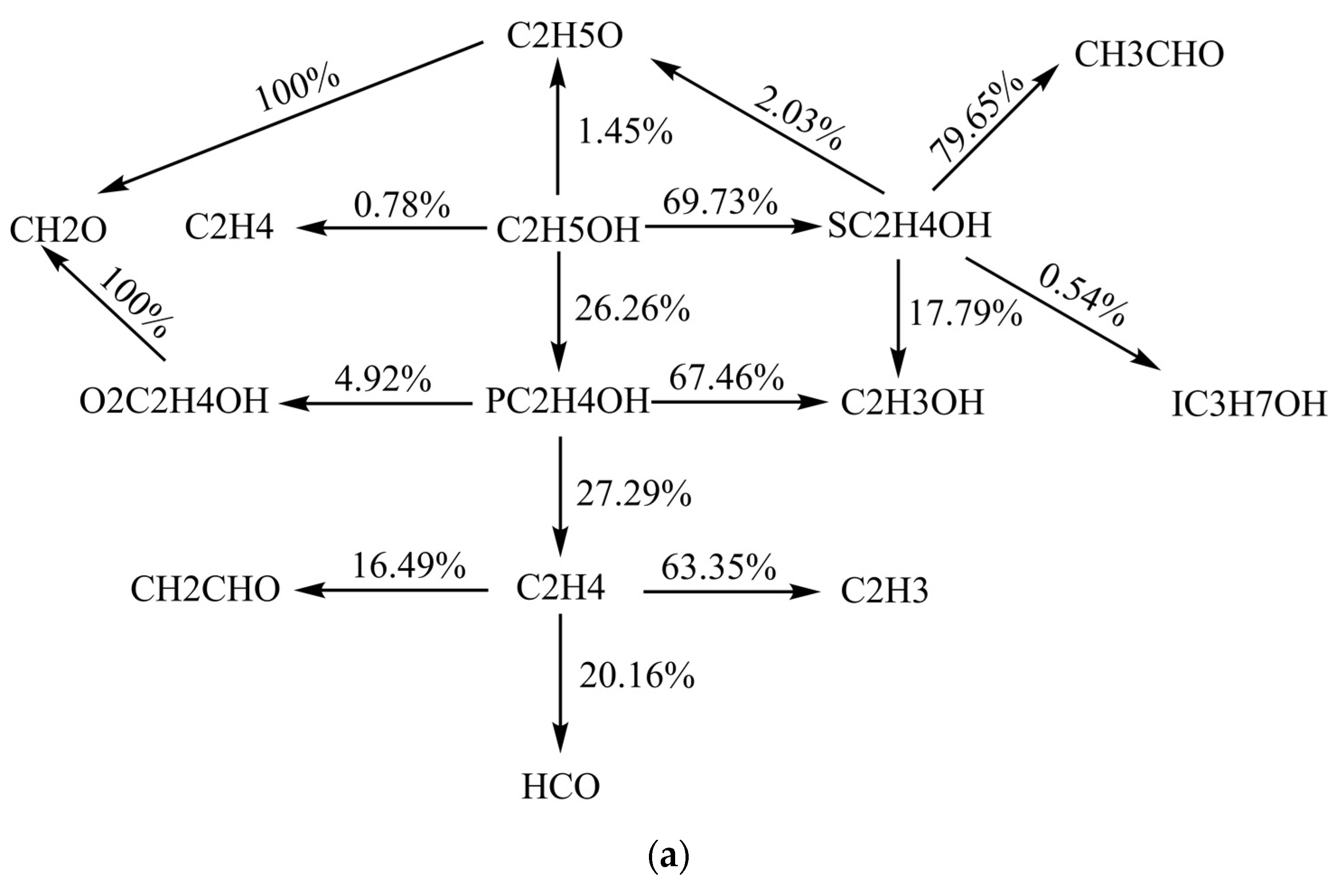
3.4. Sensitivity Analysis and Reaction Path
4. Conclusions
- Compared with pure fuel, the laminar burning velocity of mixed fuel is lower than that of ethanol and higher than the laminar burning velocity of acetone. The laminar burning velocity increases with increasing ethanol content.
- Through a three-step reduction process, the best simplification method is selected for each step and species, and the number of reactions of the detailed mechanism have been reduced by 90%.
- Multiple reduction methods were combined to alternately simplify the detailed mechanisms to skeletal mechanisms that are suitable for different mixed fuels. The calculation results of the skeletal mechanisms and the experimental results have similar trends.
- C2H2+H(+M)<=>C2H3(+M) has a clear consumption effect on ethanol and acetone of the mixtures, which means it leads to advancing effects on the laminar burning velocities.
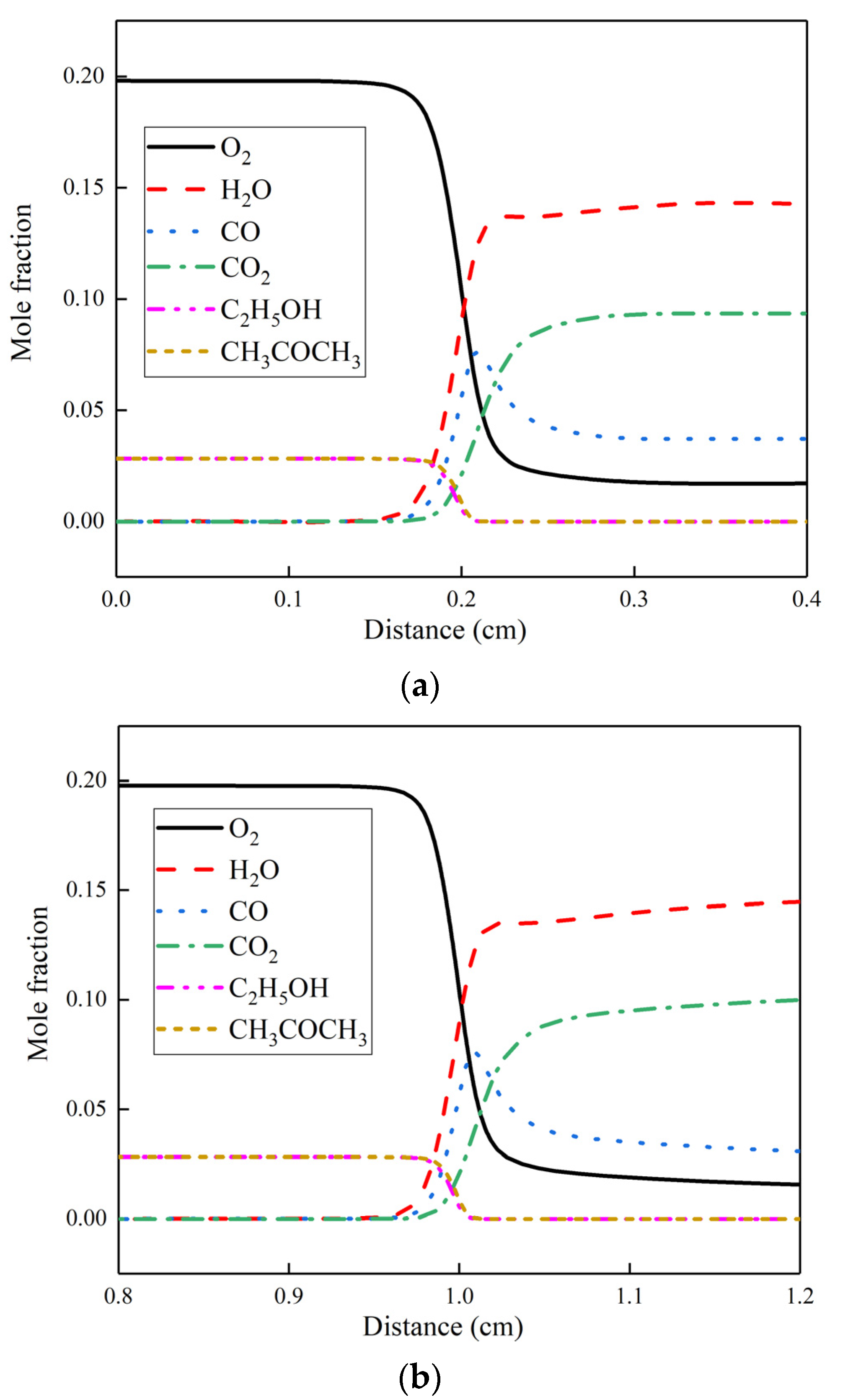
- The flame structure of the skeletal mechanism did not change significantly, and the concentration of each species remained basically the same value after the reaction.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Nomenclature
| CVCC | Constant volume combustion chamber |
| DRG | Directed relation graph |
| DRGEP | DRG with error propagation |
| SA | Sensitivity analysis |
| FSSA | Full species sensitivity analysis |
| HF | Heat flux |
| SF | Stagnation flame |
| BF | Bunsen flame |
| Markstein length | |
| Density of unburned gas | |
| R | Uncertainty of laminar burning velocity |
| Uncertainty of laminar burning velocity caused by radiation | |
| p | Pressure of bomb |
| SM | Skeletal mechanism |
| ETAC11 | 50% vol. ethanol/50% vol. acetone |
| ETAC31 | 75% vol. ethanol/25% vol. acetone |
| MBMS | Molecular beam mass spectrometry |
| Radius of the flame | |
| Flame surface-surrounded pixels | |
| Total pixel points contained in the window | |
| Actual radius of the window | |
| Stretched laminar flame speed | |
| Flame stretch rate | |
| Unstretched laminar flame speed | |
| Density of burned gas | |
| Laminar burning velocity | |
| Standard deviations of experiment | |
| T | Temperature of bomb |
| DM | Detailed mechanism |
| ET | 100% vol. ethanol |
| ETAC13 | 25% vol. ethanol/75% vol. acetone |
| AC | 100% vol. acetone |
References
- Kontses, A.; Triantafyllopoulos, G.; Ntziachristos, L.; Samaras, Z. Particle number (PN) emissions from gasoline, diesel, L.P.G., C.N.G and hybrid-electric light-duty vehicles under real-world driving conditions. Atmos. Environ. 2020, 222, 117126. [Google Scholar] [CrossRef]
- Gao, D.; Li, Z.; Liu, P.; Zhao, J.; Zhang, Y.; Li, C. A coordinated energy security model taking strategic petroleum reserve and al-ternative fuels into consideration. Energy 2018, 145, 171–181. [Google Scholar] [CrossRef]
- Kroyan, Y.; Wojcieszyk, M.; Kaario, O.; Larmi, M.; Zenger, K. Modeling the end-use performance of alternative fuels in light-duty vehicles. Energy 2020, 205, 117854. [Google Scholar] [CrossRef]
- Asad, U.; Kumar, R.; Zheng, M.; Tjong, J. Ethanol-fueled low temperature combustion: A pathway to clean and efficient diesel engine cycles. Appl. Energy 2015, 157, 838–850. [Google Scholar] [CrossRef]
- Thangavelu, S.K.; Ahmed, A.S.; Ani, F.N. Review on bioethanol as alternative fuel for spark ignition engines. Renew. Sustain. Energy Rev. 2016, 56, 820–835. [Google Scholar] [CrossRef]
- Compagnoni, M.; Mostafavi, E.; Tripodi, A.; Mahinpey, N.; Rossetti, I. Techno-economic Analysis of a Bioethanol to Hydrogen Centralized Plant. Energy Fuels 2017, 31, 12988–12996. [Google Scholar] [CrossRef]
- Matzen, M.; Demirel, Y. Methanol and dimethyl ether from renewable hydrogen and carbon dioxide: Alternative fuels pro-duction and life-cycle assessment. J. Clean. Prod. 2016, 139, 1068–1077. [Google Scholar] [CrossRef] [Green Version]
- Wei, H.; Yao, C.; Pan, W.; Han, G.; Dou, Z.; Wu, T.; Liu, M.; Wang, B.; Gao, J.; Chen, C.; et al. Experimental investigations of the effects of pilot injection on combustion and gaseous emission characteristics of diesel/methanol dual fuel engine. Fuel 2017, 188, 427–441. [Google Scholar] [CrossRef]
- Anderson, J.E.; Kramer, U.; Mueller, S.A.; Wallington, T.J. Octane Numbers of Ethanol− and Methanol−Gasoline Blends Estimated from Molar Concentrations. Energy Fuels 2010, 24, 6576–6585. [Google Scholar] [CrossRef]
- Vancoillie, J.; Christensen, M.; Nilsson, E.J.K.; Verhelst, S.; Konnov, A.A. Temperature Dependence of the Laminar Burning Veloc-ity of Methanol Flames. Energy Fuels 2012, 26, 1557–1564. [Google Scholar] [CrossRef] [Green Version]
- Park, S.H.; Lee, C.S. Applicability of dimethyl ether (DME) in a compression ignition engine as an alternative fuel. Energy Convers. Manag. 2014, 86, 848–863. [Google Scholar] [CrossRef]
- Wu, H.; Nithyanandan, K.; Zhang, J.; Lin, Y.; Lee, T.H.; Chia-fon, F.L.; Zhang, C. Impacts of Acetone–Butanol–Ethanol (ABE) ratio on spray and combustion characteristics of ABE–diesel blends. Appl. Energy 2015, 149, 367–378. [Google Scholar] [CrossRef]
- Wu, H.; Nithyanandan, K.; Zhou, N.; Lee, T.H.; Lee, C.-F.F.; Zhang, C. Impacts of acetone on the spray combustion of Acetone–Butanol–Ethanol (ABE)-Diesel blends under low ambient temperature. Fuel 2015, 142, 109–116. [Google Scholar] [CrossRef]
- Zhao, Z.; Wu, H.; Wang, M.; Lee, C.-F.; Liu, J.; Fu, J.; Chang, W. Computational Investigation of Oxygen Concentration Effects on a Soot Mechanism with a Phenomenological Soot Model of Acetone–Butanol–Ethanol (ABE). Energy Fuels 2015, 29, 1710–1721. [Google Scholar] [CrossRef]
- Li, Y.; Ning, Z.; Chia-fon, F.L.; Yan, J.; Lee, T.H. Effect of acetone-butanol-ethanol (ABE)–gasoline blends on regulated and unregulat-ed emissions in spark-ignition engine. Energy 2019, 168, 1157–1167. [Google Scholar] [CrossRef]
- Bhowmik, S.; Paul, A.; Panua, R.; Ghosh, S.K. Performance, combustion and emission characteristics of a diesel engine fueled with diesel-kerosene-ethanol: A multi-objective optimization study. Energy 2020, 211, 118305. [Google Scholar] [CrossRef]
- Bradley, D.; Lawes, M.; Mansour, M. Explosion bomb measurements of ethanol–air laminar gaseous flame characteristics at pressures up to 1.4MPa. Combust. Flame 2009, 156, 1462–1470. [Google Scholar] [CrossRef] [Green Version]
- Broustail, G.; Seers, P.; Halter, F.; Moréac, G.; Mounaim-Rousselle, C. Experimental determination of laminar burning velocity for butanol and ethanol iso-octane blends. Fuel 2011, 90, 1–6. [Google Scholar] [CrossRef]
- Broustail, G.; Halter, F.; Seers, P.; Moréac, G.; Mounaïm-Rousselle, C. Experimental determination of laminar burning velocity for butanol/iso-octane and ethanol/iso-octane blends for different initial pressures. Fuel 2013, 106, 310–317. [Google Scholar] [CrossRef]
- Dirrenberger, P.; Glaude, P.; Bounaceur, R.; Le Gall, H.; da Cruz, A.P.; Konnov, A.; Battin-Leclerc, F. Laminar burning velocity of gasolines with addition of ethanol. Fuel 2014, 115, 162–169. [Google Scholar] [CrossRef] [Green Version]
- Varea, E.; Modica, V.; Renou, B.; Boukhalfa, A.M. Pressure effects on laminar burning velocities and Markstein lengths for Iso-octane–Ethanol–Air mixtures. Proc. Combust. Inst. 2013, 34, 735–744. [Google Scholar] [CrossRef]
- Nilsson, E.; de Goey, L.; Konnov, A. Laminar burning velocities of acetone in air at room and elevated temperatures. Fuel 2013, 105, 496–502. [Google Scholar] [CrossRef]
- Burluka, A.A.; Harker, M.; Osman, H.; Sheppard, C.G.W.; Konnov, A.A. Laminar burning velocities of three C3H6O isomers at at-mospheric pressure. Fuel 2010, 89, 2864–2872. [Google Scholar] [CrossRef]
- Pichon, S.; Black, G.; Chaumeix, N.; Yahyaoui, M.; Simmie, J.; Curran, H.; Donohue, R. The combustion chemistry of a fuel tracer: Meas-ured flame speeds and ignition delays and a detailed chemical kinetic model for the oxidation of acetone. Combust. Flame 2009, 156, 494–504. [Google Scholar] [CrossRef]
- Xu, C.; Wang, H.; Oppong, F.; Li, X.; Zhou, K.; Zhou, W.; Wu, S.; Wang, C. Determination of laminar burning characteristics of a surrogate for a pyrolysis fuel using constant volume method. Energy 2020, 190, 116315. [Google Scholar] [CrossRef]
- Zhang, R.; Sun, W.; Tao, T.; Yang, B. Species diagnostics and modeling study of laminar premixed flames fueled by acetone–butanol–ethanol (ABE). Proc. Combust. Inst. 2017, 36, 1303–1310. [Google Scholar] [CrossRef]
- Bendu, H.; Deepak, B.; Murugan, S. Application of G.R.NN for the prediction of performance and exhaust emissions in HCCI engine using ethanol. Energy Convers. Manag. 2016, 122, 165–173. [Google Scholar] [CrossRef]
- Zhang, C.; Wu, H. Combustion characteristics and performance of a methanol fueled homogenous charge compression igni-tion (HCCI) engine. J. Energy Inst. 2016, 89, 346–353. [Google Scholar] [CrossRef]
- Homayoun, M.R.; Moradikazerouni, A. Vibro-acoustic Analysis of a Single Cylinder in Multi-stage Reciprocating Compres-sors. In Proceedings of the 3rd International Conference on Mechanical and Aerospace Engineering, Tehran, Iran, April 2018. [Google Scholar]
- Selle, L. Compressible large eddy simulation of turbulent combustion in complex geometry on unstructured meshes. Combust. Flame 2004, 137, 489–505. [Google Scholar] [CrossRef]
- Zhou, C.-W.; Li, Y.; Burke, U.; Banyon, C.; Somers, K.P.; Ding, S.; Khan, S.; Hargis, J.W.; Sikes, T.; Mathieu, O.; et al. An experimental and chemical kinetic modeling study of 1,3-butadiene combustion: Ignition delay time and laminar flame speed measurements. Combust. Flame 2018, 197, 423–438. [Google Scholar] [CrossRef]
- Sileghem, L.; Alekseev, V.A.; Vancoillie, J.; Nilsson, E.J.K.; Verhelst, S.; Konnov, A.A. Laminar burning velocities of primary ref-erence fuels and simple alcohols. Fuel 2014, 115, 32–40. [Google Scholar] [CrossRef]
- Rau, F.; Hartl, S.; Voss, S.; Still, M.; Hasse, C.; Trimis, D. Laminar burning velocity measurements using the Heat Flux method and numerical predictions of iso-octane/ethanol blends for different preheat temperatures. Fuel 2015, 140, 10–16. [Google Scholar] [CrossRef]
- Chong, C.T.; Hochgreb, S. Measurements of laminar flame speeds of acetone/methane/air mixtures. Combust. Flame 2011, 158, 490–500. [Google Scholar] [CrossRef]
- Gong, C.; Li, Z.; Li, D.; Liu, J.; Si, X.; Yu, J.; Huang, W.; Liu, F.; Han, Y. Numerical investigation of hydrogen addition effects on methanol-air mixtures combustion in premixed laminar flames under lean burn conditions. Renew. Energy 2018, 127, 56–63. [Google Scholar] [CrossRef]
- Wu, Y.; Modica, V.; Rossow, B.; Grisch, F. Effects of pressure and preheating temperature on the laminar flame speed of me-thane/air and acetone/air mixtures. Fuel 2016, 185, 577–588. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhu, S.; Liang, J.; Tian, L.; Li, G. Experimental and kinetic studies of premixed laminar flame of ace-tone-butanol-ethanol (ABE)/air. Fuel 2018, 211, 95–101. [Google Scholar] [CrossRef]
- Zhang, S.; Lee, T.H.; Wu, H.; Pei, J.; Wu, W.; Liu, F.; Zhang, C. Experimental and kinetic studies on laminar flame characteristics of ace-tone-butanol-ethanol (ABE) and toluene reference fuel (TRF) blends at atmospheric pressure. Fuel 2018, 232, 755–768. [Google Scholar] [CrossRef]
- Xu, C.; Wang, H.; Zhou, K.; Li, X.; Zhou, W.; Liu, W.; Wang, C. Laminar Burning Velocity of Premixed Ethanol–Air Mixtures with La-ser-Induced Spark Ignition Using the Constant-Volume Method. Energy Fuels 2019, 33, 7749–7758. [Google Scholar] [CrossRef]
- Xu, C.; Wu, S.; Oppong, F.; Xie, C.; Wei, L.; Zhou, J. Experimental and numerical studies of laminar flame characteristics of ethyl acetate with or without hydrogen addition. Int. J. Hydrog. Energy 2020, 45, 20391–20399. [Google Scholar] [CrossRef]
- Xu, C.; Zhong, A.; Li, X.; Wang, C.; Sahu, A.; Xu, H.; Lattimore, T.; Zhou, K.; Huang, Y. Laminar burning characteristics of upgraded biomass pyrolysis fuel de-rived from rice husk at elevated pressures and temperatures. Fuel 2017, 210, 249–261. [Google Scholar] [CrossRef]
- Xu, C.; Zhong, A.; Wang, H.; Jiang, C.; Sahu, A.; Zhou, W.; Wang, C. Laminar burning velocity of 2-methylfuran-air mixtures at ele-vated pressures and temperatures: Experimental and modeling studies. Fuel 2018, 231, 215–223. [Google Scholar] [CrossRef]
- Xu, C.; Wang, H.; Li, X.; Zhou, W.; Wang, C.; Wang, S. Explosion characteristics of a pyrolysis biofuel derived from rice husk. J. Hazard. Mater. 2019, 369, 324–333. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Han, W.; Santner, J.; Gou, X.; Sohn, C.H.; Ju, Y.; Chen, Z. Radiation-induced uncertainty in laminar flame speed measured from propagating spherical flames. Combust. Flame 2014, 161, 2815–2824. [Google Scholar] [CrossRef]
- Liao, S.; Jiang, D.; Huang, Z.; Zeng, K.; Cheng, Q. Determination of the laminar burning velocities for mixtures of ethanol and air at elevated temperatures. Appl. Therm. Eng. 2007, 27, 374–380. [Google Scholar] [CrossRef]
- Law, C.K. Combustion Physics; Cambridge University Press (CUP): Cambridge, UK, 2006. [Google Scholar]
- Van Geem, K.M.; Cuoci, A.; Frassoldati, A.; Pyl, S.P.; Marin, G.B.; Ranzi, E. An Experimental and Kinetic Modeling Study of Pyrolysis and Combustion of Acetone-Butanol-Ethanol (Abe) Mixtures. Combust. Sci. Technol. 2012, 184, 942–955. [Google Scholar] [CrossRef]
- Yan, B.; Cheng, Y.; Li, T.; Cheng, Y. Detailed kinetic modeling of acetylene decomposition/soot formation during quenching of coal pyrolysis in thermal plasma. Energy 2017, 121, 10–20. [Google Scholar] [CrossRef]
- Jiang, Y.; del Alamo, G.; Gruber, A.; Bothien, M.R.; Seshadri, K.; Williams, F.A. A skeletal mechanism for prediction of ignition delay times and laminar premixed flame velocities of hydrogen-methane mixtures under gas turbine conditions. Int. J. Hydrog. Energy 2019, 44, 18573–18585. [Google Scholar] [CrossRef]
- Di Sarli, V.; Di Benedetto, A. Laminar burning velocity of hydrogen-methane/air premixed flames. Int. J. Hydrog. Energy 2007, 32, 637–646. [Google Scholar] [CrossRef]
- Tsang, W. Energy transfer effects during the multichannel decomposition of ethanol. Int. J. Chem. Kinet. 2004, 36, 456–465. [Google Scholar] [CrossRef]
- Tran, L.-S.; Glaude, P.-A.; Fournet, R.; Battin-Leclerc, F. Experimental and Modeling Study of Premixed Laminar Flames of Ethanol and Methane. Energy Fuels 2013, 27, 2226–2245. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.; Chen, Y.; Wu, G. Skeletal Mechanism Generation and Validation for Acetone–n-butanol–ethanol (ABE) Combustion in Diesel Engine. Energy Fuels 2020, 34, 965–980. [Google Scholar] [CrossRef]

| Researchers | Temperature | Pressure | Equivalence Ratio | Method | Fuel |
|---|---|---|---|---|---|
| Drenberger et al. [20] | 258, 358, 398 K | 1 atm | 0.5–1.6 | HF | ethanol |
| Sileghem et al. [32] | 298–358 K | 1 atm | 0.7–1.5 | HF | ethanol |
| Rau et al. [33] | 298, 323, 348, 373 K | 1 atm | 0.7–1.4 | HF | ethanol |
| Broustail et al. [19] | 423 K | 0.1–1.0 MPa | 0.7–1.4 | CVCC | ethanol |
| Chong et al. [34] | 298 K | 1 atm | 0.6–1.4 | SF | acetone |
| Nilsson et al. [22] | 298, 318, 338, 358 K | 1 atm | 0.6–1.4 | HF | acetone |
| Gong et al. [35] | 343, 393 K | 1 atm | 0.7–1.6 | CVCC | acetone |
| Wu et al. [36] | 375–523 K | 0.1–1.0 MPa | 0.6–1.3 | BF | acetone |
| Zhang et al. [26] | 363 K | 30 Torr | 1.0 | MBMS | ABE |
| Zhang et al. [37] | 358, 393, 428 K | 0.1, 0.2, 0.4 MPa | 0.7–1.5 | CVCC | ABE |
| Zhang et al. [38] | 400 K | 0.1 MPa | 0.6–1.6 | CVCC | ABE |
| Step | ET | ETAC31 | ETAC11 | ETAC13 | AC |
|---|---|---|---|---|---|
| 1 | DRG 111/740 | DRGEP 87/493 | DRGEP 123/854 | DRGEP 117/759 | DRGEP 119/825 |
| 2 | DRGEPSA 64/383 | DRGSA 82/468 | DRGSA 65/385 | DRGSA 70/387 | DRGSA 94/618 |
| 3 | FSSA 44/270 | FSSA 66/398 | FSSA 54/327 | FSSA 65/330 | FSSA 47/274 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Liu, W.; Liao, H.; Zhou, W.; Xu, C. An Experimental and Kinetic Modelling Study on Laminar Premixed Flame Characteristics of Ethanol/Acetone Mixtures. Energies 2021, 14, 6713. https://doi.org/10.3390/en14206713
Liu Y, Liu W, Liao H, Zhou W, Xu C. An Experimental and Kinetic Modelling Study on Laminar Premixed Flame Characteristics of Ethanol/Acetone Mixtures. Energies. 2021; 14(20):6713. https://doi.org/10.3390/en14206713
Chicago/Turabian StyleLiu, Yangxun, Weinan Liu, Huihong Liao, Wenhua Zhou, and Cangsu Xu. 2021. "An Experimental and Kinetic Modelling Study on Laminar Premixed Flame Characteristics of Ethanol/Acetone Mixtures" Energies 14, no. 20: 6713. https://doi.org/10.3390/en14206713
APA StyleLiu, Y., Liu, W., Liao, H., Zhou, W., & Xu, C. (2021). An Experimental and Kinetic Modelling Study on Laminar Premixed Flame Characteristics of Ethanol/Acetone Mixtures. Energies, 14(20), 6713. https://doi.org/10.3390/en14206713





