Experimental Research on Detonation Cell Size of a Purified Biogas-Oxygen Mixture
Abstract
:1. Introduction
2. Materials and Methods
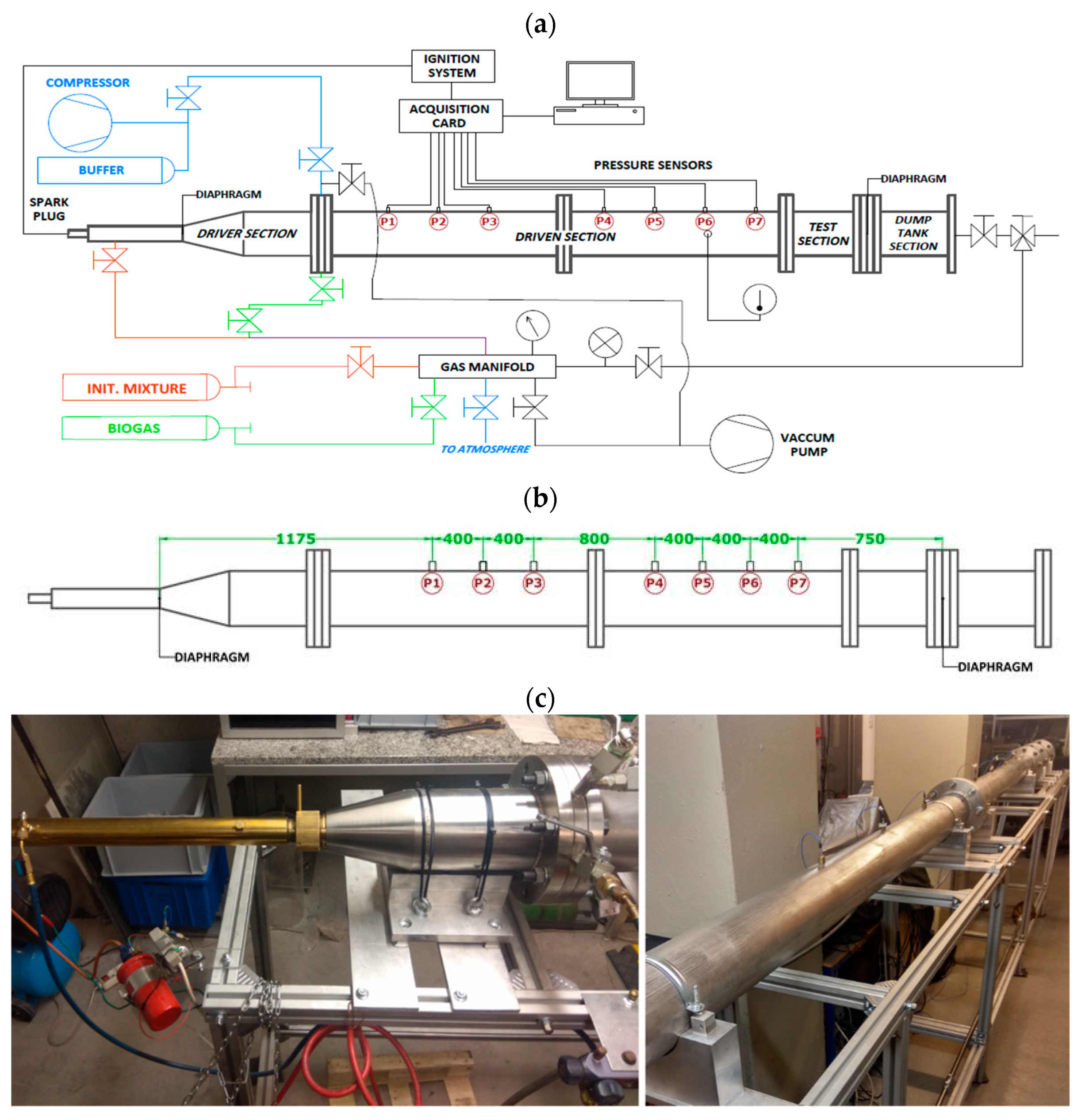
2.1. Experimental Setup
2.2. Experimental Uncertainty
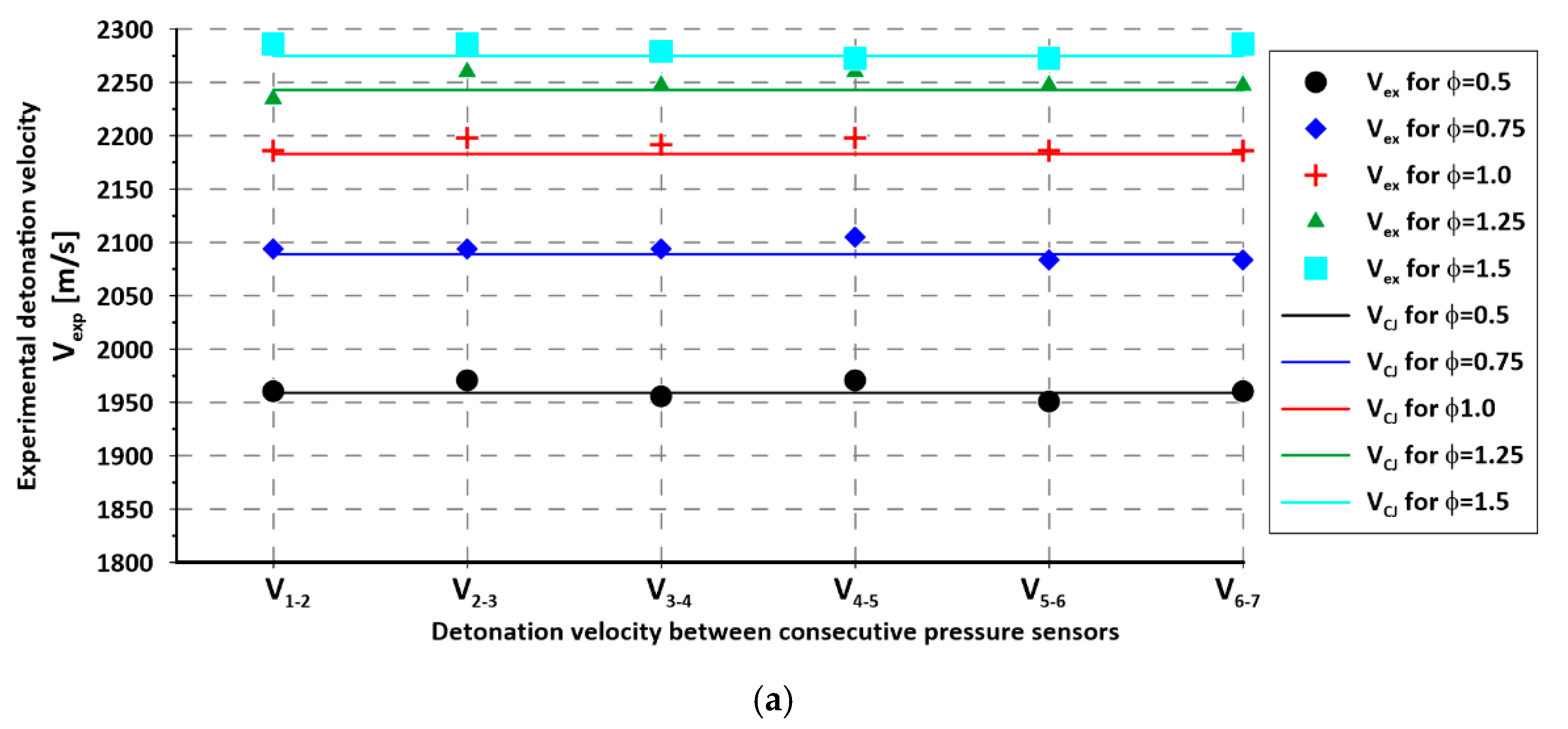
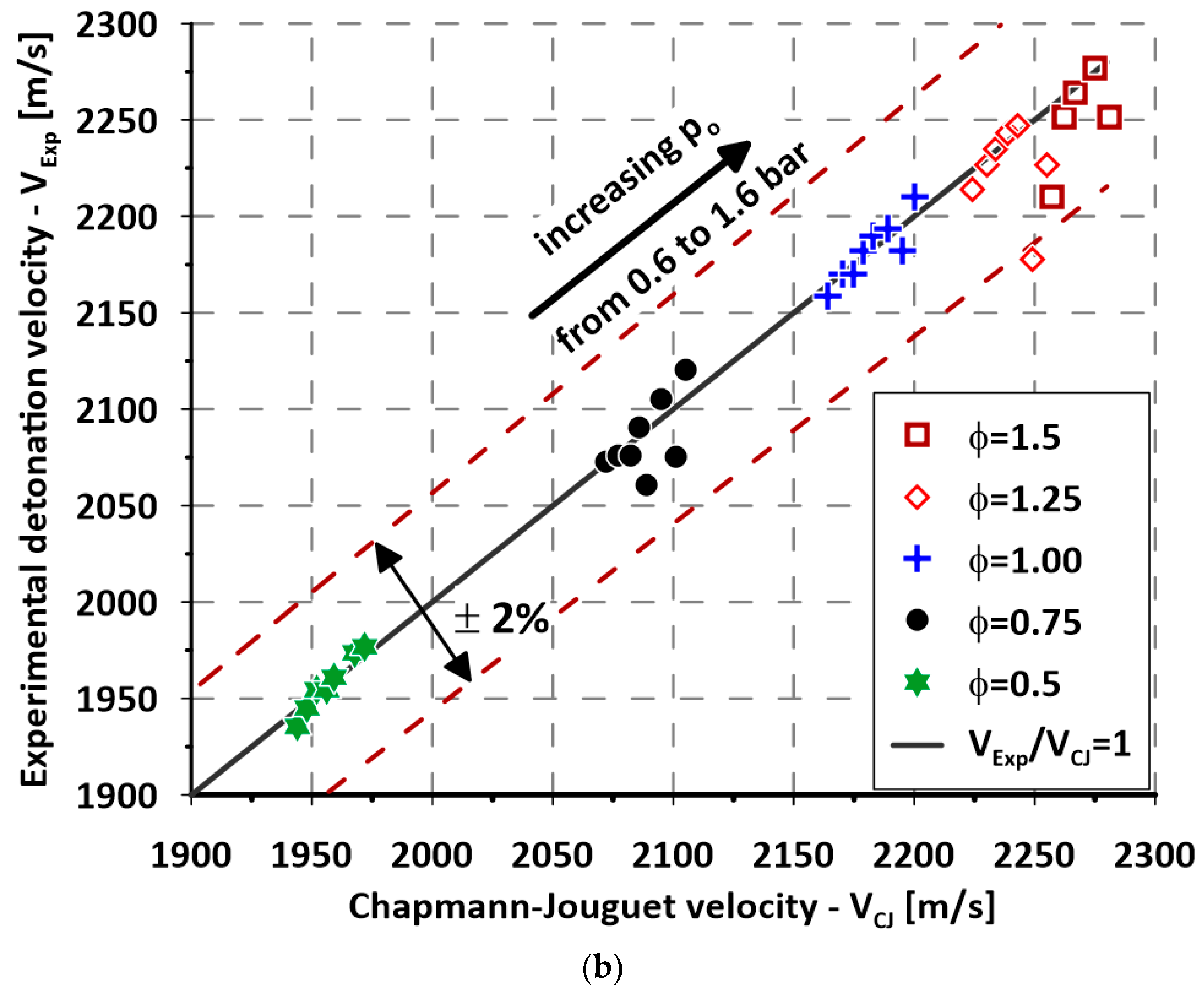
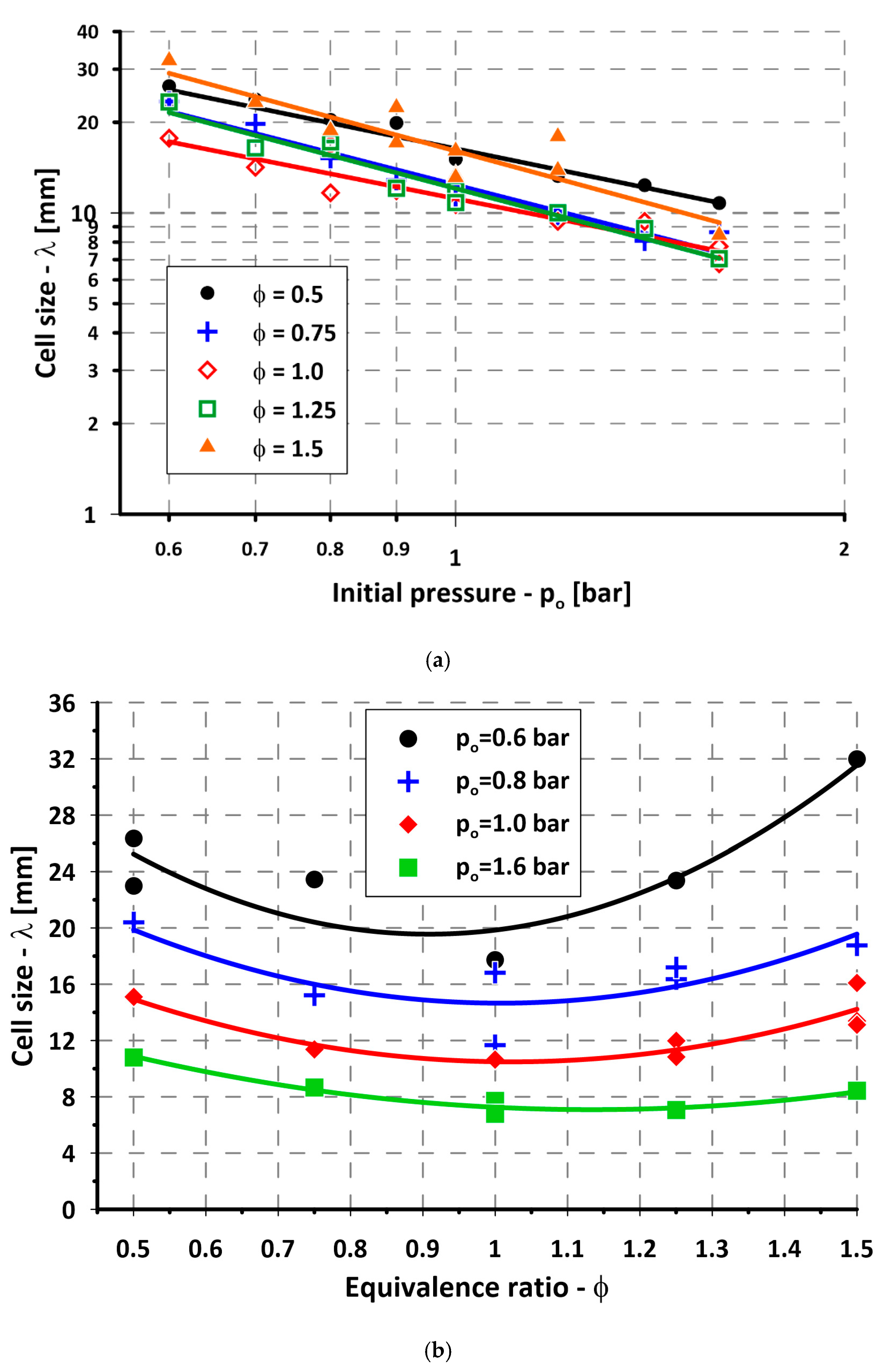
3. Experimental Results
4. Discussion
5. Summary and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Nomenclature
| p0 | initial pressure in the detonation tube [bar] |
| Φ | equivalence ratio [-] |
| λ | detonation cell size [mm] |
| CV | Coefficient of Variation |
| CJ | Chapman-Jouguet |
| FS | Full Scale |
| LHV | Lower Heating Value [MJ/Nm3] |
| PDE | Pulsed Detonation Engine |
| RDE | Rotating Detonation Engine |
| Std | Standard Deviation |
| SEM | Standard Error of Mean |
| Var | Variation |
| VExp | Experimental detonation velocity [m/s] |
| VCJ | Chapman-Jouguet detonation velocity [m/s] |
References
- Kyoto Protocol: A Guide and Assessment (Book)|ETDEWEB. Available online: https://www.osti.gov/etdeweb/biblio/20730601 (accessed on 14 September 2020).
- The Paris Agreement|UNFCCC. Available online: https://unfccc.int/process-and-meetings/the-paris-agreement/the-paris-agreement (accessed on 9 September 2020).
- United Nations Framework Convention on Climate Change Katowice Climate Package|UNFCCC. Available online: https://unfccc.int/process-and-meetings/the-paris-agreement/paris-agreement-work-programme/katowice-climate-package (accessed on 14 September 2020).
- Balta, M.O.; Eke, F. Spatial Reflection of Urban Planning in Metropolitan Areas and Urban Rent; a Case Study of Cayyolu, Ankara. Eur. Plan. Stud. 2011, 19, 1817–1838. [Google Scholar] [CrossRef]
- Benato, A.; Macor, A. Italian Biogas Plants: Trend, Subsidies, Cost, Biogas Composition and Engine Emissions. Energies 2019, 12, 979. [Google Scholar] [CrossRef] [Green Version]
- Gupta, K.K.; Rehman, A.; Sarviya, R.M. Bio-Fuels for the Gas Turbine: A Review. Renew. Sustain. Energy Rev. 2010, 14, 2946–2955. [Google Scholar] [CrossRef]
- Baccioli, A.; Antonelli, M.; Frigo, S.; Desideri, U.; Pasini, G. Small Scale Bio-LNG Plant: Comparison of Different Biogas Upgrading Techniques. Appl. Energy 2018, 217, 328–335. [Google Scholar] [CrossRef]
- Sun, Q.; Li, H.; Yan, J.; Liu, L.; Yu, Z.; Yu, X. Selection of Appropriate Biogas Upgrading Technology-a Review of Biogas Cleaning, Upgrading and Utilisation. Renew. Sustain. Energy Rev. 2015, 51, 521–532. [Google Scholar] [CrossRef]
- Shiratori, Y.; Oshima, T.; Sasaki, K. Feasibility of Direct-Biogas SOFC. Int. J. Hydrog. Energy 2008, 33, 6316–6321. [Google Scholar] [CrossRef]
- Rodrigues, M.; Walter, A.; Faaij, A. Co-Firing of Natural Gas and Biomass Gas in Biomass Integrated Gasification/Combined Cycle Systems. Energy 2003, 28, 1115–1131. [Google Scholar] [CrossRef]
- Kindracki, J. Badania Eksperymentalne i Symulacje Numeryczne Procesu Wirującej Detonacji Gazowej [Experimental Research and Numerical Calculation of the Rotating Detonatino]. Ph.D. Thesis, Warsaw University of Technology, Warszawa, Poland, 2008. (In Polish). [Google Scholar]
- Wolanski, P. Detonative Propulsion. Proc. Combust. Inst. 2013, 34, 125–158. [Google Scholar] [CrossRef]
- Xie, Q.; Wen, H.; Li, W.; Ji, Z.; Wang, B.; Wolanski, P. Analysis of Operating Diagram for H2/Air Rotating Detonation Combustors under Lean Fuel Condition. Energy 2018, 151, 408–419. [Google Scholar] [CrossRef]
- Kindracki, J.; Wolański, P.; Gut, Z. Experimental Research on the Rotating Detonation in Gaseous Fuels-Oxygen Mixtures. Shock Waves 2011, 21, 75–84. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Ma, H.; Shen, Z.; Xue, B.; Cheng, Y.; Fan, Z. Experimental Investigation of Methane-Oxygen Detonation Propagation in Tubes. Appl. Therm. Eng. 2017, 123, 1300–1307. [Google Scholar] [CrossRef]
- Lefebvre, A.H. Gas Turbine Combustion, 2nd ed.; CRC Press: Boca Raton, FL, USA, 1998; ISBN 978-1-56032-673-1. [Google Scholar]
- Schwer, D.A.; Kailasanath, K. Characterizing NOx Emissions for Air-Breathing Rotating Detonation Engines. In Proceedings of the 52nd AIAA/SAE/ASEE Joint Propulsion Conference, Salt Lake City, UT, USA, 25–27 July 2016; American Institute of Aeronautics and Astronautics: Reston, VA, USA. [Google Scholar]
- Shepherd, J.E. Detonation in Gases. Proc. Combust. Inst. 2009, 32, 83–98. [Google Scholar] [CrossRef]
- Bykovskii, F.A.; Zhdan, S.A.; Vedernikov, E.F. Continuous Spin Detonations. J. Propuls. Power 2006, 22, 1204–1216. [Google Scholar] [CrossRef]
- Kaneshige, M.; Shepherd, J.E.; Detonation Database. Technical Report FM97-8, GALCIT. July 1997. Available online: https://shepherd.caltech.edu/detn_db/html/db.html (accessed on 1 December 2019).
- Hu, X.Y.; Khoo, B.C.; Zhang, D.L.; Jiang, Z.L. The Cellular Structure of a Two-Dimensional H2/O2/Ar Detonation Wave. Combust. Theory Model. 2004, 8, 339–359. [Google Scholar] [CrossRef] [Green Version]
- Zhou, R.; Wu, D.; Wang, J. Progress of Continuously Rotating Detonation Engines. Chin. J. Aeronaut. 2016, 29, 15–29. [Google Scholar] [CrossRef] [Green Version]
- Kindracki, J.; Kobiera, A.; Wolanski, P.; Gut, Z.; Folusiak, M.; Swiderski, K. Experimental and Numerical Study of the Rotating Detonation Engine in Hydrogen-Air Mixtures. In Proceedings of the Progress in Propulsion Physics, Les Ulis, France, 1 October 2011; Volume 2, pp. 555–582. [Google Scholar]
- Kindracki, J.; Siatkowski, S.; Lukasik, B. Influence of Inlet Flow Parameters on Rotating Detonation. AIAA J. 2019, 1–6. [Google Scholar] [CrossRef]
- Wahid, M.A.; Ujir, H.; Saqr, K.M.; Sies, M.M. Experimental Study of Confined Biogas Pulse Detonation Combustion. In Proceedings of the 2nd International Meeting on Advances in Thermo-Fluids, Barcelona, Spain, 15–17 July 2009; pp. 71–77. [Google Scholar]
- Saqr, K.M.; Kassem, H.I.; Sies, M.M.; Wahid, M.A. Ideal Detonation Characteristics of Biogashydrogen and -Hydrogen Peroxide Mixtures. In Proceedings of the International Conference on Fluid Mechanics and Heat & Mass Transfer, Corfu Island, Greece, 22–24 July 2010; pp. 69–72. [Google Scholar]
- Dairobi, A.G.; Wahid, M.A.; Inuwa, I.M. Feasibility Study of Pulse Detonation Engine Fueled by Biogas. Available online: https://www.scientific.net/AMM.388.257 (accessed on 21 September 2020).
- Elhawary, S.; Saat, A.; Wahid, M.A.; Ghazali, A.D. Experimental Study of Using Biogas in Pulse Detonation Engine with Hydrogen Enrichment. Int. J. Hydrog. Energy 2020, 45, 15414–15424. [Google Scholar] [CrossRef]
- Tieszen, S.R.; Stamps, D.W.; Westbrook, C.K.; Pitz, W.J. Gaseous Hydrocarbonair Detonations. Combust. Flame 1991, 84, 376–390. [Google Scholar] [CrossRef]
- Ng, H.D. The Effect of Chemical Reaction Kinetics on the Structure of Gaseous Detonations; McGill University: Montréal, QC, Canada, 2005. [Google Scholar]
- Pintgen, F.; Austin, J.M.; Shepherd, J.E. Detonation Front Structure: Variety and Characterization. In Confined Detonations and Pulse Detonation Engines; TORUS PRESS Ltd.: Moscow, Russia, 2003; pp. 105–116. ISBN 5-94588-012-4. [Google Scholar]



| p0 [bar] | 0.6 | 0.7 | 0.8 | 0.9 | 1.0 | 1.2 | 1.4 | 1.6 | |
|---|---|---|---|---|---|---|---|---|---|
| Φ [-] | |||||||||
| 0.5 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |
| 0.75 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |
| 1.0 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |
| 1.25 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |
| 1.5 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |
| Φ | 0.5 | 0.75 | 1.0 | 1.25 | 1.5 |
| ΔΦ | ±0.01 | ±0.01 | ±0.02 | ±0.02 | ±0.03 |
| Figure 5a: λ = A + B ∗ ln(p0) | ||||
| Φ | A | B | R2 | |
| 0.50 | 2.794 | −0.881 | 0.96 | |
| 0.75 | 2.517 | −1.101 | 0.95 | |
| 1.00 | 2.412 | −0.852 | 0.89 | |
| 1.25 | 2.490 | −1.137 | 0.95 | |
| 1.50 | 2.776 | −1.164 | 0.79 | |
| Figure 5b: λ = A + B ∗ Φ + C ∗ Φ2 | ||||
| p0 [bar] | A | B | C | R2 |
| 0.6 | 47.750 | −62.099 | 34.199 | 0.82 |
| 0.8 | 35.212 | −40.801 | 20.248 | 0.62 |
| 1.0 | 27.560 | −33.417 | 16.351 | 0.78 |
| 1.6 | 19.251 | −21.461 | 9.466 | 0.95 |
| p0 [bar] | N | Min | Max | Mean | Median | Std | Var | SEM | CV |
|---|---|---|---|---|---|---|---|---|---|
| 0.6 | 89 | 13.17 | 33.41 | 23.38 | 23.13 | 4.95 | 24.53 | 0.53 | 0.21 |
| 0.7 | 201 | 10.43 | 24.14 | 16.43 | 16.31 | 3.47 | 12.02 | 0.24 | 0.21 |
| 0.8 | 119 | 11.43 | 21.87 | 16.82 | 17.07 | 2.45 | 6.00 | 0.22 | 0.15 |
| 0.9 | 104 | 8.26 | 18.62 | 12.06 | 11.70 | 2.63 | 6.93 | 0.26 | 0.22 |
| 1.0 | 160 | 7.35 | 17.10 | 11.26 | 11.19 | 2.22 | 4.91 | 0.18 | 0.20 |
| 1.2 | 445 | 6.30 | 14.35 | 10.03 | 9.94 | 2.02 | 4.07 | 0.10 | 0.20 |
| 1.4 | 321 | 6.12 | 12.08 | 8.87 | 8.77 | 1.43 | 2.03 | 0.08 | 0.16 |
| 1.6 | 369 | 4.63 | 10.20 | 7.05 | 6.89 | 1.40 | 1.95 | 0.07 | 0.20 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Siatkowski, S.; Wacko, K.; Kindracki, J. Experimental Research on Detonation Cell Size of a Purified Biogas-Oxygen Mixture. Energies 2021, 14, 6605. https://doi.org/10.3390/en14206605
Siatkowski S, Wacko K, Kindracki J. Experimental Research on Detonation Cell Size of a Purified Biogas-Oxygen Mixture. Energies. 2021; 14(20):6605. https://doi.org/10.3390/en14206605
Chicago/Turabian StyleSiatkowski, Stanislaw, Krzysztof Wacko, and Jan Kindracki. 2021. "Experimental Research on Detonation Cell Size of a Purified Biogas-Oxygen Mixture" Energies 14, no. 20: 6605. https://doi.org/10.3390/en14206605
APA StyleSiatkowski, S., Wacko, K., & Kindracki, J. (2021). Experimental Research on Detonation Cell Size of a Purified Biogas-Oxygen Mixture. Energies, 14(20), 6605. https://doi.org/10.3390/en14206605








