Combustion of Fuel Surrogates: An Application to Gas Turbine Engines
Abstract
:1. Introduction
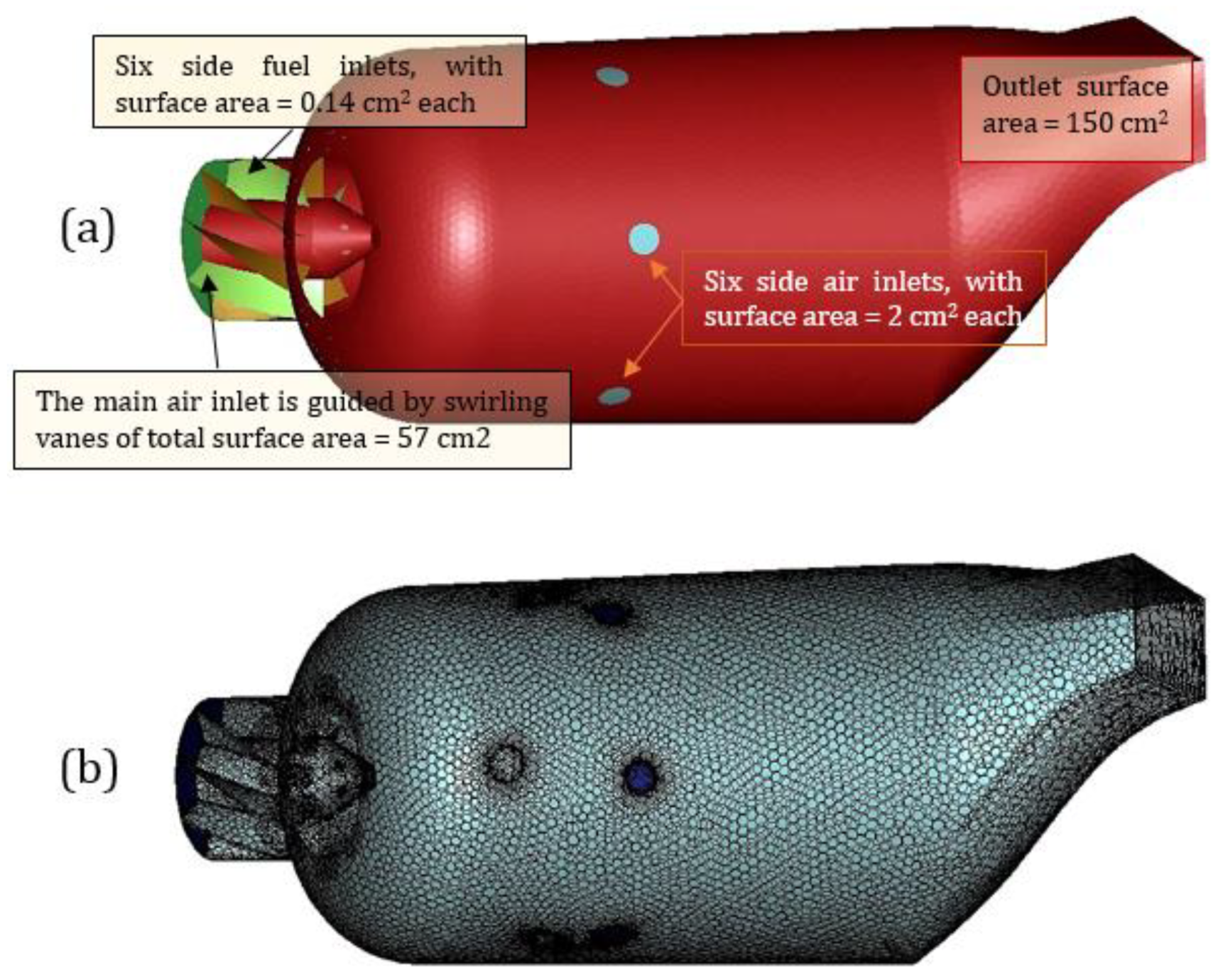
2. Method
3. Fuel Composition
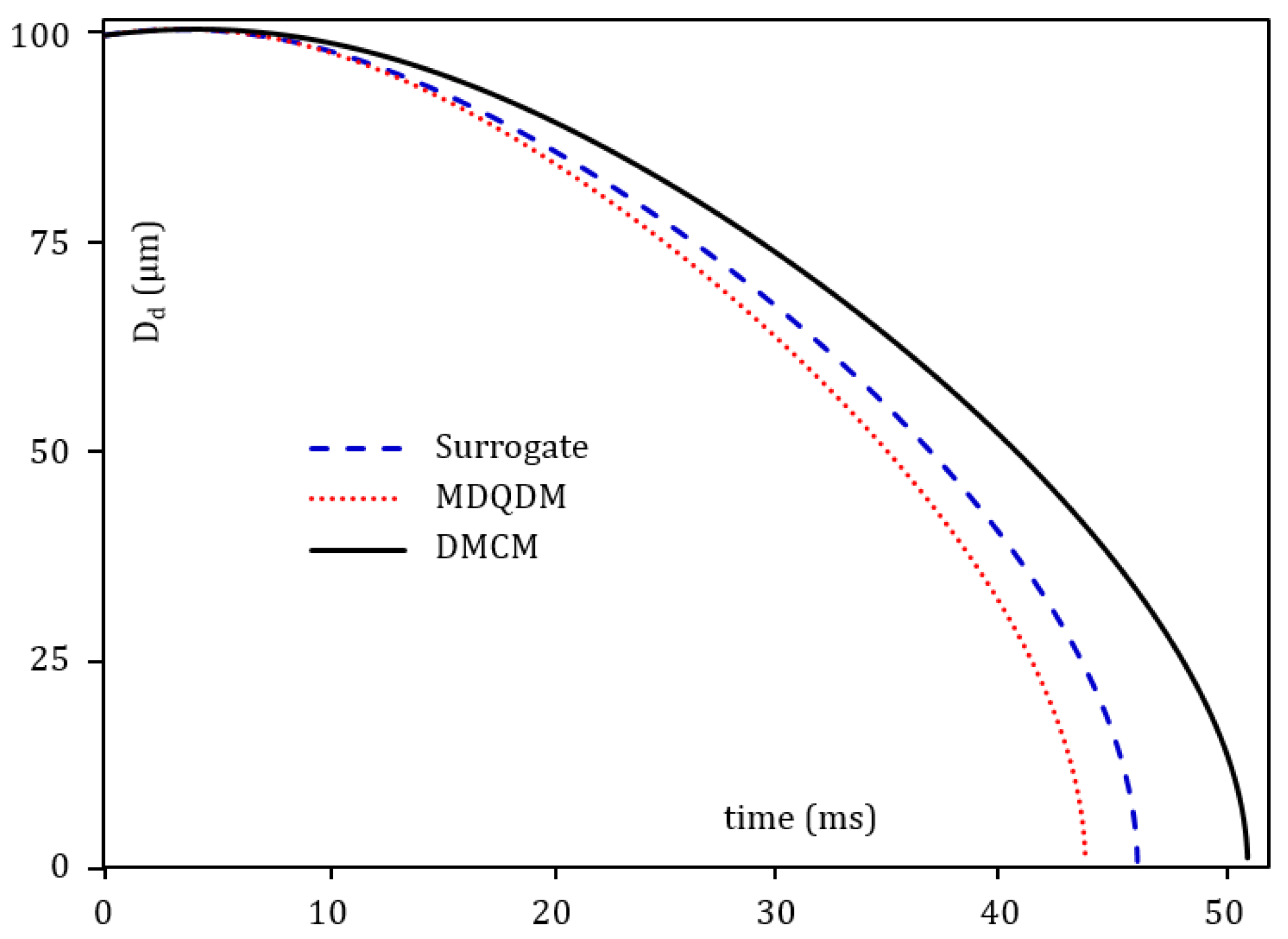
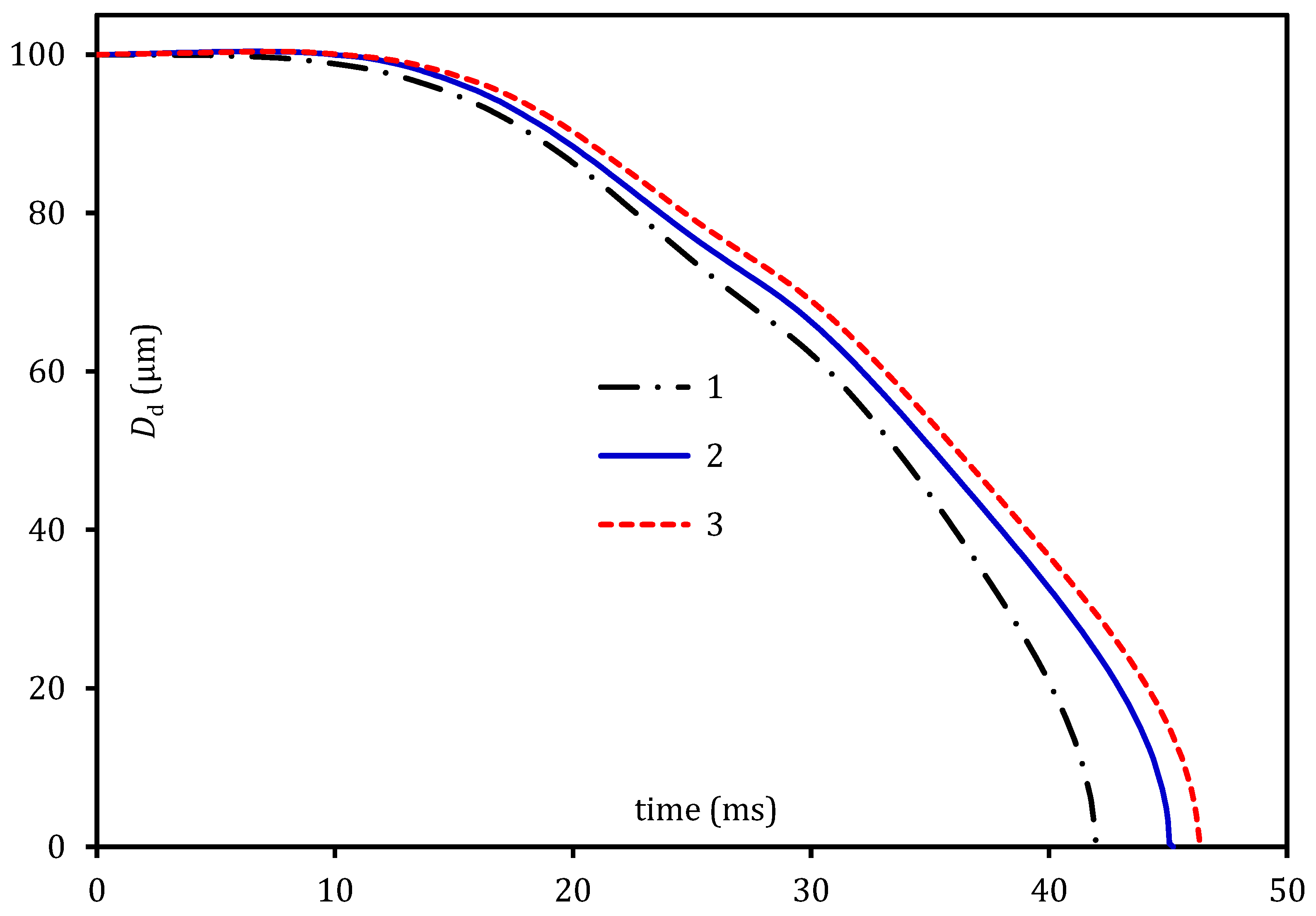
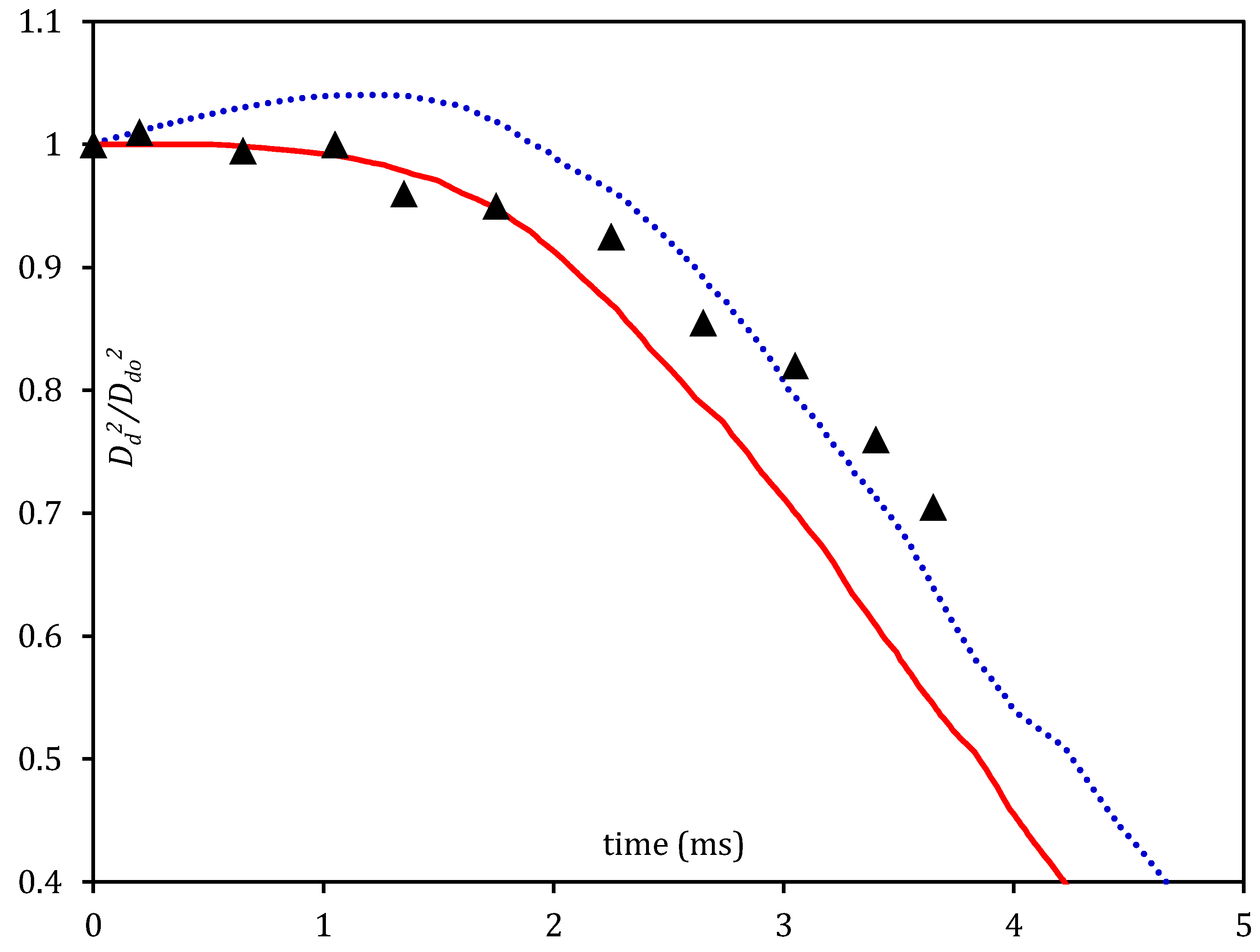
4. Pre-Combustion Analysis
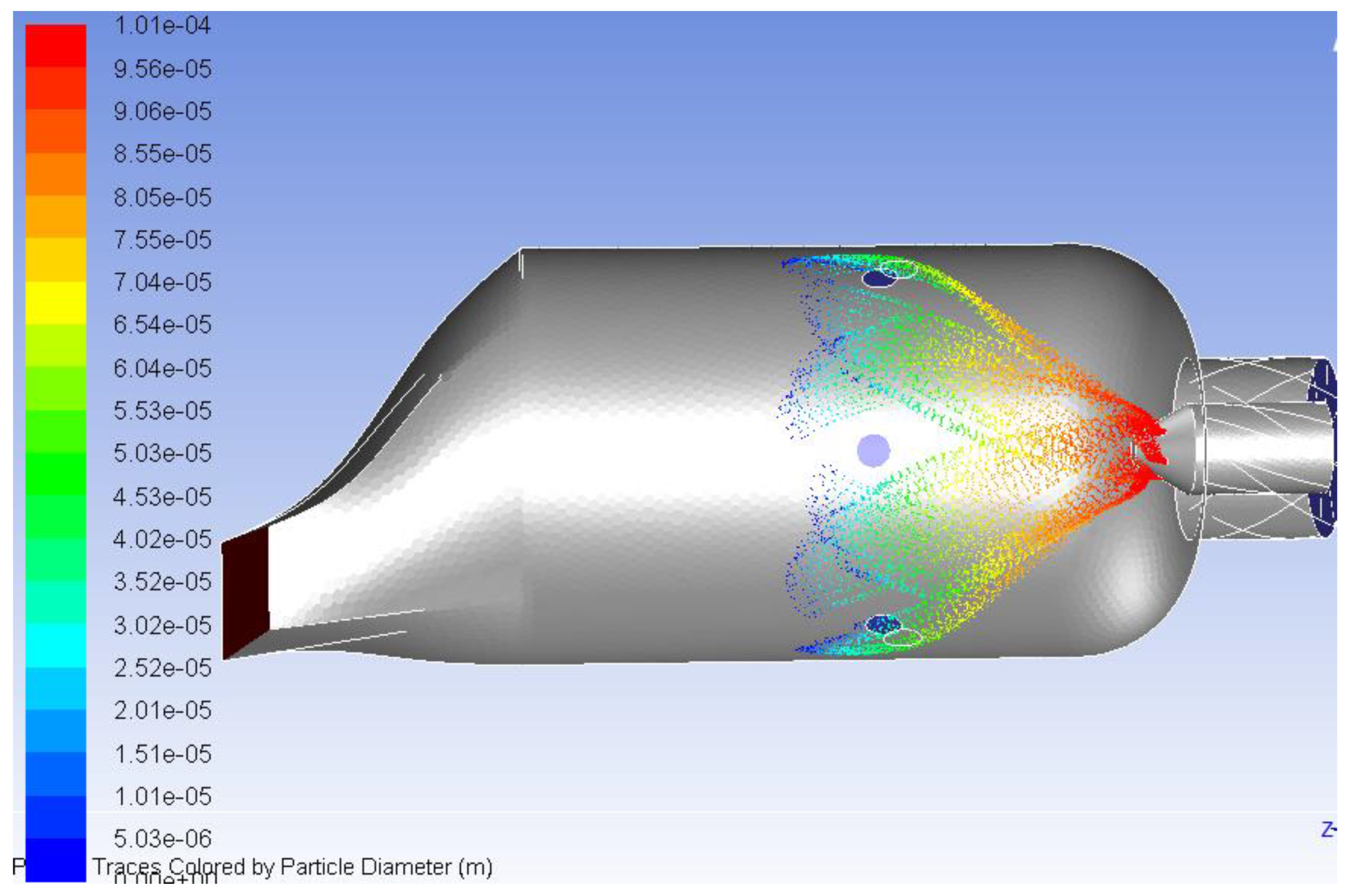
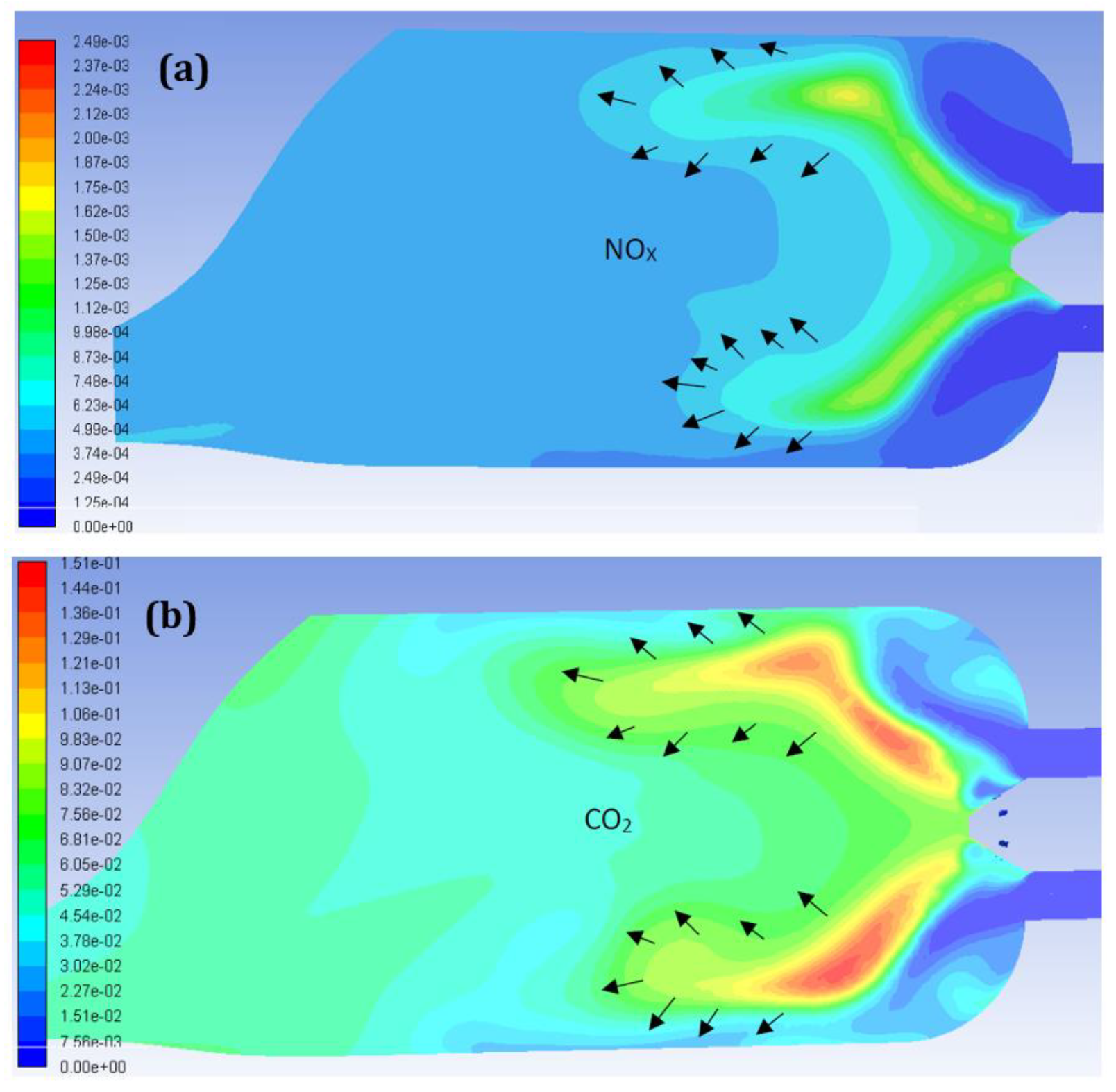
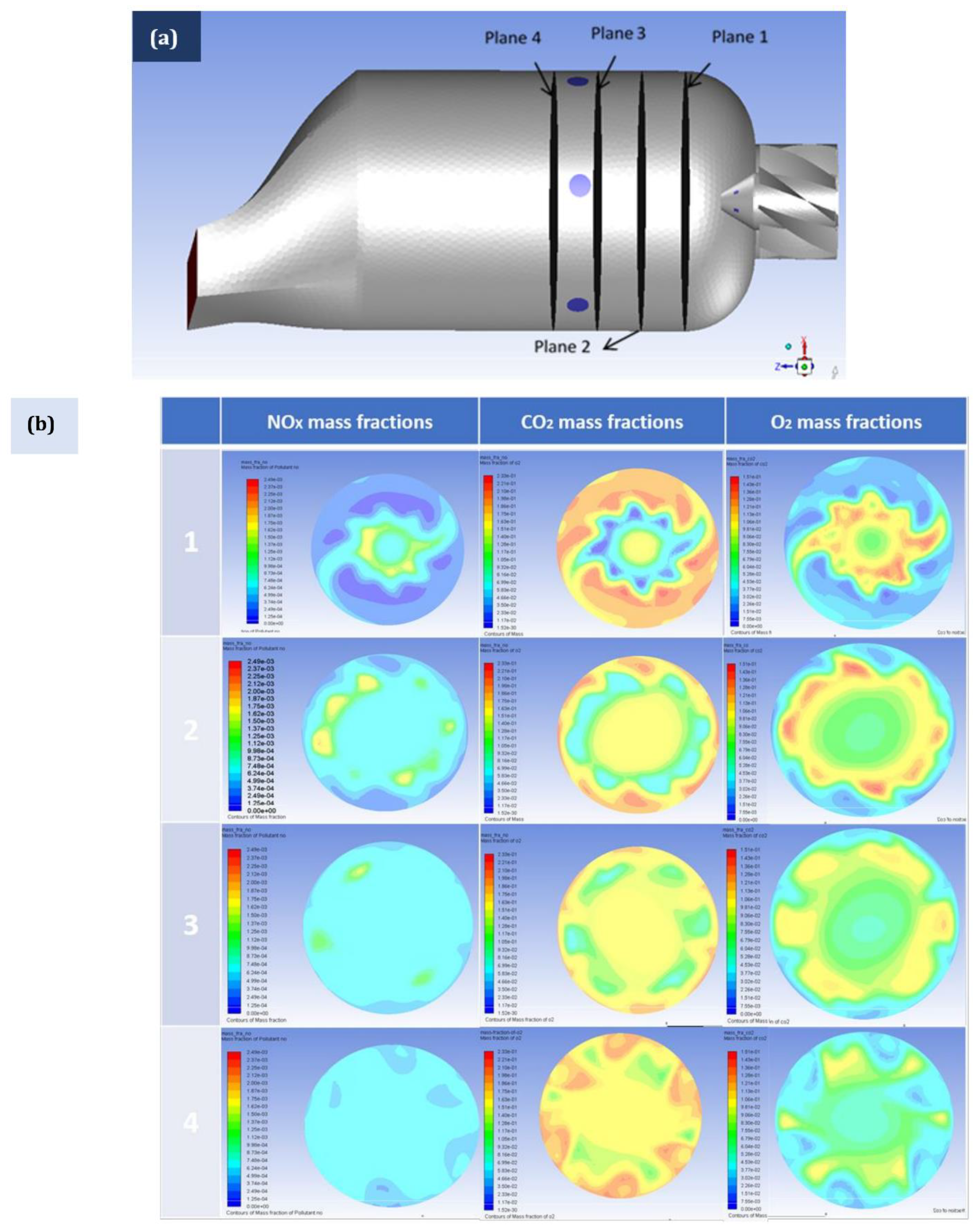
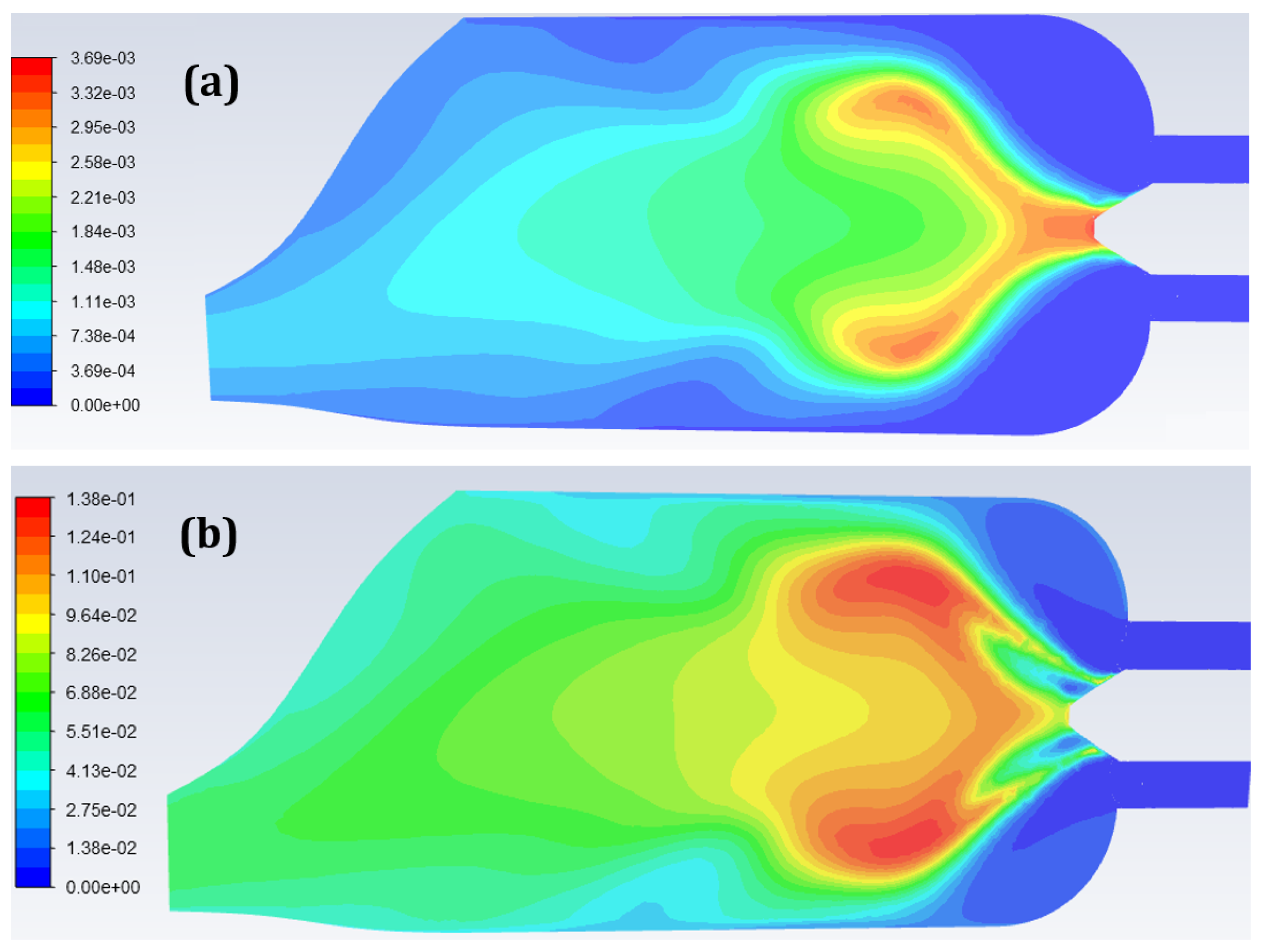
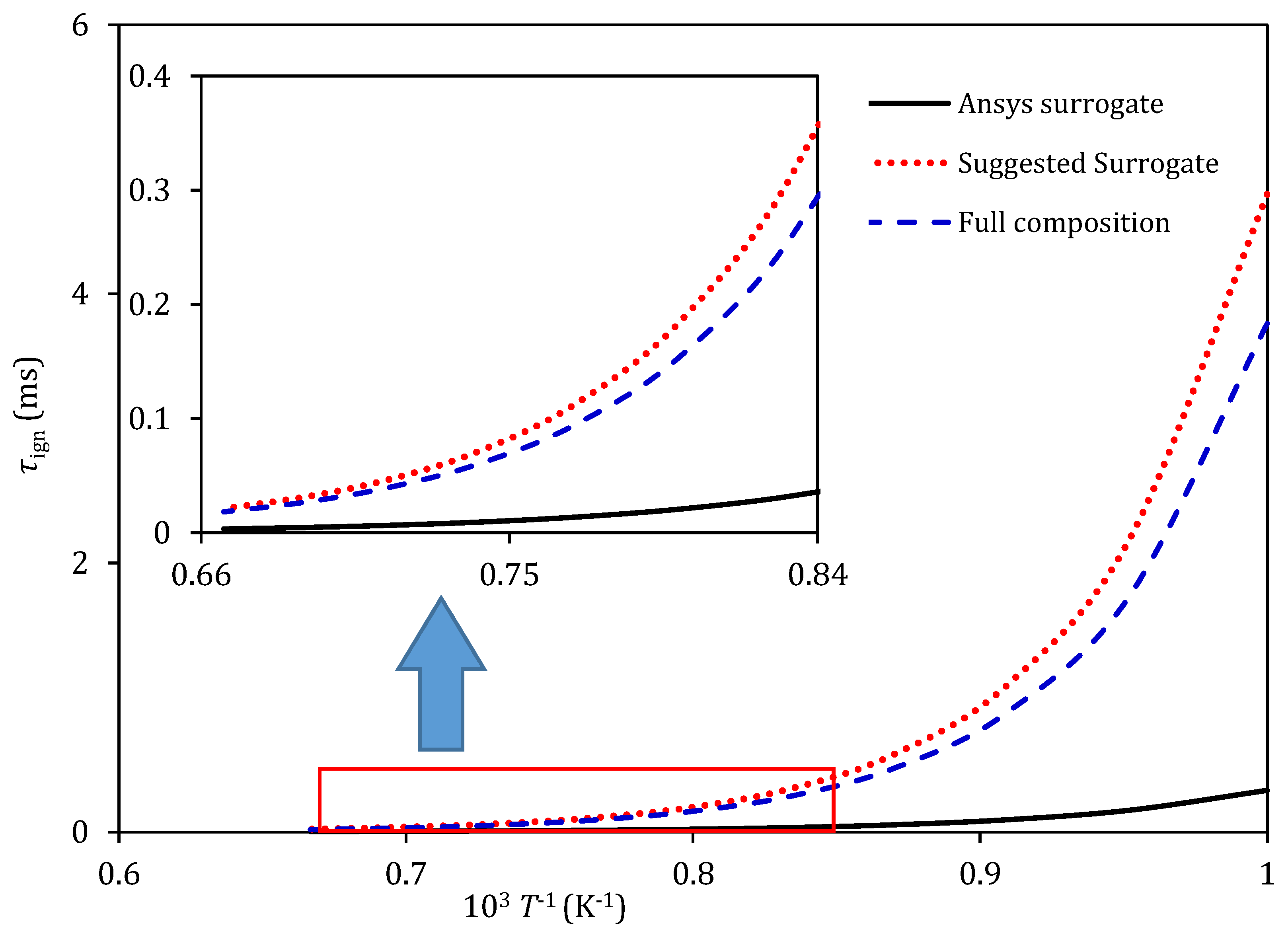
5. Combustion Analysis
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kalghatgi, G. Is it really the end of internal combustion engines and petroleum in transport? Appl. Energy 2018, 225, 965–974. [Google Scholar] [CrossRef]
- Al Qubeissi, M.; El-kharouf, A.; Serhad Soyhan, H. Renewable Energy—Resources, Challenges and Applications; IntechOpen: London, UK, 2020; ISBN 978-1-78984-283-8. [Google Scholar]
- Kalghatgi, G.; Levinsky, H.; Colket, M. Future transportation fuels. Prog. Energy Combust. Sci. 2018, 69, 103–105. [Google Scholar] [CrossRef]
- Ali, O.; Mamat, R.; Najafi, G.; Yusaf, T.; Safieddin Ardebili, S. Optimization of biodiesel-diesel blended fuel properties and engine performance with ether additive using statistical analysis and response surface methods. Energies 2015, 8, 14136–14150. [Google Scholar] [CrossRef] [Green Version]
- Al Qubeissi, M. Biofuels—Challenges and Opportunities; IntechOpen: London, UK, 2019; ISBN 978-1-78985-535-7. [Google Scholar]
- Qasim, M.; Ansari, T.M.; Hussain, M. Combustion, performance, and emission evaluation of a diesel engine with biodiesel like fuel blends derived from a mixture of Pakistani waste canola and waste transformer oils. Energies 2017, 10, 1023. [Google Scholar] [CrossRef] [Green Version]
- US Environmental Protection Agency. Available online: http://www.epa.gov/ (accessed on 15 November 2017).
- Masum, B.M.; Masjuki, H.H.; Kalam, M.A.; Rizwanul Fattah, I.M.; Palash, S.M.; Abedin, M.J. Effect of ethanol–Gasoline blend on NOx emission in SI engine. Renew. Sustain. Energy Rev. 2013, 24, 209–222. [Google Scholar] [CrossRef]
- Pure European Renewavle Ethanol. Available online: https://epure.org/ (accessed on 5 January 2020).
- U.S. Alternative Fuels Data Center. Department of Energy: Energy Efficiency and Renewable Energy. Available online: http://www.afdc.energy.gov (accessed on 12 February 2017).
- Torres-Jimenez, E.; Jerman, M.S.; Gregorc, A.; Lisec, I.; Dorado, M.P.; Kegl, B. Physical and chemical properties of ethanol–diesel fuel blends. Fuel 2011, 90, 795–802. [Google Scholar] [CrossRef]
- Padala, S.; Woo, C.; Kook, S.; Hawkes, E.R. Ethanol utilisation in a diesel engine using dual-fuelling technology. Fuel 2013, 109, 597–607. [Google Scholar] [CrossRef]
- Al Qubeissi, M. Heating and Evaporation of Multi-Component Fuel Droplets; WiSa: Stuttgart, Germany, 2015; ISBN 978-3-95538-023-6. [Google Scholar]
- Department for Transport, UK New Regulations to Double the Use of Sustainable Renewable Fuels by 2020. Available online: https://www.gov.uk/government/news/new-regulations-to-double-the-use-of-sustainable-renewable-fuels-by-2020 (accessed on 22 February 2019).
- Eller, D. Trump Administration Gives Final Approval for Year-Round E15 Use. Available online: https://eu.desmoinesregister.com/story/money/agriculture/2019/05/31/trump-administration-approves-year-round-e-15-use-biofuels-ethanol/1297055001/ (accessed on 16 August 2019).
- Sirignano, W.A. Fluid Dynamics and Transport of Droplets and Sprays; Cambridge University Press: Cambridge, UK, 2010; ISBN 978-0-521-88489-1. [Google Scholar]
- Al-Esawi, N.; Al Qubeissi, M.; Sazhin, S.S.; Whitaker, R. The impacts of the activity coefficient on heating and evaporation of ethanol/gasoline fuel blends. Int. Commun. Heat Mass Transf. 2018, 98, 177–182. [Google Scholar] [CrossRef]
- Stojkovic, B.D.; Sick, V. Evolution and impingement of an automotive fuel spray investigated with simultaneous Mie/LIF techniques. Appl Phys. B 2001, 73, 75–83. [Google Scholar] [CrossRef] [Green Version]
- Ott, L.S.; Smith, B.L.; Bruno, T.J. Composition-explicit distillation curves of waste lubricant oils and resourced crude oil: A diagnostic for re-refining and evaluation. Am. J. Environ. Sci. 2010, 6, 523–534. [Google Scholar] [CrossRef] [Green Version]
- Smith, B.L.; Bruno, T.J. Advanced distillation curve measurement with a model predictive temperature controller. Int. J. Thermophys. 2006, 27, 1419–1434. [Google Scholar] [CrossRef]
- Abdel-Qader, Z.; Hallett, W.L.H. The role of liquid mixing in evaporation of complex multicomponent mixtures: Modelling using continuous thermodynamics. Chem. Eng. Sci. 2005, 60, 1629–1640. [Google Scholar] [CrossRef] [Green Version]
- Zhu, G.-S.; Reitz, R.D. A model for high-pressure vaporization of droplets of complex liquid mixtures using continuous thermodynamics. Int. J. Heat Mass Transf. 2002, 45, 495–507. [Google Scholar] [CrossRef]
- Davidson, D.F.; Shao, J.K.; Choudhary, R.; Mehl, M.; Obrecht, N.; Hanson, R.K. Ignition delay time measurements and modeling for gasoline at very high pressures. Proc. Combust. Inst. 2018, 37, 4885–4892. [Google Scholar] [CrossRef]
- Sazhina, E.M.; Sazhin, S.S.; Heikal, M.R.; Babushok, V.I.; Johns, R.J.R. A detailed modelling of the spray ignition process in diesel engines. Combust. Sci. Technol. 2000, 160, 317–344. [Google Scholar] [CrossRef]
- Sazhin, S.S.; Krutitskii, P.A. A conduction model for transient heating of fuel droplets. In Proceedings of the 3rd ISAAC Congress, Berlin, Germany, 20–25 August 2001; World Scientific: River Edge, NJ, USA, 2003; pp. 1231–1240. [Google Scholar]
- Hallett, W.L.H.; Legault, N.V. Modelling biodiesel droplet evaporation using continuous thermodynamics. Fuel 2011, 90, 1221–1228. [Google Scholar] [CrossRef]
- Saha, K.; Abu-Ramadan, E.; Li, X. Multicomponent evaporation model for pure and blended biodiesel droplets in high temperature convective environment. Appl. Energy 2012, 93, 71–79. [Google Scholar] [CrossRef]
- Sazhin, S.S.; Elwardany, A.; Krutitskii, P.A.; Castanet, G.; Lemoine, F.; Sazhina, E.M.; Heikal, M.R. A simplified model for bi-component droplet heating and evaporation. Int. J. Heat Mass Transf. 2010, 53, 4495–4505. [Google Scholar] [CrossRef] [Green Version]
- Sazhin, S.S. Droplets and Sprays; Springer: London, UK, 2014; ISBN 978-1-4471-6385-5. [Google Scholar]
- Sazhin, S.S. Modelling of fuel droplet heating and evaporation: Recent results and unsolved problems. Fuel 2017, 196, 69–101. [Google Scholar] [CrossRef]
- Elwardany, A.E.; Sazhin, S.S.; Im, H.G. A new formulation of physical surrogates of FACE A gasoline fuel based on heating and evaporation characteristics. Fuel 2016, 176, 56–62. [Google Scholar] [CrossRef]
- Sarathy, S.M.; Kukkadapu, G.; Mehl, M.; Wang, W.; Javed, T.; Park, S.; Oehlschlaeger, M.A.; Farooq, A.; Pitz, W.J.; Sung, C.-J. Ignition of alkane-rich FACE gasoline fuels and their surrogate mixtures. Proc. Combust. Inst. 2015, 35, 249–257. [Google Scholar] [CrossRef]
- Poulton, L.; Rybdylova, O.; Zubrilin, I.A.; Matveev, S.G.; Gurakov, N.I.; Al Qubeissi, M.; Al-Esawi, N.; Khan, T.; Gun’ko, V.M.; Sazhin, S.S. Modelling of multi-component kerosene and surrogate fuel droplet heating and evaporation characteristics: A comparative analysis. Fuel 2020, 269, 117115. [Google Scholar] [CrossRef]
- Al-Esawi, N.; Al Qubeissi, M. A new approach to formulation of complex fuel surrogates. Fuel 2021, 283, 118923. [Google Scholar] [CrossRef]
- Sazhin, S.S.; Al Qubeissi, M.; Xie, J.-F. Two approaches to modelling the heating of evaporating droplets. Int. Commun. Heat Mass Transf. 2014, 57, 353–356. [Google Scholar] [CrossRef] [Green Version]
- Sazhin, S.S.; Al Qubeissi, M.; Kolodnytska, R.; Elwardany, A.E.; Nasiri, R.; Heikal, M.R. Modelling of biodiesel fuel droplet heating and evaporation. Fuel 2014, 115, 559–572. [Google Scholar] [CrossRef] [Green Version]
- Al Qubeissi, M. Proposing a numerical solution for the 3D heat conduction equation. In Proceedings of the 2012 Sixth Asia Modelling Symposium, Bali, Indonesia, 29–31 May 2012; IEEE: Piscataway, NJ, USA, 2012; pp. 144–149. [Google Scholar] [CrossRef]
- Wilke, C.R.; Chang, P. Correlation of diffusion coefficients in dilute solutions. AIChE J. 1955, 1, 264–270. [Google Scholar] [CrossRef]
- Sirignano, W.A. Fluid Dynamics and Transport of Droplets and Sprays; Cambridge University Press: Cambridge, UK, 1999; ISBN 0-521-63036-3. [Google Scholar]
- Poling, B.E.; Prausnitz, J.M.; O’Connell, J.P. The Properties of Gases and Liquids; McGraw-Hill: New York, NY, USA, 2001; ISBN 0-07-011682-2. [Google Scholar]
- Liu, J.; Hu, E.; Zeng, W.; Zheng, W. A new surrogate fuel for emulating the physical and chemical properties of RP-3 kerosene. Fuel 2020, 259, 116210. [Google Scholar] [CrossRef]
- Franzelli, B.; Riber, E.; Sanjosé, M.; Poinsot, T. A two-step chemical scheme for kerosene–Air premixed flames. Combust. Flame 2010, 157, 1364–1373. [Google Scholar] [CrossRef] [Green Version]
- Al Qubeissi, M.; Al-Esawi, N.; Sazhin, S.S. Auto-selection of quasi-components/components in the multi-dimensional quasi-discrete model. Fuel 2021, 294, 120245. [Google Scholar] [CrossRef]
- Lissitsyna, K.; Huertas, S.; Quintero, L.C.; Polo, L.M. PIONA analysis of kerosene by comprehensive two-dimensional gas chromatography coupled to time of flight mass spectrometry. Fuel 2014, 116, 716–722. [Google Scholar] [CrossRef]
- Kabil, I.; Al Qubeissi, M.; Badra, J.; Abdelghaffar, W.; Eldrainy, Y.; Sazhin, S.S.; Im, H.G.; Elwardany, A. An improved prediction of pre-combustion processes, using the discrete multicomponent model. Sustainability 2021, 13, 2937. [Google Scholar] [CrossRef]
- Rybdylova, O.; Al Qubeissi, M.; Braun, M.; Crua, C.; Manin, J.; Pickett, L.M.; de Sercey, G.; Sazhina, E.M.; Sazhin, S.S.; Heikal, M. A model for droplet heating and its implementation into ANSYS fluent. Int. Commun. Heat Mass Transf. 2016, 76, 265–270. [Google Scholar] [CrossRef] [Green Version]
- Rybdylova, O.; Poulton, L.; Al Qubeissi, M.; Elwardany, A.E.; Crua, C.; Khan, T.; Sazhin, S.S. A model for multi-component droplet heating and evaporation and its implementation into ANSYS fluent. Int. Commun. Heat Mass Transf. 2018, 90, 29–33. [Google Scholar] [CrossRef]
- Wang, F.; Liu, R.; Li, M.; Yao, J.; Jin, J. Kerosene evaporation rate in high temperature air stationary and convective environment. Fuel 2018, 211, 582–590. [Google Scholar] [CrossRef]
- Wang, H.; Hanson, R.K.; Bowman, C.T.; Davidson, D.F.; Pitsch, H.; Tsang, W.; Cernansky, N.P.; Miller, D.L.; Violi, A.; Lindstedt, R.P. A High-Temperature Chemical Kinetic Model of Nalkane, Cyclohexane, and Methyl-, Ethyl-, N-Propyl and N-Butyl-Cyclohexane Oxidation at High Temperatures, Version 1.1; JetSurF. 2010. Available online: https://web.stanford.edu/group/haiwanglab/JetSurF/JetSurF1.1/howtocite.html (accessed on 11 March 2021).
| C No | N-Alkanes | Iso-Alkanes | Cycloalkanes/Olefins | Alkylbenzenes | Naphtobenzenes | Diaromatics |
|---|---|---|---|---|---|---|
| C7 | 0.19 | 0.23 | 0.17 | 0.09 | - | - |
| C8 | 0.19 | 0.39 | 0.63 | 0.61 | - | - |
| C9 | 0.49 | 1.72 | 2.38 | 1.56 | 0.22 | - |
| C10 | 0.7 | 4.09 | 5.83 | 2.72 | 1.06 | 0.09 |
| C11 | 0.75 | 5.33 | 6.93 | 2.19 | 1.81 | 0.25 |
| C12 | 1.15 | 6.67 | 7.4 | 3 | 3.48 | 0.3 |
| C13 | 0.87 | 5.06 | 4.49 | 2.91 | 0.9 | 0.06 |
| C14 | 0.89 | 5.14 | 3.78 | 1.74 | 0.24 | - |
| C15 | 0.57 | 5.63 | 1.67 | 0.35 | - | - |
| C16 | 0.05 | 2.11 | 0.74 | - | - | - |
| C17 | - | - | 0.48 | - | - | - |
| Total % | 5.84 | 36.09 | 34.52 | 15.16 | 7.7 | 0.7 |
| Parameter | Value | Unit |
|---|---|---|
| Primary injection air velocity | 10 | m/s |
| Secondary injection air velocity | 6 | m/s |
| Fuel mass flowrate | 0.003 | Kg/s |
| Ambient pressure | 0.4 | MPa |
| Air temp | 293 | K |
| Fuel temp | 375 | K |
| Oxidation temp | 800 | K |
| Parameter | ANSYS Surrogate | Suggested Surrogate |
|---|---|---|
| Total reaction heat (MJ/kg) | −4.03 | |
| Internal energy (MJ/kg) | 3.11 | 3.05 |
| Total enthalpy at the outlet (MJ/kg) | 3.48 | 3.41 |
| Evaporation enthalpy (MJ/kg) | −1.61 | −1.473 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al Qubeissi, M.; Al-Esawi, N.; Soyhan, H.S. Combustion of Fuel Surrogates: An Application to Gas Turbine Engines. Energies 2021, 14, 6545. https://doi.org/10.3390/en14206545
Al Qubeissi M, Al-Esawi N, Soyhan HS. Combustion of Fuel Surrogates: An Application to Gas Turbine Engines. Energies. 2021; 14(20):6545. https://doi.org/10.3390/en14206545
Chicago/Turabian StyleAl Qubeissi, Mansour, Nawar Al-Esawi, and Hakan Serhad Soyhan. 2021. "Combustion of Fuel Surrogates: An Application to Gas Turbine Engines" Energies 14, no. 20: 6545. https://doi.org/10.3390/en14206545
APA StyleAl Qubeissi, M., Al-Esawi, N., & Soyhan, H. S. (2021). Combustion of Fuel Surrogates: An Application to Gas Turbine Engines. Energies, 14(20), 6545. https://doi.org/10.3390/en14206545








