Simultaneous Synergy in CH4 Yield and Kinetics: Criteria for Selecting the Best Mixtures during Co-Digestion of Wastewater and Manure from a Bovine Slaughterhouse
Abstract
1. Introduction
2. Materials and Methods
2.1. Evaluation of Anaerobic Co-Digestion (AcoD)
2.1.1. Substrates and Inoculum Origin
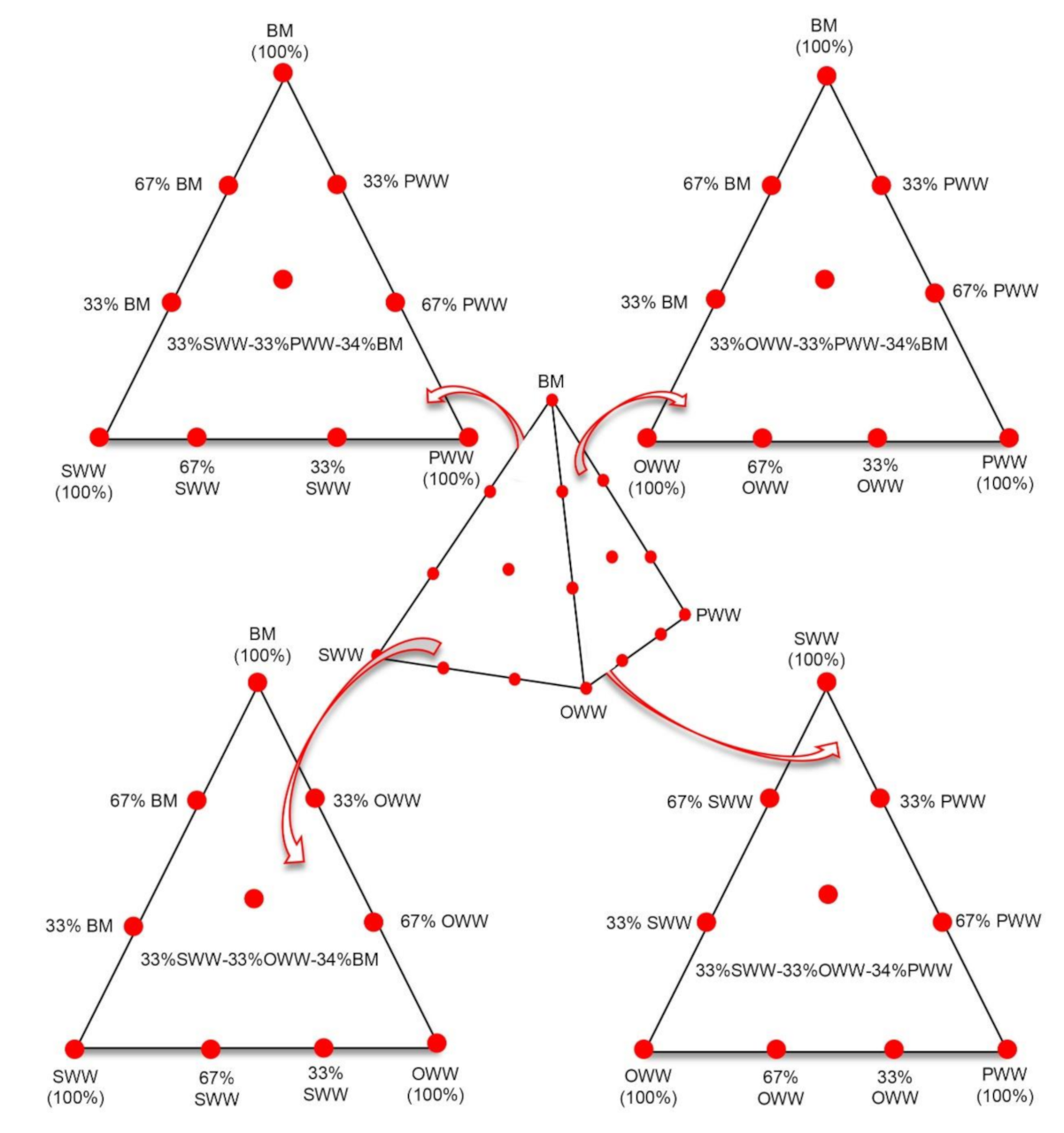
2.1.2. Experimental Mixture Design
2.1.3. Ultimate Experimental Specific CH4 Yield (Bo)
2.1.4. Theoretical Specific CH4 Yield (Both)
2.1.5. The Extent of Degradation (fd)
2.1.6. Analytical Procedures
2.2. Kinetic Modeling
2.3. Evaluation of Synergistic Effects
2.4. Energy and Economic Considerations
2.5. Statistical Analysis
3. Results and Discussion
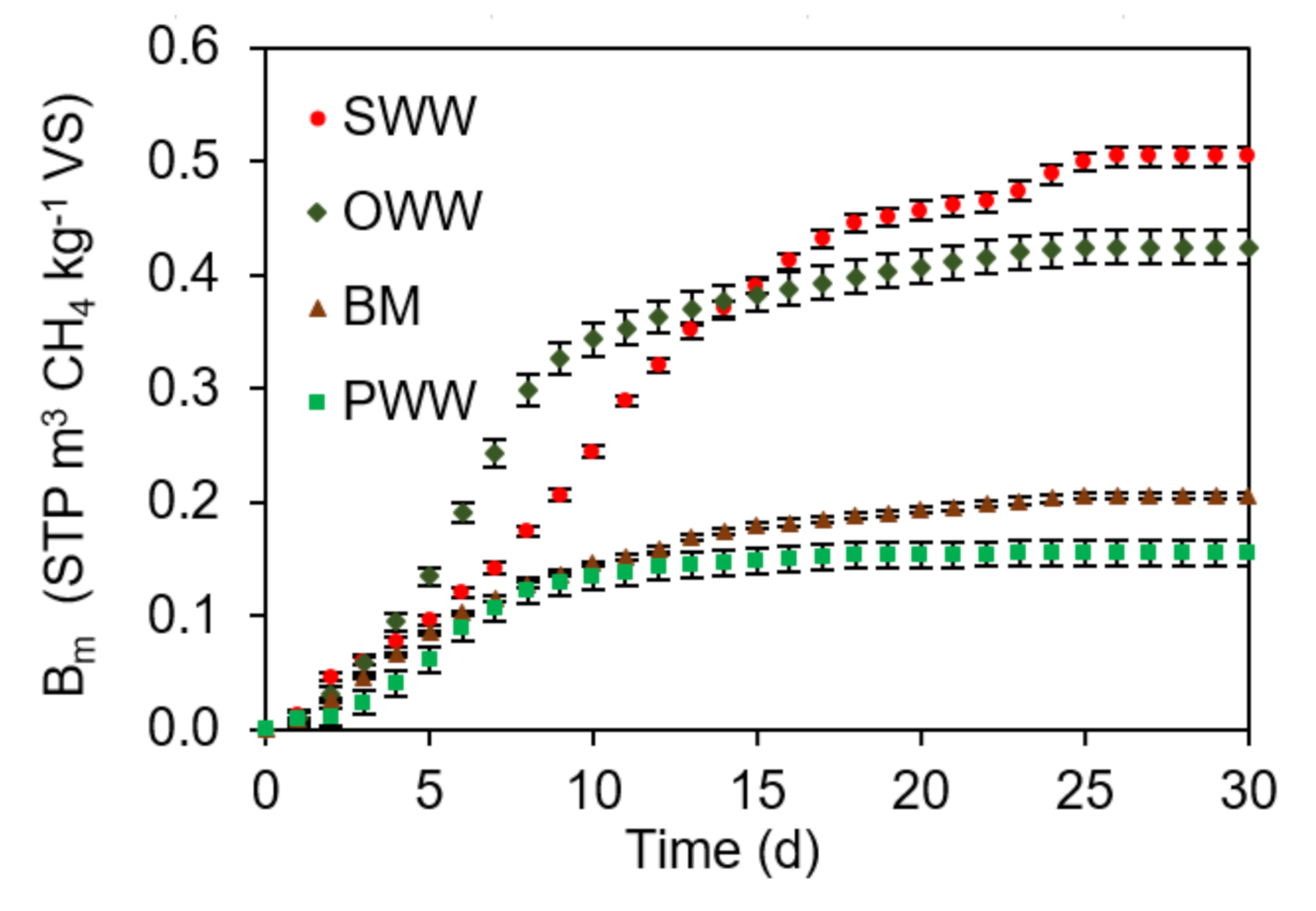
3.1. Ultimate Experimental Specific CH4 Yield (Bo) of Single Substrates
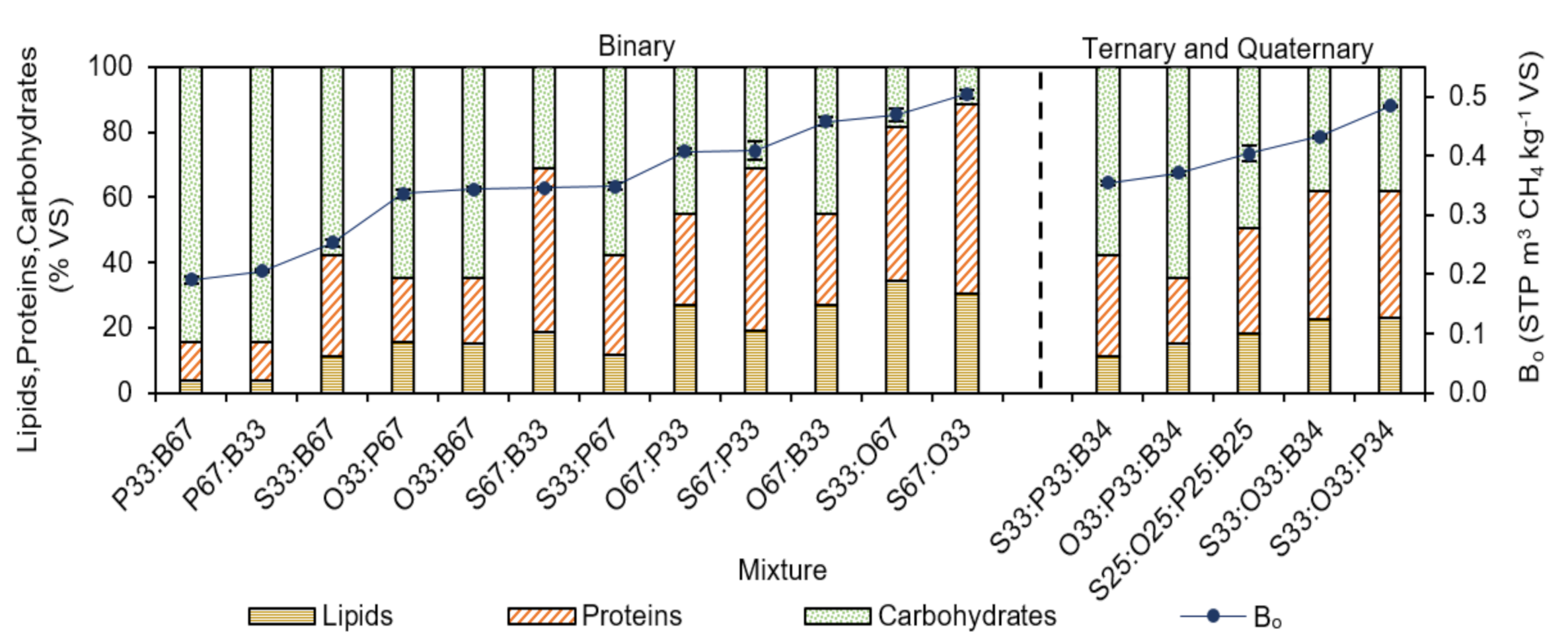
3.2. Experimental Ultimate Specific CH4 Yield of AcoD
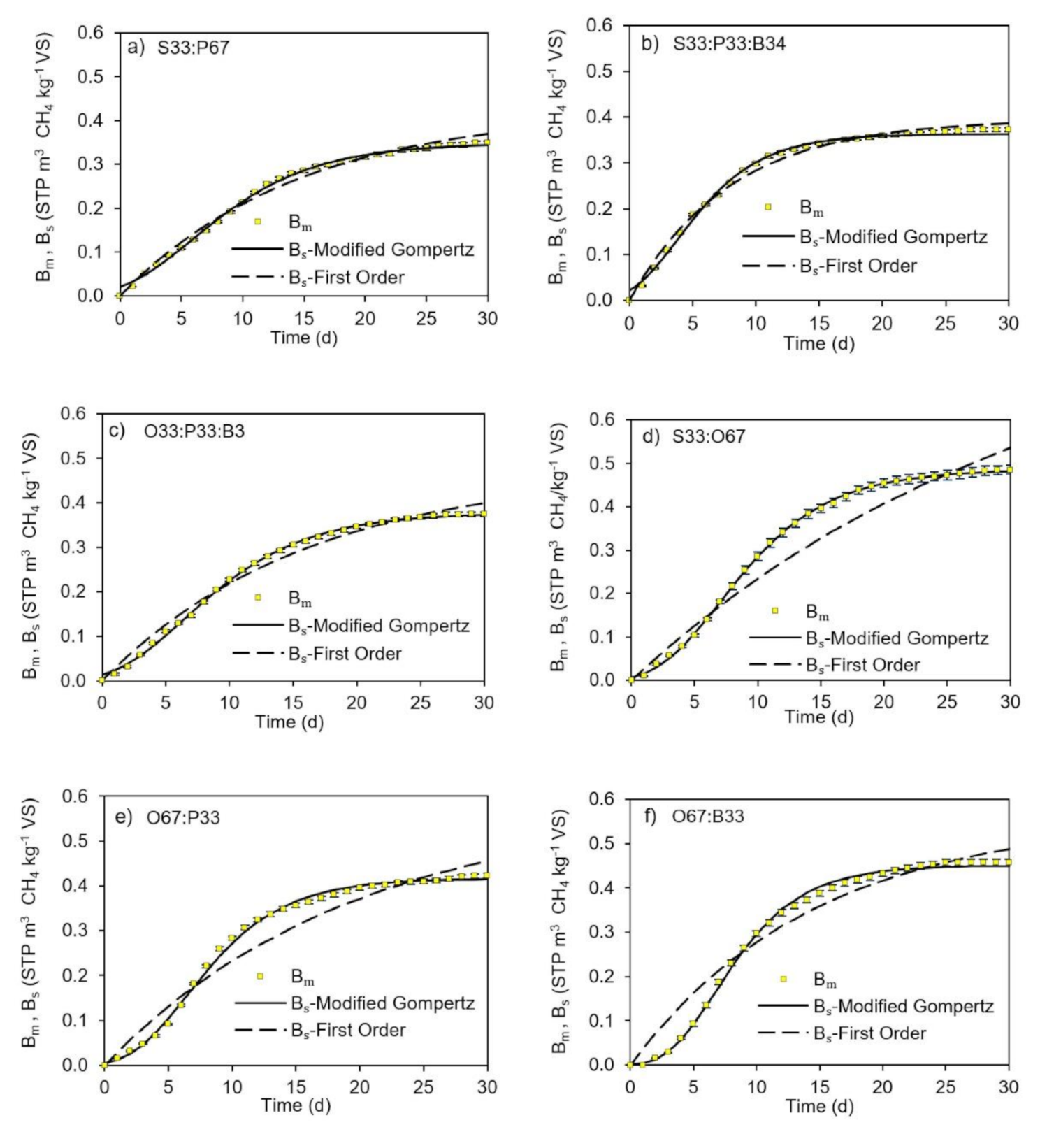
3.3. Kinetic Model Selection
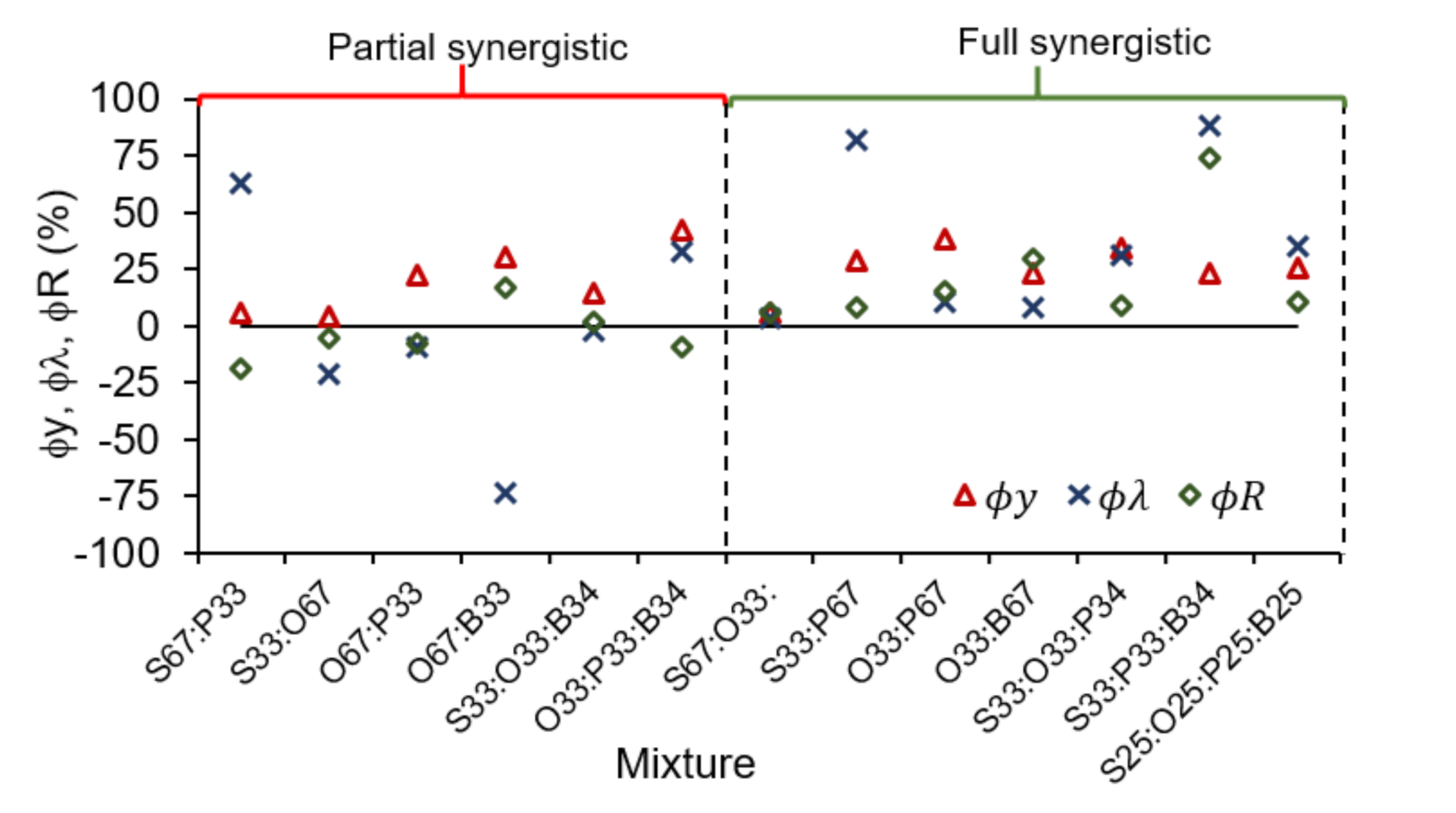
3.4. Evaluation of Synergy Effects
3.5. Energy and Economic Feasibility
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bustillo-Lecompte, C.; Mehrvar, M. Slaughterhouse wastewater characteristics, treatment, and management in the meat processing industry: A review on trends and advances. J. Environ. Manag. 2015, 161, 287–302. [Google Scholar] [CrossRef] [PubMed]
- Mekonnen, M.M.; Hoekstra, A.Y. A Global Assessment of the Water Footprint of Farm Animal Products. Ecosystems 2012, 15, 401–415. [Google Scholar] [CrossRef]
- Geraghty, R. Sustainable Practices in Irish Beef Processing; Enterprise Ireland: Dublin, Ireland, 2009. [Google Scholar]
- Jensen, P.; Sullivan, T.W.; Carney, C.; Batstone, D.J. Analysis of the potential to recover energy and nutrient resources from cattle slaughterhouses in Australia by employing anaerobic digestion. Appl. Energy 2014, 136, 23–31. [Google Scholar] [CrossRef]
- Caixeta, C.E.; Cammarota, M.C.; Xavier, A.M. Slaughterhouse wastewater treatment: Evaluation of a new three-phase separation system in a UASB reactor. Bioresour. Technol. 2002, 81, 61–69. [Google Scholar] [CrossRef]
- Saddoud, A.; Sayadi, S. Application of acidogenic fixed-bed reactor prior to anaerobic membrane bioreactor for sustainable slaughterhouse wastewater treatment. J. Hazard. Mater. 2007, 149, 700–706. [Google Scholar] [CrossRef]
- World Bank. Global Study of Livestock Markets, Slaughterhouses and Related Waste Management Systems; Final Report; World Bank: Tokyo, Japan, 2009. [Google Scholar]
- Kinyua, M.N.; Rowse, L.E.; Ergas, S. Review of small-scale tubular anaerobic digesters treating livestock waste in the developing world. Renew. Sustain. Energy Rev. 2016, 58, 896–910. [Google Scholar] [CrossRef]
- Martí-Herrero, J.; Alvarez, R.; Flores, T. Evaluation of the low technology tubular digesters in the production of biogas from slaughterhouse wastewater treatment. J. Clean. Prod. 2018, 199, 633–642. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, Y.; Angelidaki, I. Ammonia inhibition on hydrogen enriched anaerobic digestion of manure under mesophilic and thermophilic conditions. Water Res. 2016, 105, 314–319. [Google Scholar] [CrossRef]
- Zonta, Z.J.; Alves, M.M.; Flotats, X.; Palatsi, J. Modelling inhibitory effects of long chain fatty acids in the anaerobic digestion process. Water Res. 2013, 47, 1369–1380. [Google Scholar] [CrossRef]
- Astals, S.; Batstone, D.; Mata-Alvarez, J.; Jensen, P. Identification of synergistic impacts during anaerobic co-digestion of organic wastes. Bioresour. Technol. 2014, 169, 421–427. [Google Scholar] [CrossRef]
- Jensen, P.; Mehta, C.M.; Carney, C.; Batstone, D.J. Recovery of energy and nutrient resources from cattle paunch waste using temperature phased anaerobic digestion. Waste Manag. 2016, 51, 72–80. [Google Scholar] [CrossRef] [PubMed]
- Kafle, G.K.; Kim, S.-H. Kinetic Study of the Anaerobic Digestion of Swine Manure at Mesophilic Temperature: A Lab Scale Batch Operation. J. Biosyst. Eng. 2012, 37, 233–244. [Google Scholar] [CrossRef]
- Fogler, S. Conversion and reactor sizing. In Elements of Chemical Reaction Engineering, 5th ed.; Prentice Hall: Kendallville, IN, USA, 2016; pp. 31–68. [Google Scholar]
- Devuyst, E.; Pryor, S.W.; Lardy, G.; Eide, W.; Wiederholt, R. Cattle, ethanol, and biogas: Does closing the loop make economic sense? Agric. Syst. 2011, 104, 609–614. [Google Scholar] [CrossRef]
- Andriamanohiarisoamanana, F.J.; Saikawa, A.; Tarukawa, K.; Qi, G.; Pan, Z.; Yamashiro, T.; Iwasaki, M.; Ihara, I.; Nishida, T.; Umetsu, K. Anaerobic co-digestion of dairy manure, meat and bone meal, and crude glycerol under mesophilic conditions: Synergistic effect and kinetic studies. Energy Sustain. Dev. 2017, 40, 11–18. [Google Scholar] [CrossRef]
- Ara, E.; Sartaj, M.; Kennedy, K. Enhanced biogas production by anaerobic co-digestion from a trinary mix substrate over a binary mix substrate. Waste Manag. Res. 2015, 33, 578–587. [Google Scholar] [CrossRef]
- Wang, X.; Yang, G.; Li, F.; Feng, Y.; Ren, G.; Han, X. Evaluation of two statistical methods for optimizing the feeding composition in anaerobic co-digestion: Mixture design and central composite design. Bioresour. Technol. 2013, 131, 172–178. [Google Scholar] [CrossRef]
- Dennehy, C.; Lawlor, P.G.; Croize, T.; Jiang, Y.; Morrison, L.; Gardiner, G.E.; Zhan, X. Synergism and effect of high initial volatile fatty acid concentrations during food waste and pig manure anaerobic co-digestion. Waste Manag. 2016, 56, 173–180. [Google Scholar] [CrossRef]
- Pagés-Díaz, J.; Pereda-Reyes, I.; Taherzadeh, M.J.; Sárvári-Horváth, I.; Lundin, M. Anaerobic co-digestion of solid slaughterhouse wastes with agro-residues: Synergistic and antagonistic interactions determined in batch digestion assays. Chem. Eng. J. 2014, 245, 89–98. [Google Scholar] [CrossRef]
- Ebner, J.H.; Labatut, R.A.; Lodge, J.S.; Williamson, A.A.; Trabold, T.A. Anaerobic co-digestion of commercial food waste and dairy manure: Characterizing biochemical parameters and synergistic effects. Waste Manag. 2016, 52, 286–294. [Google Scholar] [CrossRef]
- Donoso-Bravo, A.; Ortega, V.; Lesty, Y.; Bossche, H.V.; Olivares, D. Addressing the synergy determination in anaerobic co-digestion and the inoculum activity impact on BMP test. Water Sci. Technol. 2019, 80, 387–396. [Google Scholar] [CrossRef]
- Mendieta, O.; Castro, L.; Rodríguez, J.; Escalante, H. Synergistic effect of sugarcane scum as an accelerant co-substrate on anaerobic co-digestion with agricultural crop residues from non-centrifugal cane sugar agribusiness sector. Bioresour. Technol. 2020, 303, 122957. [Google Scholar] [CrossRef] [PubMed]
- Holliger, C.; Alves, M.; Andrade, D.; Angelidaki, I.; Astals, S.; Baier, U.; Bougrier, C.; Buffière, P.; Carballa, M.; De Wilde, V.; et al. Towards a standardization of biomethane potential tests. Water Sci. Technol. 2016, 74, 2515–2522. [Google Scholar] [CrossRef] [PubMed]
- Callaghan, F.; Wase, D.; Thayanithy, K.; Forster, C. Continuous co-digestion of cattle slurry with fruit and vegetable wastes and chicken manure. Biomass Bioenergy 2002, 22, 71–77. [Google Scholar] [CrossRef]
- Angelidaki, I.; Alves, M.M.; Bolzonella, D.; Borzacconi, L.; Campos, J.L.; Guwy, A.J.; Kalyuzhnyi, S.; Jenicek, P.; Van Lier, J.B. Defining the biomethane potential (BMP) of solid organic wastes and energy crops: A proposed protocol for batch assays. Water Sci. Technol. 2009, 59, 927–934. [Google Scholar] [CrossRef] [PubMed]
- Maya-Altamira, L.; Baun, A.; Angelidaki, I.; Schmidt, J.E. Influence of wastewater characteristics on methane potential in food-processing industry wastewaters. Water Res. 2008, 42, 2195–2203. [Google Scholar] [CrossRef]
- Raposo, F.; Fernández-Cegrí, V.; De La Rubia, M.; Ángeles Borja, R.; Béline, F.; Cavinato, C.; Demirer, G.N.; Fernández, B.; Fernández-Polanco, M.; Frigon, J.C.; et al. Biochemical methane potential (BMP) of solid organic substrates: Evaluation of anaerobic biodegradability using data from an international interlaboratory study. J. Chem. Technol. Biotechnol. 2011, 86, 1088–1098. [Google Scholar] [CrossRef]
- APHA-AWWA-WEF. Standard Methods for the Examination of Water and Wastewater, 23rd ed.; American Public Health Association: Washington, D.C., USA, 2017. [Google Scholar]
- Lahav, O.; Morgan, B. Titration methodologies for monitoring of anaerobic digestion in developing countries? A review. J. Chem. Technol. Biotechnol. 2004, 79, 1331–1341. [Google Scholar] [CrossRef]
- Van Soest, P.; Robertson, J.; Lewis, B. Methods for Dietary Fiber, Neutral Detergent Fiber, and Nonstarch Polysaccharides in Relation to Animal Nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef]
- Passos, F.; Ortega, V.; Donoso-Bravo, A. Thermochemical pretreatment and anaerobic digestion of dairy cow manure: Experimental and economic evaluation. Bioresour. Technol. 2017, 227, 239–246. [Google Scholar] [CrossRef]
- Astals, S.; Batstone, D.; Tait, S.; Jensen, P. Development and validation of a rapid test for anaerobic inhibition and toxicity. Water Res. 2015, 81, 208–215. [Google Scholar] [CrossRef]
- Astals, S.; Peces, M.; Batstone, D.; Jensen, P.; Tait, S. Characterising and modelling free ammonia and ammonium inhibition in anaerobic systems. Water Res. 2018, 143, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Yan, H.; Zhang, R.; Liu, G.; Chen, C. Characterization and methane production of different nut residue wastes in anaerobic digestion. Renew. Energy 2018, 116, 835–841. [Google Scholar] [CrossRef]
- Pan, X.; Wang, L.; Lv, N.; Ning, J.; Zhou, M.; Wang, T.; Li, C.; Zhu, G. Impact of physical structure of granular sludge on methanogenesis and methanogenic community structure. RSC Adv. 2019, 9, 29570–29578. [Google Scholar] [CrossRef]
- Poggio, D.; Walker, M.; Nimmo, W.; Ma, L.; Pourkashanian, M. Modelling the anaerobic digestion of solid organic waste–Substrate characterisation method for ADM1 using a combined biochemical and kinetic parameter estimation approach. Waste Manag. 2016, 53, 40–54. [Google Scholar] [CrossRef]
- Molano, L.C.; Escalante, H.; Lambis-Benítez, L.E.; Marín-Batista, J.D. Synergistic effects in anaerobic codigestion of chicken manure with industrial wastes. DYNA 2018, 85, 135–141. [Google Scholar] [CrossRef]
- Escalante, H.; Castro, L.; Amaya, M.; Jaimes, L.; Jaimes-Estévez, J. Anaerobic digestion of cheese whey: Energetic and nutritional potential for the dairy sector in developing countries. Waste Manag. 2018, 71, 711–718. [Google Scholar] [CrossRef]
- Ali, M.M.; Ndongo, M.; Bilal, B.; Yetilmezsoy, K.; Youm, I.; Bahramian, M. Mapping of biogas production potential from livestock manures and slaughterhouse waste: A case study for African countries. J. Clean. Prod. 2020, 256, 120499. [Google Scholar] [CrossRef]
- Haitai Power Machinery. Available online: http://www.hitepower.com/index.jsp (accessed on 18 November 2020).
- González-González, A.; Collares-Pereira, M.; Cuadros, F.; Fartaria, T. Energy self-sufficiency through hybridization of biogas and photovoltaic solar energy: An application for an Iberian pig slaughterhouse. J. Clean. Prod. 2014, 65, 318–323. [Google Scholar] [CrossRef]
- Jin, Y.; Hu, Z.; Wen, Z. Enhancing anaerobic digestibility and phosphorus recovery of dairy manure through microwave-based thermochemical pretreatment. Water Res. 2009, 43, 3493–3502. [Google Scholar] [CrossRef]
- Zheng, Z.; Liu, J.; Yuan, X.; Wang, X.; Zhu, W.; Yang, F.; Cui, Z. Effect of dairy manure to switchgrass co-digestion ratio on methane production and the bacterial community in batch anaerobic digestion. Appl. Energy 2015, 151, 249–257. [Google Scholar] [CrossRef]
- Matthews, C.; Crispie, F.; Lewis, E.; Reid, M.; O’Toole, P.W.; Cotter, P.D. The rumen microbiome: A crucial consideration when optimising milk and meat production and nitrogen utilisation efficiency. Gut Microbes 2019, 10, 115–132. [Google Scholar] [CrossRef] [PubMed]
- RSPCA. How Are Animals Killed for Food? Available online: https://kb.rspca.org.au/knowledge-base/how-are-animals-killed-for-food/ (accessed on 1 October 2020).
- Ministry of Health and Social Protection. Resolution No. 240/2013; Ministry of Health and Social Protection: Bogota, Colombia, 2013. [Google Scholar]
- Harris, P.W.; Schmidt, T.; McCabe, B.K. Bovine bile as a bio-surfactant pre-treatment option for anaerobic digestion of high-fat cattle slaughterhouse waste. J. Environ. Chem. Eng. 2018, 6, 444–450. [Google Scholar] [CrossRef]
- Nielsen, H.B.; Angelidaki, I. Strategies for optimizing recovery of the biogas process following ammonia inhibition. Bioresour. Technol. 2008, 99, 7995–8001. [Google Scholar] [CrossRef] [PubMed]
- Cuetos, M.J.; Martinez, E.J.; Moreno, R.; Gonzalez, R.; Otero, M.; Gómez, X. Enhancing anaerobic digestion of poultry blood using activated carbon. J. Adv. Res. 2017, 8, 297–307. [Google Scholar] [CrossRef]
- Jensen, P.; Yap, S.; Boyle-Gotla, A.; Janoschka, J.; Carney, C.; Pidou, M.; Batstone, D.J. Anaerobic membrane bioreactors enable high rate treatment of slaughterhouse wastewater. Biochem. Eng. J. 2015, 97, 132–141. [Google Scholar] [CrossRef]
- Neshat, S.A.; Mohammadi, M.; Darzi, G.N.; Lahijani, P. Anaerobic co-digestion of animal manures and lignocellulosic residues as a potent approach for sustainable biogas production. Renew. Sustain. Energy Rev. 2017, 79, 308–322. [Google Scholar] [CrossRef]
- López, I.; Passeggi, M.; Borzacconi, L. Co-digestion of ruminal content and blood from slaughterhouse industries: Influence of solid concentration and ammonium generation. Water Sci. Technol. 2006, 54, 231–236. [Google Scholar] [CrossRef]
- Cuetos, M.; Gómez, X.; Martínez, E.J.; Fierro, J.; Otero, M. Feasibility of anaerobic co-digestion of poultry blood with maize residues. Bioresour. Technol. 2013, 144, 513–520. [Google Scholar] [CrossRef]
- Zhang, Y.; Banks, C. Co-digestion of the mechanically recovered organic fraction of municipal solid waste with slaughterhouse wastes. Biochem. Eng. J. 2012, 68, 129–137. [Google Scholar] [CrossRef]
- Hernández-Fydrych, V.C.; Benítez-Olivares, G.; Meraz-Rodríguez, M.A.; Salazar-Peláez, M.L.; Fajardo-Ortiz, M.C. Methane production kinetics of pretreated slaughterhouse wastewater. Biomass Bioenergy 2019, 130, 105385. [Google Scholar] [CrossRef]
- Koch, K.; Hafner, S.D.; Weinrich, S.; Astals, S.; Holliger, C. Power and Limitations of Biochemical Methane Potential (BMP) Tests. Front. Energy Res. 2020, 8, 8. [Google Scholar] [CrossRef]
- Feleban; Eco Business Fund; IFC. What Is the Latin American Banking Sector Doing to Mitigate Climate Change? Report 2017; Feleban; Eco Business Fund; IFC: Washington, DC, USA, 2017. [Google Scholar]
- Portafolio. Colombia Emitirá Bonos Verdes Desde El Próximo Año. 2020. Available online: https://www.portafolio.co/economia/finanzas/colombia-emitira-bonos-verdes-desde-el-proximo-ano-538462 (accessed on 17 November 2020).
| Parameter a | Unit | Value |
|---|---|---|
| Average slaughter capacity | Bovines d−1 | 327 |
| Flow of SWW | m3 d−1 | 45.34 |
| Flow of OWW | m3 d−1 | 111.60 |
| Flow of PWW | m3 d−1 | 139.50 |
| Flow of BM | t d−1 | 7.70 |
| Thermal energy consumption | kWh d−1 | 8594.90 |
| Electrical energy consumption | kWh d−1 | 4743.28 |
| Parameter a | Unit | SWW b | OWW b | PWW b | BM b |
|---|---|---|---|---|---|
| pH | -- | 6.72 ± 0.08 | 6.90 ± 0.08 | 7.80 ± 0.08 | 7.38 ± 0.06 |
| TS | kg m−3 | 8.28 ± 0.12 | 12.53 ± 0.22 | 18.23 ± 0.93 | 242.14 ± 1.04 |
| VS | kg m−3 | 7.63 ± 0.21 | 10.96 ± 0.23 | 15.99 ± 0.98 | 154.22 ± 1.50 |
| COD | kg m−3 | 9.39 ± 0.04 | 9.75 ± 0.08 | 8.35 ± 0.14 | 37.06 ± 1.66 |
| TVFAs | kg CH3COOH m−3 | 0.72 ± 0.00 | 0.88 ± 0.07 | 1.25 ± 0.07 | 2.40 ± 0.00 |
| TA | Kg CaCO3 m−3 | 0.80 ± 0.00 | 1.38 ± 0.18 | 1.75 ± 0.05 | 3.25 ± 0.35 |
| Lipids | %VS | 26.5 | 38.6 | 4.1 | 3.3 |
| Proteins | %VS | 69.3 | 36.1 | 11.6 | 12.1 |
| Carb | %VS | 4.2 | 12.0 | 8.8 | 21.4 |
| Cell | %VS | -- | 2.3 | 21.9 | 24.8 |
| Hem | %VS | -- | 6.4 | 32.0 | 22.1 |
| Lig | %VS | -- | 4.6 | 21.6 | 16.3 |
| Mixture | SWW a (% VS) | OWW a (%VS) | PWW a (%VS) | BM a (%VS) | Mixture Type |
|---|---|---|---|---|---|
| S100 | 100 | 0 | 0 | 0 | Single Substrates |
| O100 | 0 | 100 | 0 | 0 | |
| P100 | 0 | 0 | 100 | 0 | |
| B100 | 0 | 0 | 0 | 100 | |
| S67:O33 | 67 | 33 | 0 | 0 | Binary |
| S67:P33 | 67 | 0 | 33 | 0 | |
| S67:B33 | 67 | 0 | 0 | 33 | |
| S33:O67 | 33 | 67 | 0 | 0 | |
| S33:P67 | 33 | 0 | 67 | 0 | |
| S33:B67 | 33 | 0 | 0 | 67 | |
| O67:P33 | 0 | 67 | 33 | 0 | |
| O67:B33 | 0 | 67 | 0 | 33 | |
| O33:P67 | 0 | 33 | 67 | 0 | |
| O33:B67 | 0 | 33 | 0 | 67 | |
| P67:B33 | 0 | 0 | 67 | 33 | |
| P33:B67 | 0 | 0 | 33 | 67 | |
| S33:O33:P34 | 33 | 33 | 34 | 0 | Ternary |
| S33:P33:B34 | 33 | 0 | 33 | 34 | |
| S33:O33:B34 | 33 | 33 | 0 | 34 | |
| O33:P33:B34 | 0 | 33 | 33 | 34 | |
| S25:O25:P25:B25 | 25 | 25 | 25 | 25 | Quaternary |
| Synergistic Factor a | Equation | Evaluation |
|---|---|---|
| ϕy,kh,R,λ > 0: the mixture has a synergistic effect. ϕy,kh,R,λ < 0: the mixture has an antagonistic effect. ϕy,kh,R,λ = 0: the mixture does not affect the performance of the substrates. | ||
| Unit | Value | |
|---|---|---|
| Design Assumptions a | ||
| Flow of SWW to be treated | m3 d−1 | 4.5 |
| Flow of OWW to be treated | m3 d−1 | 11.2 |
| Flow of PWW to be treated | m3 d−1 | 14.0 |
| Flow of BM to be treated | t d−1 | 0.8 |
| Operational volume of digester (liquid fraction) | % | 75 b |
| CAPEX | ||
| Anaerobic digester | US$ m−3 | 96 |
| Electricity generator | US$ | 5640 c |
| OPEX | ||
| Labour | US$ year−1 | 4380 |
| Electricity generator maintenance | US$ MWh−1 | 14.82 d |
| Benefits | ||
| Electricity saving | US$ kWh−1 | 0.114 |
| Natural gas saving | US$ m−3 | 0.323 |
| Wastewater treatment saving | US$ m−3 | 1.30 |
| Mixture a | Initial Lignocellulosic Material (%VS) | Final NH3 (mg/L) | Reduction of Lignocellulosic Material Composition b | Reduction of Inhibition Risk by NH3 a | fd c |
|---|---|---|---|---|---|
| S100 | 0.0 | 21.82 ± 0.25 | n/a | n/a | 0.80 ± 0.01 |
| O100 | 13.3 | 10.62 ± 0.27 | n/a | n/a | 0.63 ± 0.02 |
| P100 | 75.5 | 7.48 ± 0.25 | n/a | n/a | 0.34 ± 0.01 |
| B100 | 63.2 | 7.24 ± 0.21 | n/a | n/a | 0.46 ± 0.00 |
| S67:O33 | 4.4 | 15.89 ± 0.37 | + | + | 0.78 ± 0.01 |
| S67:P33 | 25.2 | 16.73 ± 0.35 | + | + | 0.72 ± 0.03 |
| S67:B33 | 21.1 | 23.73 ± 0.33 | + | - | 0.61 ± 0.01 |
| S33:O67 | 8.9 | 15.71 ± 0.37 | + | + | 0.71 ± 0.02 |
| S33:P67 | 50.4 | 9.91 ± 0.25 | + | + | 0.68 ± 0.01 |
| S33:B67 | 42.1 | 22.01 ± 0.33 | + | - | 0.50 ± 0.01 |
| O67:P33 | 34.1 | 8.64 ± 0.37 | + | + | 0.68 ± 0.01 |
| O67:B33 | 29.9 | 6.32 ± 0.34 | + | + | 0.77 ± 0.01 |
| O33:P67 | 54.8 | 6.62 ± 0.37 | + | + | 0.64 ± 0.01 |
| O33:B67 | 46.6 | 10.22 ± 0.34 | + | + | 0.66 ± 0.01 |
| P67:B33 | 71.4 | 10.48 ± 0.33 | - | + | 0.45 ± 0.01 |
| P33:B67 | 67.3 | 8.37 ± 0.33 | - | + | 0.43 ± 0.01 |
| S33:O33:P34 | 29.6 | 2.30 ± 0.44 | + | + | 0.83 ± 0.00 |
| S33:P33:B34 | 46.2 | 5.43 ± 0.41 | + | + | 0.70 ± 0.01 |
| S33:O33:B34 | 25.5 | 2.16 ± 0.42 | + | + | 0.74 ± 0.00 |
| O33:P33:B34 | 50.7 | 1.79 ± 0.42 | + | + | 0.71 ± 0.01 |
| S25:O25:P25:B25 | 38.0 | 3.56 ± 0.49 | + | + | 0.73 ± 0.01 |
| Model | Mixture | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| S67:O33 | S67:P33 | S33:O67 | S33:P67 | O67:P33 | O67:B33 | O33:P67 | O33:B67 | S33:O33:P34 | S33:P33:B34 | S33:O33:B34 | O33:P33:B34 | S25:O25:P25:B25 | |
| First Order | |||||||||||||
| P | 0.637 | 0.560 | 0.904 | 0.424 | 0.580 | 0.558 | 0.453 | 0.401 | 0.657 | 0.395 | 0.715 | 0.473 | 0.555 |
| kh | 0.057 | 0.047 | 0.030 | 0.068 | 0.051 | 0.069 | 0.060 | 0.095 | 0.055 | 0.126 | 0.040 | 0.062 | 0.055 |
| NRMSE | 0.075 | 0.072 | 0.134 | 0.047 | 0.113 | 0.102 | 0.115 | 0.068 | 0.082 | 0.037 | 0.097 | 0.058 | 0.086 |
| R2 | 0.969 | 0.974 | 0.918 | 0.987 | 0.930 | 0.937 | 0.919 | 0.968 | 0.965 | 0.988 | 0.958 | 0.981 | 0.961 |
| Modified Gompertz | |||||||||||||
| P | 0.494 | 0.435 | 0.486 | 0.349 | 0.417 | 0.451 | 0.342 | 0.344 | 0.504 | 0.363 | 0.450 | 0.377 | 0.411 |
| λ | 1.734 | 0.697 | 2.142 | 0.281 | 2.152 | 2.982 | 1.690 | 1.187 | 1.145 | 0.152 | 1.466 | 1.045 | 0.934 |
| Rmax | 0.036 | 0.022 | 0.037 | 0.023 | 0.036 | 0.044 | 0.034 | 0.036 | 0.033 | 0.036 | 0.030 | 0.026 | 0.029 |
| NRMSE | 0.022 | 0.044 | 0.011 | 0.024 | 0.026 | 0.027 | 0.029 | 0.025 | 0.033 | 0.018 | 0.031 | 0.016 | 0.026 |
| R2 | 0.998 | 0.992 | 0.999 | 0.998 | 0.997 | 0.997 | 0.995 | 0.996 | 0.995 | 0.997 | 0.996 | 0.999 | 0.997 |
| Scenario a | Unit | CH4 for Thermal Energy Production | CH4 for Electrical Energy Production |
|---|---|---|---|
| Mono-digestion | |||
| Potential | kWh m−3 | 33.74 | 10.54 |
| Total volume of digesters | m3 | 888 | 888 |
| PBP | years | 5 | 5 |
| NPV | US$ | 50,894.00 | 56,962.88 |
| IRR | % | 22.77 | 23.28 |
| AcoD | |||
| Potential | kWh m−3 | 42.69 | 13.34 |
| Total volume of digesters | m3 | 858 | 858 |
| PBP | years | 4 | 4 |
| NPV | US$ | 70,636.35 | 79,675.98 |
| IRR | % | 27.71 | 28.48 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sánchez, Z.; Poggio, D.; Castro, L.; Escalante, H. Simultaneous Synergy in CH4 Yield and Kinetics: Criteria for Selecting the Best Mixtures during Co-Digestion of Wastewater and Manure from a Bovine Slaughterhouse. Energies 2021, 14, 384. https://doi.org/10.3390/en14020384
Sánchez Z, Poggio D, Castro L, Escalante H. Simultaneous Synergy in CH4 Yield and Kinetics: Criteria for Selecting the Best Mixtures during Co-Digestion of Wastewater and Manure from a Bovine Slaughterhouse. Energies. 2021; 14(2):384. https://doi.org/10.3390/en14020384
Chicago/Turabian StyleSánchez, Zamir, Davide Poggio, Liliana Castro, and Humberto Escalante. 2021. "Simultaneous Synergy in CH4 Yield and Kinetics: Criteria for Selecting the Best Mixtures during Co-Digestion of Wastewater and Manure from a Bovine Slaughterhouse" Energies 14, no. 2: 384. https://doi.org/10.3390/en14020384
APA StyleSánchez, Z., Poggio, D., Castro, L., & Escalante, H. (2021). Simultaneous Synergy in CH4 Yield and Kinetics: Criteria for Selecting the Best Mixtures during Co-Digestion of Wastewater and Manure from a Bovine Slaughterhouse. Energies, 14(2), 384. https://doi.org/10.3390/en14020384






