Numerical Investigation of the Impact of H2 Enrichment on Lean Biogas/Air Flames: An Analytical Modelling Approach
Abstract
1. Introduction
2. Methods
2.1. Modelling
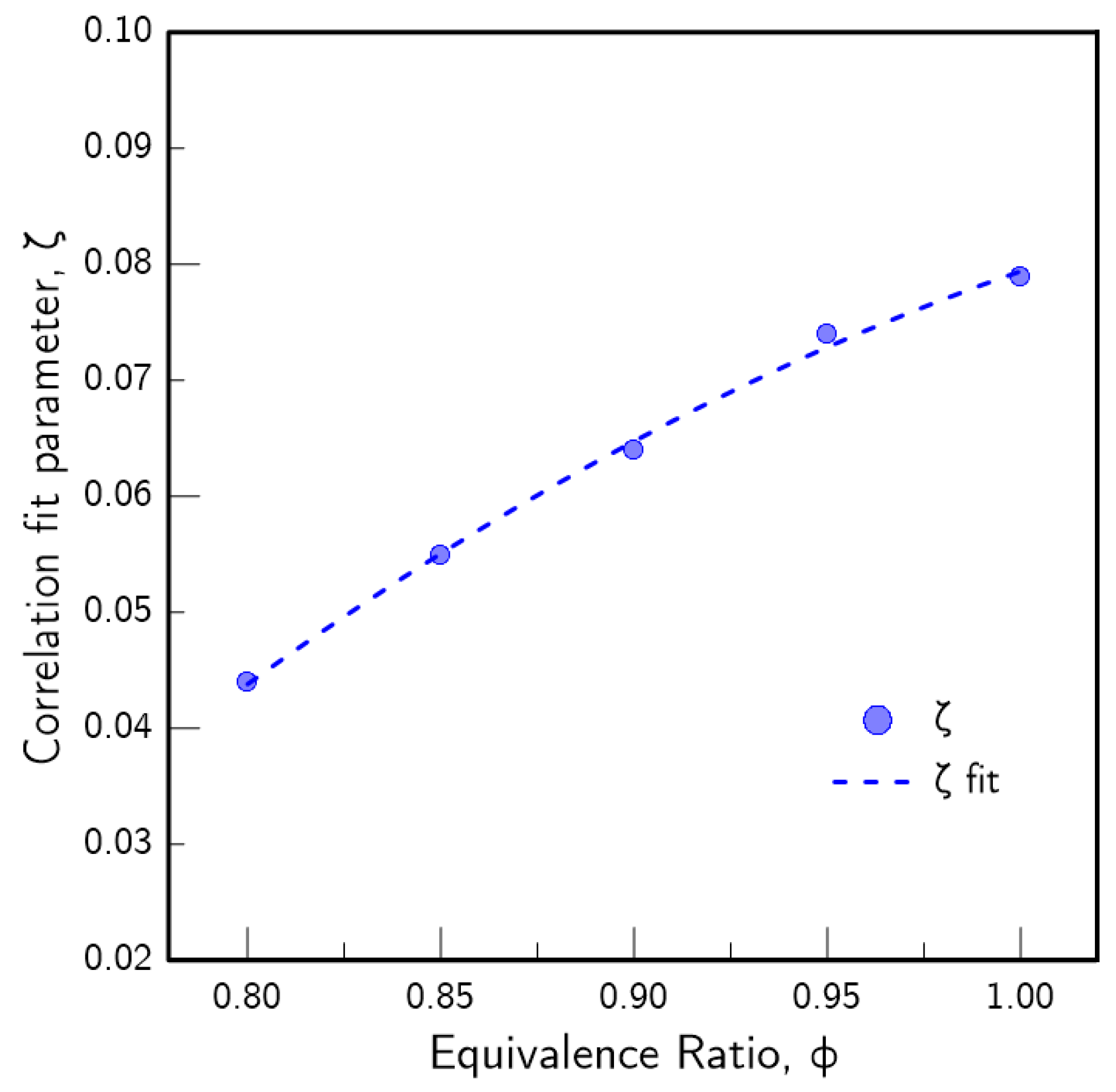
2.2. Correlation
3. Results and Discussion
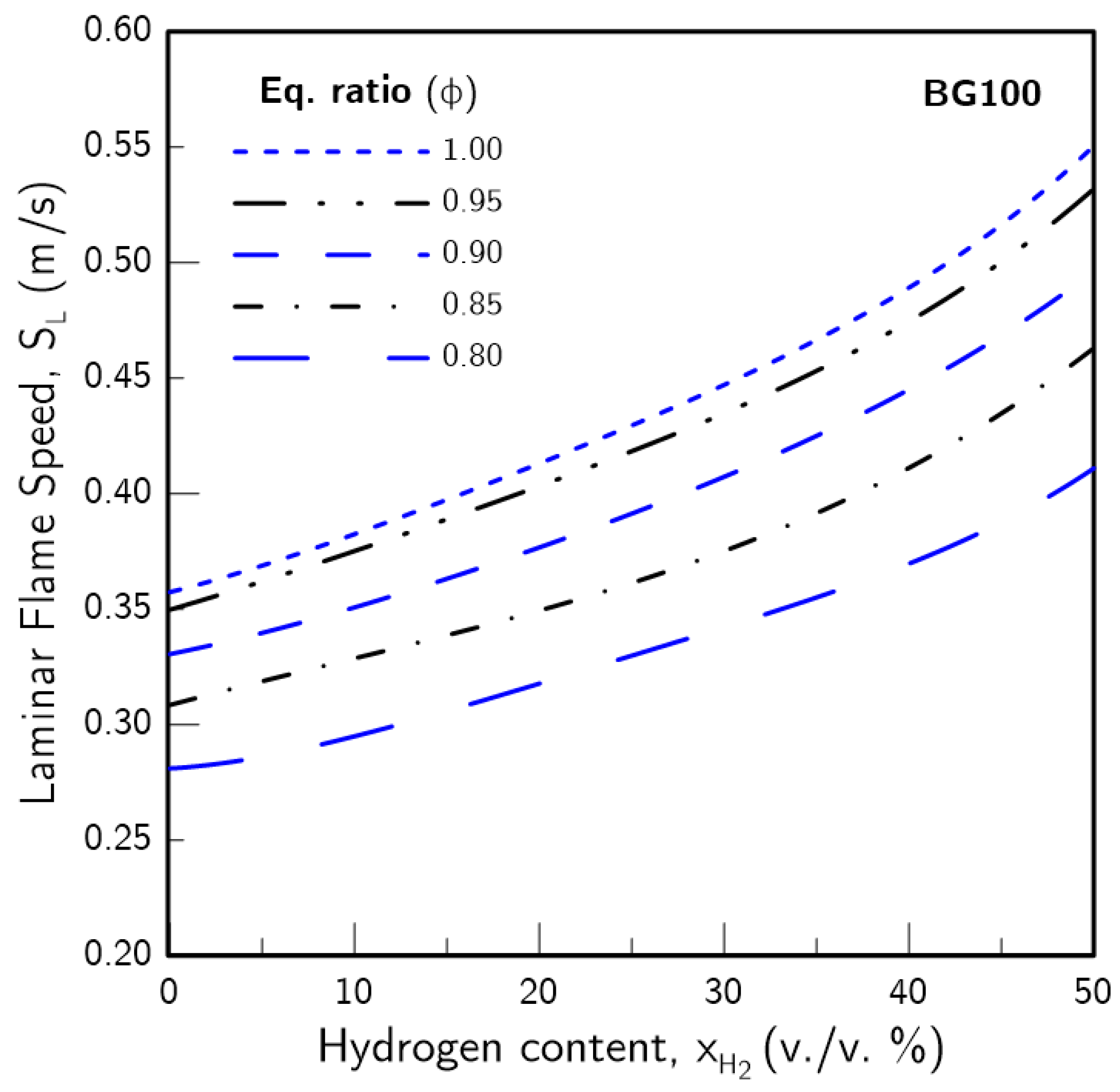
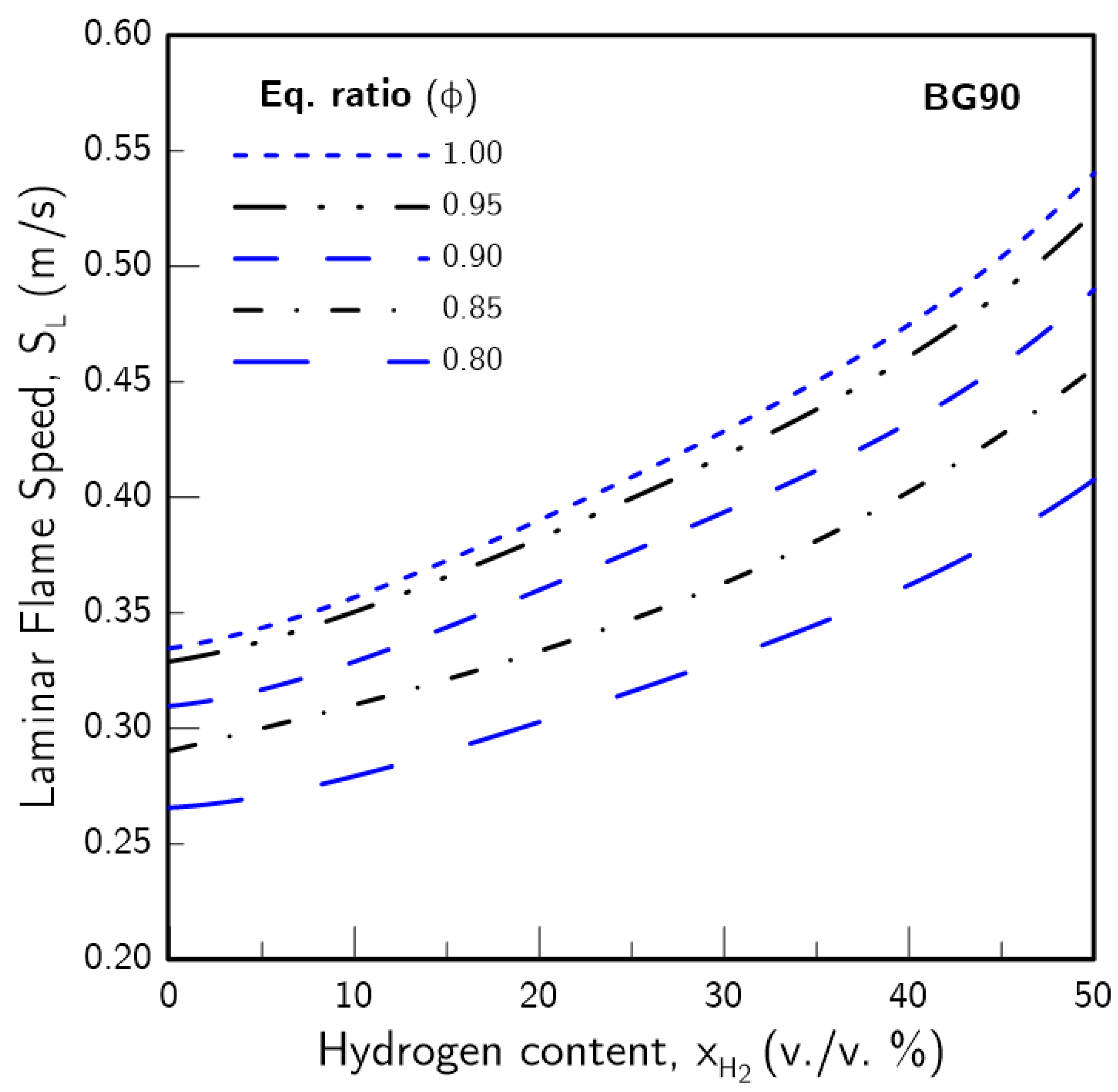
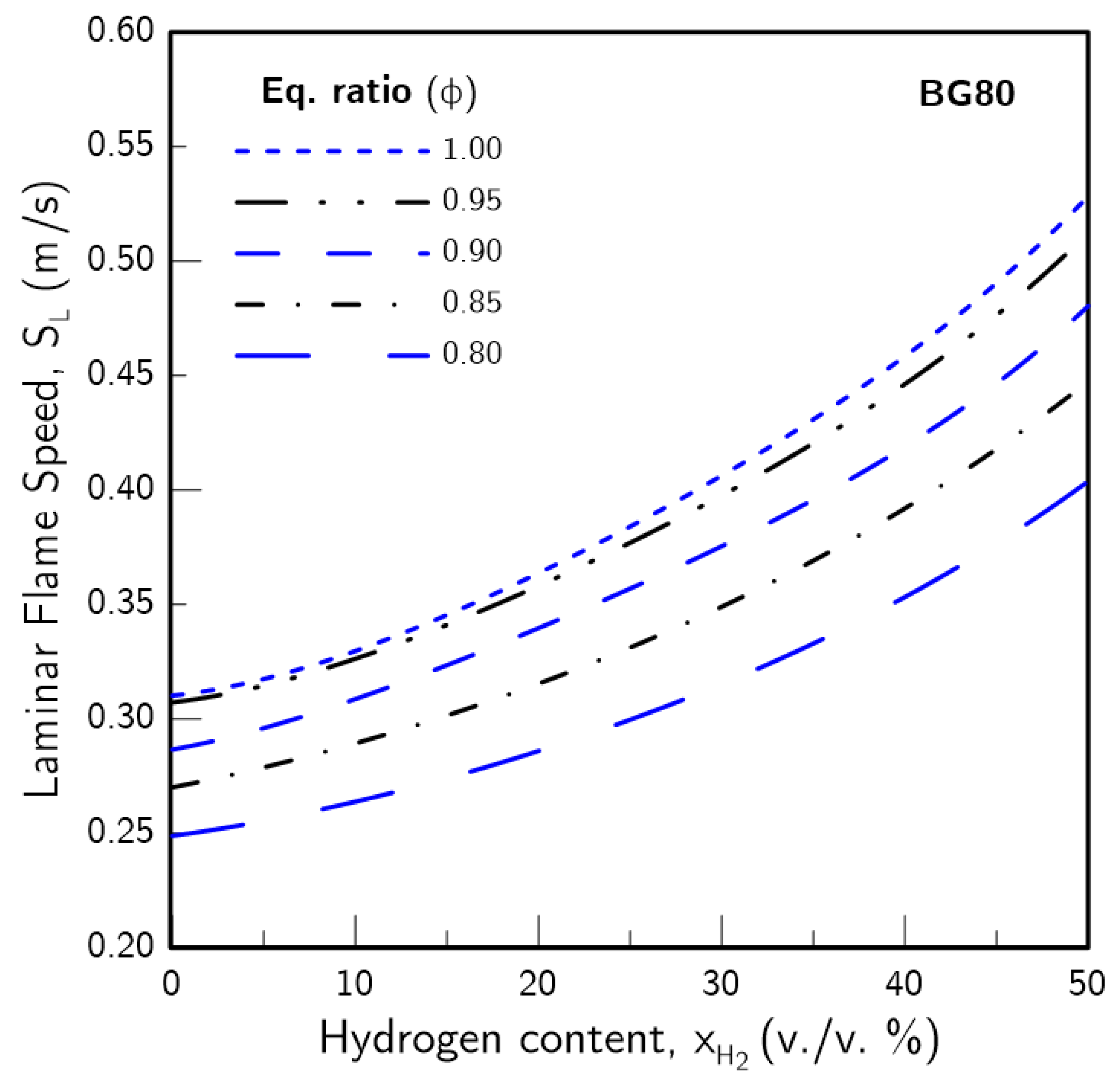
3.1. Hydrogen Content Impact on
3.2. Analytical Model
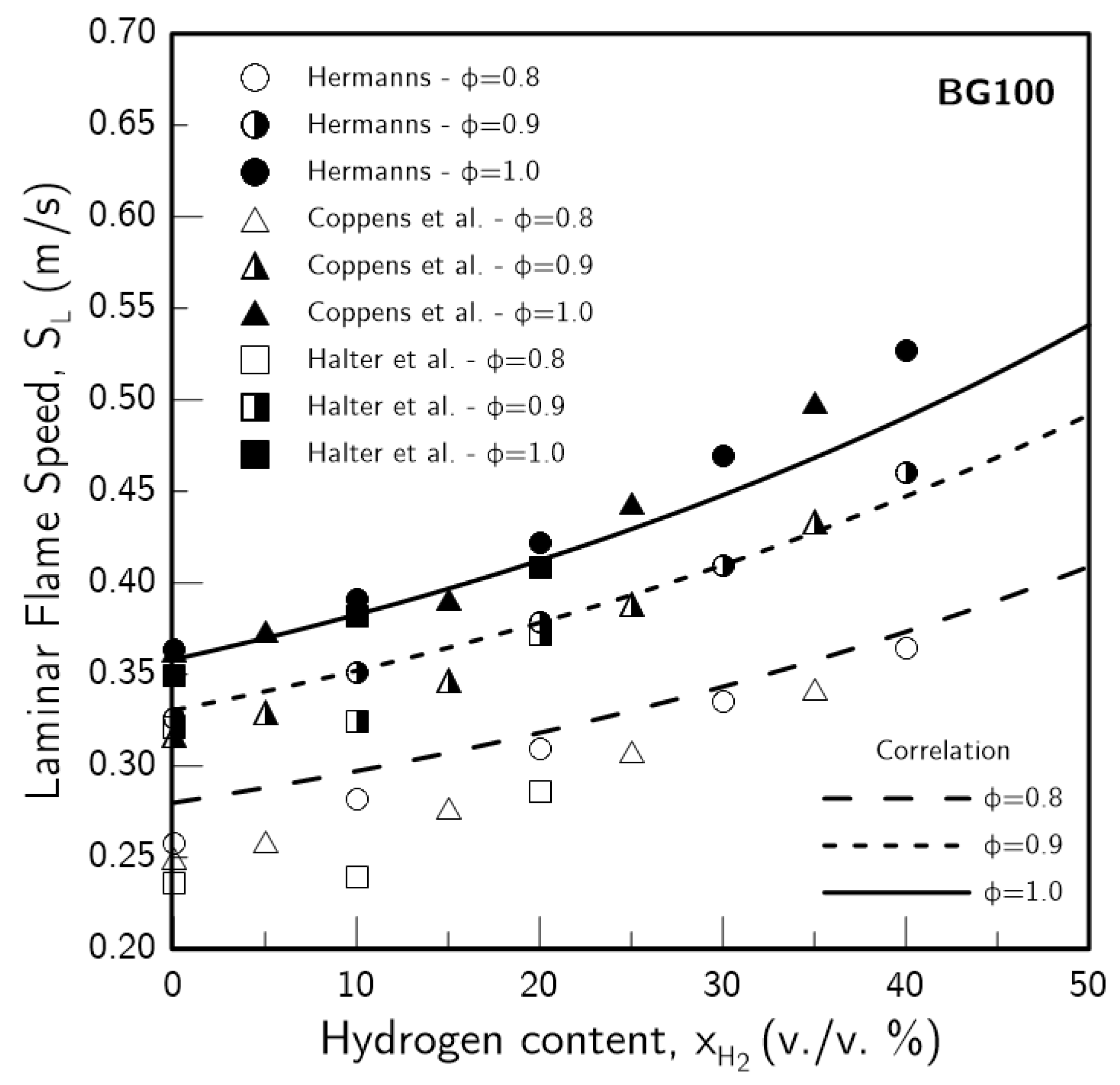
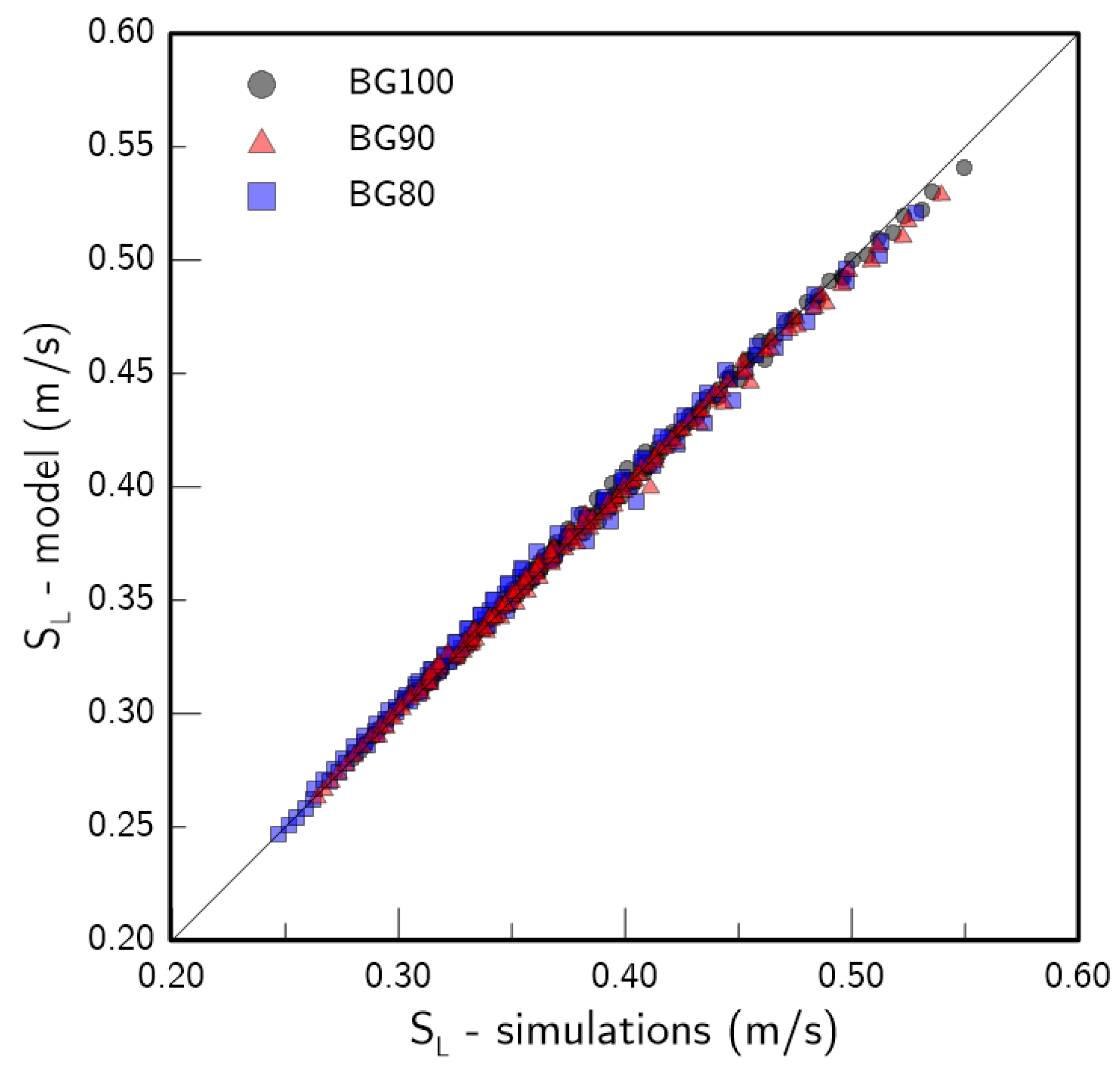
3.3. Validation
4. Conclusions
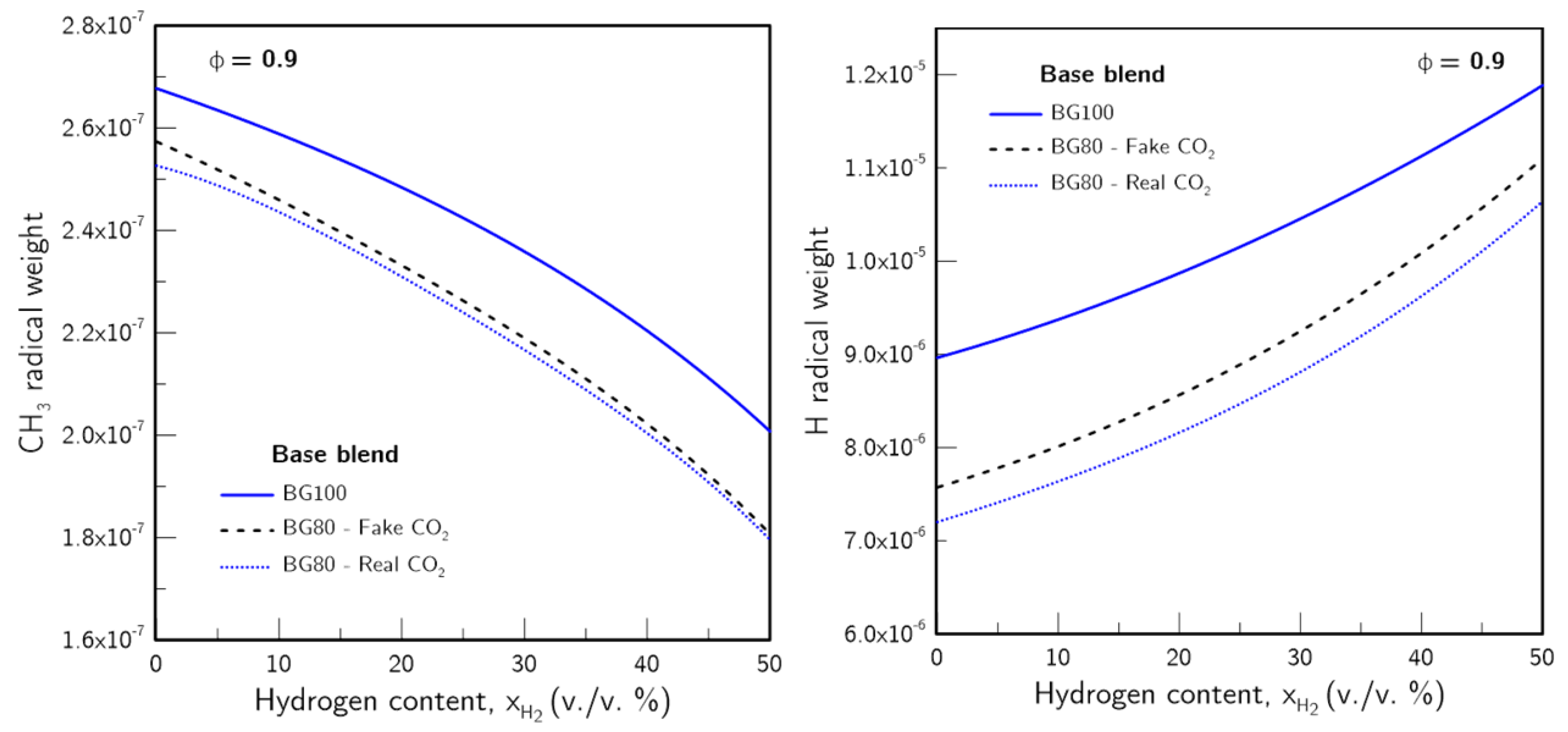
- The introduction of H2 in the fuel blends produced a greater effect on the higher the CO2 content was. Furthermore, the increase in due to H2 enrichment was non-linear, with a more pronounced effect the higher was. This was found to be linked with an increase in the H radical pool, which resulted from greater H production through the sub-mechanism of the water-gas shift (WGS) reaction.
- The presence of H2 in the fuel blend tended to mitigate the thermal-diffusive and concentration effects of CO2 on . These effects were the highest contributor for reduction for low . However, these gradually subsided as increased, while the CO2 chemistry maintained a consistent impact. Thus, for practical applications, higher amounts of H2-enrichment may avoid the need to completely remove carbon dioxide from biogas blends.
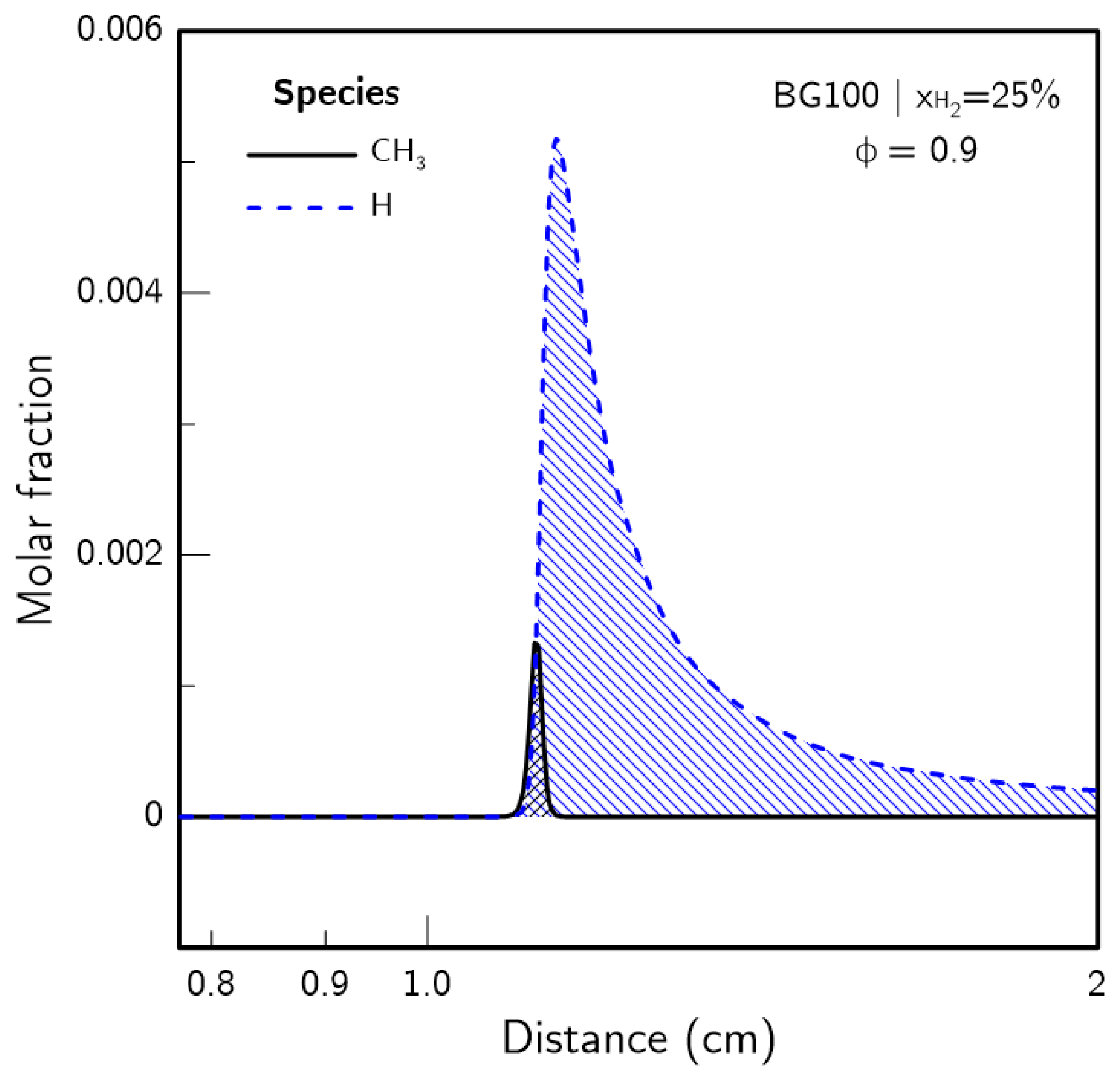
- H2 enrichment was found to weaken the CH4 related chemistry as the CH3 radical pool was significantly impacted. The reduction of the CH3 radical presence was found to be not directly proportional to the CH4 concentration, with an acceleration of its reduction with increasing .
- Based on the numerical results, a new correlation to model the impact of H2 enrichment on the of lean biogas/air flames was proposed:where is the laminar flame speed of the H2-enriched flame mixture, is the intermediate flame speed computed with Equation (4), is the CO2 content of the base biogas blend, is the hydrogen fraction in the flame mixture (Equation (5)) and is a fit parameter. The correlation exhibited good agreement with literature data and with simulations for all of the tested conditions. This new model isolates the contribution of H2 and CO2 as both are arguments of the function that yield . Thus, the final model can be directly used to estimate without the need for a priori adaptations of fit parameters for varying base blends as the correlation itself accomplishes that goal. The fast and reliable estimation of for H2-enriched biogas blends makes this model useful for the adaptation or development of existing devices that rely on these blends.
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| 1D | One-dimensional |
| BG | Biogas |
| EGR | Exhaust gas recirculation |
| FPF | Freely propagating flame |
| USC | University of Southern California |
| WGS | Water-gas shift |
References
- Gillingham, K.; Huang, P. Is abundant natural gas a bridge to a low-carbon future or a dead-end? Energy J. 2019, 40. [Google Scholar] [CrossRef]
- Speirs, J.; Balcombe, P.; Johnson, E.; Martin, J.; Brandon, N.; Hawkes, A. A greener gas grid: What are the options. Energy Policy 2018, 118, 291–297. [Google Scholar] [CrossRef]
- Glenk, G.; Reichelstein, S. Economics of converting renewable power to hydrogen. Nat. Energy 2019, 4, 216–222. [Google Scholar] [CrossRef]
- Lantz, M. Biogas in Sweden-Opportunities and Challenges from a Systems Perspective; Lund University: Lund, Sweden, 2013. [Google Scholar]
- Patricio, R.; Sales, A.; Sacramento, E.; De Lima, L.; Veziroglu, T. Wind hydrogen energy system and the gradual replacement of natural gas in the State of Ceará–Brazil. Int. J. Hydrogen Energy 2012, 37, 7355–7364. [Google Scholar] [CrossRef]
- Roubík, H.; Mazancová, J.; Le Dinh, P.; Dinh Van, D.; Banout, J. Biogas quality across small-scale biogas plants: A case of central Vietnam. Energies 2018, 11, 1794. [Google Scholar] [CrossRef]
- Korberg, A.D.; Skov, I.R.; Mathiesen, B.V. The role of biogas and biogas-derived fuels in a 100% renewable energy system in Denmark. Energy 2020, 199, 117426. [Google Scholar] [CrossRef]
- Saccani, C.; Pellegrini, M.; Guzzini, A. Analysis of the Existing Barriers for the Market Development of Power to Hydrogen (P2H) in Italy. Energies 2020, 13, 4835. [Google Scholar] [CrossRef]
- Quintino, F.M.; Fernandes, E.C. Interchangeability Analysis of Biogas and Hydrogen Blends. In Proceedings of the 27th European Biomass Conference and Exhibition Proceedings, Lisbon, Portugal, 27–30 May 2019; pp. 736–741. [Google Scholar] [CrossRef]
- Barlow, R.; Frank, J.; Karpetis, A.; Chen, J.Y. Piloted methane/air jet flames: Transport effects and aspects of scalar structure. Combust. Flame 2005, 143, 433–449. [Google Scholar] [CrossRef]
- Turns, S.R. Introduction to Combustion; McGraw-Hill Companies: New York, NY, USA, 1996; Volume 287. [Google Scholar]
- Lu, T.; Law, C.K. Toward accommodating realistic fuel chemistry in large-scale computations. Prog. Energy Combust. Sci. 2009, 35, 192–215. [Google Scholar] [CrossRef]
- Lu, T.; Law, C.K. A directed relation graph method for mechanism reduction. Proc. Combust. Inst. 2005, 30, 1333–1341. [Google Scholar] [CrossRef]
- Metghalchi, M.; Keck, J.C. Burning velocities of mixtures of air with methanol, isooctane, and indolene at high pressure and temperature. Combust. Flame 1982, 48, 191–210. [Google Scholar] [CrossRef]
- Gülder, O. Burning velocities of ethanol–air and ethanol–water–air mixtures. AIAA Progr Astronaut Aeronaut 1984, 95, 181–197. [Google Scholar]
- Liao, S.; Jiang, D.; Cheng, Q. Determination of laminar burning velocities for natural gas. Fuel 2004, 83, 1247–1250. [Google Scholar] [CrossRef]
- Han, P.; Checkel, M.D.; Fleck, B.A.; Nowicki, N.L. Burning velocity of methane/diluent mixture with reformer gas addition. Fuel 2007, 86, 585–596. [Google Scholar] [CrossRef]
- Elia, M.; Ulinski, M.; Metghalchi, M. Laminar burning velocity of methane–air–diluent mixtures. J. Eng. Gas Turbines Power 2001, 123, 190–196. [Google Scholar] [CrossRef]
- Bougrine, S.; Richard, S.; Nicolle, A.; Veynante, D. Numerical study of laminar flame properties of diluted methane-hydrogen-air flames at high pressure and temperature using detailed chemistry. Int. J. Hydrogen Energy 2011, 36, 12035–12047. [Google Scholar] [CrossRef]
- Quintino, F.; Fernandes, E. Analytical correlation to model diluent concentration repercussions on the burning velocity of biogas lean flames: Effect of CO2 and N2. Biomass Bioenergy 2018, 119, 354–363. [Google Scholar] [CrossRef]
- Goodwin, D.G.; Moffat, H.K.; Speth, R.L. Cantera: An Object-Oriented Software Toolkit for Chemical Kinetics, Thermodynamics, and Transport Processes; Caltech: Pasadena, CA, USA, 2009. [Google Scholar]
- Wang, H.; You, X.; Joshi, A.V.; Davis, S.G.; Laskin, A.; Egolfopoulos, F.; Law, C.K.; Version II, U.M. High-Temperature Combustion Reaction Model of H2. Technical Report; CO/C1–C4 Compounds. 2007. Available online: http://ignis.usc.edu/Mechanisms/USC-MechII/USC_MechII.htm (accessed on 14 December 2020).
- Zhang, Y.; Jiang, X.; Wei, L.; Zhang, J.; Tang, C.; Huang, Z. Experimental and modeling study on auto-ignition characteristics of methane/hydrogen blends under engine relevant pressure. Int. J. Hydrogen Energy 2012, 37, 19168–19176. [Google Scholar] [CrossRef]
- Ren, F.; Chu, H.; Xiang, L.; Han, W.; Gu, M. Effect of hydrogen addition on the laminar premixed combustion characteristics the main components of natural gas. J. Energy Inst. 2019, 92, 1178–1190. [Google Scholar] [CrossRef]
- Qian, Y.; Sun, S.; Ju, D.; Shan, X.; Lu, X. Review of the state-of-the-art of biogas combustion mechanisms and applications in internal combustion engines. Renew. Sustain. Energy Rev. 2017, 69, 50–58. [Google Scholar] [CrossRef]
- Xie, Y.; Wang, J.; Xu, N.; Yu, S.; Huang, Z. Comparative study on the effect of CO2 and H2O dilution on laminar burning characteristics of CO/H2/air mixtures. Int. J. Hydrogen Energy 2014, 39, 3450–3458. [Google Scholar] [CrossRef]
- Chen, Z.; Tang, C.; Fu, J.; Jiang, X.; Li, Q.; Wei, L.; Huang, Z. Experimental and numerical investigation on diluted DME flames: Thermal and chemical kinetic effects on laminar flame speeds. Fuel 2012, 102, 567–573. [Google Scholar] [CrossRef]
- Stylianidis, N.; Azimov, U.; Birkett, M. Investigation of the effect of hydrogen and methane on combustion of multicomponent syngas mixtures using a constructed reduced chemical kinetics mechanism. Energies 2019, 12, 2442. [Google Scholar] [CrossRef]
- Law, C.K. Combustion Physics; Cambridge University Press: Cambridge, UK, 2010. [Google Scholar]
- Wei, Z.; He, Z.; Zhen, H.; Zhang, X.; Chen, Z.; Huang, Z. Kinetic modeling investigation on the coupling effects of H2 and CO2 addition on the laminar flame speed of hydrogen enriched biogas mixture. Int. J. Hydrogen Energy 2020, 45, 27891–27903. [Google Scholar] [CrossRef]
- Glarborg, P.; Bentzen, L.L. Chemical effects of a high CO2 concentration in oxy-fuel combustion of methane. Energy Fuels 2008, 22, 291–296. [Google Scholar] [CrossRef]
- Xie, Y.; Wang, J.; Zhang, M.; Gong, J.; Jin, W.; Huang, Z. Experimental and numerical study on laminar flame characteristics of methane oxy-fuel mixtures highly diluted with CO2. Energy Fuels 2013, 27, 6231–6237. [Google Scholar] [CrossRef]
- Westbrook, C.K.; Dryer, F.L. Chemical kinetic modeling of hydrocarbon combustion. Prog. Energy Combust. Sci. 1984, 10, 1–57. [Google Scholar] [CrossRef]
- Halter, F.; Foucher, F.; Landry, L.; Mounaïm-Rousselle, C. Effect of dilution by nitrogen and/or carbon dioxide on methane and iso-octane air flames. Combust. Sci. Technol. 2009, 181, 813–827. [Google Scholar] [CrossRef]
- Rocha, N.; Quintino, F.; Fernandes, E. H2 enrichment impact on the chemiluminescence of biogas/air premixed flames. Int. J. Hydrogen Energy 2020, 45, 3233–3250. [Google Scholar] [CrossRef]
- Zhang, X.; Mei, B.; Ma, S.; Pan, H.; Wang, H.; Li, Y. Experimental and kinetic modeling investigation on laminar flame propagation of CH4/CO mixtures at various pressures: Insight into the transition from CH4 related chemistry to CO related chemistry. Combust. Flame 2019, 209, 481–492. [Google Scholar] [CrossRef]
- Coppens, F.; De Ruyck, J.; Konnov, A.A. Effects of hydrogen enrichment on adiabatic burning velocity and NO formation in methane+ air flames. Exp. Therm. Fluid Sci. 2007, 31, 437–444. [Google Scholar] [CrossRef]
- Hermanns, R.T.E. Laminar Burning Velocities of Methane-Hydrogen-Air Mixtures. Ph.D. Thesis, Technische Universiteit Eindhoven, Eindhoven, The Netherlands, 2007. [Google Scholar]
- Halter, F.; Chauveau, C.; Djebaïli-Chaumeix, N.; Gökalp, I. Characterization of the effects of pressure and hydrogen concentration on laminar burning velocities of methane–hydrogen–air mixtures. Proc. Combust. Inst. 2005, 30, 201–208. [Google Scholar] [CrossRef]
- Yadav, V.K.; Ray, A.; Ravi, M. Experimental and computational investigation of the laminar burning velocity of hydrogen-enriched biogas. Fuel 2019, 235, 810–821. [Google Scholar] [CrossRef]
- Smith, G.P.; Golden, D.M.; Frenklach, M.; Moriarty, N.W.; Eiteneer, B.; Goldenberg, M.; Bowman, C.T.; Hanson, R.K.; Song, S.; Gardiner, W., Jr.; et al. GRI-Mech 3.0, 1999. 2011. Available online: http://www.me.berkeley.edu/gri_mech (accessed on 14 December 2020).
- Jones, D.R.; Al-Masry, W.A.; Dunnill, C.W. Hydrogen-enriched natural gas as a domestic fuel: An analysis based on flash-back and blow-off limits for domestic natural gas appliances within the UK. Sustain. Energy Fuels 2018, 2, 710–723. [Google Scholar] [CrossRef]
- De Vries, H.; Mokhov, A.V.; Levinsky, H.B. The impact of natural gas/hydrogen mixtures on the performance of end-use equipment: Interchangeability analysis for domestic appliances. Appl. Energy 2017, 208, 1007–1019. [Google Scholar] [CrossRef]


| Country | Electrolyser Capacity (GW) | Hydrogen Refuelling Stations | Hydrogen Share in Gas Networks (%) |
|---|---|---|---|
| France | 6.5 | 400–1000 | - |
| Germany | 5 | - | - |
| Japan | - | 900 | - |
| Netherlands | 3–4 | - | - |
| New Zealand | - | - | 20 |
| Portugal | 2–2.5 | 50–100 | 10–15 |
| South Korea | - | 310–1200 | - |
| Spain | 4 | 100–150 | - |
| Authors | Method | p | Ref. | ||
|---|---|---|---|---|---|
| Hermanns | Heat flux burner | 0.8, 0.9, 1.0 | atm | K | [38] |
| Coppens et al. | Heat flux burner | 0.8, 0.9, 1.0 | atm | K | [37] |
| Halter et al. | Constant volume bomb | 0.8, 0.9, 1.0 | bar | K | [39] |
| Yadav et al. | Flat flame burner and Ansys (GRI3.0) | 0.8, 0.9, 1.0 | bar | K | [40] |
| Wei et al. | Premix Code (GRI3.0) | 0.9, 1.0 | atm | K | [30] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Quintino, F.M.; Fernandes, E.C. Numerical Investigation of the Impact of H2 Enrichment on Lean Biogas/Air Flames: An Analytical Modelling Approach. Energies 2021, 14, 369. https://doi.org/10.3390/en14020369
Quintino FM, Fernandes EC. Numerical Investigation of the Impact of H2 Enrichment on Lean Biogas/Air Flames: An Analytical Modelling Approach. Energies. 2021; 14(2):369. https://doi.org/10.3390/en14020369
Chicago/Turabian StyleQuintino, Filipe M., and Edgar C. Fernandes. 2021. "Numerical Investigation of the Impact of H2 Enrichment on Lean Biogas/Air Flames: An Analytical Modelling Approach" Energies 14, no. 2: 369. https://doi.org/10.3390/en14020369
APA StyleQuintino, F. M., & Fernandes, E. C. (2021). Numerical Investigation of the Impact of H2 Enrichment on Lean Biogas/Air Flames: An Analytical Modelling Approach. Energies, 14(2), 369. https://doi.org/10.3390/en14020369






