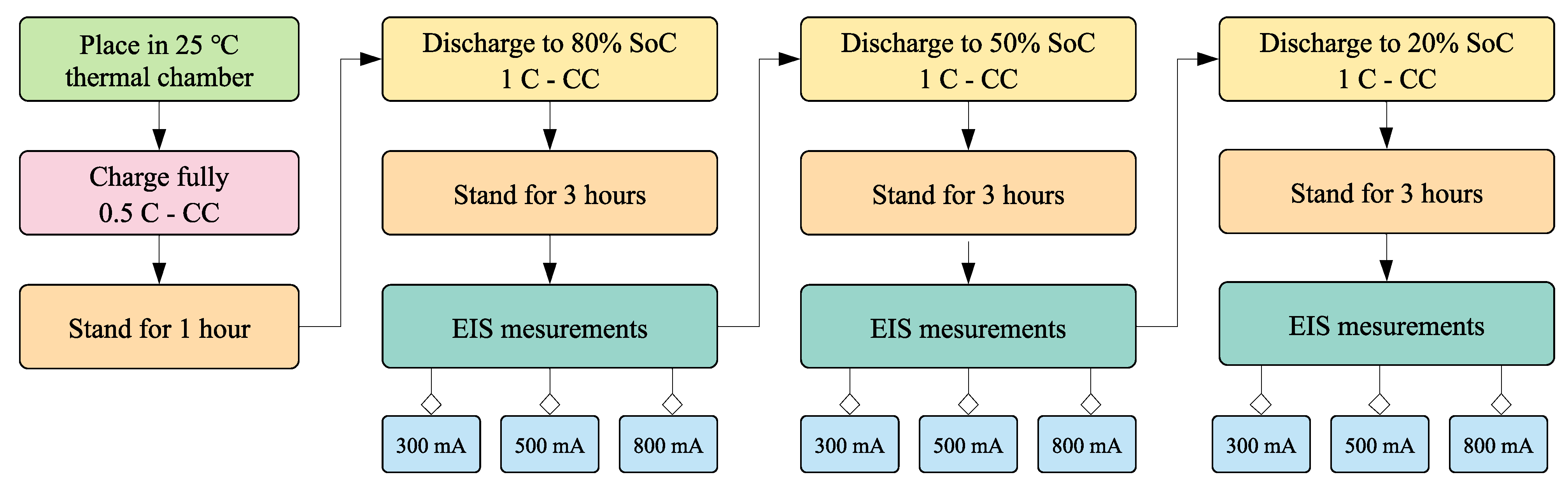
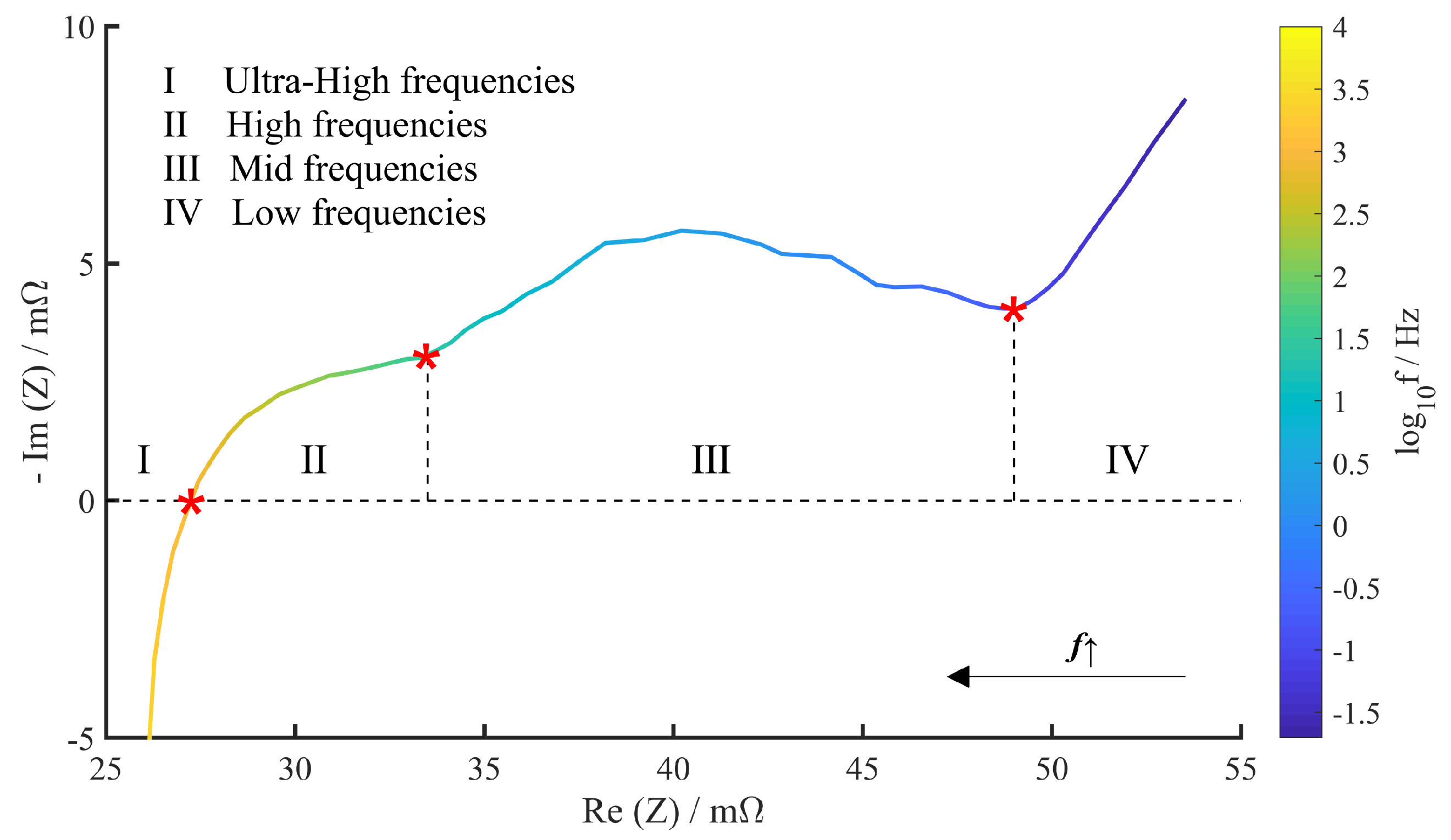
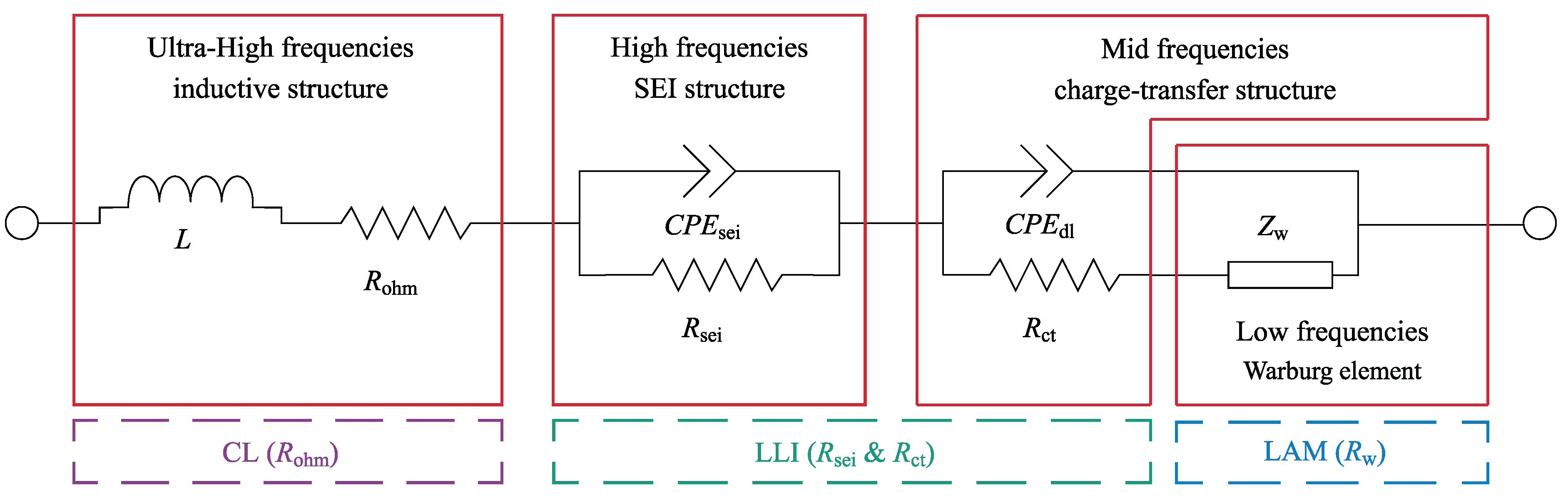
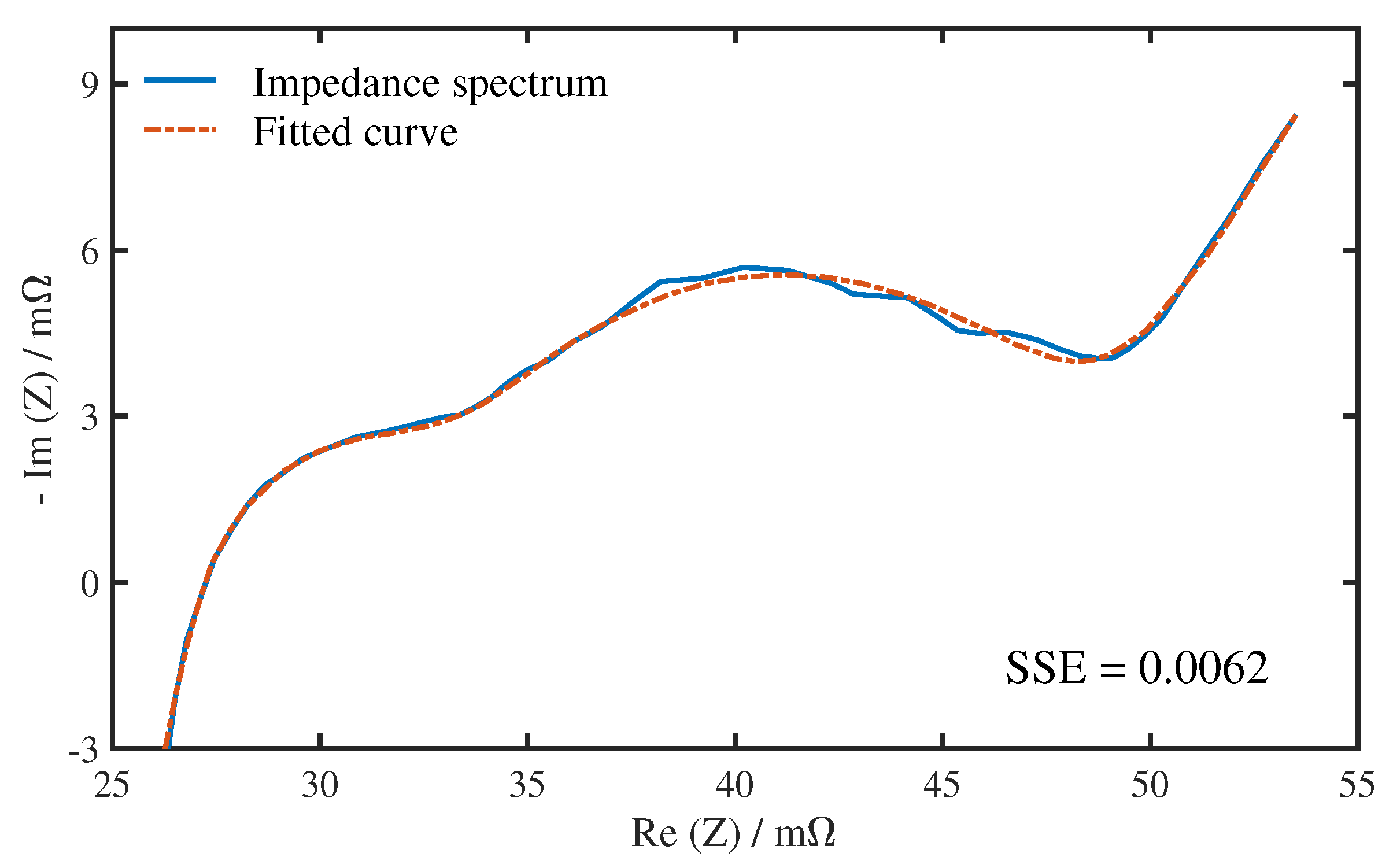
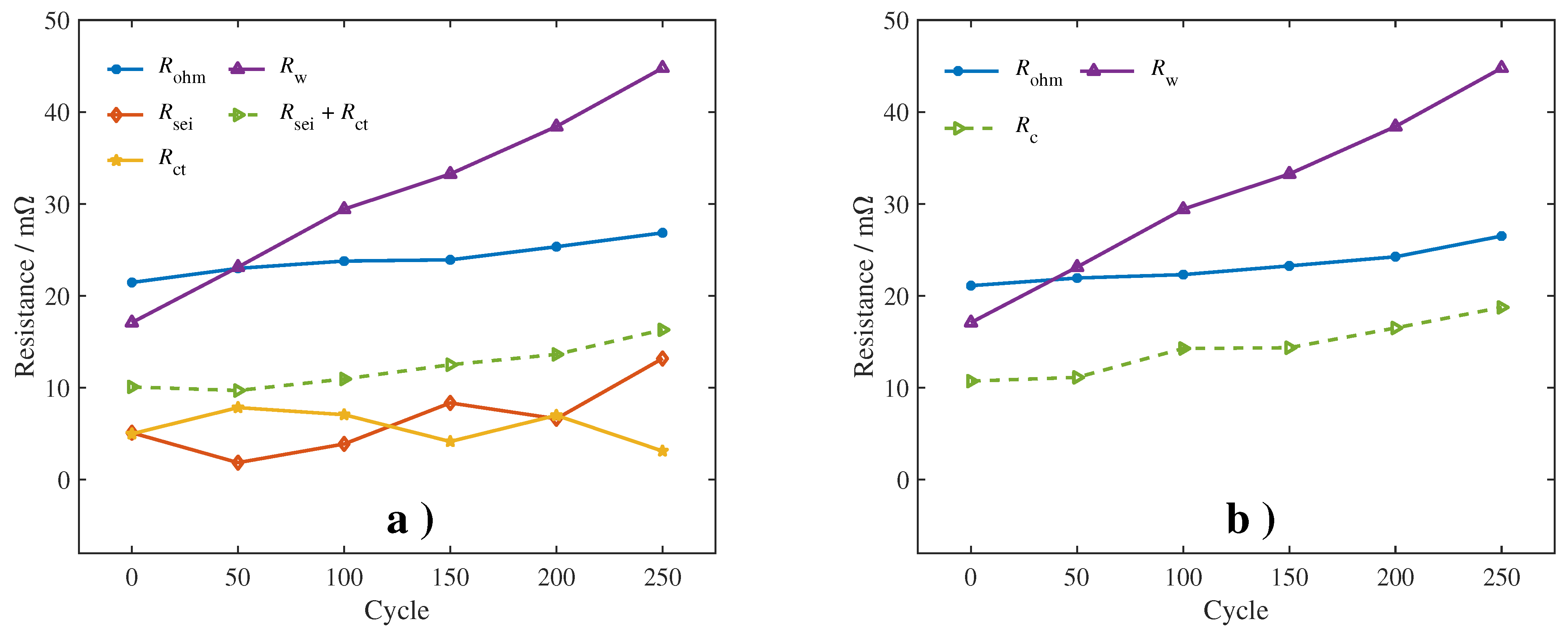
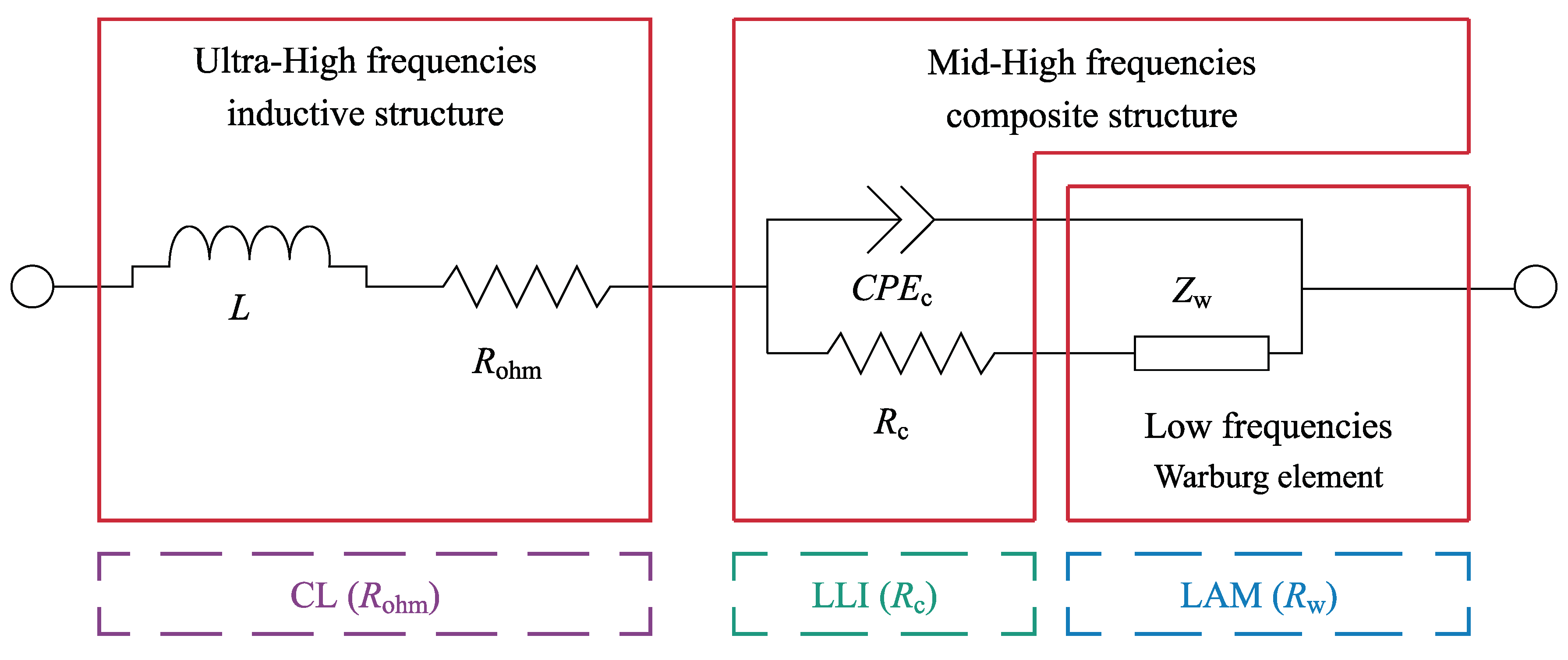
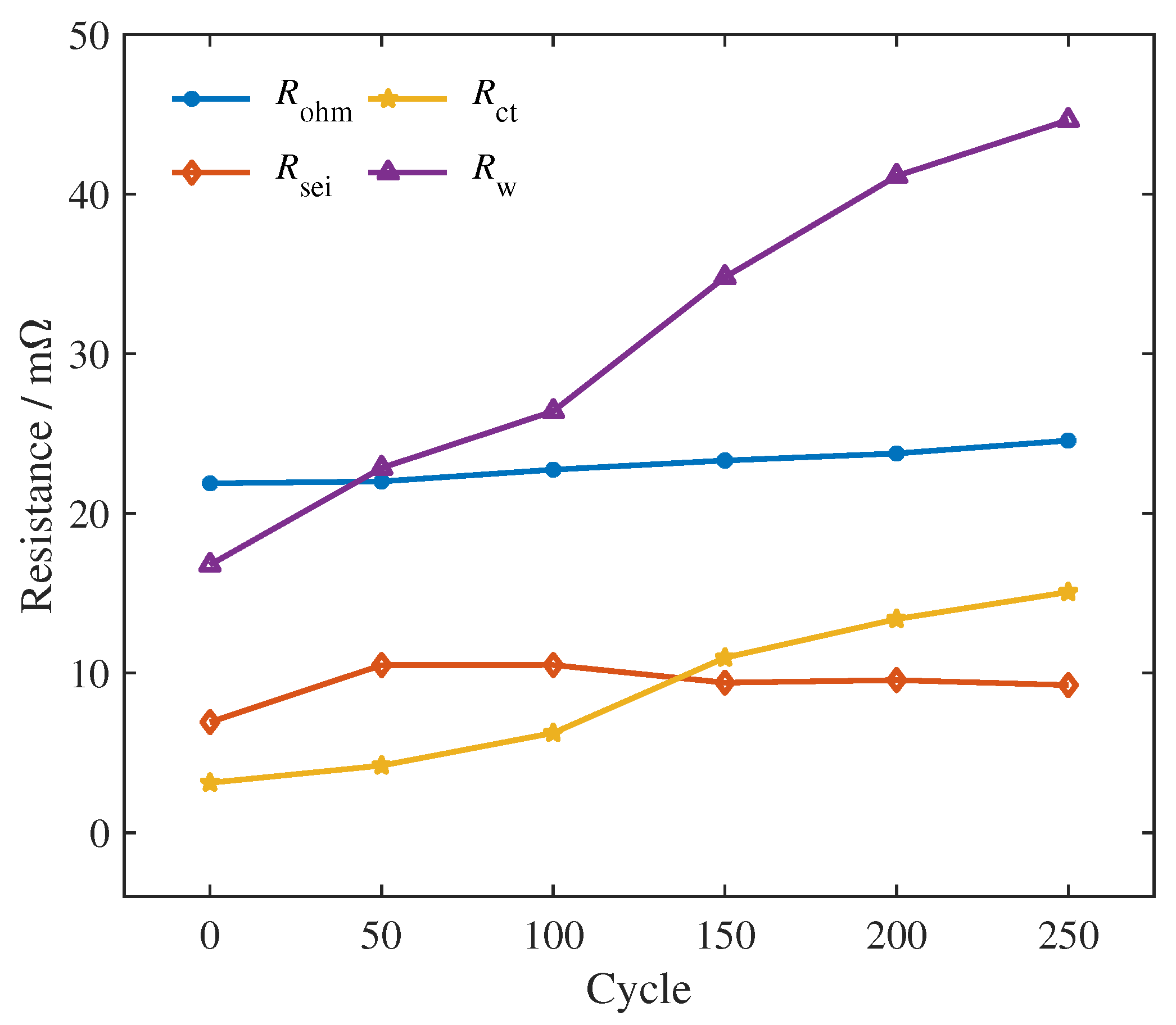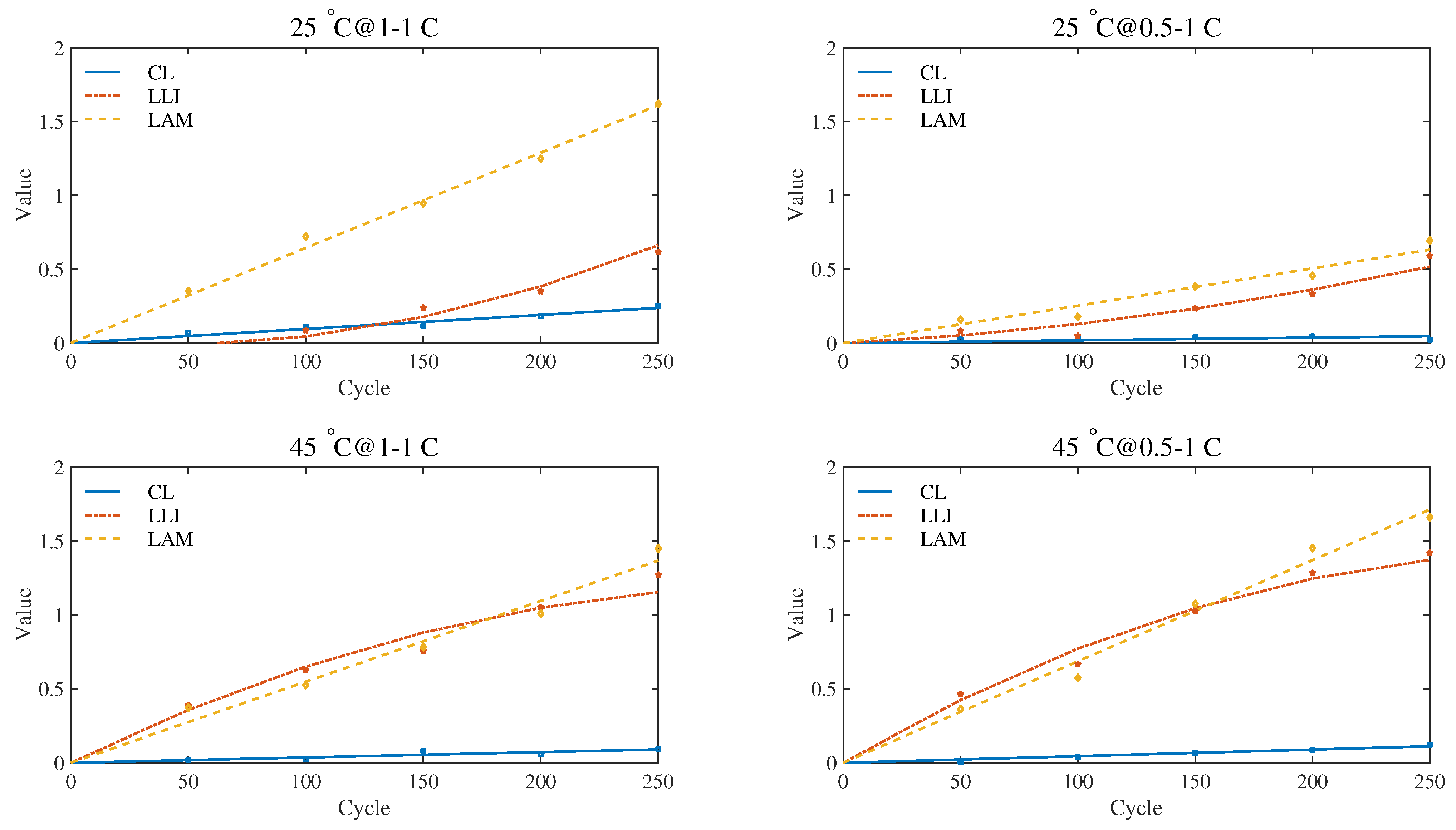4.1. Quantitative Results of Degradation Modes under Different Conditions
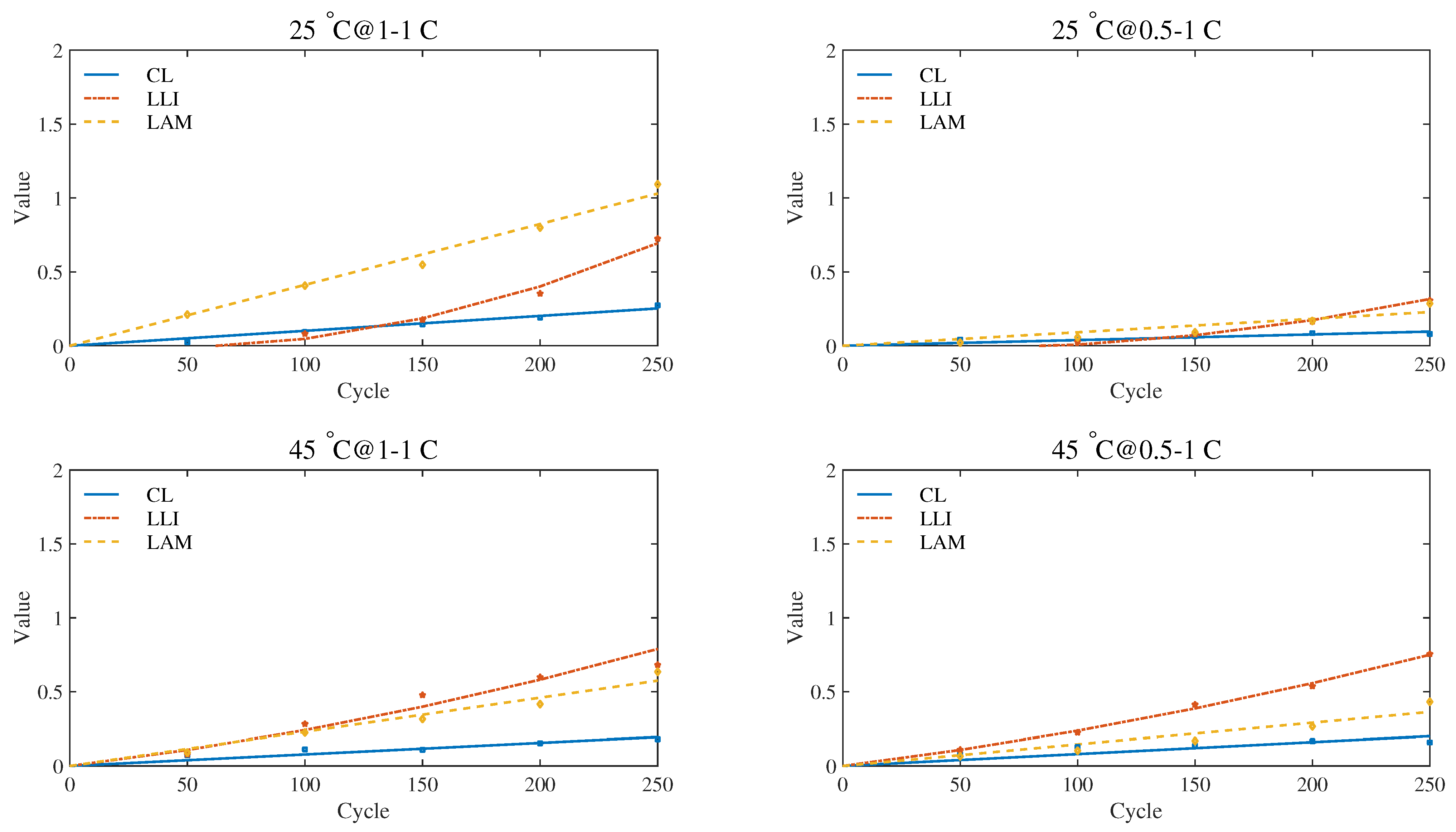
According to the quantification schemes proposed above, the quantitative values of the degradation modes in the first 250 test cycles of the four experimental groups were calculated. When EIS measurement condition is uniformly selected with 500 mA current excitation, the quantitative results recorded at 20%, 50% and 80% SoC are plotted in
Figure 10,
Figure 11 and
Figure 12, respectively.
Observing the quantitative results, we found that, as the aging process progresses, the quantitative values of the three modes show a clear upward trend. Compared with CL, LLI and LAM show more significant growth. This result is consistent with previous studies on aging mechanisms and degradation modes of lithium-ion batteries [
20,
33,
34]. The rapid rise of LLI is mainly due to the continuous growth of SEI films on the surface of the anode materials particles, which is also considered as the main reason for capacity fade in previous studies [
35]. However, we found that LAM occupied a similar proportion with LLI, sometimes even higher. This phenomenon has a lot to do with the charge and discharge mode of the cyclic life tests we set, making the batteries always work in a wide SoC range. Frequent deep insertion and extraction of lithium ions will induce greater mechanical stress within the active material particles, which further causing more damage to the electrode material, such as lithiated material loss and particle cracking [
19].
Meanwhile, the impact of the operating conditions on the aging mechanism is also well reflected by the quantitative results. Compared with the conditions at 25
C, the values of LLI change more significantly at 45
C. An important reason for that is the SEI layer on the graphite electrode grows faster at elevated temperatures [
36]. The influence of charging current mainly manifests in LAM. As the current decreases, the impact applied to the electrode material during the charging process will be weakened. Accordingly, the increase in the values of LAM is slowed down. CL also shows dependency on charging current and is accelerating at high currents. This result is similar to the ohmic resistance change for various charging rates obtained by Schuster et al. [
37]. It indicates that the reaction between the current collector and the electrolyte components is directly or indirectly promoted. Although previous studies [
38] have shown that high charging current will also aggravate some side reactions that consume the lithium inventory, such as lithium plating, there is no significant difference in LLI between 0.5 and 1 C. Some scholars [
39] have drawn a similar conclusion through ICA. One possible explanation is that a charging current setting of no more than 1 C is not enough to trigger obvious side reactions.
4.2. Effect of SoC and Current Excitation on Quantitative Results
When quantifying the degradation modes, the measuring conditions of EIS were strictly controlled. Different SoC and excitation amplitudes may affect the impedance spectrum curve, which will further affect the resistance values obtained by the equivalent circuit fitting and make the quantitative results change.
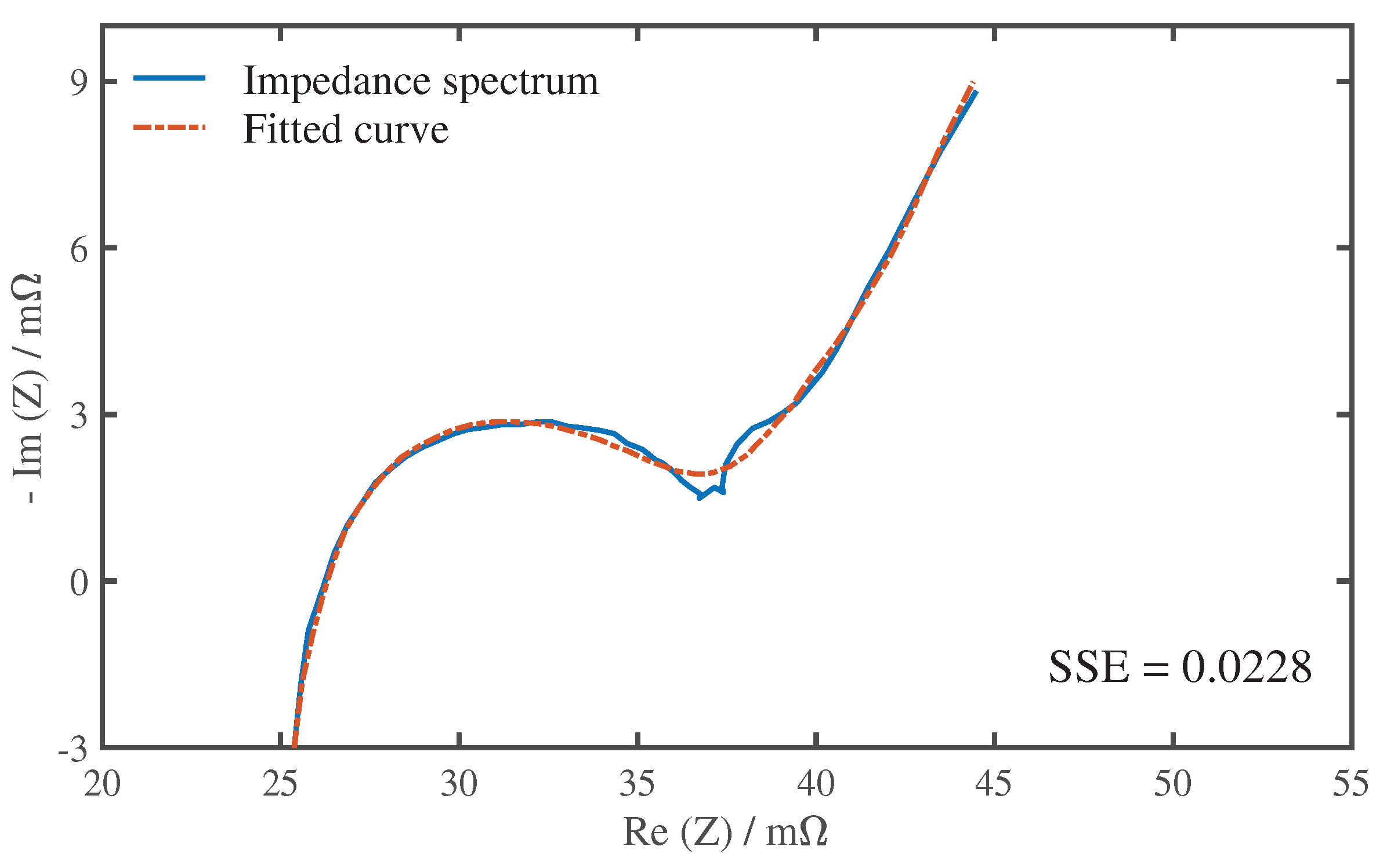
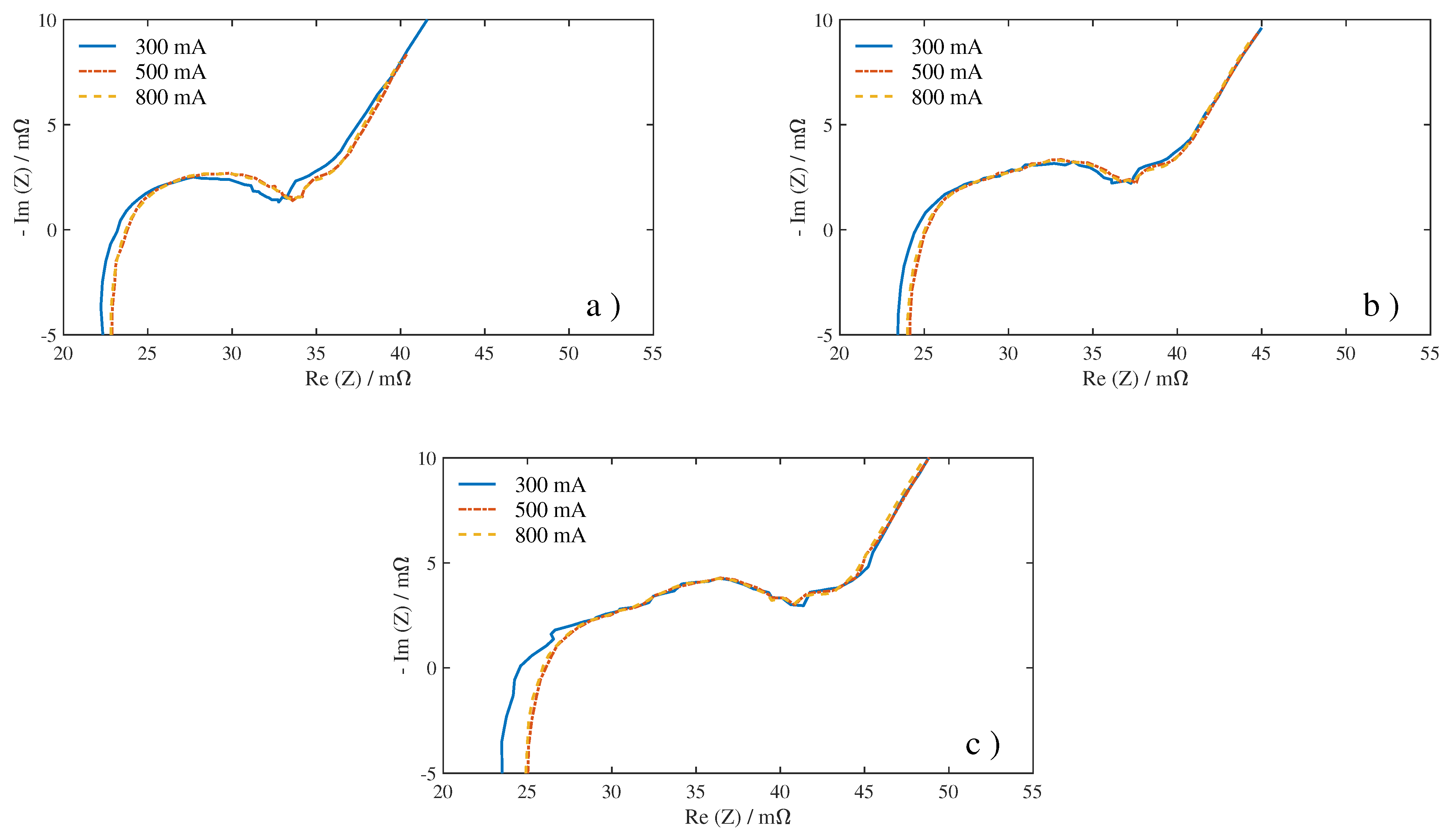
First, we analyzed the influence of SoC and excitation current amplitude on the impedance spectra. The impedance measuring results under 45
C@0.5–1 C condition at different SoCs are shown in
Figure 13, and the current excitation amplitude is uniformly selected as 500 mA. It can be seen that there is a clear difference between the curves of the three SoCs, especially in mid frequencies and low frequencies. This result is consistent with other studies on EIS. Andre et al. [
26] found that the first semi-circle of the spectrum curve shows no significant SoC dependency, but the second semi-circle will spread strongly with declining SoC for SoCs lower than 30%. Since the second semi-circle is closely related to
, this phenomenon can also be explained from the perspective of the battery model. Wang et al. [
28] conducted a model derivation on the relationship between
and SoC based on the dynamic of the electrode processes, and the results show that
will increase significantly at low SoC.
The impedance measuring results under 45
C@0.5–1 C condition with different current excitations are illustrated in
Figure 14, and the SoC is uniformly selected as 80%. The curves of the three excitation amplitudes roughly overlap together, which means that the excitation has little effect on the change of the impedance spectra. However, compared with 500 and 800 mA, the impedance curve under 300 mA excitation has obvious glitches and bulges. It indicates that the measurement under low current excitation is more sensitive to external noise signals. To avoid the deviation caused by noise, we only consider 500 and 800 mA in the subsequent analysis of the effect of excitation on the quantitative results.
Then, based on the analysis of the impedance spectra, we used two-way analysis of variance (ANOVA) to study and analyze the effect of SoC and current excitation on the quantitative results. ANOVA is a procedure for determining whether variation in the response variable arises within or among different experimental groups [
40]. Here, we have two potential influence factors: SoC and excitation current. ANOVA takes the variation due to the factor or interaction and compares it to the variation due to error. If the ratio of the two variations is high, then the effect of the factor or the interaction effect is statistically significant. The statistical significance can be measured using an
F-statistic that has an
F-distribution [
41,
42]. A
p-value < 0.05 for the
F-statistic was considered to be significant. The statistical analysis was accomplished using the function
anova2 in Matlab R2018a (The MathWorks, Inc., Natick, MA, USA). For the quantitative results calculated every 50 cycles in the first 400 cycles under 45
C@0.5–1 C condition, the statistical results are summarized in
Table 4. The definitions of the output arguments are listed in
Table 5.
For all degradation modes, the F-statistics corresponding to the SoC factor are far more than one, indicating that the influence of SoC is out of the range of random errors. The p-value for CL is between 0.01 and 0.05, which corresponds to a significant level of more than 95%. The F-statistics and significant level (99%) for LLI and LAM are much higher.
Regarding the excitation factor, the F-statistics of the three modes are all less than one, and the corresponding p-values also far exceed 0.05, which shows that the change of excitation has no significant effect on the quantitative results of each mode.
In addition, for the interaction between SoC and excitation, although the F-statistic value exceeds 1 in CL, the corresponding p-value is higher than 0.05. Therefore, it can be considered that there is no evidence of the interaction effect of the two.
The results of two-way ANOVA show that there is a correlation between SoC and the quantitative results of the degradation modes, especially for LAM and LLI. Next, we further analyzed the specific differences between different SoCs through multiple comparisons using the function
multcompare in Matlab R2018a (The MathWorks, Inc., Natick, MA, USA). Multiple comparisons were used to judge whether there is a difference between the estimated group means [
43], giving a 95% confidence interval for the true mean difference. The
p-value is still used for a hypothesis test that the corresponding mean difference is equal to zero. A
p-value < 0.05 was considered a significant difference between the two groups. The results under 45
C@0.5–1 C condition and 500 mA excitation are summarized in
Table 6.
Observing the data, we found that the difference mainly existed between low SoC (20%) and medium-high SoC (50%, 80%). There is a very significant statistical difference in LLI and LAM values between 20% SoC and the other two SoCs, featured with the confidence intervals excluding zero and the
p-values less than 0.01. In contrast, the difference between 50% SoC and 80% SoC is not significant (
p-value ≫ 0.05). Combined with the quantitative results of the same condition at different SoCs shown in
Figure 10,
Figure 11 and
Figure 12, this difference is mainly manifested in the larger LAM and LLI values at 20% SoC, which is in agreement with the study of Pastor-Fernández et al. [
10] under 25
C@0.5–1 C condition. This phenomenon is related to the characteristics of the impedance spectrum, as described above. Due to the expansion of the second semi-circle, the fitted values of
(
) and
are significantly different at 20% SoC, and then bring the difference in the quantified values of LLI and LAM. For CL, the difference is principally between 20% SoC and 50% SoC (0.01 <
p-value < 0.05), but, overall, the significant level is much lower than the other two modes. Combining the quantification method, this result is determined by the characteristics of
, which shows no significant dependency on SoC [
44].