Combustion Behavior and Kinetics Analysis of Isothermal Oxidized Oils from Fengcheng Extra-Heavy Oil
Abstract
:1. Introduction
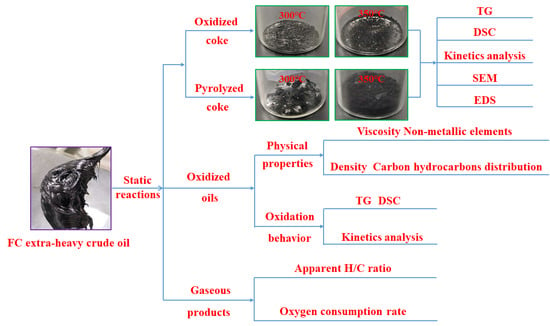
2. Experimental
2.1. Materials
2.2. Static Isothermal Oxidation Experiments and Physical Property Analysis
2.3. TG and DSC Analysis
2.4. Kinetics Theory
3. Results
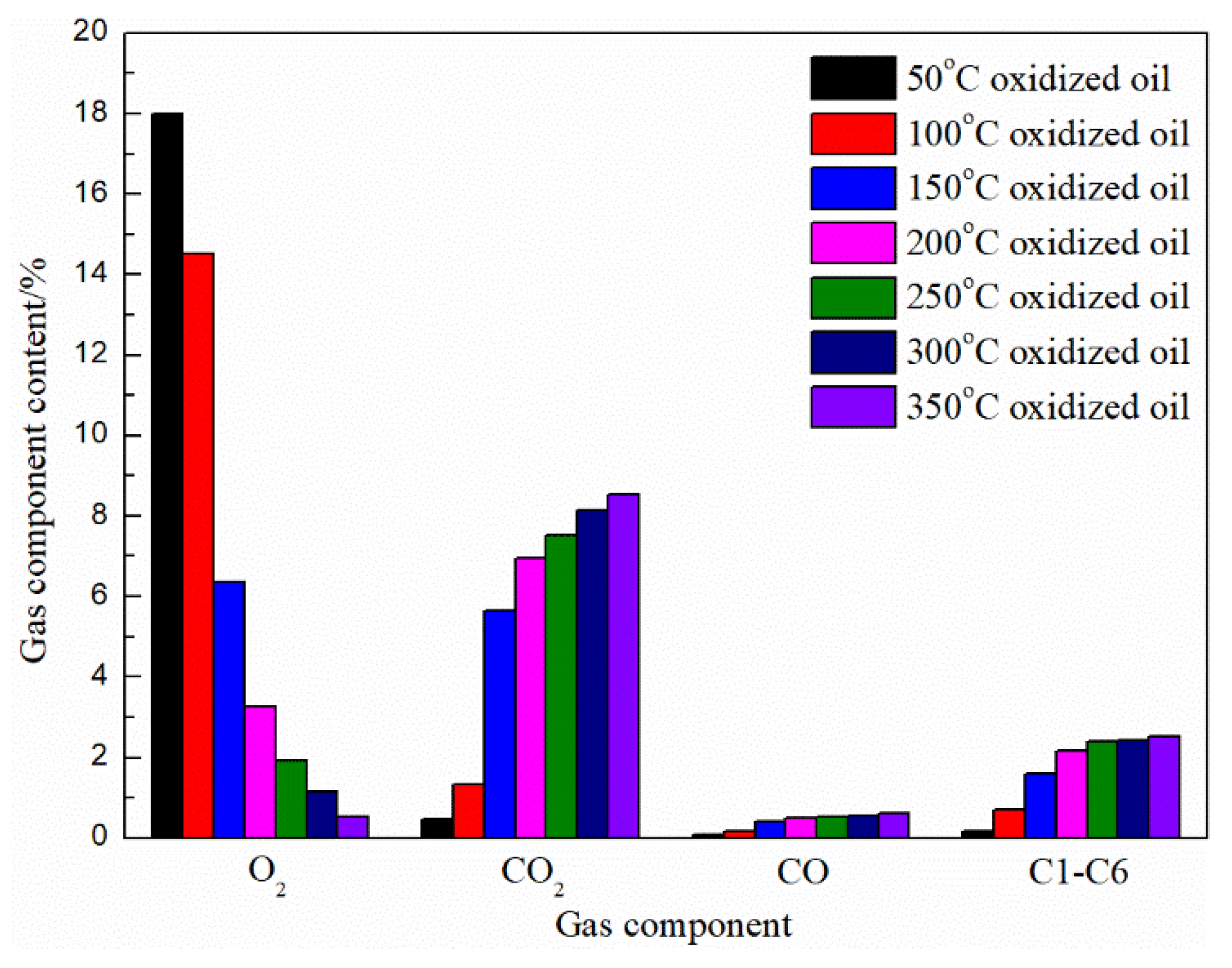
3.1. Analysis of the Gaseous Products
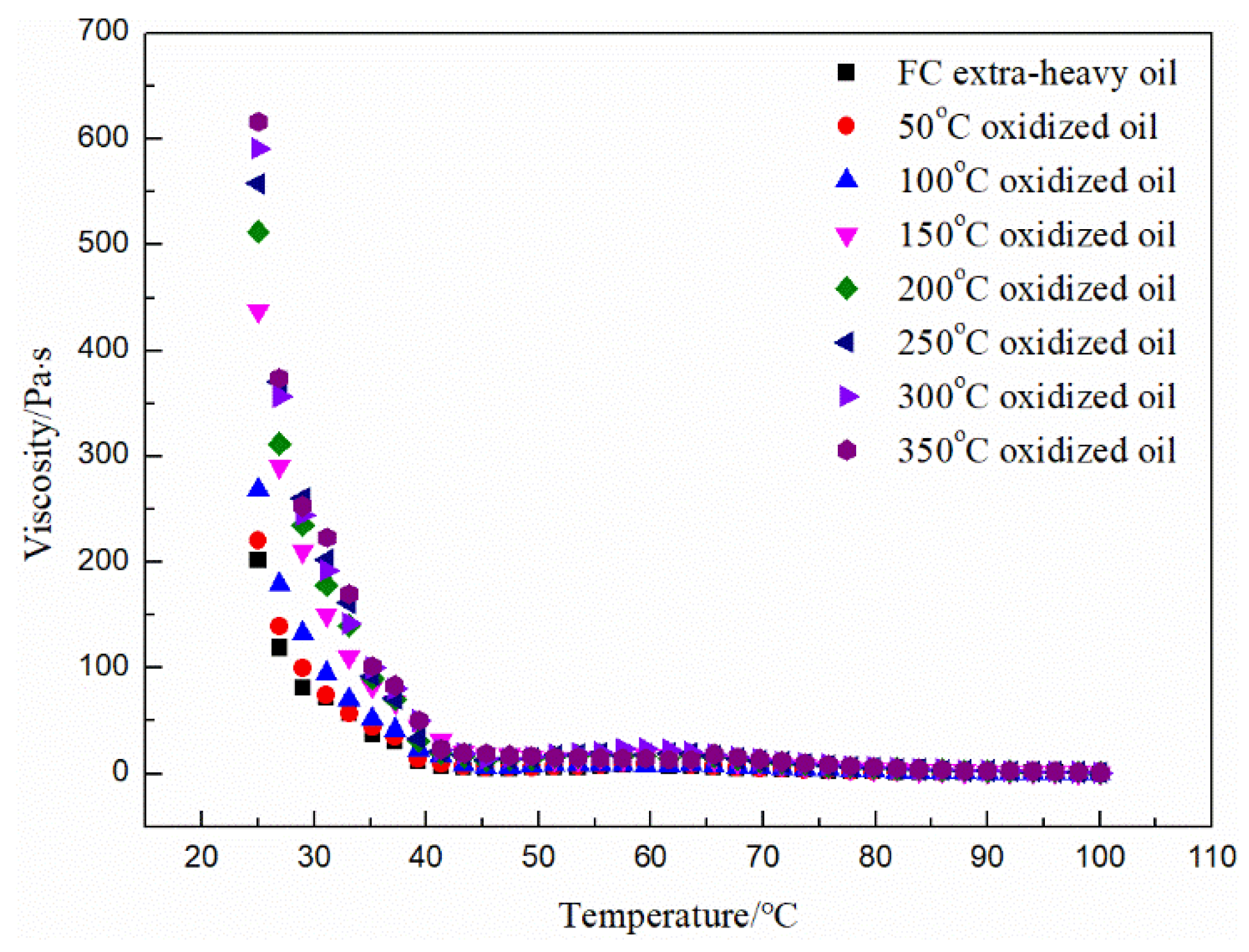
3.2. Basic Property Analysis of the Oxidized Oils
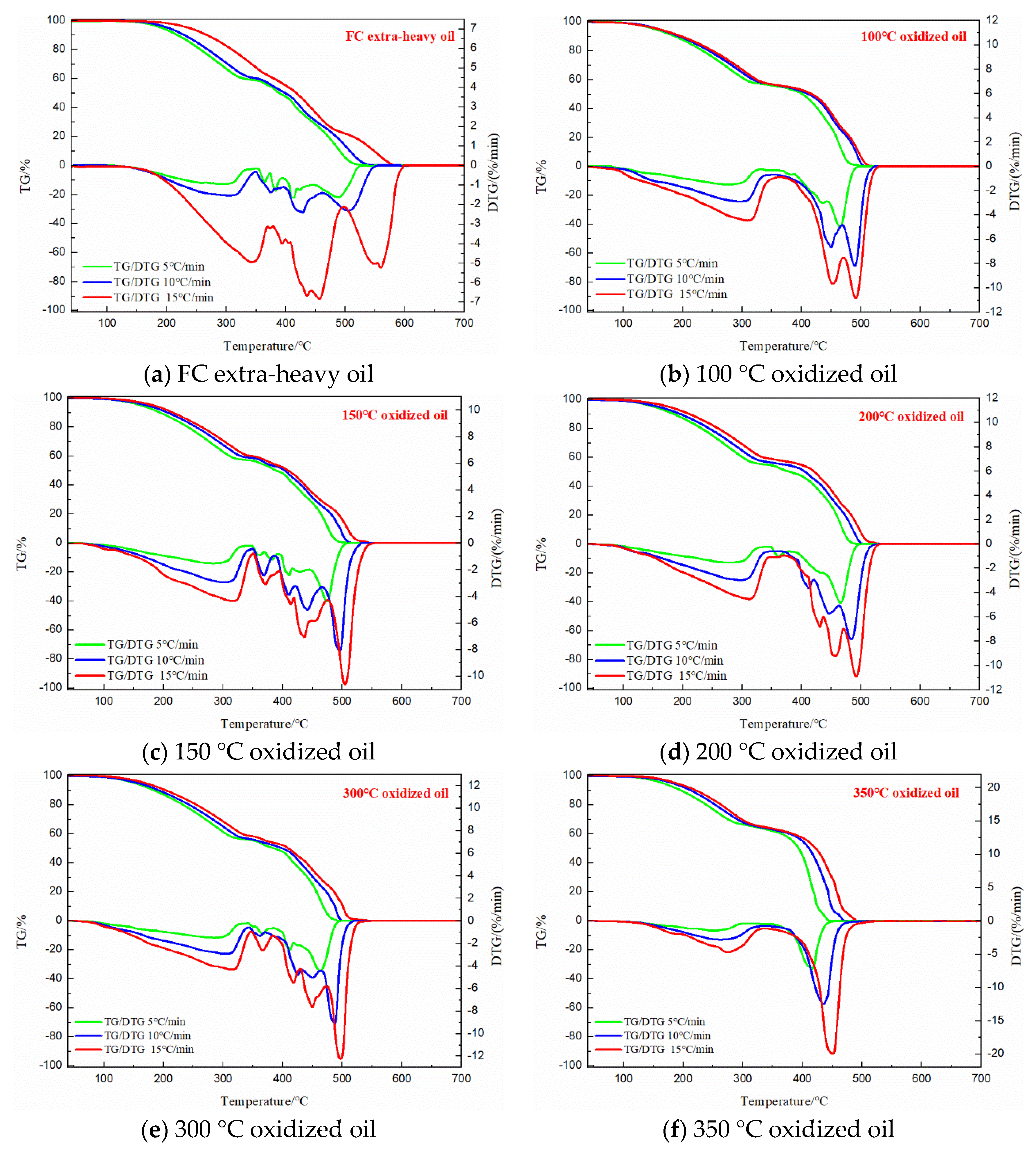
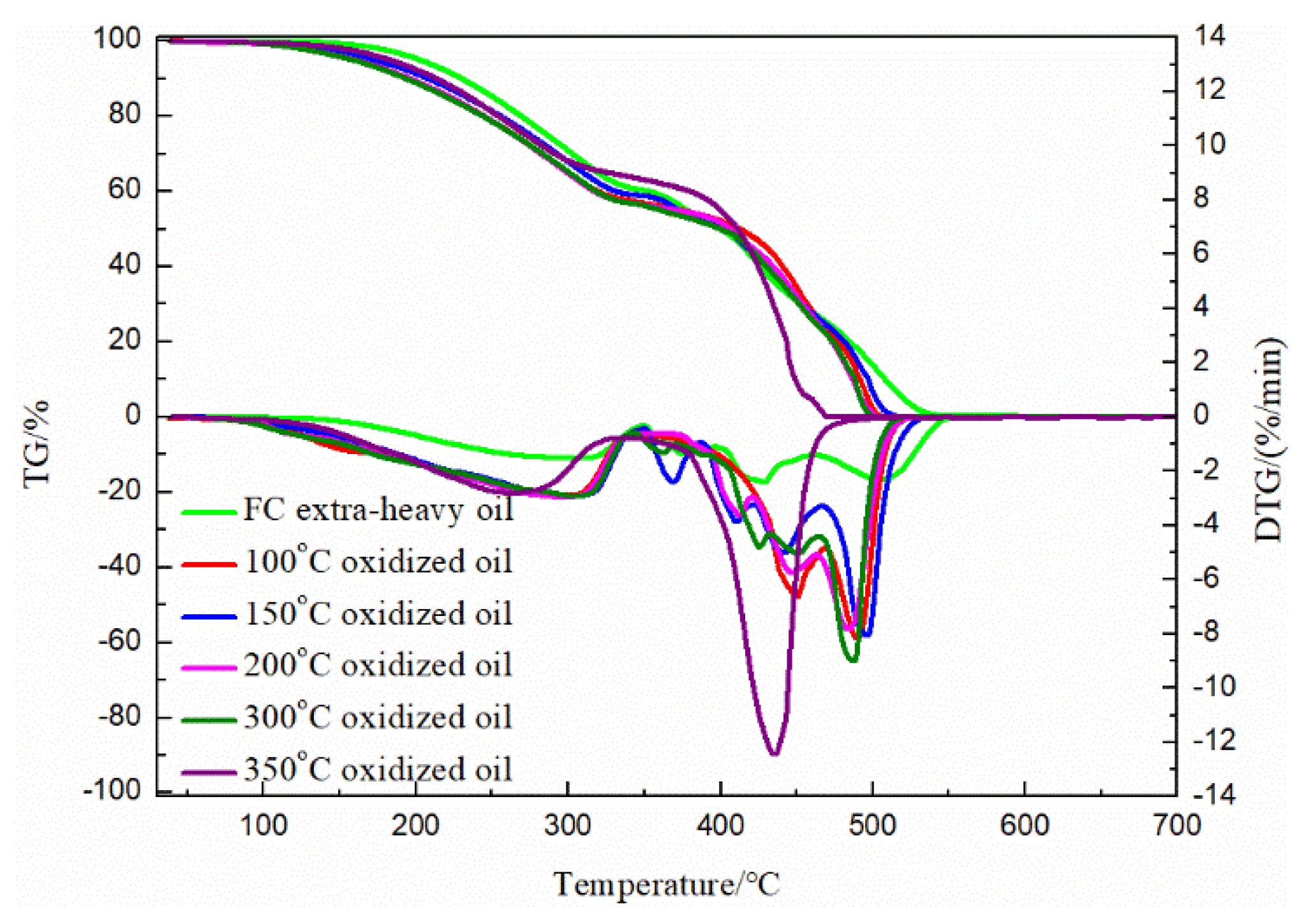
3.3. TG Analysis of the FC Extra-Heavy Oil and Oxidized Oils
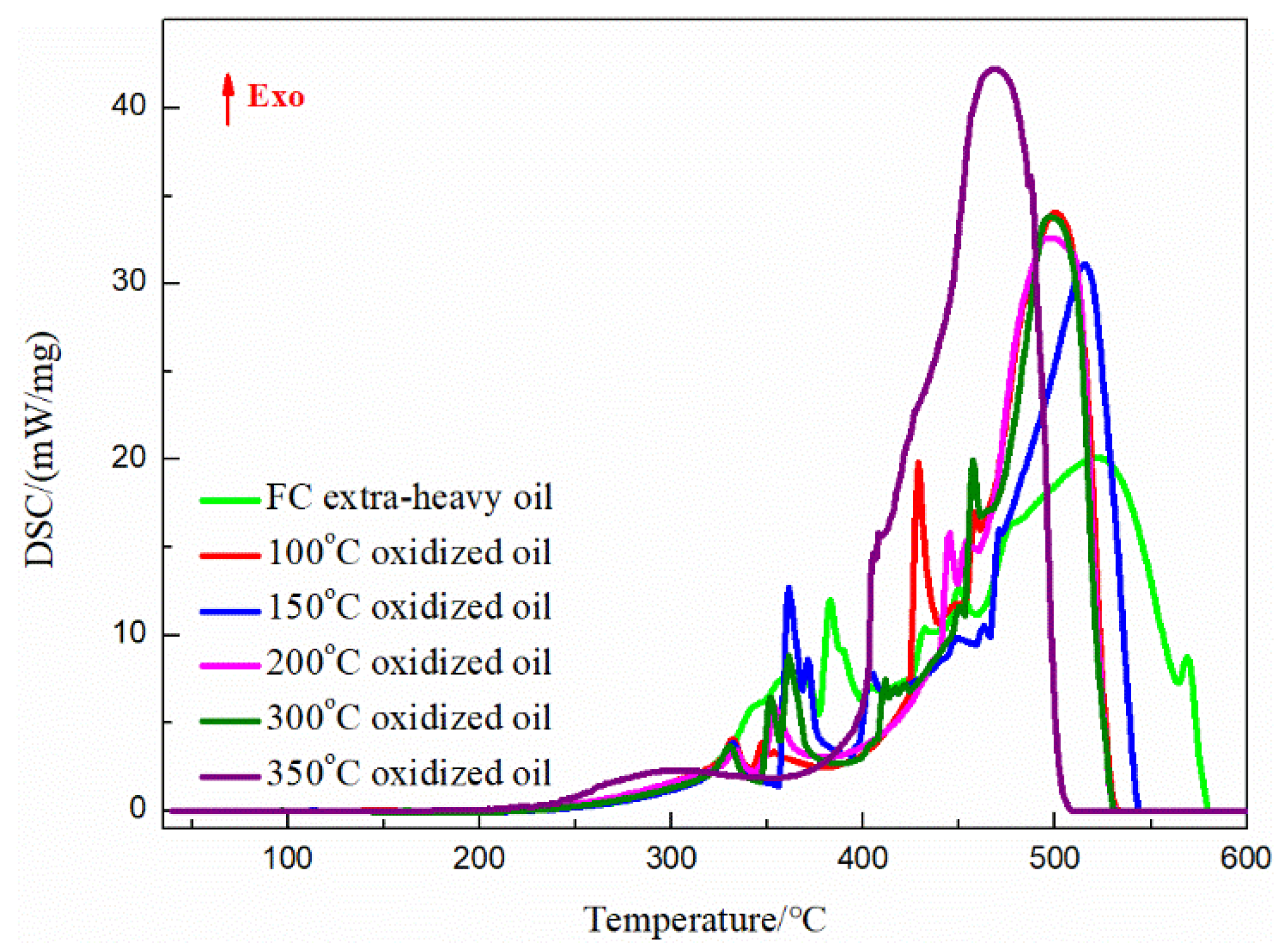
3.4. DSC Analysis of the FC Extra-Heavy Oil and Oxidized Oils
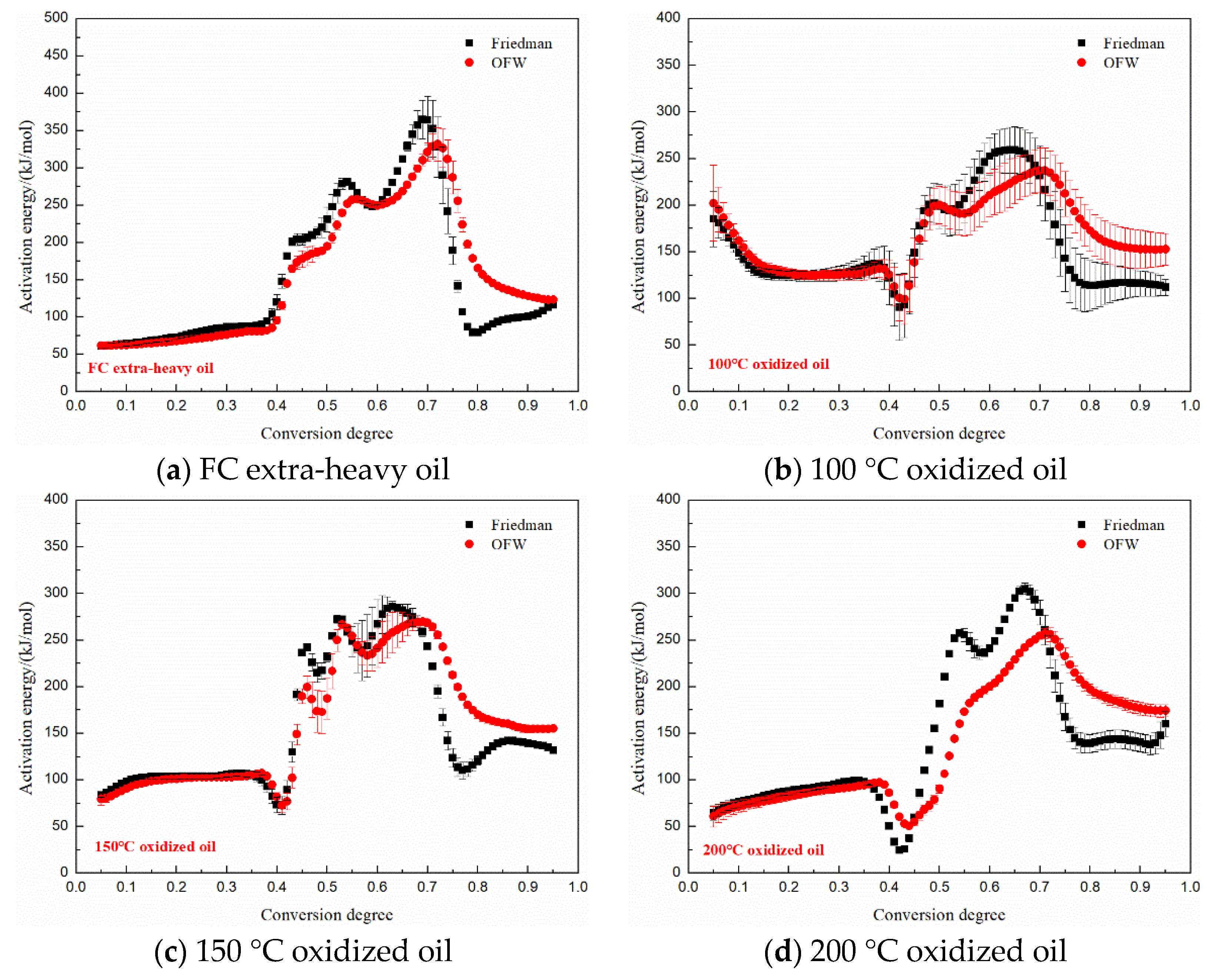
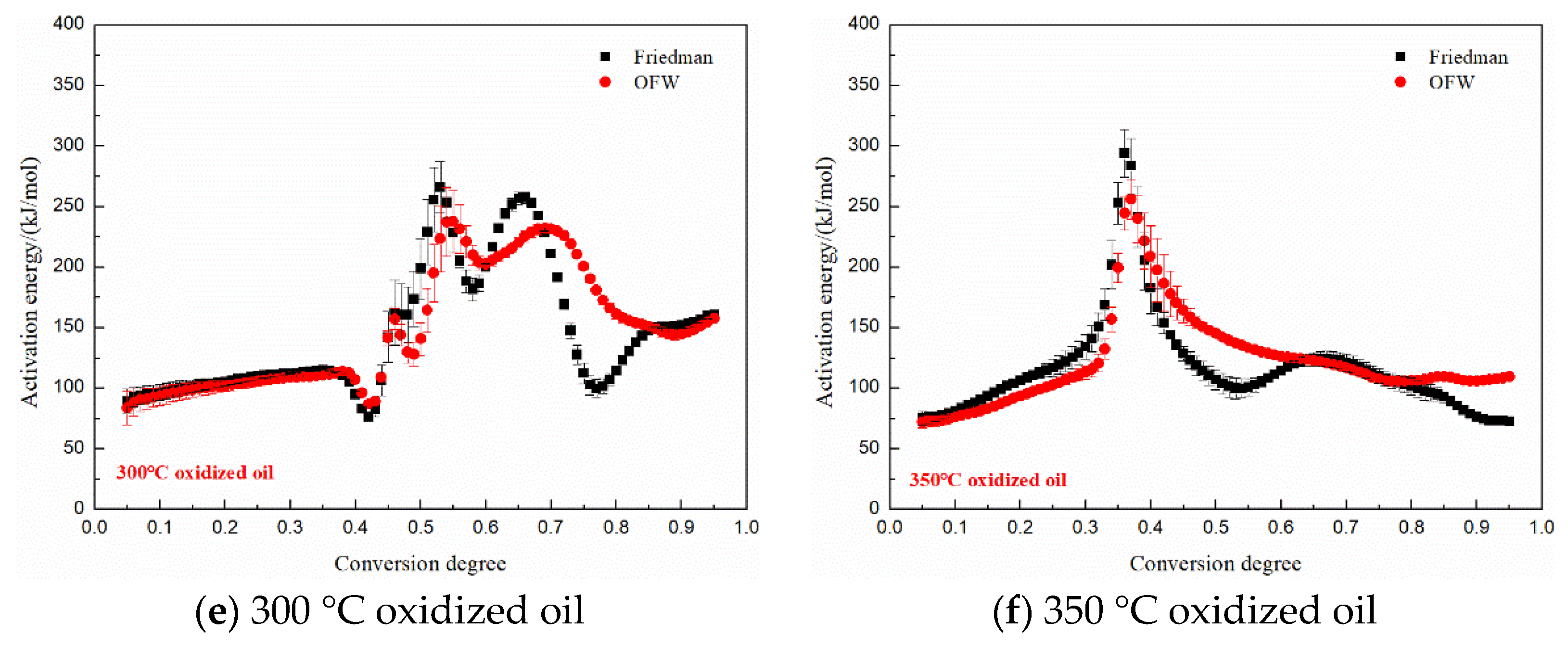
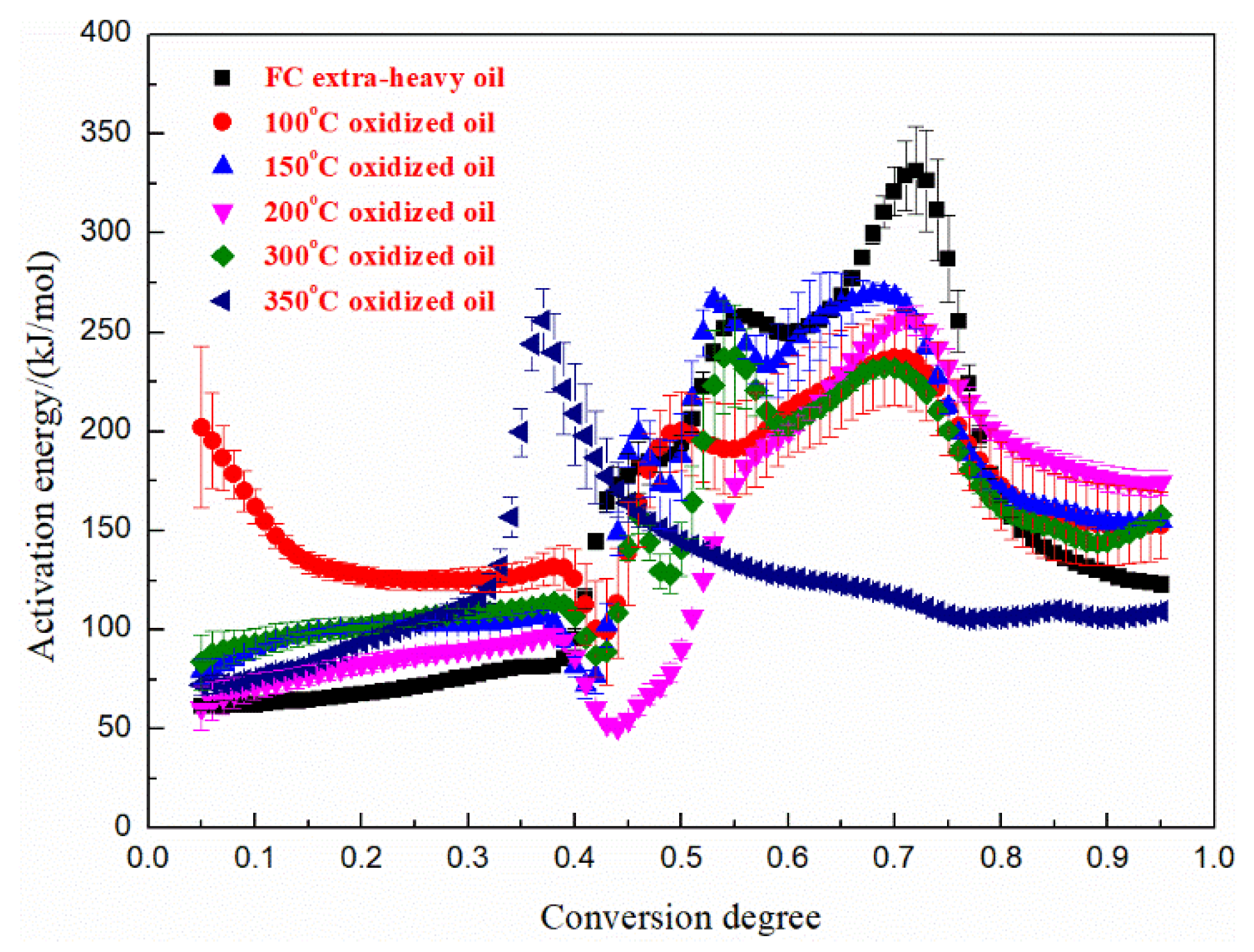
3.5. Kinetics Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chow, D.L.; Nasr, T.N.; Chow, R.S.; Sawatzky, R.P. Recovery Techniques for Canada’s Heavy Oil and Bitumen Resources. J. Can. Pet. Technol. 2009, 47, 12–17. [Google Scholar]
- Marsh, R.; Hein, F. Canada’s Extra-Heavy (Bitumen) and Heavy Oil Resources, Reserves and Development. J. Can. Pet. Technol. 2008, 47, 7–11. [Google Scholar]
- Hein, F.J. Geology of Bitumen and Heavy Oil: An Overview. J. Pet. Sci. Eng. 2017, 154, 551–563. [Google Scholar] [CrossRef]
- Davudov, D.; Moghanloo, R.G. A Systematic Comparison of various Upgrading Techniques for Heavy Oil. J. Pet. Sci. Eng. 2017, 156, 623–632. [Google Scholar] [CrossRef]
- Jiang, Y.W.; Li, Q. Heavy Oil and Bitumen Resources and Development of Liaohe and Xinjiang Oil Fields of China. Bull. Can. Pet. Geol. 2016, 64, 266–277. [Google Scholar] [CrossRef]
- Mehrabi-Kalajahi, S.; Varfolomeev, M.A.; Yuan, C.D.; Zinnatullin, A.L.; Rodionov, N.O.; Vagizov, F.G.; Osin, Y.N.; Yakimova, L.S. Improving Heavy Oil Oxidation Performance by Oil-Dispersed CoFe2O4 Nanoparticles in In-Situ Combustion Process for Enhanced Oil Recovery. Fuel 2021, 285, 119216. [Google Scholar] [CrossRef]
- Liu, D.; Tang, J.S.; Zheng, R.N.; Song, Q. Influence of Steam on the Coking Characteristics of Heavy Oil during In Situ Combustion. Fuel 2020, 264, 116904. [Google Scholar] [CrossRef]
- Yuan, C.D.; Emelianov, D.A.; Varfolomeev, M.A.; Rodionov, N.O.; Suwaid, M.A.; Vakhitov, I.R. Mechanistic and Kinetic Insight into Catalytic Oxidation Process of Heavy Oil in In-Situ Combustion Process using Copper (II) Stearate as Oil Soluble Catalyst. Fuel 2021, 284, 118981. [Google Scholar] [CrossRef]
- Pu, W.F.; Wang, L.L.; Peng, X.Q.; Li, N.; Zhao, S. Effects of Aromatics, Resins, and Asphaltenes on Oxidation Behavior and Kinetics of Heavy Crude Oil. Pet. Sci. Technol. 2020, 38, 815–822. [Google Scholar] [CrossRef]
- Varfolomeev, M.A.; Galukhin, A.; Nurgaliev, D.K.; Kok, M.V. Thermal Decomposition of Tatarstan Ashal’cha Heavy Crude Oil and its SARA Fractions. Fuel 2016, 186, 122–127. [Google Scholar] [CrossRef]
- Li, Y.B.; Chen, Y.F.; Pu, W.F.; Gao, H.; Bai, B.J. Experimental Investigation into the Oxidative Characteristics of Tahe Heavy Crude Oil. Fuel 2017, 209, 194–202. [Google Scholar] [CrossRef]
- Ma, J.F.; Liu, J.X.; Jiang, X.M.; Zhang, H. A Two-Dimensional Distributed Activation Energy Model for Pyrolysis of Solid Fuels. Energy 2021, 230, 120860. [Google Scholar] [CrossRef]
- Zhao, S.; Pu, W.F.; Varfolomeev, M.A.; Yuan, C.D.; Zhang, J.Z.; Han, X.Q.; Yang, Y.; Peng, X.Q.; Wu, J.X. Comprehensive Investigations into Low Temperature Oxidation of Heavy Crude Oil. J. Pet. Sci. Eng. 2018, 171, 835–842. [Google Scholar] [CrossRef]
- Wang, J.X.; Wang, L.L.; Wang, T.F.; Peng, X.Q. Effects of SARA Fractions on Pyrolysis Behavior and Kinetics of Heavy Crude Oil. Pet. Sci. Technol. 2020, 38, 945–954. [Google Scholar] [CrossRef]
- Kok, M.V.; Varfolomeev, M.A.; Nurgaliev, D.K. Low-Temperature Oxidation Reactions of Crude Oils using TGA–DSC Techniques. J. Therm. Anal. Calorim. 2020, 141, 775–781. [Google Scholar] [CrossRef]
- Zhao, R.B.; Xia, X.T.; Luo, W.W.; Shi, Y.L.; Diao, C.J. Alteration of Heavy Oil Properties under In-Situ Combustion: A Field Study. Energy Fuels 2015, 29, 6839–6848. [Google Scholar] [CrossRef]
- Khansari, Z.; Gates, I.D.; Mahinpey, N. Low-Temperature Oxidation of Lloydminster Heavy Oil: Kinetic Study and Product Sequence Estimation. Fuel 2014, 115, 534–538. [Google Scholar] [CrossRef]
- Zhao, S.; Pu, W.F.; Varfolomeev, M.A.; Yuan, C.D.; Pan, J.J.; Wang, R.Y.; Chen, L.; Kan, N. Low-Temperature Oxidation of Light and Heavy Oils via Thermal Analysis: Kinetic Analysis and temperature Zone Division. J. Pet. Sci. Eng. 2018, 168, 246–255. [Google Scholar] [CrossRef]
- Yuan, C.D.; Emelianov, D.; Varfolomeev, M.A. Oxidation Behaviour and Kinetics of Light, Medium and Heavy Crude Oils Characterized by Thermogravimetry Coupled with Fourier-Transform Infrared Spectroscopy (TG-FTIR). Energy Fuels 2018, 32, 5571–5581. [Google Scholar] [CrossRef]
- Zhao, S.; Pu, W.F.; Yuan, C.D.; Peng, X.Q.; Zhang, J.Z.; Wang, L.L.; Emelianov, D.A. Thermal Behavior and Kinetic Triplets of Heavy Crude Oil and its SARA Fractions during Combustion by High Pressure Differential Scanning Calorimetry. Energy Fuels 2019, 33, 3176–3186. [Google Scholar] [CrossRef]
- Li, Y.B.; Chen, Y.F.; Pu, W.F.; Dong, H.; Gao, H.; Jin, F.Y.; Wei, B. Low Temperature Oxidation Characteristics Analysis of Ultra-Heavy Oil by Thermal Methods. J. Ind. Eng. Chem. 2017, 48, 249–258. [Google Scholar] [CrossRef]
- Pu, W.F.; Zhao, S.; Pan, J.J.; Wang, R.Y.; Chen, L.; Kan, N.; Wang, L.L. Comparative Analysis of Quartz Sand and Detritus Effects on Thermal Behavior and Kinetics of Heavy Crude Oil. Thermochim. Acta 2018, 667, 153–159. [Google Scholar] [CrossRef]
- Liu, D.; Song, Q.; Tang, J.S.; Zheng, R.A.; Yao, Q. Interaction between Saturates, Aromatics and Resins during Pyrolysis and Oxidation of Heavy Oi. J. Pet. Sci. Eng. 2017, 154, 543–550. [Google Scholar] [CrossRef]
- Wang, Y.Y.; Ren, S.R.; Zhang, L.; Deng, J.Y.; Peng, X.Y.; Cheng, H.Q. New Insights into the Oxidation Behaviors of Crude Oils and Their Exothermic Characteristics: Experimental Study via Simultaneous TGA/DSC. Fuel 2018, 219, 141–150. [Google Scholar] [CrossRef]
- Zhao, S.; Pu, W.F.; Varfolomeev, M.A.; Yuan, C.D.; Rodionov, A.A. Integrative Investigation of Low-Temperature Oxidation Characteristics and Mechanisms of Heavy Crude Oil. Ind. Eng. Chem. Res. 2019, 58, 14595–14602. [Google Scholar] [CrossRef]
- Zhao, S.; Pu, W.F.; Peng, X.Q.; Zhang, J.Z.; Ren, H. Low-Temperature Oxidation of Heavy Crude Oil Characterized by TG, DSC, GC-MS, and Negative Ion ESI FT-ICR MS. Energy 2021, 214, 119004. [Google Scholar] [CrossRef]
- Wang, J.X.; Wang, L.L.; Wang, T.F.; Pu, W.F. Exothermal Property and Kinetics Analysis of Oxidized Coke and Pyrolyzed Coke from Fengcheng Extra-Heavy Oil. Ind. Eng. Chem. Res. 2021, 60, 7014–7023. [Google Scholar] [CrossRef]
- Wang, T.F.; Wang, J.X.; Meng, X.B.; Chu, G.Y.; Liu, C. The low Temperature Oxidation Characteristics of SARA Fractions in Air Flooding Process. Pet. Sci. Technol. 2018, 36, 1125–1130. [Google Scholar] [CrossRef]
- Pu, W.F.; Yuan, C.D.; Jin, F.Y.; Wang, L.; Qian, Z.; Li, Y.B.; Li, D.; Chen, Y.F. Low-Temperature Oxidation and Characterization of Heavy Oil via Thermal Analysis. Energy Fuels 2015, 29, 1151–1159. [Google Scholar] [CrossRef]
- Li, Y.B.; Luo, C.; Lin, X.; Li, K.; Xiao, Z.R.; Wang, Z.Q.; Pu, W.F. Characteristics and Properties of Coke Formed by Low-Temperature Oxidation and Thermal Pyrolysis during in Situ Combustion. Ind. Eng. Chem. Res. 2020, 59, 2171–2180. [Google Scholar] [CrossRef]
- Niu, B.; Ren, S.; Liu, Y.; Wang, D.; Tang, L.; Chen, B. Low-Temperature Oxidation of Oil Components in an Air Injection Process for Improved Oil Recovery. Energy Fuels 2011, 25, 4299–4304. [Google Scholar] [CrossRef]
- Yuan, C.D.; Varfolomeev, M.A.; Emelianov, D.A.; Eskin, A.A.; Nagrimanov, R.N.; Kok, M.V.; Afanasiev, I.S.; Fedorchenko, G.D.; Kopylova, E.V. Oxidation Behavior of Light Crude Oil and its SARA Fractions Characterized by TG and DSC Techniques: Differences and Connections. Energy Fuels 2018, 32, 801–808. [Google Scholar] [CrossRef]
- Li, Y.B.; Gao, H.; Pu, W.F.; Li, L.; Chen, Y.F.; Bai, B.J. Study of the Catalytic Effect of Copper Oxide on the Low-Temperature Oxidation of Tahe Ultra-Heavy Oil. J. Therm. Anal. Calorim. 2019, 135, 3353–3362. [Google Scholar] [CrossRef]
- Pu, W.F.; Gong, X.L.; Chen, Y.F.; Liu, X.L.; Hui, J.; Guo, C.; Varfolomeev, M.A. Oxidation Kinetic Evaluation of the Low Temperature Oxidized Products of Tahe Heavy Oil Characterized by the Distributed Activation Energy Model. J. Pet. Sci. Eng. 2019, 181, 106155. [Google Scholar] [CrossRef]
- Zhao, R.B.; Wei, Y.G.; Wang, Z.M.; Yang, W.; Yang, H.J.; Liu, S.J. Kinetics of Low-Temperature Oxidation of Light Crude Oil. Energy Fuels 2016, 30, 2647–2654. [Google Scholar] [CrossRef]
- Mothé, C.G.; Miranda, I.C.D. Study of Kinetic Parameters of Thermal Decomposition of Bagasse and Sugarcane Straw using Friedman and Ozawa–Flynn–Wall Isoconversional Methods. J. Therm. Anal. Calorim. 2013, 113, 497–505. [Google Scholar] [CrossRef]
- Karimian, M.; Schaffie, M.; Fazaelipoor, M.H. Estimation of the Kinetic Triplet for In-Situ Combustion of Crude Oil in the Presence of Limestone Matrix. Fuel 2017, 209, 203–210. [Google Scholar] [CrossRef]
- Vyazovkin, S.; Burnham, A.K.; Criado, J.M.; Pérez-Maqueda, L.A.; Popescu, C.; Sbirrazzuoli, N. ICTAC Kinetics Committee Recommendations for Performing Kinetic Computations on Thermal Analysis Data. Thermochim. Acta 2011, 520, 1–19. [Google Scholar] [CrossRef]
- Akin, S.; Kok, M.V.; Bagci, S.; Karacan, O. Oxidation of Heavy Oil and Their SARA Fractions: Its Role in Modeling In-Situ Combustion. SPE Reserv. Eng. 2000, 757–768. [Google Scholar] [CrossRef]
- Kok, M.V.; Gul, K.G. Thermal Characteristics and Kinetics of Crude Oils and SARA Fractions. Thermochim. Acta 2013, 569, 66–70. [Google Scholar] [CrossRef]
- Al-Saffar, H.B.; Hasanin, H.; Price, D.; Hughes, R. Oxidation of a Light Crude Oil and Its SARA Fractions in Consolidated Cores. Energy Fuels 2001, 15, 182–188. [Google Scholar] [CrossRef]
- Wang, T.F.; Yang, W.P.; Wang, J.X.; Shem, K.; Deng, Z.Y. Low Temperature Oxidation of Crude Oil: Reaction Progress and Catalytic Mechanism of Metallic Salts. Fuel 2018, 225, 336–342. [Google Scholar] [CrossRef]
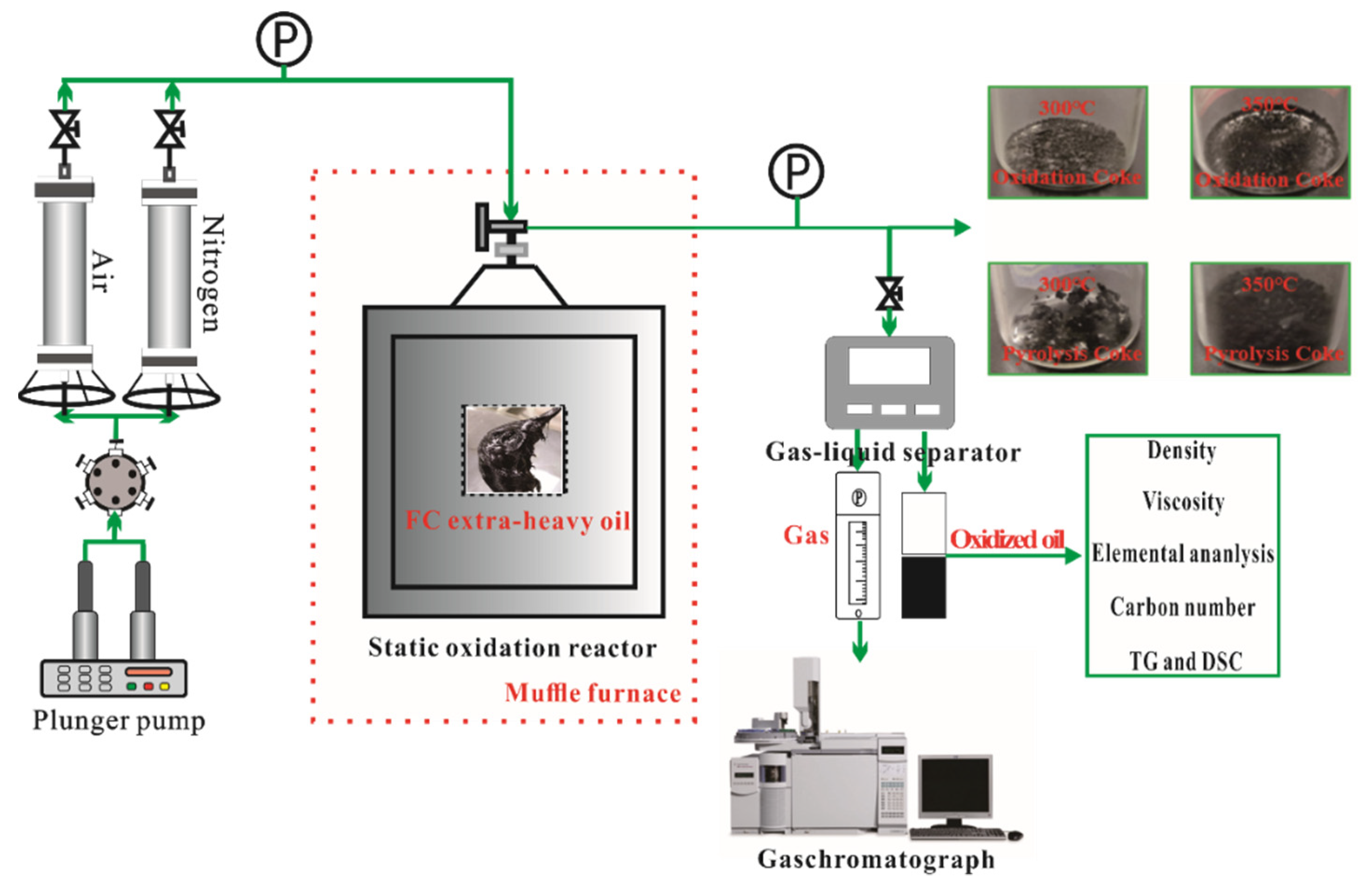

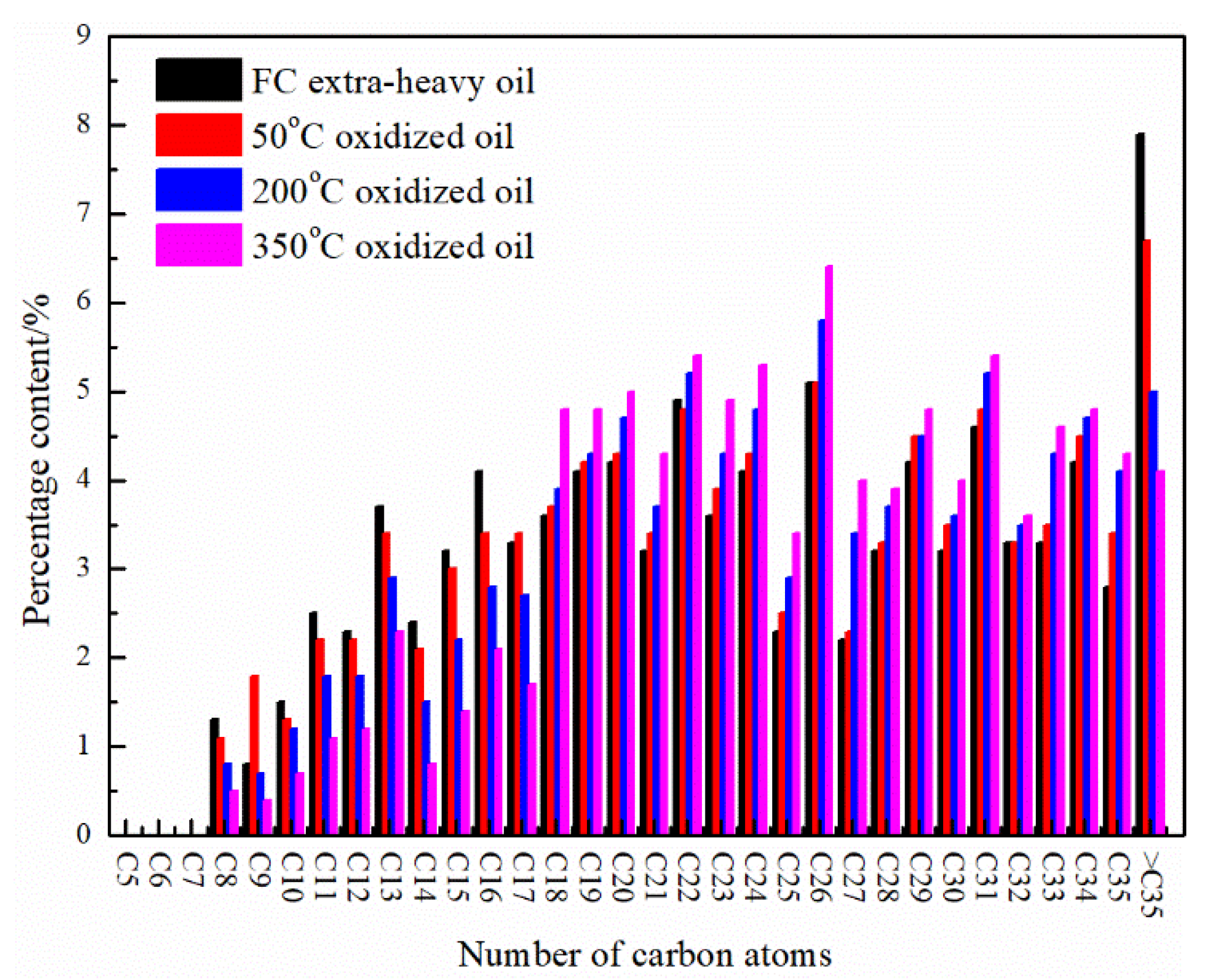
| Density (g/cm3, 20 °C) | Viscosity (mPa·s, 50 °C) | Gravity (°API) (25 °C) | SARA Fractions (wt%) | Elemental Analysis (wt%) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| S | A1 | R | A2 | C | H | O | N | S | |||
| 0.9517 | 6118 | 17 | 35.64 | 20.78 | 28.30 | 15.28 | 82.3 | 11.9 | 3.47 | 0.41 | 1.64 |
| T/°C | Gas Phase Composition | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| O2 /% | N2 /% | CO2 /% | CO /% | C1–C6 /% | H/C /% | O2/N2 | CO/CO2 | OCR /(%/d) | |
| 50 | 18.27 ± 0.26 | 81.05 ± 0.20 | 0.46 ± 0.02 | 0.07 ± 0.02 | 0.15 ± 0.02 | 20.94 ± 0.56 | 0.225 ± 0.004 | 0.151 ± 0.037 | 0.91 ± 0.09 |
| 100 | 15.10 ± 0.57 | 82.71 ± 0.53 | 1.32 ± 0.02 | 0.18 ± 0.00 | 0.70 ± 0.02 | 14.63 ± 1.59 | 0.183 ± 0.008 | 0.133 ± 0.002 | 1.97 ± 0.19 |
| 150 | 7.86 ± 1.49 | 85.67 ± 0.30 | 4.66 ± 0.98 | 0.36 ± 0.06 | 1.47 ± 0.15 | 8.31 ± 1.28 | 0.092 ± 0.018 | 0.077 ± 0.005 | 4.38 ± 0.50 |
| 200 | 4.52 ± 1.25 | 86.74 ± 0.35 | 6.13 ± 0.84 | 0.52 ± 0.01 | 2.09 ± 0.07 | 7.38 ± 0.62 | 0.052 ± 0.015 | 0.087 ± 0.014 | 5.49 ± 0.42 |
| 250 | 2.93 ± 1.01 | 87.21 ± 0.43 | 6.91 ± 0.61 | 0.55 ± 0.02 | 2.40 ± 0.01 | 7.03 ± 0.27 | 0.034 ± 0.012 | 0.080 ± 0.010 | 6.02 ± 0.34 |
| 300 | 1.85 ± 0.70 | 87.42 ± 0.29 | 7.68 ± 0.47 | 0.58 ± 0.03 | 2.48 ± 0.03 | 6.51 ± 0.18 | 0.021 ± 0.008 | 0.076 ± 0.009 | 6.38 ± 0.23 |
| 350 | 0.75 ± 0.22 | 87.68 ± 0.14 | 8.26 ± 0.28 | 0.62 ± 0.01 | 2.55 ± 0.04 | 6.31 ± 0.21 | 0.009 ± 0.003 | 0.075 ± 0.004 | 6.75 ± 0.07 |
| Samples | Viscosity/Pa·s (50 °C) | Density/g/cm3 (20 °C) |
|---|---|---|
| FC extra-heavy oil | 6.1175 ± 0.0005 | 0.9519 ± 0.0002 |
| 50 °C oxidized oil | 7.2155 ± 0.0005 | 0.9535 ± 0.0003 |
| 100 °C oxidized oil | 8.4360 ± 0.0010 | 0.9557 ± 0.0006 |
| 150 °C oxidized oil | 13.6440 ± 0.0010 | 0.9608 ± 0.0003 |
| 200 °C oxidized oil | 15.9715 ± 0.0015 | 0.9629 ± 0.0002 |
| 250 °C oxidized oil | 17.1125 ± 0.0025 | 0.9641 ± 0.0001 |
| 300 °C oxidized oil | 18.4310 ± 0.0010 | 0.9656 ± 0.0001 |
| 350 °C oxidized oil | 19.1060 ± 0.0010 | 0.9662 ± 0.0001 |
| Samples | Heating Rate (°C /min) | LTO | FD | HTC | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Region (°C) | Peak Temperature (°C) | Mass Loss (%) | Region (°C) | Peak Temperature (°C) | Mass Loss (%) | Region (°C) | Peak Temperature (°C) | Mass Loss (%) | ||
| FC extra-heavy oil | 5 | 40–353 | 295 | 41.4 | 353–447 | 413 | 30.0 | 447–528 | 488 | 28.6 |
| 10 | 40–348 | 311 | 39.6 | 348–461 | 428 | 33.1 | 461–557 | 505 | 27.3 | |
| 15 | 40–379 | 341 | 40.1 | 379–497 | 457 | 37.3 | 497–601 | 560 | 22.6 | |
| 100 °C oxidized oil | 5 | 40–331 | 275 | 42.7 | 331–443 | 435 | 26.7 | 443–504 | 464 | 30.6 |
| 10 | 40–353 | 295 | 43.6 | 353–467 | 450 | 31.4 | 467–524 | 488 | 25.0 | |
| 15 | 40–362 | 312 | 43.9 | 362–470 | 453 | 30.9 | 470–530 | 492 | 25.2 | |
| 150 °C oxidized oil | 5 | 40–348 | 284 | 43.1 | 348–438 | 410 | 24.7 | 438–512 | 474 | 32.2 |
| 10 | 40–350 | 301 | 41.5 | 350–467 | 441 | 33.2 | 467–533 | 496 | 25.3 | |
| 15 | 40–352 | 320 | 40.2 | 352-476 | 435 | 33.9 | 476-552 | 505 | 25.9 | |
| 200 °C oxidized oil | 5 | 40-348 | 278 | 45.4 | 348-435 | 360 | 20.4 | 435-505 | 465 | 34.1 |
| 10 | 40-350 | 301 | 43.5 | 350–462 | 447 | 30.7 | 462–525 | 484 | 25.8 | |
| 15 | 40–359 | 312 | 41.7 | 359–471 | 455 | 32.0 | 471–535 | 491 | 26.3 | |
| 300 °C oxidized oil | 5 | 40–335 | 289 | 43.5 | 335–417 | 410 | 16.3 | 417–497 | 460 | 40.2 |
| 10 | 40–342 | 305 | 43.2 | 342–464 | 449 | 32.7 | 464–521 | 488 | 24.1 | |
| 15 | 40–347 | 316 | 41.6 | 347–474 | 450 | 33.0 | 474–544 | 497 | 25.4 | |
| 350 °C oxidized oil | 5 | 40–312 | 253 | 34.4 | / | / | / | 312–473 | 417 | 65.6 |
| 10 | 40–338 | 261 | 36.1 | / | / | / | 338–511 | 437 | 63.9 | |
| 15 | 40–335 | 272 | 35.2 | / | / | / | 335–532 | 451 | 64.8 | |
| Samples | LTO | HTC | ||||||
|---|---|---|---|---|---|---|---|---|
| Region (°C) | Peak Temperature (°C) | Peak Heat Flow (mW/mg) | Enthalpy (kJ/mol) | Region (°C) | Peak Temperature (°C) | Peak Heat Flow (mW/mg) | Enthalpy (kJ/mol) | |
| FC extra-heavy oil | 40–399 | 382 | 11.7 | 3.54 | 399–579 | 521 | 20.1 | 13.97 |
| 100 °C oxidized oil | 40–380 | 332 | 3.9 | 1.63 | 380–534 | 501 | 34.1 | 13.99 |
| 150 °C oxidized oil | 40–393 | 362 | 12.5 | 2.17 | 393–546 | 517 | 31.1 | 12.94 |
| 200 °C oxidized oil | 40–380 | 355 | 5.9 | 1.76 | 380–534 | 497 | 32.6 | 13.38 |
| 300 °C oxidized oil | 40–388 | 362 | 8.6 | 1.89 | 388–530 | 494 | 33.8 | 12.81 |
| 350 °C oxidized oil | 40–360 | 295 | 2.3 | 1.35 | 360–510 | 467 | 42.4 | 17.77 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, L.; Wang, J.; Pu, W.; Wang, T. Combustion Behavior and Kinetics Analysis of Isothermal Oxidized Oils from Fengcheng Extra-Heavy Oil. Energies 2021, 14, 6294. https://doi.org/10.3390/en14196294
Wang L, Wang J, Pu W, Wang T. Combustion Behavior and Kinetics Analysis of Isothermal Oxidized Oils from Fengcheng Extra-Heavy Oil. Energies. 2021; 14(19):6294. https://doi.org/10.3390/en14196294
Chicago/Turabian StyleWang, Liangliang, Jiexiang Wang, Wanfen Pu, and Tengfei Wang. 2021. "Combustion Behavior and Kinetics Analysis of Isothermal Oxidized Oils from Fengcheng Extra-Heavy Oil" Energies 14, no. 19: 6294. https://doi.org/10.3390/en14196294
APA StyleWang, L., Wang, J., Pu, W., & Wang, T. (2021). Combustion Behavior and Kinetics Analysis of Isothermal Oxidized Oils from Fengcheng Extra-Heavy Oil. Energies, 14(19), 6294. https://doi.org/10.3390/en14196294