Effects of Surfactant and Hydrophobic Nanoparticles on the Crude Oil-Water Interfacial Tension
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Experimental Method
3. Results and Discussion
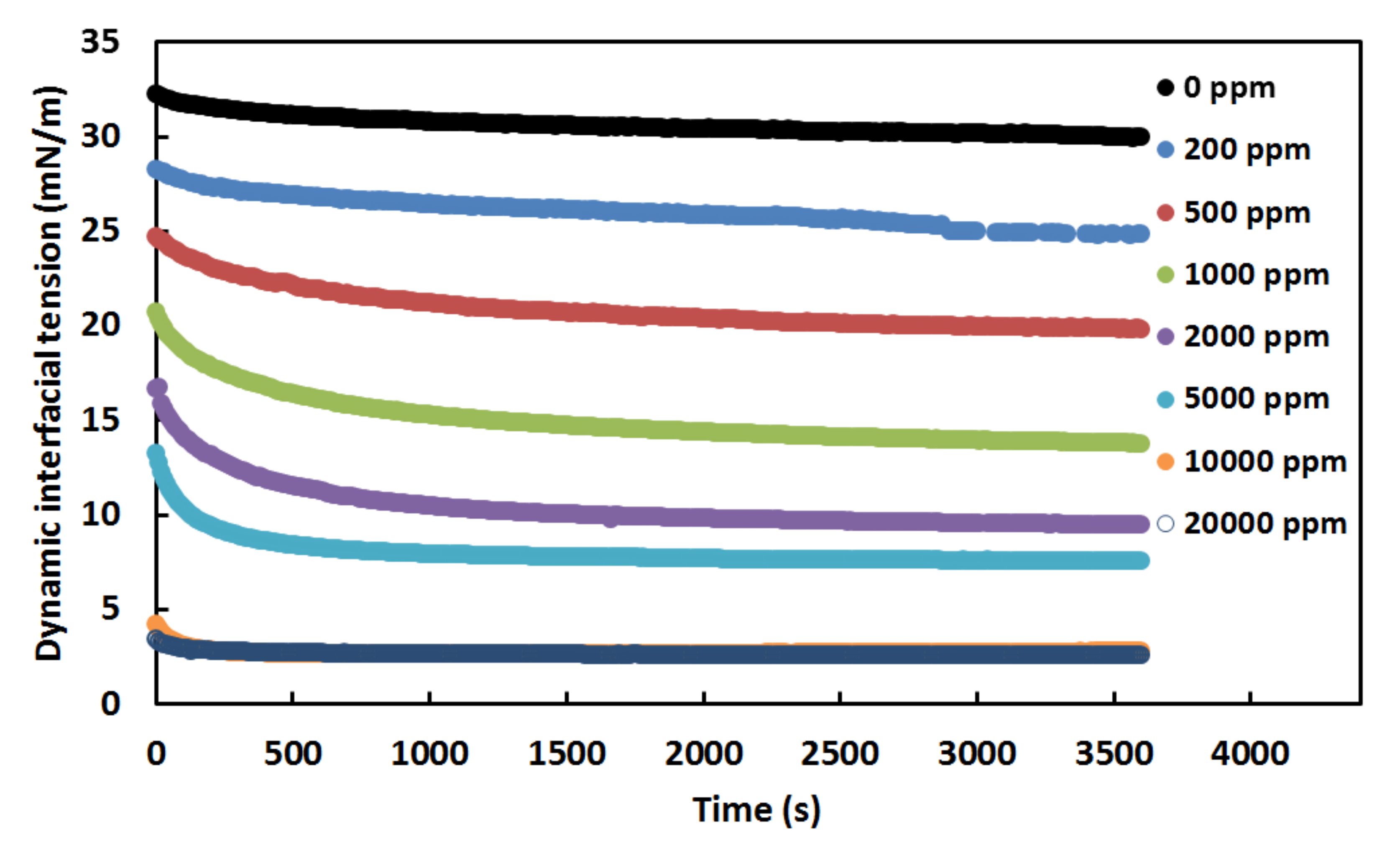
3.1. Effect of Surfactant Concentration on the Crude Oil-Water Interfacial Tension
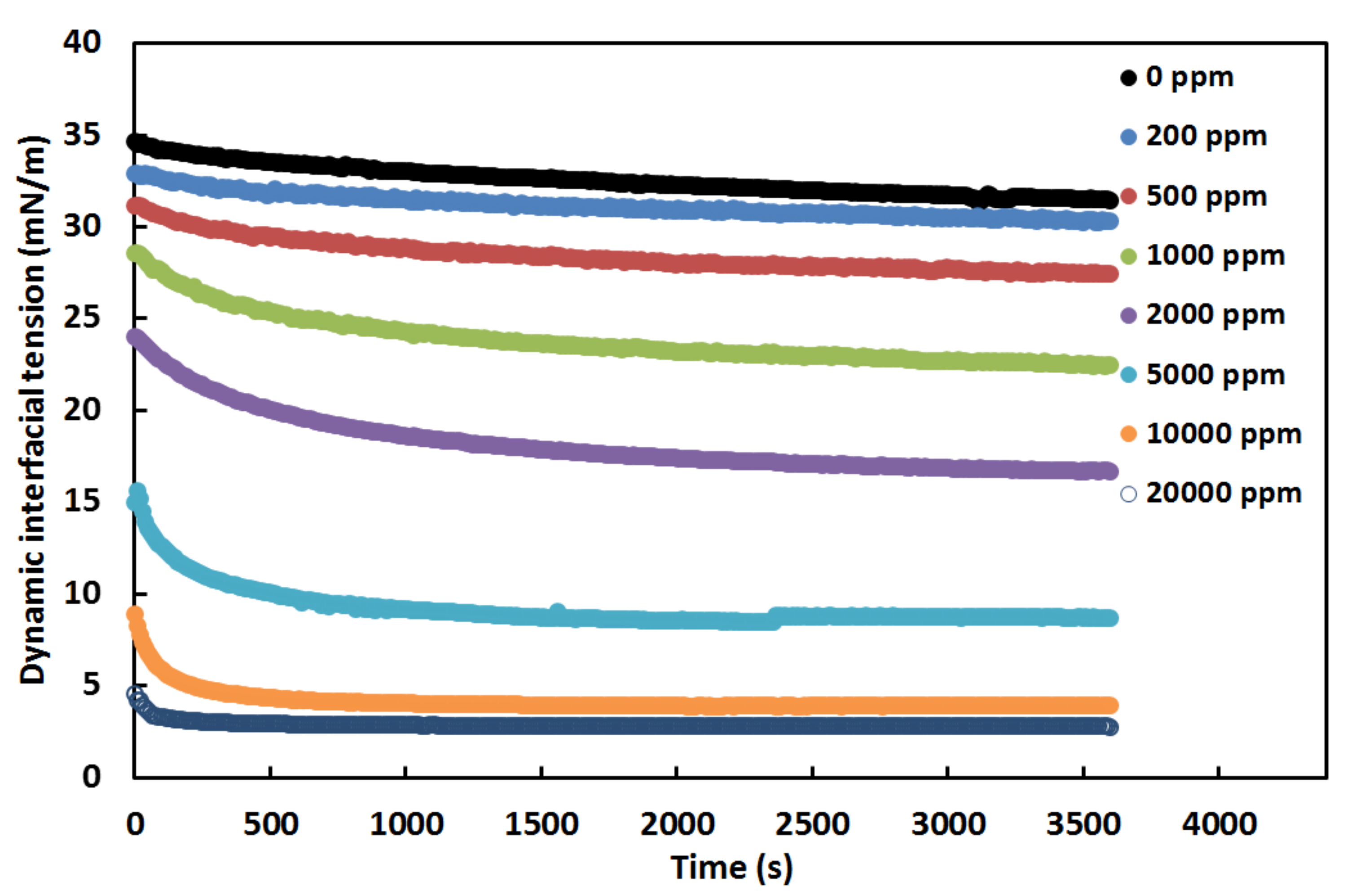
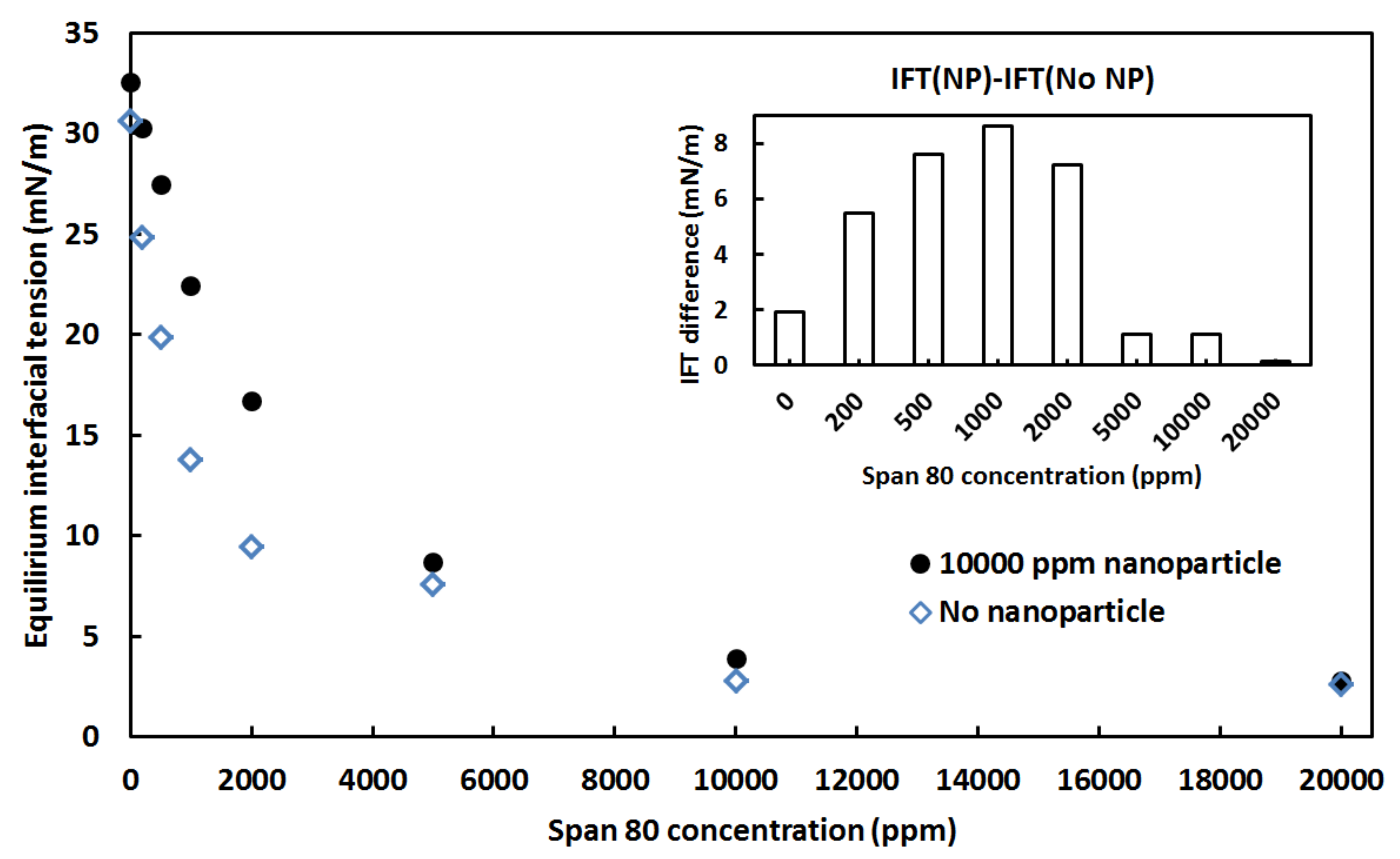
3.2. Effect of Surfactant Concentration on the Crude Oil-Water Interfacial Tension in the Presence of Nanoparticles
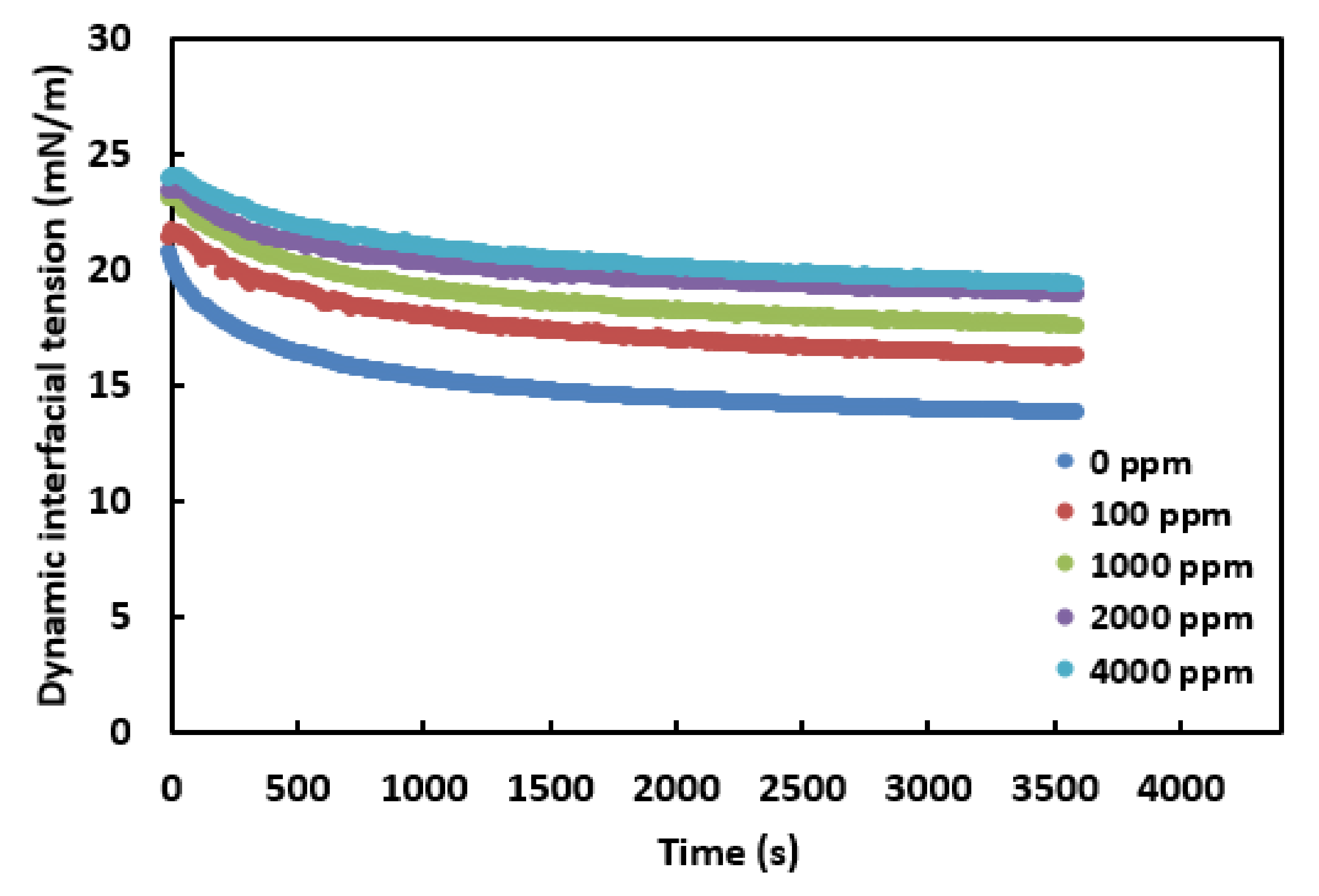
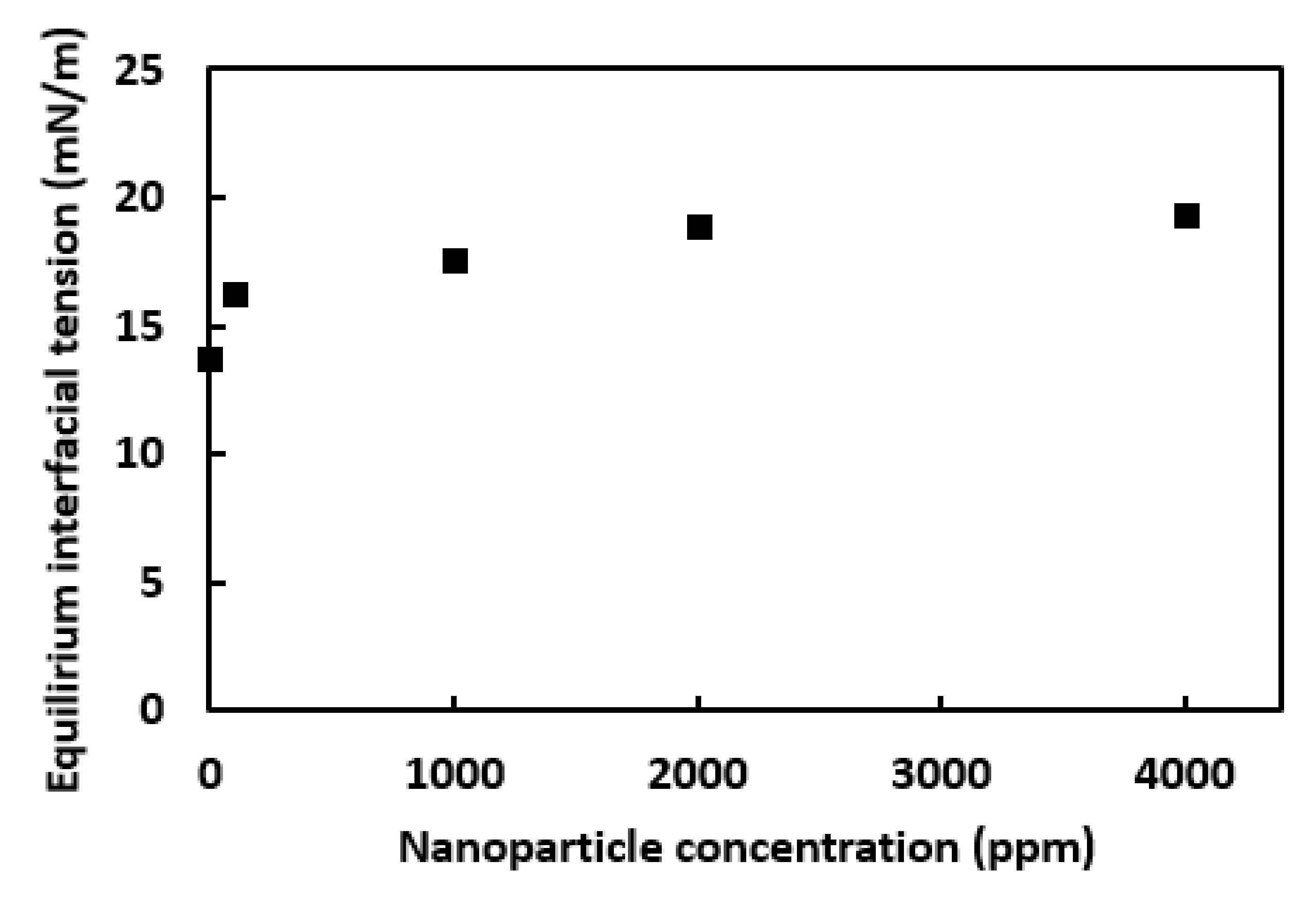
3.3. Effect of Nanoparticle Concentration on the Crude Oil-Water Interfacial Tension in the Presence of Surfactant
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Maurya, N.K.; Mandal, A. Investigation of synergistic effect of nanoparticle and surfactant in macro emulsion based EOR application in oil reservoirs. Chem. Eng. Res. Des. 2018, 132, 370–384. [Google Scholar] [CrossRef]
- Pei, H.; Shu, Z.; Zhang, G.; Ge, J.; Jiang, P.; Qin, Y.; Cao, X. Experimental study of nanoparticle and surfactant stabilized emulsion flooding to enhance heavy oil recovery. J. Pet. Sci. Eng. 2018, 163, 476–483. [Google Scholar] [CrossRef]
- Yegya Raman, A.K.; Aichele, C.P. Influence of non-ionic surfactant addition on the stability and rheology of particle-stabilized emulsions. Colloids Surf. A Physicochem. Eng. Asp. 2020, 585, 124084. [Google Scholar] [CrossRef]
- Zheng, B.; Zheng, B.; Carr, A.J.; Yu, X.; McClements, D.J.; Bhatia, S.R. Emulsions stabilized by inorganic nanoclays and surfactants: Stability, viscosity, and implications for applications. Inorg. Chim. Acta 2020, 508, 119566. [Google Scholar] [CrossRef]
- He, L.-M.; Yang, D.-H.; Gong, R.-N.; Ye, T.-J.; Lü, Y.-L.; Luo, X.-M. An investigation into the deformation, movement and coalescence characteristics of water-in-oil droplets in an AC electric field. Pet. Sci. 2013, 10, 548–561. [Google Scholar] [CrossRef] [Green Version]
- Hong, J.S.; Bergfreund, J.; Fischer, P. Complex emulsion stabilization behavior of clay particles and surfactants based on an interfacial rheological study. Colloids Surf. A Physicochem. Eng. Asp. 2020, 602, 125121. [Google Scholar] [CrossRef]
- Mahmoudi, S.; Jafari, A.; Javadian, S. Temperature effect on performance of nanoparticle/surfactant flooding in enhanced heavy oil recovery. Pet. Sci. 2019, 16, 1387–1402. [Google Scholar] [CrossRef] [Green Version]
- Betancur, S.; Giraldo, L.J.; Carrasco-Marín, F.; Riazi, M.; Manrique, E.J.; Quintero, H.; García, H.A.; Franco-Ariza, C.A.; Cortés, F.B. Importance of the Nanofluid Preparation for Ultra-Low Interfacial Tension in Enhanced Oil Recovery Based on Surfactant–Nanoparticle–Brine System Interaction. ACS Omega 2019, 4, 16171–16180. [Google Scholar] [CrossRef] [Green Version]
- AlamiNia, H.; Khalilinezhad, S.S. Application of hydrophilic silica nanoparticles in chemical enhanced heavy oil recovery processes. Energy Sources Part A Recovery Util. Environ. Eff. 2017. [Google Scholar] [CrossRef]
- Li, Y.; Di, Q.; Hua, S.; Jia, X. The effect of foam system containing surfactant and silica nanoparticles on oil recovery of carbonate rocks. Energy Sources Part A Recovery Util. Environ. Eff. 2020. [Google Scholar] [CrossRef]
- Afekare, D.; Garno, J.C.; Rao, D. Insights into Nanoscale Wettability Effects of Low Salinity and Nanofluid Enhanced Oil Recovery Techniques. Energies 2020, 13, 4443. [Google Scholar] [CrossRef]
- Irfan, S.A.; Shafie, A.; Yahya, N.; Zainuddin, N. Mathematical Modeling and Simulation of Nanoparticle-Assisted Enhanced Oil Recovery—A Review. Energies 2019, 12, 1575. [Google Scholar] [CrossRef] [Green Version]
- Pickering, S.U. CXCVI.—Emulsions. J. Chem. Soc. Trans. 1907, 91, 2001–2021. [Google Scholar] [CrossRef] [Green Version]
- Binks, B.P. Particles as surfactants—similarities and differences. Curr. Opin. Colloid Interface Sci. 2002, 7, 21–41. [Google Scholar] [CrossRef]
- Saleh, N.; Sarbu, T.; Sirk, K.; Lowry, G.V.; Matyjaszewski, K.; Tilton, R.D. Oil-in-Water Emulsions Stabilized by Highly Charged Polyelectrolyte-Grafted Silica Nanoparticles. Langmuir 2005, 21, 9873–9878. [Google Scholar] [CrossRef]
- Saha, A.; Nikova, A.; Venkataraman, P.; John, V.T.; Bose, A. Oil Emulsification Using Surface-Tunable Carbon Black Particles. ACS Appl. Mater. Interfaces 2013, 5, 3094–3100. [Google Scholar] [CrossRef]
- Dargahi-Zaboli, M.; Sahraei, E.; Pourabbas, B. Hydrophobic silica nanoparticle-stabilized invert emulsion as drilling fluid for deep drilling. Pet. Sci. 2017, 14, 105–115. [Google Scholar] [CrossRef] [Green Version]
- Whitby, C.P.; Fornasiero, D.; Ralston, J. Effect of adding anionic surfactant on the stability of Pickering emulsions. J. Colloid Interface Sci. 2009, 329, 173–181. [Google Scholar] [CrossRef]
- Wang, J.; Yang, F.; Tan, J.; Liu, G.; Xu, J.; Sun, D. Pickering Emulsions Stabilized by a Lipophilic Surfactant and Hydrophilic Platelike Particles. Langmuir 2010, 26, 5397–5404. [Google Scholar] [CrossRef]
- Yekeen, N.; Padmanabhan, E.; Syed, A.H.; Sevoo, T.; Kanesen, K. Synergistic influence of nanoparticles and surfactants on interfacial tension reduction, wettability alteration and stabilization of oil-in-water emulsion. J. Pet. Sci. Eng. 2020, 186, 106779. [Google Scholar] [CrossRef]
- Powell, K.C.; Chauhan, A. Interfacial Tension and Surface Elasticity of Carbon Black (CB) Covered Oil–Water Interface. Langmuir 2014, 30, 12287–12296. [Google Scholar] [CrossRef]
- Soleimani, H.; Baig, M.K.; Yahya, N.; Khodapanah, L.; Sabet, M.; Demiral, B.M.R.; Burda, M. Synthesis of ZnO nanoparticles for oil–water interfacial tension reduction in enhanced oil recovery. Appl. Phys. A 2018, 124, 128. [Google Scholar] [CrossRef]
- Panahpoori, D.; Rezvani, H.; Parsaei, R.; Riazi, M. A pore-scale study on improving CTAB foam stability in heavy crude oil−water system using TiO2 nanoparticles. J. Pet. Sci. Eng. 2019, 183, 106411. [Google Scholar] [CrossRef]
- Rezaei, A.; Riazi, M.; Escrochi, M.; Elhaei, R. Integrating surfactant, alkali and nano-fluid flooding for enhanced oil recovery: A mechanistic experimental study of novel chemical combinations. J. Mol. Liq. 2020, 308, 113106. [Google Scholar] [CrossRef]
- Pichot, R.; Spyropoulos, F.; Norton, I.T. Competitive adsorption of surfactants and hydrophilic silica particles at the oil–water interface: Interfacial tension and contact angle studies. J. Colloid Interface Sci. 2012, 377, 396–405. [Google Scholar] [CrossRef]
- Biswal, N.R.; Rangera, N.; Singh, J.K. Effect of Different Surfactants on the Interfacial Behavior of the n-Hexane–Water System in the Presence of Silica Nanoparticles. J. Phys. Chem. B 2016, 120, 7265–7274. [Google Scholar] [CrossRef]
- Zhang, H.; Zhou, M.; Guo, Z.; Lan, X.; Zhang, L.; Zhang, L. Effect of hydrophobicity on the interfacial rheological behaviors of nanoparticles at decane-water interface. J. Mol. Liq. 2019, 294, 111618. [Google Scholar] [CrossRef]
- Emadi, S.; Shadizadeh, S.R.; Manshad, A.K.; Rahimi, A.M.; Mohammadi, A.H. Effect of nano silica particles on Interfacial Tension (IFT) and mobility control of natural surfactant (Cedr Extraction) solution in enhanced oil recovery process by nano-surfactant flooding. J. Mol. Liq. 2017, 248, 163–167. [Google Scholar] [CrossRef]
- Nesterenko, A.; Drelich, A.; Lu, H.; Clausse, D.; Pezron, I. Influence of a mixed particle/surfactant emulsifier system on water-in-oil emulsion stability. Colloids Surf. A Physicochem. Eng. Asp. 2014, 457, 49–57. [Google Scholar] [CrossRef]
- Saien, J.; Fadaei, V. The study of interfacial tension of kerosene-water under influence of CTAB surfactant and different size silica nanoparticles. J. Mol. Liq. 2018, 255, 439–446. [Google Scholar] [CrossRef]
- Jafarnezhad, M.; Giri, M.S.; Alizadeh, M. Impact of SnO2 nanoparticles on enhanced oil recovery from carbonate media. Energy Sources Part A Recovery Util. Environ. Eff. 2017, 39, 121–128. [Google Scholar] [CrossRef]
- Hong, J.S.; Rühs, P.A.; Fischer, P. Localization of clay particles at the oil–water interface in the presence of surfactants. Rheol. Acta 2015, 54, 725–734. [Google Scholar] [CrossRef]
- Kazemzadeh, Y.; Dehdari, B.; Etemadan, Z.; Riazi, M.; Sharifi, M. Experimental investigation into Fe3O4/SiO2 nanoparticle performance and comparison with other nanofluids in enhanced oil recovery. Pet. Sci. 2019, 16, 578–590. [Google Scholar] [CrossRef] [Green Version]
- de Oliveira, F.C.; Khani, S.; Maia, J.M.; Tavares, F.W. Concentration and Solvent Effects on Structural, Dynamical, and Rheological Properties of Asphaltene Suspensions. Energy Fuels 2020, 34, 1071–1081. [Google Scholar] [CrossRef]
- Nguele, R.; Sasaki, K. Asphaltene behavior at the interface oil-nanofluids: Implications to adsorption. Colloids Surf. A Physicochem. Eng. Asp. 2021, 622, 126630. [Google Scholar] [CrossRef]
- Forny, L.; Pezron, I.; Saleh, K.; Guigon, P.; Komunjer, L. Storing water in powder form by self-assembling hydrophobic silica nanoparticles. Powder Technol. 2007, 171, 15–24. [Google Scholar] [CrossRef]
- Fereidooni Moghadam, T.; Azizian, S. Effect of ZnO Nanoparticle and Hexadecyltrimethylammonium Bromide on the Dynamic and Equilibrium Oil–Water Interfacial Tension. J. Phys. Chem. B 2014, 118, 1527–1534. [Google Scholar] [CrossRef]
- Akhlaghi, N.; Riahi, S.; Parvaneh, R. Interfacial tension behavior of a nonionic surfactant in oil/water system; salinity, pH, temperature, and ionic strength effects. J. Pet. Sci. Eng. 2021, 198, 108177. [Google Scholar] [CrossRef]
- Lashkarbolooki, M.; Ayatollahi, S. The effects of pH, acidity, asphaltene and resin fraction on crude oil/water interfacial tension. J. Pet. Sci. Eng. 2018, 162, 341–347. [Google Scholar] [CrossRef]
- Hauswirth, S.C.; Schultz, P.B.; Miller, C.T. Compositional and pH Effects on the Interfacial Tension Between Complex Tar Mixtures and Aqueous Solutions. Environ. Sci. Technol. 2012, 46, 10214–10221. [Google Scholar] [CrossRef]
- Soleymanzadeh, A.; Rahmati, A.; Yousefi, M.; Roshani, B. Theoretical and experimental investigation of effect of salinity and asphaltene on IFT of brine and live oil samples. J. Pet. Explor. Prod. 2021, 11, 769–781. [Google Scholar] [CrossRef]
- Farhadi, H.; Ayatollahi, S.; Fatemi, M. The effect of brine salinity and oil components on dynamic IFT behavior of oil-brine during low salinity water flooding: Diffusion coefficient, EDL establishment time, and IFT reduction rate. J. Pet. Sci. Eng. 2021, 196, 107862. [Google Scholar] [CrossRef]
- Moeini, F.; Hemmati-Sarapardeh, A.; Ghazanfari, M.-H.; Masihi, M.; Ayatollahi, S. Toward mechanistic understanding of heavy crude oil/brine interfacial tension: The roles of salinity, temperature and pressure. Fluid Phase Equilibria 2014, 375, 191–200. [Google Scholar] [CrossRef]


| Component | Mass Fraction (%) |
|---|---|
| Saturates | 61.09 |
| Aromatics | 16.77 |
| Resins | 11.12 |
| Asphaltenes | 1.96 |
| Test Name | Mass Fraction (%) |
|---|---|
| C | 83.64 |
| H | 12.51 |
| O | 3.92 |
| N | 0.71 |
| S | 0.06 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, X.; Liu, M.; Li, X.; Wang, L.; Liang, S.; Guo, X. Effects of Surfactant and Hydrophobic Nanoparticles on the Crude Oil-Water Interfacial Tension. Energies 2021, 14, 6234. https://doi.org/10.3390/en14196234
Jiang X, Liu M, Li X, Wang L, Liang S, Guo X. Effects of Surfactant and Hydrophobic Nanoparticles on the Crude Oil-Water Interfacial Tension. Energies. 2021; 14(19):6234. https://doi.org/10.3390/en14196234
Chicago/Turabian StyleJiang, Xu, Ming Liu, Xingxun Li, Li Wang, Shuang Liang, and Xuqiang Guo. 2021. "Effects of Surfactant and Hydrophobic Nanoparticles on the Crude Oil-Water Interfacial Tension" Energies 14, no. 19: 6234. https://doi.org/10.3390/en14196234
APA StyleJiang, X., Liu, M., Li, X., Wang, L., Liang, S., & Guo, X. (2021). Effects of Surfactant and Hydrophobic Nanoparticles on the Crude Oil-Water Interfacial Tension. Energies, 14(19), 6234. https://doi.org/10.3390/en14196234








