Progress in Catalytic Decomposition and Removal of N2O in Fluidized Bed
Abstract
1. Introduction
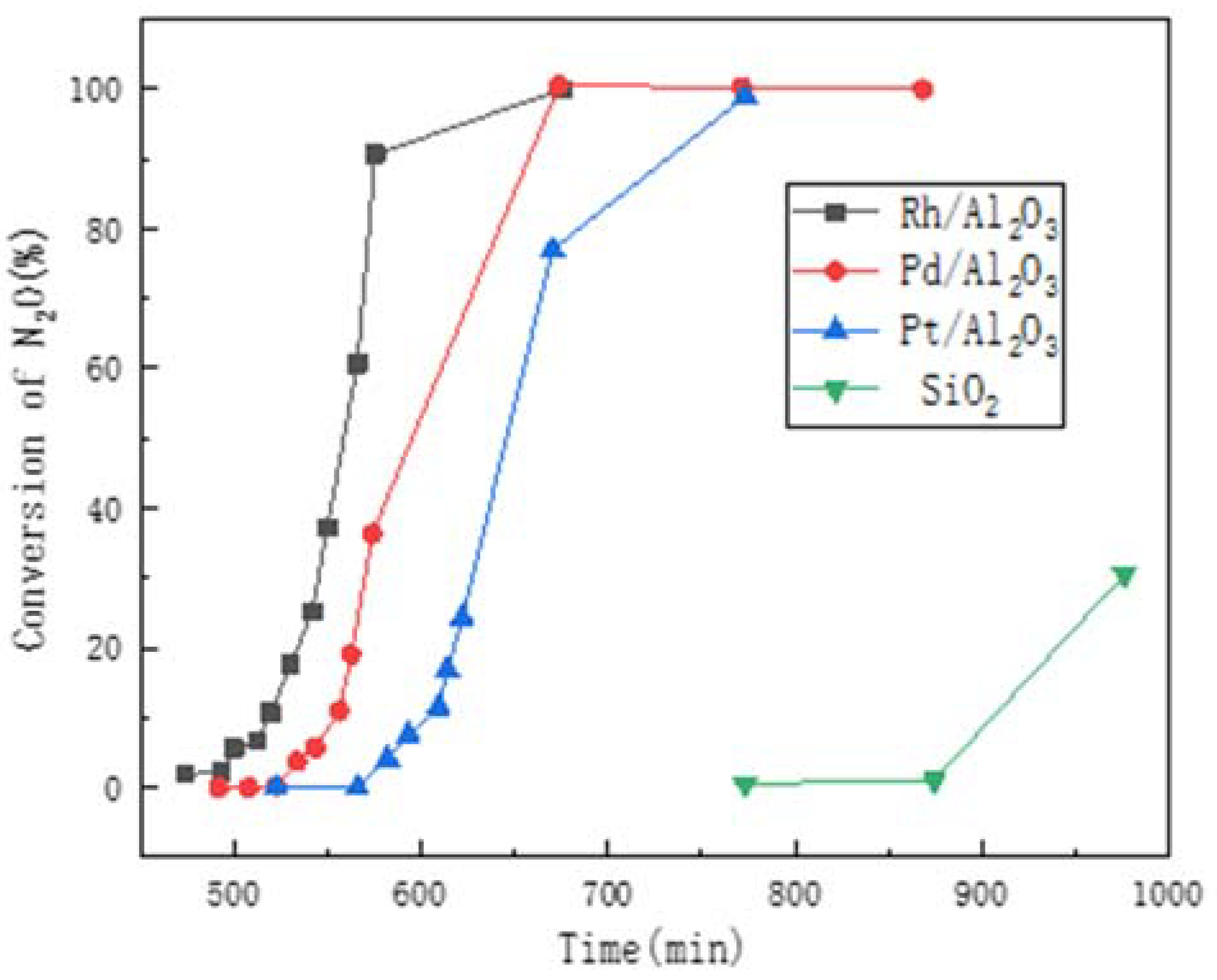
2. The Noble Metal
2.1. Catalytic Mechanism
2.2. Research Progress
2.3. Effect of Support
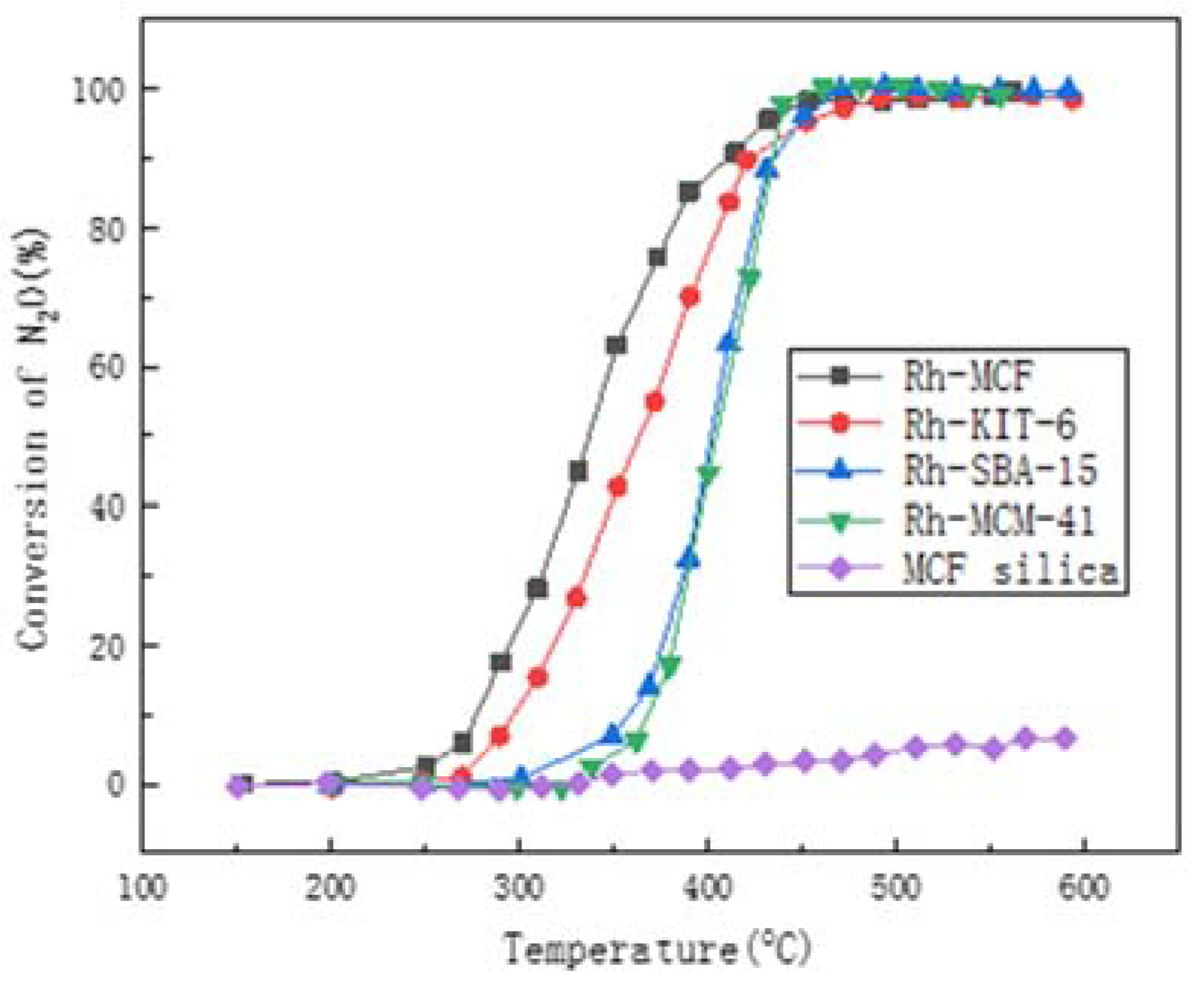
3. The Molecular Sieve
3.1. Research Progress
3.2. Effect of the Preparation Method
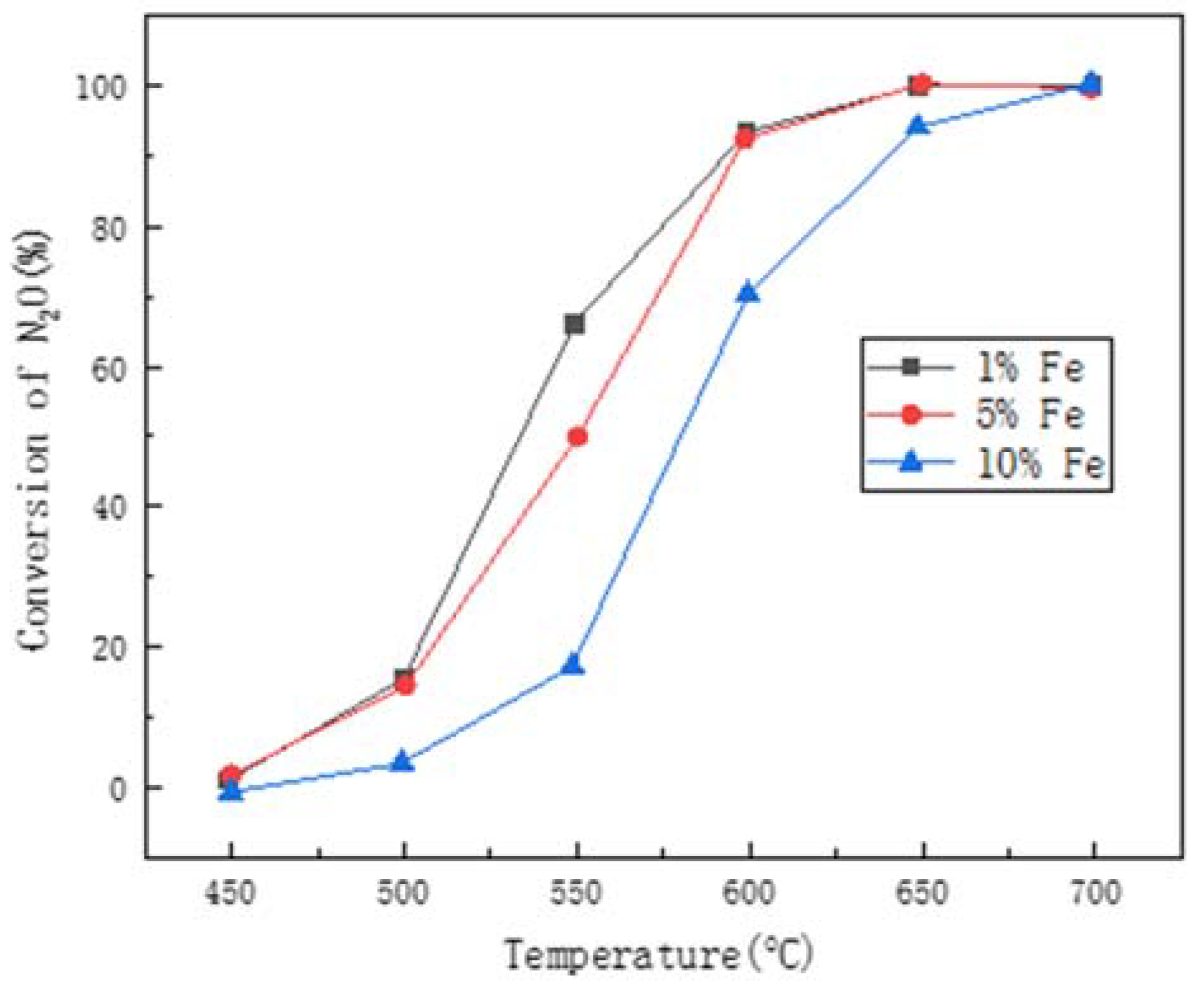
4. The Metal Oxide
4.1. Single Metal Oxide (Bare Oxide)
4.2. Mixed Metal Oxide
4.3. Oxygen Carrier-Aided Combustion (OCAC)
5. Other Catalytic Removal Methods
6. Conclusions
- Noble metals show good N2O decomposition performance at low temperatures, but they have high costs, a narrow temperature window and can easily lead to poisoning by oxygen and water vapor.
- A metal-ion-modified molecular sieve has higher activity and better low-temperature activity, but its hydrothermal stability is poor.
- Metal oxides have high thermal stability, so they are more suitable for use in CFB. Among them, hexaaluminate oxide has a large high-temperature specific surface area, excellent sintering resistance and thermal shock resistance and has high removal efficiency and selectivity for N2O. It is a very promising high-temperature N2O decomposition catalyst.
- Partial or total replacement of silica sand with an oxygen carrier may help to promote oxygen distribution in the combustion chamber, which is called OCAC. OCAC can significantly reduce the concentrations of carbon monoxide and nitric oxide in the furnace. Therefore, it can also be considered to reduce N2O emissions in CFB boilers.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Blaszczuk, A.; Pogorzelec, M.; Shimizu, T. Heat transfer characteristics in a large-scale bubbling fluidized bed with immersed horizontal tube bundles. Energy 2018, 162, 10–19. [Google Scholar] [CrossRef]
- Scala, F. Attrition during steam gasification of lignite char in a fluidized bed reactor. Fuel Process. Technol. 2016, 141, 38–43. [Google Scholar] [CrossRef]
- Yang, H.; Lu, J.; Zhang, H.; Yue, G.; Guo, Y. Coal ignition characteristics in CFB boiler. Fuel 2005, 84, 1849–1853. [Google Scholar] [CrossRef]
- Yue, G.; Cai, R.; Lu, J.; Zhang, H. From a CFB reactor to a CFB boiler—The review of R&D progress of CFB coal combustion technology in China. Powder Technol. 2017, 316, 18–28. [Google Scholar] [CrossRef]
- Leckner, B. Fluidized bed combustion: Achievements and problems. Symp. Int. Combust. 1996, 26, 3231–3241. [Google Scholar] [CrossRef]
- Scala, F.; Chirone, R.; Meloni, P.; Carcangiu, G.; Manca, M.; Mulas, G.; Mulas, A. Fluidized bed desulfurization using lime obtained after slow calcination of limestone particles. Fuel 2013, 114, 99–105. [Google Scholar] [CrossRef]
- Mei, L.; Lu, X.; Wang, Q.; Pan, Z.; Ji, X.; Hong, Y.; Fang, C.; Guo, H.; Yang, X. The experimental study of fly ash decarbonization on a circulating fluidized bed combustor. Appl. Therm. Eng. 2014, 63, 608–615. [Google Scholar] [CrossRef]
- Cai, R.; Zhang, H.; Zhang, M.; Yang, H.; Lyu, J.; Yue, G. Development and application of the design principle of fluidization state specification in CFB coal combustion. Fuel Process. Technol. 2018, 174, 41–52. [Google Scholar] [CrossRef]
- Miao, M.; Kong, H.; Deng, B.; Chen, L.; Yang, H.; Lyu, J.; Zhang, M. Experimental study on N2O and NOx emission characteristics of five high-volatile fuels in bubbling bed combustion. Fuel Process. Technol. 2020, 208, 106517. [Google Scholar] [CrossRef]
- Crutzen, P.J. The influence of nitrogen oxide on the atmospheric ozone content. Q. J. R. Meteorol. Soc. 1970, 408, 320–325. [Google Scholar] [CrossRef]
- Bernhard, B.; Gudrun, P.; Herbert, B. Formation and decomposition of N2O in fluidized bed boilers. Fuel 1995, 74, 165–171. [Google Scholar]
- Ke, X.; Cai, R.; Zhang, M.; Miao, M.; Lyu, J.; Yang, H. Application of ultra-low NOx emission control for CFB boilers based on theoretical analysis and industrial practices. Fuel Process. Technol. 2018, 181, 252–258. [Google Scholar] [CrossRef]
- Miao, M.; Zhang, M.; Lv, J.; Yang, H.; Zhang, K. N2O formation mechanism and control in circulating fluidized beds. J. Tsinghua Univ. Sci. Technol. 2020, 60, 507–517. [Google Scholar]
- Turunen, J.; Markkula, J.; Rajakallio, M.; Aroviita, J. Riparian forests mitigate harmful ecological effects of agricultural diffuse pollution in medium-sized streams. Sci. Total. Environ. 2019, 649, 495–503. [Google Scholar] [CrossRef]
- Zheng, L.; Wu, C.-C.; Xu, X.-F. Catalytic decomposition of N2O over Mg-Co and Mg-Mn-Co composite oxides. J. Fuel Chem. Technol. 2016, 44, 1494–1501. [Google Scholar] [CrossRef]
- Lew, V.; McKay, E.; Maze, M. Past, present, and future of nitrous oxide. Br. Med Bull. 2018, 125, 103–119. [Google Scholar] [CrossRef]
- Xu, C.; Zhang, Q.; Gao, L.; Zheng, M.; Qiao, L.; Cui, L.; Wang, R.; Cheng, J. Spatial distributions and transport implications of short- and medium-chain chlorinated paraffins in soils and sediments from an e-waste dismantling area in China. Sci. Total Environ. 2019, 649, 821–828. [Google Scholar] [CrossRef]
- Swamy, C.S.; Christopher, J. Decomposition of N2O on perovskite-related oxides. Catal. Rev. 1992, 34, 409–425. [Google Scholar] [CrossRef]
- Peña, M.A.; Fierro, J.L. Chemical structures and performance of perovskite oxides. Chem. Rev. 2001, 101, 1981–2017. [Google Scholar] [CrossRef]
- Sui, C.; Zhang, T.; Dong, Y.; Yuan, F.; Niu, X.; Zhu, Y. Interaction between Ru and Co3O4 for promoted catalytic decomposition of N2O over the Rux-Co3O4 catalysts. Mol. Catal. 2017, 435, 174–181. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, Z.; Sui, C.; Yuan, F.; Niu, X.; Zhu, Y. Catalytic decomposition of N2O over Co–Ti oxide catalysts: Interaction between Co and Ti oxide. ChemCatChem 2016, 8, 2155–2164. [Google Scholar] [CrossRef]
- Li, C.; Shen, Y.; Zhu, S.; Shen, S. Supported Ni-La-Ox for catalytic decomposition of N2O I: Component optimization and synergy. RSC Adv. 2014, 4, 29107–29119. [Google Scholar] [CrossRef]
- Centi, G.; Perathoner, S.; Rak, Z.S. Reduction of greenhouse gas emissions by catalytic processes. Appl. Catal. B Environ. 2003, 41, 143–155. [Google Scholar] [CrossRef]
- Kondratenko, V. Mechanistic and kinetic insights into N2O decomposition over Pt gauze. J. Catal. 2004, 225, 37–44. [Google Scholar] [CrossRef]
- Kondratenko, V.; Baerns, M. Effect of different oxygen species originating from the dissociation of O2, N2O and NO on the selectivity of the Pt-catalysed NH3 oxidation. Catal. Today 2007, 121, 210–216. [Google Scholar] [CrossRef]
- Suárez, S.; Yates, M.; Petre, A.; Martín, J.; Avila, P.; Blanco, J. Development of a new Rh/TiO2–sepiolite monolithic catalyst for N2O decomposition. Appl. Catal. B Environ. 2006, 64, 302–311. [Google Scholar] [CrossRef]
- Haber, J.; Nattich-Rak, M.; Machej, T. Alkali-metal promoted rhodium-on-alumina catalysts for nitrous oxide decomposition. Appl. Catal. B Environ. 2008, 77, 278–283. [Google Scholar] [CrossRef]
- Reddy, P.S.S.; Babu, N.S.; Pasha, N.; Lingaiah, N.; Prasad, P.S.S. Influence of microwave irradiation on catalytic decomposition of nitrous oxide over Rh/Al2O3 catalyst. Catal. Commun. 2008, 9, 2303–2307. [Google Scholar] [CrossRef]
- Ramnani, S.P.; Sabharwal, S.; Kumar, J.V.; Reddy, K.H.P.; Rao, K.S.R.; Prasad, P.S.S. Advantage of radiolysis over impregnation method for the synthesis of SiO2 supported nano-Ag catalyst for direct decomposition of N2O. Catal. Commun. 2008, 9, 756–761. [Google Scholar] [CrossRef]
- Tzitzios, V.K.; Georgakilas, V. Catalytic reduction of N2O over Ag-Pd/Al2O3 bimetallic catalysts. Chemosphere 2005, 59, 887–891. [Google Scholar] [CrossRef]
- Christoforou, S.C.; Efthimiadis, E.A.; Vasalos, I.A. Catalytic conversion of N2O to N2 over metal-based catalysts in the presence of hydrocarbons and oxygen. Catal. Lett. 2002, 79, 137–147. [Google Scholar] [CrossRef]
- Centi, G.; Dall’Olio, L.; Perathoner, S. Oscillating behavior in N2O decomposition over Rh supported on zirconia-based catalysts: The role of the reaction conditions. J. Catal. 2000, 192, 224–235. [Google Scholar] [CrossRef]
- Dacquin, J.-P.; Dujardin, C.; Granger, P. Surface reconstruction of supported Pd on LaCoO3: Consequences on the catalytic properties in the decomposition of N2O. J. Catal. 2008, 253, 37–49. [Google Scholar] [CrossRef]
- Pinna, F.; Scarpa, M.; Strukul, G.; Guglielminotti, E.; Boccuzzi, F.; Manzoli, M. Ru/ZrO2 catalysts: II. N2O adsorption and decomposition. J. Catal. 2000, 192, 158–162. [Google Scholar] [CrossRef]
- Dacquin, J.P.; Dujardin, C.; Granger, P. Catalytic decomposition of N2O on supported Pd catalysts: Support and thermal ageing effects on the catalytic performances. Catal. Today 2008, 137, 390–396. [Google Scholar] [CrossRef]
- Hinshelwood, C.N.; Prichard, C.R. LI.—A comparison between the homogeneous thermal decomposition of nitrous oxide and its heterogeneous catalytic decomposition on the surface of platinum. J. Chem. Soc. Trans. 1925, 127, 327–336. [Google Scholar] [CrossRef]
- Kim, S.S.; Lee, S.J.; Hong, S.C. Effect of CeO2 addition to Rh/Al2O3 catalyst on N2O decomposition. Chem. Eng. J. 2011, 169, 173–179. [Google Scholar] [CrossRef]
- Doi, K.; Wu, Y.Y.; Takeda, R.; Matsunami, A.; Arai, N.; Tagawa, T.; Goto, S. Catalytic decomposition of N2O in medical operating rooms over Rh/Al2O3, Pd/Al2O3, and Pt/Al2O3. Appl. Catal. B Environ. 2001, 35, 43–51. [Google Scholar] [CrossRef]
- Avilés, R.; Poulain, E.; Olvera-Neria, O.; Bertin, V. The spin significance in the capture and activation of N2O by small Rh nanoparticles. J. Mol. Catal. A Chem. 2013, 376, 22–33. [Google Scholar] [CrossRef]
- Piumetti, M.; Hussain, M.; Fino, D.; Russo, N. Mesoporous silica supported Rh catalysts for high concentration N2O decomposition. Appl. Catal. B Environ. 2015, 165, 158–168. [Google Scholar] [CrossRef]
- Beyer, H.; Emmerich, J.; Chatziapostolou, K.; Köhler, K. Decomposition of nitrous oxide by rhodium catalysts: Effect of rhodium particle size and metal oxide support. Appl. Catal. A Gen. 2011, 391, 411–416. [Google Scholar] [CrossRef]
- Parres-Esclapez, S.; Illán-Gómez, M.J.; De Lecea, C.S.; Bueno-López, A. On the importance of the catalyst redox properties in the N2O decomposition over alumina and ceria supported Rh, Pd and Pt. Appl. Catal. B Environ. 2010, 96, 370–378. [Google Scholar] [CrossRef]
- Hussain, M.; Fino, D.; Russo, N. N2O decomposition by mesoporous silica supported Rh catalysts. J. Hazard. Mater. 2012, 211–212, 255–265. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Meng, T.; Ma, Z. Catalytic decomposition of N2O over RhO supported on metal phosphates. J. Ind. Eng. Chem. 2015, 28, 138–146. [Google Scholar] [CrossRef]
- Cui, Y.; Liu, H.; Lin, Y.; Ma, Z. Metal phosphate-supported RuOx catalysts for N2O decomposition. J. Taiwan Inst. Chem. Eng. 2016, 67, 254–262. [Google Scholar] [CrossRef]
- Dou, Z.; Feng, M.; Xiu-Feng, X. Catalytic decomposition of N2O over Au/Co3O4 and Au/ZnCo2O4 catalysts. J. Fuel Chem. Technol. 2013, 41, 1234–1240. [Google Scholar] [CrossRef]
- Žemva, P.; Lesar, A.; Kobal, I.; Senegačnik, M. Interpretation of kinetic isotope effects in the decomposition of N2O over CoO. Chem. Phys. 2001, 264, 413–418. [Google Scholar] [CrossRef]
- Smeets, P.J.; Sels, B.F.; van Teeffelen, R.M.; Leeman, H.; Hensen, E.J.M.; Schoonheydt, R.A. The catalytic performance of Cu-containing zeolites in N2O decomposition and the influence of O2, NO and H2O on recombination of oxygen. J. Catal. 2008, 256, 183–191. [Google Scholar] [CrossRef]
- Park, J.-H.; Choung, J.-H.; Nam, I.-S.; Ham, S.-W. N2O decomposition over wet- and solid-exchanged Fe-ZSM-5 catalysts. Appl. Catal. B Environ. 2008, 78, 342–354. [Google Scholar] [CrossRef]
- Nobukawa, T.; Yoshida, M.; Okumura, K.; Tomishige, K.; Kunimori, K. Effect of reductants in N2O reduction over Fe-MFI catalysts. J. Catal. 2005, 229, 374–388. [Google Scholar] [CrossRef]
- Jisa, K.; Novakova, J.; Schwarze, M.; Vondrova, A.; Sklenak, S.; Sobalik, Z. Role of the Fe-zeolite structure and iron state in the N2O decomposition: Comparison of Fe-FER, Fe-BEA, and Fe-MFI catalysts. J. Catal. 2009, 262, 27–34. [Google Scholar] [CrossRef]
- Kiwi-Minsker, L. Active sites in HZSM-5 with low Fe content for the formation of surface oxygen by decomposing N2O: Is every deposited oxygen active? J. Catal. 2003, 219, 273–285. [Google Scholar] [CrossRef][Green Version]
- Pérez-Ramírez, J. Steam-activated FeMFI zeolites. Evolution of iron species and activity in direct N2O decomposition. J. Catal. 2003, 214, 33–45. [Google Scholar] [CrossRef]
- Ahrens, M.; Marie, O.; Bazin, P.; Daturi, M. Fe-H-BEA and Fe-H-ZSM-5 for NO2 removal from ambient air-A detailed in situ and operando FTIR study revealing an unexpected positive water-effect. J. Catal. 2010, 271, 1–11. [Google Scholar] [CrossRef]
- Kaucky, D.; Sobalik, Z.; Schwarze, M.; Vondrová, A.; Wichterlová, B. Effect of FeH-zeolite structure and Al-Lewis sites on N2O decomposition and NO/NO2-assisted reaction. J. Catal. 2006, 238, 293–300. [Google Scholar] [CrossRef]
- Pérez-Ramírez, J. Active iron sites associated with the reaction mechanism of N2O conversions over steam-activated FeMFI zeolites. J. Catal. 2004, 227, 512–522. [Google Scholar] [CrossRef]
- Melián-Cabrera, I.; Mentruit, C.; Pieterse, J.A.Z.; Van den Brink, R.W.; Mul, G.; Kapteijn, F.; Moulijn, J.A. Highly active and stable ion-exchanged Fe-Ferrierite catalyst for N2O decomposition under nitric acid tail gas conditions. Catal. Commun. 2005, 6, 301–305. [Google Scholar] [CrossRef]
- Held, A.; Florczak, P. Vanadium, niobium and tantalum modified mesoporous molecular sieves as catalysts for propene epoxidation. Catal. Today 2009, 142, 329–334. [Google Scholar] [CrossRef]
- Guzmanvargas, A. Catalytic decomposition of N2O and catalytic reduction of N2O and N2O + NO by NH3 in the presence of O2 over Fe-zeolite. Appl. Catal. B Environ. 2003, 42, 369–379. [Google Scholar] [CrossRef]
- Wu, M.; Wang, H.; Zhong, L.; Zhang, X.; Hao, Z.; Shen, Q.; Wei, W.; Qian, G.; Sun, Y. Effects of acid pretreatment on Fe-ZSM-5 and Fe-beta catalysts for N2O decomposition. Chin. J. Catal. 2016, 37, 898–907. [Google Scholar] [CrossRef]
- Smeets, P.J.; Meng, Q.; Corthals, S.; Leeman, H.; Schoonheydt, R.A. Co-ZSM-5 catalysts in the decomposition of N2O and the SCR of NO with CH4: Influence of preparation method and cobalt loading. Appl. Catal. B Environ. 2008, 84, 505–513. [Google Scholar] [CrossRef]
- Meng, T.; Ren, N.; Ma, Z. Silicalite-1@ Cu-ZSM-5 core-shell catalyst for N2O decomposition. J. Mol. Catal. A Chem. 2015, 404–405, 233–239. [Google Scholar] [CrossRef]
- Kondratenko, E.; Kondratenko, V.; Santiago, M.; Perezramirez, J. Mechanistic origin of the different activity of Rh-ZSM-5 and Fe-ZSM-5 in N2O decomposition. J. Catal. 2008, 256, 248–258. [Google Scholar] [CrossRef]
- Zhang, R.; Liu, N.; Lei, Z.; Chen, B. Selective transformation of various nitrogen-containing exhaust gases toward N2 over zeolite catalysts. Chem. Rev. 2016, 116, 3658–3721. [Google Scholar] [CrossRef] [PubMed]
- Pannov, G.I.; Sobolev, V.I.; Kharitonov, A.S. The role of iron in N2O decomposition on ZSM-5 zeolite and reactivity of the surface oxygen formed. J. Mol. Catal. 1990, 61, 85–97. [Google Scholar] [CrossRef]
- Pieterse, J. Evaluation of Fe-zeolite catalysts prepared by different methods for the decomposition of N2O. Appl. Catal. B Environ. 2004, 51, 215–228. [Google Scholar] [CrossRef]
- Du, J.; Kuang, W.; Xu, H.; Shen, W.; Zhao, D. The influence of precursors on Rh/SBA-15 catalysts for N2O decomposition. Appl. Catal. B Environ. 2008, 84, 490–496. [Google Scholar] [CrossRef]
- Kim, M.-Y.; Lee, K.W.; Park, J.-H.; Shin, C.-H.; Lee, J.; Seo, G. Catalytic decomposition of nitrous oxide over Fe-BEA zeolites: Essential components of iron active sites. Korean J. Chem. Eng. 2010, 27, 76–82. [Google Scholar] [CrossRef]
- Abu-Zied, B.M.; Schwieger, W.; Unger, A. Nitrous oxide decomposition over transition metal exchanged ZSM-5 zeolites prepared by the solid-state ion-exchange method. Appl. Catal. B Environ. 2008, 84, 277–288. [Google Scholar] [CrossRef]
- Yan, L.; Ren, T.; Wang, X.; Gao, Q.; Ji, D.; Suo, J. Excellent catalytic performance of Zn Co1−Co2O4 spinel catalysts for the decomposition of nitrous oxide. Catal. Commun. 2003, 4, 505–509. [Google Scholar] [CrossRef]
- Ohnishi, C.; Asano, K.; Iwamoto, S.; Chikama, K.; Inoue, M. Alkali-doped Co3O4 catalysts for direct decomposition of N2O in the presence of oxygen. Catal. Today 2007, 120, 145–150. [Google Scholar] [CrossRef]
- Asano, K.; Ohnishi, C.; Iwamoto, S.; Shioya, Y.; Inoue, M. Potassium-doped Co3O4 catalyst for direct decomposition of N2O. Appl. Catal. B Environ. 2008, 78, 242–249. [Google Scholar] [CrossRef]
- Cimino, A. Activity of Mn3+ and Mn4+ ions dispersed in MgO for N2O decomposition. J. Catal. 1970, 17, 54–70. [Google Scholar] [CrossRef]
- Giecko, G.; Borowiecki, T.; Gac, W.; Kruk, J. Fe2O3/Al2O3 catalysts for the N2O decomposition in the nitric acid industry. Catal. Today 2008, 137, 403–409. [Google Scholar] [CrossRef]
- Wu, L.; Hu, X.; Qin, W.; Gao, P.; Dong, C.; Yang, Y. Effect of CaO on the selectivity of N2O decomposition products: A combined experimental and DFT study. Surf. Sci. 2016, 651, 128–136. [Google Scholar] [CrossRef]
- Hou, X.; Zhang, H.; Pilawska, M.; Lu, J.; Yue, G. The formation of N2O during the reduction of NO by NH3. Fuel 2008, 87, 3271–3277. [Google Scholar] [CrossRef]
- Barišić, V.; Klingstedt, F.; Naydenov, A.; Stefanov, P.; Kilpinen, P.; Hupa, M. Catalytic activity of bed materials from industrial CFB boilers for the decomposition of N2O. Catal. Today 2005, 100, 337–342. [Google Scholar] [CrossRef]
- Yang, X.; Zhuo, Y.; Li, T.; Chen, C.; Xu, X. Decomposition of nitrous oxide over a low-cost catalyst. Asia-Pacific J. Chem. Eng. 2010, 5, 838–846. [Google Scholar] [CrossRef]
- Liu, Z.; He, F.; Ma, L.; Peng, S. Recent advances in catalytic decomposition of N2O on noble metal and metal oxide catalysts. Catal. Surv. Asia 2016, 20, 121–132. [Google Scholar] [CrossRef]
- Konsolakis, M. Recent advances on nitrous oxide (N2O) decomposition over non-noble-metal oxide catalysts: Catalytic performance, mechanistic considerations, and surface chemistry aspects. ACS Catal. 2015, 5, 6397–6421. [Google Scholar] [CrossRef]
- Russo, N.; Fino, D.; Saracco, G.; Specchia, V. N2O catalytic decomposition over various spinel-type oxides. Catal. Today 2007, 119, 228–232. [Google Scholar] [CrossRef]
- Pacultová, K.; Obalová, L.; Kovanda, F.; Jirátová, K. Catalytic reduction of nitrous oxide with carbon monoxide over calcined Co–Mn–Al hydrotalcite. Catal. Today 2008, 137, 385–389. [Google Scholar] [CrossRef]
- Santiago, M.; Groen, J.; Pérez-Ramírez, J. Carbon-templated hexaaluminates with enhanced surface area and catalytic performance. J. Catal. 2008, 257, 152–162. [Google Scholar] [CrossRef]
- Ruszak, M.; Inger, M.; Witkowski, S.; Wilk, M.; Kotarba, A.; Sojka, Z. Selective N2O removal from the process gas of nitric acid plants over ceramic 12CaO 7Al2O3 catalyst. Catal. Lett. 2008, 126, 72–77. [Google Scholar] [CrossRef]
- Xue, L.; Zhang, C.; He, H.; Teraoka, Y. Catalytic decomposition of N2O over CeO2 promoted Co3O4 spinel catalyst. Appl. Catal. B Environ. 2007, 75, 167–174. [Google Scholar] [CrossRef]
- Keav, S.; Matam, S.; Ferri, D.; Weidenkaff, A. Structured perovskite-based catalysts and their application as three-way catalytic con-verters—A review. Catalysts 2014, 4, 226–255. [Google Scholar] [CrossRef]
- Dacquin, J.P.; Lancelot, C.; Dujardin, C.; Da Costa, P.; Djega-Mariadassou, G.; Beaunier, P.; Kaliaguine, S.; Vaudreuil, S.; Royer, S.; Granger, P. Influence of preparation methods of LaCoO3 on the catalytic performances in the decomposition of N2O. Appl. Catal. B Environ. 2009, 91, 596–604. [Google Scholar] [CrossRef]
- Carl, K.; Marcus, H.; Henrik, L. The effect of iron and manganese-based oxygen carriers as bed materials in oxygen carrier aided combustion. Energy Technol. 2019, 7, 1–13. [Google Scholar]
- Hildor, F.; Mattisson, T.; Leion, H.; Linderholm, C.; Rydén, M. Steel converter slag as an oxygen carrier in a 12 MWth CFB boiler–Ash interaction and material evolution. Int. J. Greenh. Gas Control 2019, 88, 321–331. [Google Scholar] [CrossRef]
- Wang, P.; Leion, H.; Yang, H. Oxygen-carrier-aided combustion in a bench-scale fluidized bed. Energy Fuels 2017, 31, 6463–6471. [Google Scholar] [CrossRef]
- Thunman, H.; Lind, F.; Breitholtz, C.; Berguerand, N.; Seemann, M. Using an oxygen-carrier as bed material for combustion of biomass in a 12-MWth circulating fluidized-bed boiler. Fuel 2013, 113, 300–309. [Google Scholar] [CrossRef]
- Darwish, E.; Yılmaz, D.; Leion, H. Experimental and thermodynamic study on the interaction of copper oxygen carriers and oxide compounds commonly present in ashes. Energy Fuels 2019, 33, 2502–2515. [Google Scholar] [CrossRef]
- Xiangsong, H.; Hai, Z.; Shi, Y.; Junfu, L.; Guangxi, Y. N2O decomposition over the circulating ashes from coal-fired CFB boilers. Chem. Eng. J. 2008, 140, 43–51. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miao, M.; Zhang, M.; Kong, H.; Zhou, T.; Yang, X.; Yang, H. Progress in Catalytic Decomposition and Removal of N2O in Fluidized Bed. Energies 2021, 14, 6148. https://doi.org/10.3390/en14196148
Miao M, Zhang M, Kong H, Zhou T, Yang X, Yang H. Progress in Catalytic Decomposition and Removal of N2O in Fluidized Bed. Energies. 2021; 14(19):6148. https://doi.org/10.3390/en14196148
Chicago/Turabian StyleMiao, Miao, Man Zhang, Hao Kong, Tuo Zhou, Xinhua Yang, and Hairui Yang. 2021. "Progress in Catalytic Decomposition and Removal of N2O in Fluidized Bed" Energies 14, no. 19: 6148. https://doi.org/10.3390/en14196148
APA StyleMiao, M., Zhang, M., Kong, H., Zhou, T., Yang, X., & Yang, H. (2021). Progress in Catalytic Decomposition and Removal of N2O in Fluidized Bed. Energies, 14(19), 6148. https://doi.org/10.3390/en14196148









