Interfacial Engineering of Attractive Pickering Emulsion Gel-Templated Porous Materials for Enhanced Solar Vapor Generation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Preparation of Attractive Pickering Emulsion Gels-Templated Porous Evaporators (APEG-TPEs)
2.2.2. Hydrophilization of APEG-TPEs
2.2.3. Preparation of PPy Coating
2.2.4. Measurement of Solar Evaporation
2.2.5. Characterization
2.2.6. Simulations of Water Evaporation of APEG-TPEs with Different Porosity
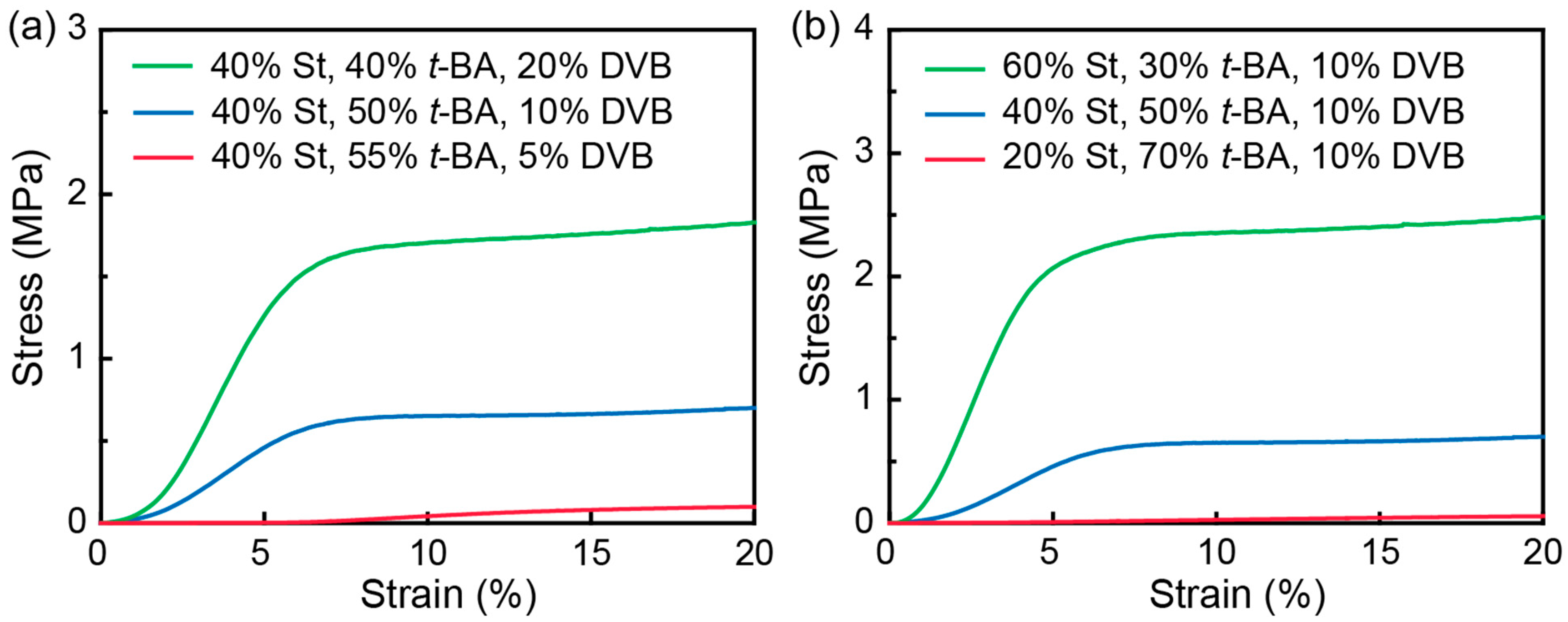
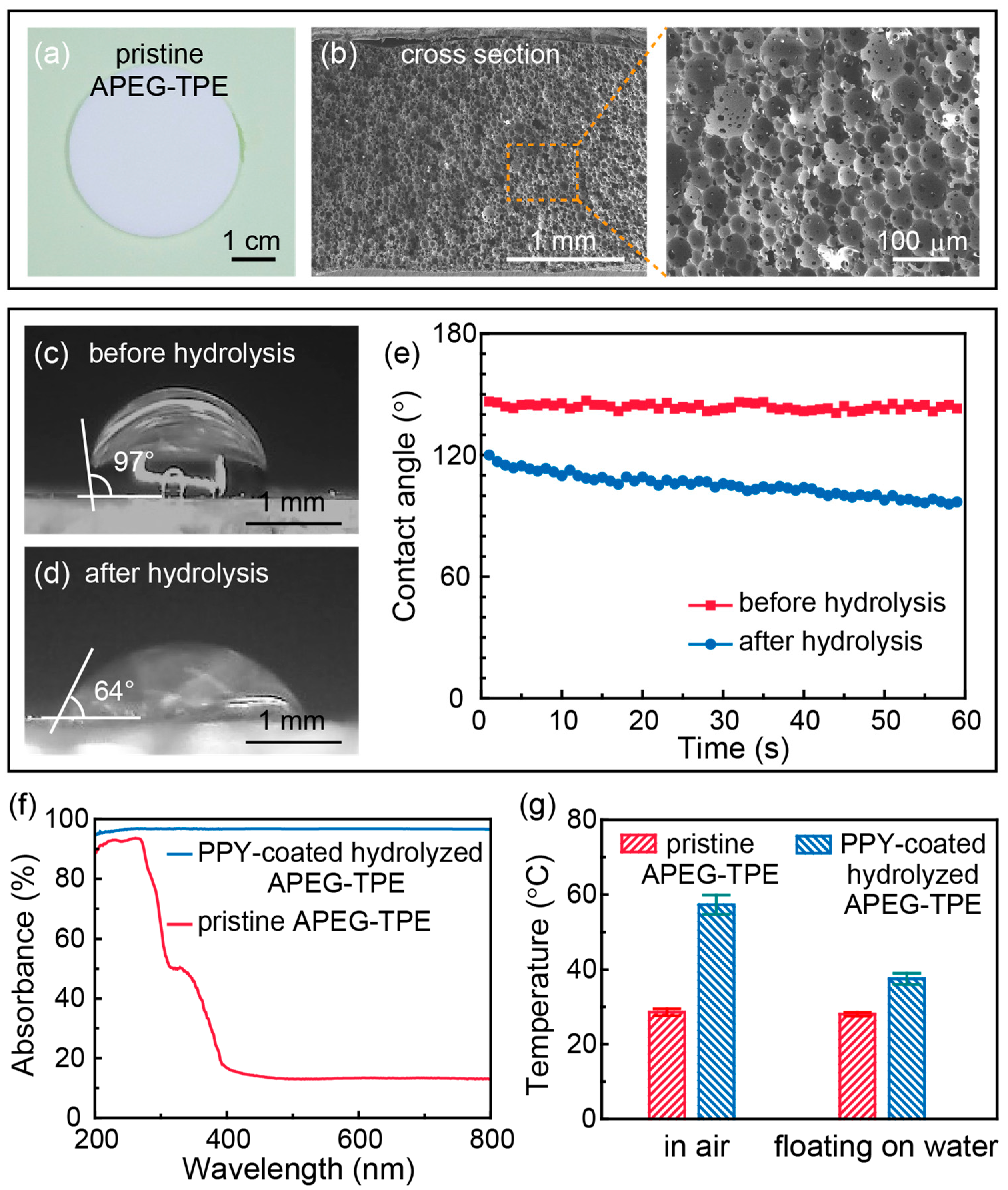
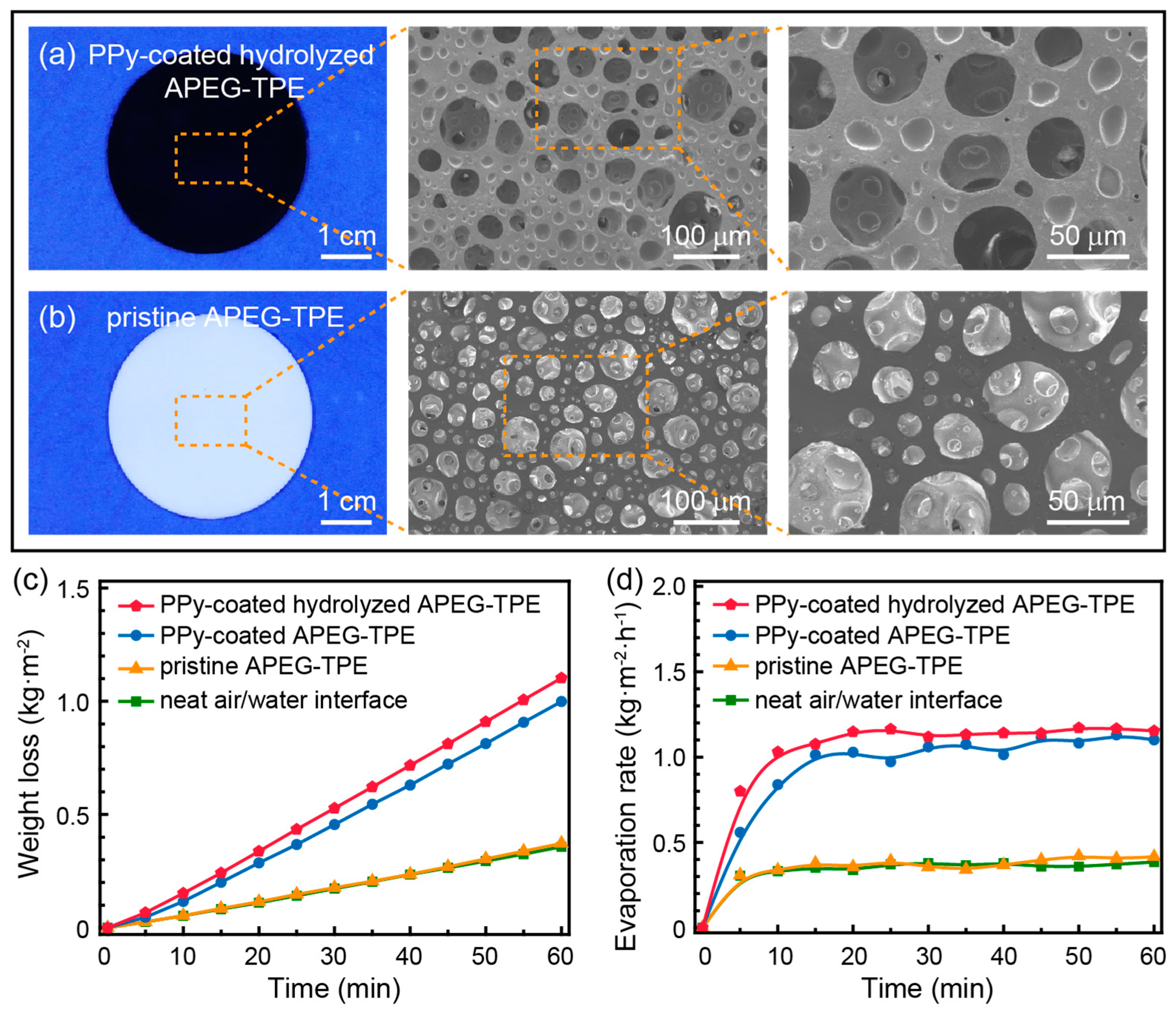
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lu, Y.; Song, S.; Wang, R.; Liu, Z.; Meng, J.; Sweetman, A.J.; Jenkins, A.; Ferrier, R.C.; Li, H.; Luo, W.; et al. Impacts of Soil and Water Pollution on Food Safety and Health Risks in China. Environ. Int. 2015, 77, 5–15. [Google Scholar] [CrossRef] [Green Version]
- Zhao, J.; Gleason, K.K. Solvent-Less Vapor-Phase Fabrication of Membranes for Sustainable Separation Processes. Engineering 2020, 6, 1432–1442. [Google Scholar] [CrossRef]
- Gong, B.; Yang, H.; Wu, S.; Yan, J.; Cen, K.; Bo, Z.; Ostrikov, K.K. Superstructure-Enabled Anti-Fouling Membrane for Efficient Photothermal Distillation. ACS Sustain. Chem. Eng. 2019, 7, 20151–20158. [Google Scholar] [CrossRef]
- Wu, L.; Dong, Z.; Cai, Z.; Ganapathy, T.; Fang, N.X.; Li, C.; Yu, C.; Zhang, Y.; Song, Y. Highly Efficient Three-Dimensional Solar Evaporator for High Salinity Desalination by Localized Crystallization. Nat. Commun. 2020, 11, 521. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bai, H.; Zhao, T.; Cao, M. Interfacial Solar Evaporation for Water Production: From Structure Design to Reliable Performance. Mol. Syst. Des. Eng. 2020, 5, 419–432. [Google Scholar] [CrossRef]
- Zhu, L.; Gao, M.; Peh, C.K.N.; Ho, G.W. Recent Progress in Solar-Driven Interfacial Water Evaporation: Advanced Designs and Applications. Nano Energy 2019, 57, 507–518. [Google Scholar] [CrossRef]
- Tao, P.; Ni, G.; Song, C.; Shang, W.; Wu, J.; Zhu, J.; Chen, G.; Deng, T. Solar-Driven Interfacial Evaporation. Nat. Energy 2018, 3, 1031–1041. [Google Scholar] [CrossRef]
- Lin, Y.; Xu, H.; Shan, X.; Di, Y.; Zhao, A.; Hu, Y.; Gan, Z. Solar Steam Generation Based on the Photothermal Effect: From Designs to Applications, and Beyond. J. Mater. Chem. A 2019, 7, 19203–19227. [Google Scholar] [CrossRef]
- Cao, S.; Jiang, Q.; Wu, X.; Ghim, D.; Derami, H.G.; Chou, P.-I.; Jun, Y.-S.; Singamaneni, S. Advances in Solar Evaporator Materials for Freshwater Generation. J. Mater. Chem. A 2019, 7, 24092–24123. [Google Scholar] [CrossRef]
- Mirmanto; Sayoga, I.M.A.; Wijayanta, A.T.; Sasmito, A.P.; Aziz, M. Enhancement of Continuous-Feed Low-Cost Solar Distiller: Effects of Various Fin Designs. Energies 2021, 14, 4844. [Google Scholar] [CrossRef]
- Zhou, L.; Li, X.; Ni, G.W.; Zhu, S.; Zhu, J. The Revival of Thermal Utilization from the Sun: Interfacial Solar Vapor Generation. Natl. Sci. Rev. 2019, 6, 562–578. [Google Scholar] [CrossRef] [Green Version]
- Zhao, F.; Guo, Y.; Zhou, X.; Shi, W.; Yu, G. Materials for Solar-Powered Water Evaporation. Nat. Rev. Mater. 2020, 5, 388–401. [Google Scholar] [CrossRef]
- Chen, L.; Yang, C.; Xiao, Y.; Yan, X.; Hu, L.; Eggersdorfer, M.; Chen, D.; Weitz, D.; Ye, F. Millifluidics, Microfluidics, and Nanofluidics: Manipulating Fluids at Varying Length Scales. Mater. Today Nano 2021, 16, 100136. [Google Scholar] [CrossRef]
- Alvi, J.Z.; Feng, Y.; Wang, Q.; Imran, M.; Khan, L.A.; Pei, G. Effect of Phase Change Material Storage on the Dynamic Performance of a Direct Vapor Generation Solar Organic Rankine Cycle System. Energies 2020, 13, 5904. [Google Scholar] [CrossRef]
- Zhou, L.; Tan, Y.; Ji, D.; Zhu, B.; Zhang, P.; Xu, J.; Gan, Q.; Yu, Z.; Zhu, J. Self-Assembly of Highly Efficient, Broadband Plasmonic Absorbers for Solar Steam Generation. Sci. Adv. 2016, 2, e1501227. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, L.; Tan, Y.; Wang, J.; Xu, W.; Yuan, Y.; Cai, W.; Zhu, S.; Zhu, J. 3D Self-Assembly of Aluminium Nanoparticles for Plasmon-Enhanced Solar Desalination. Nat. Photonics 2016, 10, 393–398. [Google Scholar] [CrossRef]
- Yang, Y.; Zhao, H.; Yin, Z.; Zhao, J.; Yin, X.; Li, N.; Yin, D.; Li, Y.; Lei, B.; Du, Y. A General Salt-Resistant Hydrophilic/Hydrophobic Nanoporous Double Layer Design for Efficient and Stable Solar Water Evaporation Distillation. Mater. Horiz. 2018, 5, 1143–1150. [Google Scholar] [CrossRef]
- Wu, T.; Li, H.; Xie, M.; Shen, S.; Wang, W.; Zhao, M.; Mo, X.; Xia, Y. Incorporation of Gold Nanocages into Electrospun Nanofibers for Efficient Water Evaporation through Photothermal Heating. Mater. Today Energy 2019, 12, 129–135. [Google Scholar] [CrossRef]
- Chen, Z.; Jin, L.; Hao, W.; Ren, W.; Cheng, H.-M. Synthesis and Applications of Three-Dimensional Graphene Network Structures. Mater. Today Nano 2019, 5, 100027. [Google Scholar] [CrossRef]
- Zhao, Y.; Yuan, H.; Zhang, X.; Xue, G.; Tang, J.; Chen, Y.; Zhang, X.; Zhou, W.; Liu, H. Laser-Assisted Synthesis of Cobalt@N-Doped Carbon Nanotubes Decorated Channels and Pillars of Wafer-Sized Silicon as Highly Efficient Three-Dimensional Solar Evaporator. Chin. Chem. Lett. 2021. [Google Scholar] [CrossRef]
- Chen, W.; Chen, S.; Liang, T.; Zhang, Q.; Fan, Z.; Yin, H.; Huang, K.-W.; Zhang, X.; Lai, Z.; Sheng, P. High-Flux Water Desalination with Interfacial Salt Sieving Effect in Nanoporous Carbon Composite Membranes. Nat. Nanotechnol. 2018, 13, 345–350. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ogieglo, W.; Puspasari, T.; Hota, M.K.; Wehbe, N.; Alshareef, H.N.; Pinnau, I. Nanohybrid Thin-Film Composite Carbon Molecular Sieve Membranes. Mater. Today Nano 2020, 9, 100065. [Google Scholar] [CrossRef]
- Zhang, C.; Wu, M.-B.; Wu, B.-H.; Yang, J.; Xu, Z.-K. Solar-Driven Self-Heating Sponges for Highly Efficient Crude Oil Spill Remediation. J. Mater. Chem. A 2018, 6, 8880–8885. [Google Scholar] [CrossRef]
- Yu, H.-H.; Yan, L.-J.; Shen, Y.-C.; Chen, S.-Y.; Li, H.-N.; Yang, J.; Xu, Z.-K. Janus Poly(Vinylidene Fluoride) Membranes with Penetrative Pores for Photothermal Desalination. Research 2020, 2020, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, S.; He, Y.; Guan, Y.; Liu, X.; Liu, H.; Xie, M.; Zhou, L.; Wei, C.; Yu, C.; Chen, Y. Cellulose Nanofibril-Stabilized Pickering Emulsion and in Situ Polymerization Lead to Hybrid Aerogel for High-Efficiency Solar Steam Generation. ACS Appl. Polym. Mater. 2020, 2, 4581–4591. [Google Scholar] [CrossRef]
- Huang, S.; Li, Q.; Xiao, Y.; Chen, X.; Cui, Y.; Meng, X.; Hu, X.; Liu, Z.; Li, C.; Zhang, C. Improvement in Power Conversion Efficiency of All-Polymer Solar Cells Enabled by Ultrafast Channels for Charge Dynamics. Mater. Today Nano 2021, 100133. [Google Scholar] [CrossRef]
- Wu, S.; Xiong, G.; Yang, H.; Tian, Y.; Gong, B.; Wan, H.; Wang, Y.; Fisher, T.S.; Yan, J.; Cen, K. Scalable Production of Integrated Graphene Nanoarchitectures for Ultrafast Solar-Thermal Conversion and Vapor Generation. Matter 2019, 1, 1017–1032. [Google Scholar] [CrossRef] [Green Version]
- Wu, S.; Xiong, G.; Yang, H.; Gong, B.; Tian, Y.; Xu, C.; Wang, Y.; Fisher, T.; Yan, J.; Cen, K. Multifunctional Solar Waterways: Plasma-Enabled Self-Cleaning Nanoarchitectures for Energy-Efficient Desalination. Adv. Energy Mater. 2019, 9, 1901286. [Google Scholar] [CrossRef]
- Wu, S.-L.; Chen, H.; Wang, H.-L.; Chen, X.; Yang, H.-C.; Darling, S.B. Solar-Driven Evaporators for Water Treatment: Challenges and Opportunities. Environ. Sci. Water Res. Technol. 2021, 7, 24–39. [Google Scholar] [CrossRef]
- Xu, W.; Xing, Y.; Liu, J.; Wu, H.; Cui, Y.; Li, D.; Guo, D.; Li, C.; Liu, A.; Bai, H. Efficient Water Transport and Solar Steam Generation via Radially, Hierarchically Structured Aerogels. ACS Nano 2019, 13, 7930–7938. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Xu, W.; Tang, M.; Zhou, L.; Zhu, B.; Zhu, S.; Zhu, J. Graphene Oxide-Based Efficient and Scalable Solar Desalination under One Sun with a Confined 2D Water Path. Proc. Natl. Acad. Sci. USA 2016, 113, 13953–13958. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, T.; Li, Y.; Chen, C.; Yang, Z.; Kuang, Y.; Jia, C.; Song, J.; Hitz, E.M.; Liu, B.; Huang, H. Architecting a Floatable, Durable, and Scalable Steam Generator: Hydrophobic/Hydrophilic Bifunctional Structure for Solar Evaporation Enhancement. Small Methods 2019, 3, 1800176. [Google Scholar] [CrossRef]
- Xu, N.; Hu, X.; Xu, W.; Li, X.; Zhou, L.; Zhu, S.; Zhu, J. Mushrooms as Efficient Solar Steam-Generation Devices. Adv. Mater. 2017, 29, 1606762. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Zhao, X.; Zhao, F.; Jiao, Z.; Zhou, X.; Yu, G. Tailoring Surface Wetting States for Ultrafast Solar-Driven Water Evaporation. Energy Environ. Sci. 2020, 13, 2087–2095. [Google Scholar] [CrossRef]
- Wu, B.; Sun, Z.; Wu, J.; Ruan, J.; Zhao, P.; Liu, K.; Zhao, C.; Sheng, J.; Liang, T.; Chen, D. Nanoparticle-Stabilized Oxygen Microcapsules Prepared by Interfacial Polymerization for Enhanced Oxygen Delivery. Angew. Chem. Int. Ed. 2021, 60, 9284–9289. [Google Scholar] [CrossRef]
- Chen, L.; Xiao, Y.; Wu, Q.; Yan, X.; Zhao, P.; Ruan, J.; Shan, J.; Chen, D.; Weitz, D.A.; Ye, F. Emulsion Designer Using Microfluidic Three-Dimensional Droplet Printing in Droplet. Small 2021, 2102579. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Wu, B.; Ren, Y.; Wang, Z.; Zhao, C.-X.; Hai, M.; Weitz, D.A.; Chen, D. Diverse Particle Carriers Prepared by Co-Precipitation and Phase Separation: Formation and Applications. ChemPlusChem 2021, 86, 49–58. [Google Scholar] [CrossRef]
- Wu, B.; Yang, C.; Xin, Q.; Kong, L.; Eggersdorfer, M.; Ruan, J.; Zhao, P.; Shan, J.; Liu, K.; Chen, D.; et al. Attractive Pickering Emulsion Gels. Adv. Mater. 2021, 2102362. [Google Scholar] [CrossRef] [PubMed]
- Datta, A.K. Porous Media Approaches to Studying Simultaneous Heat and Mass Transfer in Food Processes. I: Problem Formulations. J. Food Eng. 2007, 80, 80–95. [Google Scholar] [CrossRef]
- Datta, A.K. Porous Media Approaches to Studying Simultaneous Heat and Mass Transfer in Food Processes. II: Property Data and Representative Results. J. Food Eng. 2007, 80, 96–110. [Google Scholar] [CrossRef]
- Evaporation in Porous Media with Large Evaporation Rates. Available online: http://cn.comsol.com/model/evaporation-in-porous-media-with-large-evaporation-rates-33731 (accessed on 21 April 2021).
- Kong, L.; Chen, R.; Wang, X.; Zhao, C.-X.; Chen, Q.; Hai, M.; Chen, D.; Yang, Z.; Weitz, D.A. Controlled Co-Precipitation of Biocompatible Colorant-Loaded Nanoparticles by Microfluidics for Natural Color Drinks. Lab Chip 2019, 19, 2089–2095. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Yang, C.; Wang, F.; Wu, B.; Shao, B.; Li, Z.; Chen, D.; Yang, Z.; Liu, K. Biocompatible and PH-Responsive Colloidal Surfactants with Tunable Shape for Controlled Interfacial Curvature. Angew. Chem. Int. Ed. 2020, 59, 9365–9369. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.; Yang, C.; Li, B.; Feng, L.; Hai, M.; Zhao, C.-X.; Chen, D.; Liu, K.; Weitz, D.A. Active Encapsulation in Biocompatible Nanocapsules. Small 2020, 16, 2002716. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Yang, C.; Eggersdorfer, M.; Cui, J.; Li, Y.; Hai, M.; Chen, D.; Weitz, D.A. A General Strategy for One-Step Fabrication of Biocompatible Microcapsules with Controlled Active Release. Chin. Chem. Lett. 2020, 31, 249–252. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, X.; Wu, B.; Wu, Q.; Chen, L.; Ye, F.; Chen, D. Interfacial Engineering of Attractive Pickering Emulsion Gel-Templated Porous Materials for Enhanced Solar Vapor Generation. Energies 2021, 14, 6077. https://doi.org/10.3390/en14196077
Yan X, Wu B, Wu Q, Chen L, Ye F, Chen D. Interfacial Engineering of Attractive Pickering Emulsion Gel-Templated Porous Materials for Enhanced Solar Vapor Generation. Energies. 2021; 14(19):6077. https://doi.org/10.3390/en14196077
Chicago/Turabian StyleYan, Xiaoxiao, Baiheng Wu, Qinglin Wu, Li Chen, Fangfu Ye, and Dong Chen. 2021. "Interfacial Engineering of Attractive Pickering Emulsion Gel-Templated Porous Materials for Enhanced Solar Vapor Generation" Energies 14, no. 19: 6077. https://doi.org/10.3390/en14196077
APA StyleYan, X., Wu, B., Wu, Q., Chen, L., Ye, F., & Chen, D. (2021). Interfacial Engineering of Attractive Pickering Emulsion Gel-Templated Porous Materials for Enhanced Solar Vapor Generation. Energies, 14(19), 6077. https://doi.org/10.3390/en14196077





