Microalgal Hydrogen Production in Relation to Other Biomass-Based Technologies—A Review
Abstract
:1. Introduction
2. Thermochemical Methods of Producing Hydrogen from Conventional Fuels
3. Thermochemical Methods of Producing Hydrogen from Biomass
4. Biological Methods of Producing Hydrogen from Biomass
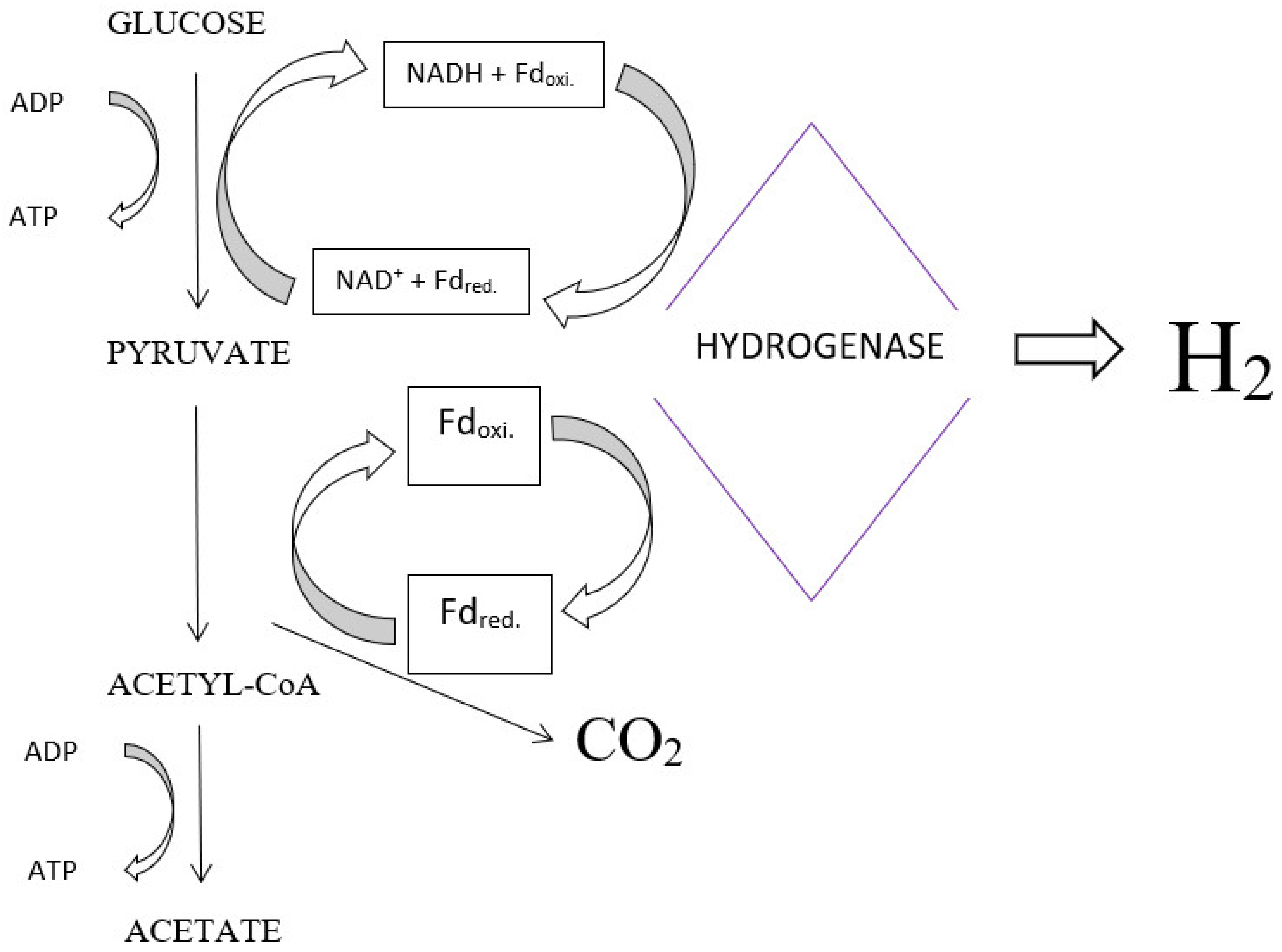
4.1. Fermentation
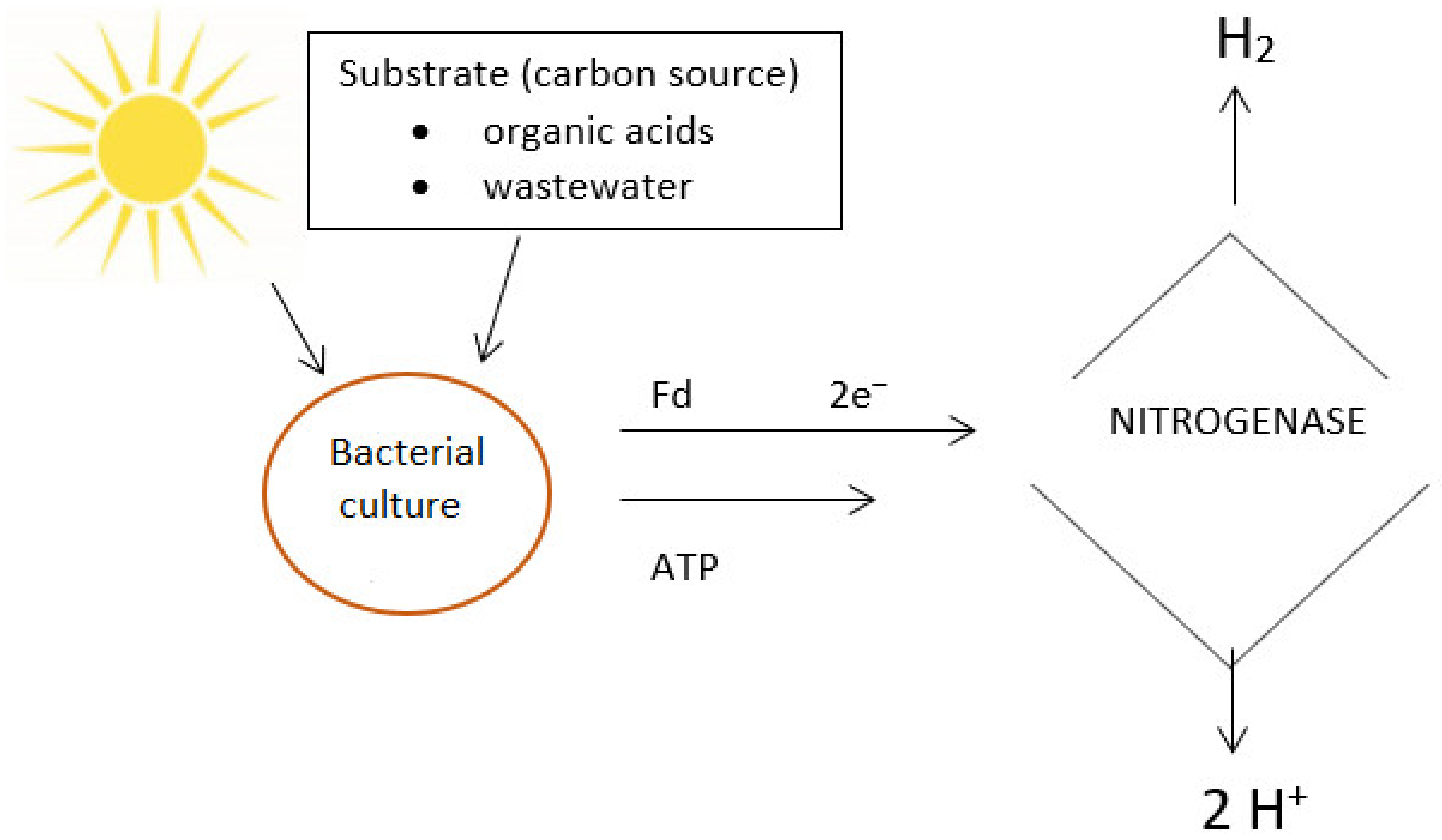
4.2. Photofermentation
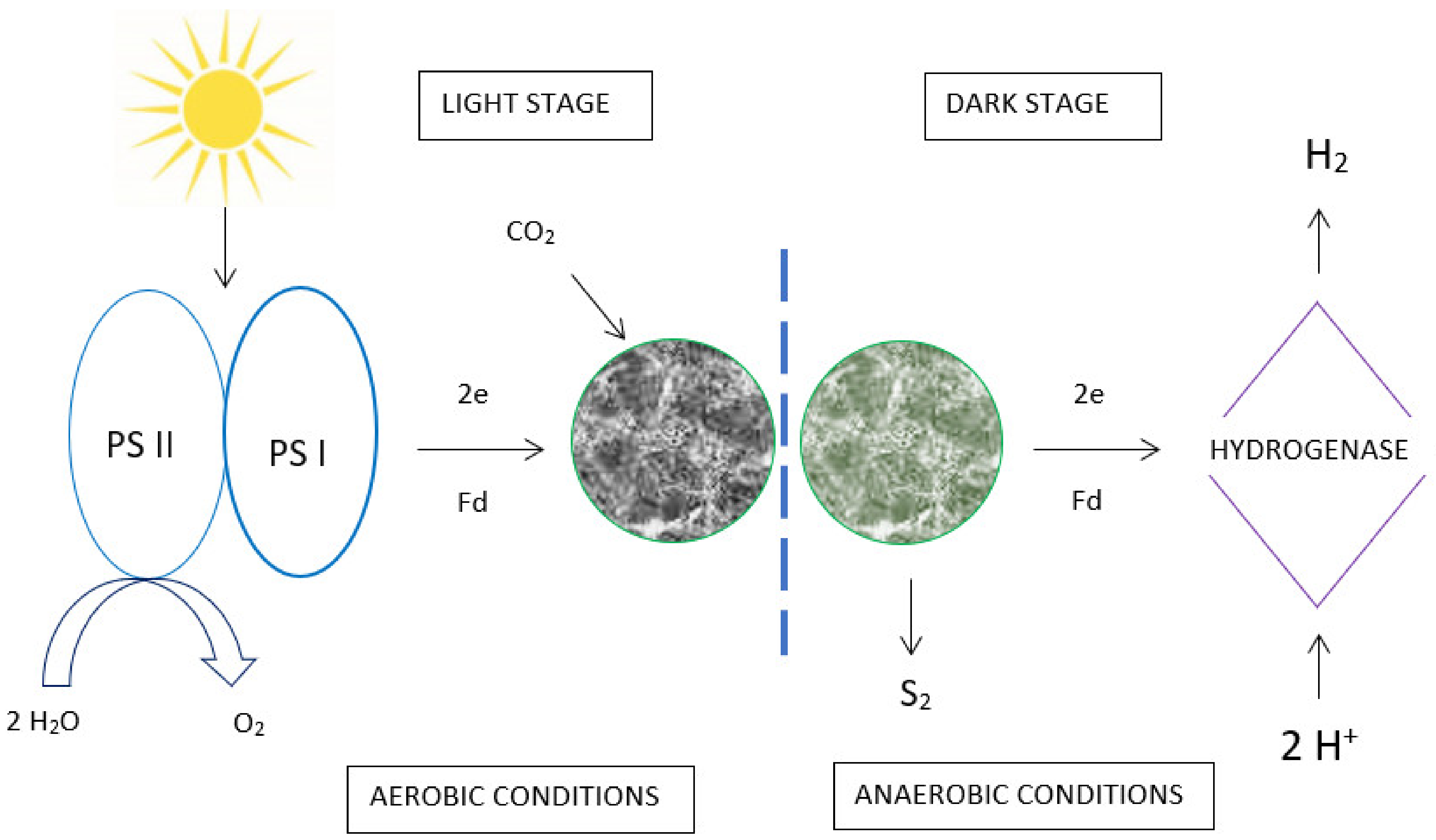
5. Hydrogen Production Using Algae
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yu, M.; Wang, K.; Vredenburg, V. Insights into low-carbon hydrogen production methods: Green, blue and aqua hydrogen. Int. J. Hydrogen Energy 2021, 46, 21261–21273. [Google Scholar] [CrossRef]
- Meier, K.; Kurtz, C.; Weckerle, C.; Hubner, M.; Bürger, I. Air-conditioning system for vehicles with on-board hydrogen. Appl. Therm. Eng. 2018, 129, 1150–1159. [Google Scholar] [CrossRef]
- Bizon, N.; Raceanu, M.; Koudoumas, E.; Marinoiu, A.; Karapidakis, E.; Carcadea, E. Renewable/Fuel Cell Hybrid Power System Operation Using Two Search Controllers of the Optimal Power Needed on the DC Bus. Energies 2020, 13, 6111. [Google Scholar] [CrossRef]
- Chen, S.; Kumar, A.; Wong, W.C.; Chiu, M.-S.; Wang, X. Hydrogen value chain and fuel cells within hybrid renewable energy systems: Advanced operation and control strategies. Appl. Energy 2019, 233, 321–337. [Google Scholar] [CrossRef]
- Hu, G.; Chen, C.; Lu, H.T.; Wu, Y.; Liu, C.; Tao, L.; Men, Y.; He, G.; Li, K.G. A Review of Technical Advances, Barriers, and Solutions in the Power to Hydrogen (P2H) Roadmap. Engineering 2020, 6, 1364–1380. [Google Scholar] [CrossRef]
- Cao, L.; Yu, I.K.M.; Xiong, X.; Tsang, D.C.W.; Zhang, S.; Clark, J.H.; Hu, C.; Ng, Y.H.; Shang, J.; Ok, Y.S. Biorenewable hydrogen production through biomass gasification: A review and future prospects. Environ. Res. 2020, 186, 109547. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Fan, Y.; Jiaqiang, E.; Cao, W.; Zhang, F.; Gong, J.; Liu, G.; Xu, W. Effects analysis on the gasification kinetic characteristics of food waste in supercritical water. Fuel 2019, 241, 94–104. [Google Scholar] [CrossRef]
- Fakeeha, A.; Ibrahim, A.A.; Aljuraywi, H.; Alqahtani, Y.; Alkhodair, A.; Alswaidan, S.; Abasaeed, A.E.; Kasim, S.O.; Mahmud, S.; Al-Fatesh, A.S. Hydrogen production by partial oxidation reforming of methane over Ni catalysts supported on high and low surface area alumina and zirconia. Processes 2020, 8, 499. [Google Scholar] [CrossRef]
- Parkinson, B.; Balcombe, P.; Speirs, J.F.; Hawkes, A.D.; Hellgardt, K. Levelized cost of CO2 mitigation from hydrogen production routes. Energy Environ. Sci. 2019, 12, 19–40. [Google Scholar] [CrossRef]
- Rajabi Hamedani, S.; Villarini, M.; Colantoni, A.; Moretti, M.; Bocci, E. Life Cycle Performance of Hydrogen Production via Agro-Industrial Residue Gasification—A Small Scale Power Plant Study. Energies 2018, 11, 675. [Google Scholar] [CrossRef] [Green Version]
- Shahbaz, M.; Al-Ansari, T.; Aslam, M.; Khan, Z.; Inayat, A.; Athar, M.; Naqvi, S.R.; Ahmed, M.A.; McKay, G. A state of the art review on biomass processing and conversion technologies to produce hydrogen and its recovery via membrane separation. Int. J. Hydrogen Energy 2020, 45, 15166–15195. [Google Scholar] [CrossRef]
- Mahata, C.; Dhar, S.; Ray, S.; Das, D. Effect of thermal pretreated organic wastes on the dark fermentative hydrogen production using mixed microbial consortia. Fuel 2021, 284, 119062. [Google Scholar] [CrossRef]
- Tsegaye, B.; Jaiswal, S.; Jaiswal, A.K. Food Waste Biorefinery: Pathway towards Circular Bioeconomy. Foods 2021, 10, 1174. [Google Scholar] [CrossRef]
- Nagarajan, D.; Chang, J.-S.; Lee, D.-J. Pretreatment of microalgal biomass for efficient biohydrogen production—Recent insights and future perspectives. Bioresour. Technol. 2020, 302, 122871–122885. [Google Scholar] [CrossRef]
- Shobana, S.; Kumar, G.; Bakonyi, P.; Saratale, G.D.; Al-Muhtaseb, A.a.H.; Nemestóthy, N.; Bélafi-Bakó, K.; Xia, A.; Chang, J.-S. A review on the biomass pretreatment and inhibitor removal methods as key-steps towards efficient macroalgae-based biohydrogen production. Bioresour. Technol. 2017, 244, 1341–1348. [Google Scholar] [CrossRef]
- Cordier, C.; Guyomard, K.; Stavrakakis, C.; Sauvade, P.; Coelho, F.; Moulin, P. Culture of Microalgae with Ultrafiltered Seawater: A Feasibility Study. Sci. Med. J. 2020, 2, 56–62. [Google Scholar] [CrossRef]
- Dębowski, M.; Zieliński, M.; Kazimierowicz, J.; Kujawska, N.; Talbierz, S. Microalgae Cultivation Technologies as an Opportunity for Bioenergetic System Development—Advantages and Limitations. Sustainability 2020, 12, 9980. [Google Scholar] [CrossRef]
- Barbosa, R.C.; Soares, J.; Martins, M.A. Low-cost and versatile sensor based on multi-wavelengths for real-time estimation of microalgal biomass concentration in open and closed cultivation systems. Comput. Electron. Agric. 2020, 176, 105641. [Google Scholar] [CrossRef]
- Dębowski, M.; Zieliński, M.; Krzemieniewski, M.; Dudek, M.; Grala, A. Microalgae—Cultivation methods. Pol. J. Nat. Sci. 2012, 27, 151–164. [Google Scholar]
- Dębowski, M.; Zieliński, M.; Grala, A.; Dudek, M. Algae biomass as an alternative substrate in biogas production technologies—Review. Renew. Sustain. Energy Rev. 2013, 27, 596–604. [Google Scholar] [CrossRef]
- Xiaogang, H.; Jalalah, M.; Jingyuan, W.; Zheng, Y.; Li, X.; Salama, E.-S. Microalgal growth coupled with wastewater treatment in open and closed systems for advanced biofuel generation. Biomass Convers. Biorefin. 2020, 17, 1–20. [Google Scholar] [CrossRef]
- Yen, H.-W.; Hu, I.-C.; Chen, C.-Y.; Nagarajan, D. Design of photobioreactors for algal cultivation. Biofuels Algae 2019, 225–256. [Google Scholar] [CrossRef]
- Mohan, S.V.; Rohit, M.V.; Subhash, G.V.; Chandra, R.; Devi, M.P.; Butti, S.K.; Rajesh, K. Biofuels from algae. In Algal Oils as Biodiesel; Pandey, A., Chang, J.-S., Soccol, C.R., Lee, D.-J., Chisti, Y., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 287–323. [Google Scholar] [CrossRef]
- Ji, C.F.; Legrand, J.; Pruvost, J.; Chen, Z.A.; Zhang, W. Characterization of hydrogen production by Platymonas Subcordiformis in torus photobioreactor. Int. J. Hydrogen Energy 2010, 35, 7200–7205. [Google Scholar] [CrossRef]
- Song, W.; Rashid, N.; Choi, W.; Lee, K. Biohydrogen production by immobilized Chlorella sp. using cycles of oxygenic photo-synthesis and anaerobiosis. Bioresour. Technol. 2011, 102, 8676–8681. [Google Scholar] [CrossRef]
- Franchi, G.; Capocelli, M.; De Falco, M.; Piemonte, V.; Barba, D. Hydrogen Production via Steam Reforming: A Critical Analysis of MR and RMM Technologies. Membranes 2020, 10, 10. [Google Scholar] [CrossRef] [Green Version]
- Park, H.-G.; Han, S.-Y.; Jun, K.-W.; Woo, Y.; Park, M.-J.; Kim, S.K. Bench-Scale Steam Reforming of Methane for Hydrogen Production. Catalysts 2019, 9, 615. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.; Qi, Z.; Zhang, S.; Su, J.; Somorjai, G.A. Catalytic Hydrogen Production from Methane: A Review on Recent Progress and Prospect. Catalysts 2020, 10, 858. [Google Scholar] [CrossRef]
- LeValley, T.L.; Richard, A.R.; Fan, M. The progress in water gas shift and steam reforming hydrogen production technologies—A review. Int. J. Hydrogen Energy 2014, 39, 16983–17000. [Google Scholar] [CrossRef]
- Goicoechea, S.; Ehrich, H.; Arias, P.L.; Kockmann, N. Thermodynamic analysis of acetic acid steam reforming for hydrogen production. J. Power Sources 2015, 279, 312–322. [Google Scholar] [CrossRef]
- Pashchenko, D. Thermochemical waste-heat recuperation by steam methane reforming with flue gas addition. Int. J. Energy Res. 2019, 43, 2216–2226. [Google Scholar] [CrossRef]
- Wang, G.; Wang, F.; Chen, B. Performance Study on Methanol Steam Reforming Rib Micro-Reactor with Waste Heat Recovery. Energies 2020, 13, 1564. [Google Scholar] [CrossRef] [Green Version]
- Mosinska, M.; Szynkowska, M.I.; Mierczynski, P. Oxy-Steam Reforming of Natural Gas on Ni Catalysts—A Minireview. Catalysts 2020, 10, 896. [Google Scholar] [CrossRef]
- Sadooghi, P.; Rauch, R. Experimental and modeling study of catalytic steam reforming of methane mixture with propylene in a packed bed reactor. Int. J. Heat Mass Transf. 2014, 78, 515–521. [Google Scholar] [CrossRef]
- Nahar, G.; Dupont, V.; Twigg, M.V.; Dvininov, E. Feasibility of hydrogen production from steam reforming of biodiesel (FAME) feedstock on Ni-supported catalysts. Appl. Catal. B Environ. 2015, 168-169, 228–242. [Google Scholar] [CrossRef] [Green Version]
- Hammoud, D.; Gennequin, C.; Aboukaïs, A.; Abi Aad, E. Steam reforming of methanol over x % Cu/Zn–Al 400 500 based catalysts for production of hydrogen: Preparation by adopting memory effect of hydrotalcite and behavior evaluation. Int. J. Hydrogen Energy 2015, 40, 1283–1297. [Google Scholar] [CrossRef]
- Charisiou, N.D.; Siakavelas, G.I.; Papageridis, K.N.; Motta, D.; Dimitratos, N.; Sebastian, V.; Polychronopoulou, K.; Goula, M.A. The Effect of Noble Metal (M: Ir, Pt, Pd) on M/Ce2O3-γ-Al2O3 Catalysts for Hydrogen Production via the Steam Reforming of Glycerol. Catalysts 2020, 10, 790. [Google Scholar] [CrossRef]
- Remón, J.; Jarauta-Córdoba, C.; García, L.; Arauzo, J. Analysis and optimisation of H2 production from crude glycerol by steam reforming using a novel two step process. Fuel Process. Technol. 2016, 145, 130–147. [Google Scholar] [CrossRef] [Green Version]
- Palo, E.; Salladini, A.; Morico, B.; Palma, V.; Ricca, A.; Iaquaniello, G. Application of Pd-Based Membrane Reactors: An Industrial Perspective. Membranes 2018, 8, 101. [Google Scholar] [CrossRef] [Green Version]
- Moharana, M.K.; Peela, N.R.; Khandekar, S.; Kunzru, D. Distributed hydrogen production from ethanol in a microfuel pro-cessor: Issues and challenges. Renew. Sustain. Energy Rev. 2011, 15, 524–533. [Google Scholar] [CrossRef]
- Yerga, R.M.N. Catalysts for Production and Conversion of Syngas. Catalysts 2021, 11, 752. [Google Scholar] [CrossRef]
- Lopez-Urionabarrenechea, A.; Acha, E.; Adrados, A.; Solar, J.; Caballero, B.M.; De Marco, I. Use of a Reforming Catalyst for Hydrogen Production in the Carbonization Process of Torrefied Biomass. Catalysts 2020, 10, 1300. [Google Scholar] [CrossRef]
- Molino, A.; Larocca, V.; Chianese, S.; Musmarra, D. Biofuels Production by Biomass Gasification: A Review. Energies 2018, 11, 811. [Google Scholar] [CrossRef] [Green Version]
- Safarian, S.; Unnthorsson, R.; Richter, C. Simulation and Performance Analysis of Integrated Gasification–Syngas Fermentation Plant for Lignocellulosic Ethanol Production. Fermentation 2020, 6, 68. [Google Scholar] [CrossRef]
- Huang, L.; Zhou, J.; Hsu, A.T.; Chen, R. Catalytic partial oxidation of n-butanol for hydrogen production over LDH-derived Ni-based catalysts. Int. J. Hydrogen Energy 2013, 38, 14550–14558. [Google Scholar] [CrossRef]
- Chen, Y.; Zhou, W.; Shao, Z.; Xu, N. Nickel catalyst prepared via glycine nitrate process for partial oxidation of methane to syngas. Catal. Commun. 2008, 9, 1418–1425. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, Z.; Wen, X.; Liu, Y. Partial oxidation of methane over Ni/Ce-Ti-O catalysts Chem. Eng. J. 2006, 121, 115–123. [Google Scholar]
- Shan, W.; Fleys, M.; Lapicque, F.; Swierczynski, D.; Kiennemann, A.; Simon, Y.; Marquaire, P. Syngas production from par-tial oxidation of methane over Ce1−XNiXOY catalysts prepared by complexation–combustion method. Appl. Catal. A Gen. 2006, 311, 24–33. [Google Scholar] [CrossRef]
- Pelletier, L.; Liu, D.D.S. Stable nickel catalysts with alumina-aluminum phosphate supports for partial oxidation and carbon dioxide reforming of methane. Appl. Catal. A Gen. 2007, 317, 293–298. [Google Scholar] [CrossRef]
- Dashliborun, A.M.; Fatemi, S.; Najafabadi, A.T. Hydrogen production through partial oxidation of methane in a new reactor configuration. Int. J. Hydrogen Energy 2013, 38, 1901–1909. [Google Scholar] [CrossRef]
- Martínez-Rodríguez, A.; Abánades, A. Comparative Analysis of Energy and Exergy Performance of Hydrogen Production Methods. Entropy 2020, 22, 1286. [Google Scholar] [CrossRef] [PubMed]
- Jimmy, U.; Mohamedali, M.; Ibrahim, H. Thermodynamic Analysis of Autothermal Reforming of Synthetic Crude Glycerol (SCG) for Hydrogen Production. Chem. Eng. 2017, 1, 4. [Google Scholar] [CrossRef] [Green Version]
- Xiang, D.; Li, P.; Yuan, X. Process Modeling, Optimization, and Heat Integration of Ethanol Reforming Process for Syngas Production with High H2/CO Ratio. Processes 2019, 7, 960. [Google Scholar] [CrossRef] [Green Version]
- Goula, M.A.; Polychronopoulou, K. Editorial—Special Issue “Catalysis for Energy Production”. Catalysts 2021, 11, 785. [Google Scholar] [CrossRef]
- Meloni, E.; Martino, M.; Palma, V. A Short Review on Ni Based Catalysts and Related Engineering Issues for Methane Steam Reforming. Catalysts 2020, 10, 352. [Google Scholar] [CrossRef] [Green Version]
- Ghaffari Saeidabad, N.G.; Noh, Y.S.; Alizadeh Eslami, A.; Song, H.T.; Kim, H.D.; Fazeli, A.; Moon, D.J. A Review on Catalysts Development for Steam Reforming of Biodiesel Derived Glycerol; Promoters and Supports. Catalysts 2020, 10, 910. [Google Scholar] [CrossRef]
- Yan, Y.; Zhan, J.; Zhang, L. Properties of thermodynamic equilibrium-based methane autothermal reforming to generate hydrogen. Int. J. Hydrogen Energy 2013, 38, 15744–15750. [Google Scholar] [CrossRef]
- Palma, V.; Ricca, A.; Ciambelli, P. Structured catalysts for methane auto-thermal reforming in a compact thermal integrated reaction system. Appl. Therm. Eng. 2013, 61, 128–133. [Google Scholar] [CrossRef]
- Czernik, S.; French, R. Distributed production of hydrogen by auto-thermal reforming of fast pyrolysis bio-oil. Int. J. Hydrogen Energy 2014, 39, 744–750. [Google Scholar] [CrossRef]
- Gallucci, F.; Annaland, M.V.S.; Kuipers, J.A.M. Pure hydrogen production via autothermal reforming of ethanol in a fluidized bed membrane reactor: A simulation study. Int. J. Hydrogen Energy 2010, 35, 1659–1668. [Google Scholar] [CrossRef]
- Ju, D.G.; Jo, S.B.; Ha, D.S.; Kim, T.Y.; Jung, S.Y.; Chae, H.J.; Lee, S.C.; Kim, J.C. Enhanced Ni-Al-Based Catalysts and Influence of Aromatic Hydrocarbon for Autothermal Reforming of Diesel Surrogate Fuel. Catalysts 2019, 9, 573. [Google Scholar] [CrossRef] [Green Version]
- Abejón, R.; Fernández-Ríos, A.; Domínguez-Ramos, A.; Laso, J.; Ruiz-Salmón, I.; Yáñez, M.; Ortiz, A.; Gorri, D.; Donzel, N.; Jones, D.; et al. Hydrogen Recovery from Waste Gas Streams to Feed (High-Temperature PEM) Fuel Cells: Environmental Performance under a Life-Cycle Thinking Approach. Appl. Sci. 2020, 10, 7461. [Google Scholar] [CrossRef]
- Li, W.; He, S.; Li, S. Experimental Study and Thermodynamic Analysis of Hydrogen Production through a Two-Step Chemical Regenerative Coal Gasification. Appl. Sci. 2019, 9, 3035. [Google Scholar] [CrossRef] [Green Version]
- Porada, S.; Czerski, G.; Dziok, T.; Grzywacz, P.; Makowska, D. Kinetics of steam gasification of bituminous coals in terms of their use for underground coal gasification. Fuel Process. Technol. 2015, 130, 282–291. [Google Scholar] [CrossRef]
- Seyednejadian, S.; Rauch, R.; Bensaid, S.; Hofbauer, H.; Weber, G.; Saracco, G. Power to Fuels: Dynamic Modeling of a Slurry Bubble Column Reactor in Lab-Scale for Fischer Tropsch Synthesis under Variable Load of Synthesis Gas. Appl. Sci. 2018, 8, 514. [Google Scholar] [CrossRef] [Green Version]
- Barisano, D.; Canneto, G.; Nanna, F.; Villone, A.; Fanelli, E.; Freda, C.; Grieco, M.; Cornacchia, G.; Braccio, G.; Marcantonio, V.; et al. Investigation of an Intensified Thermo-Chemical Experimental Set-Up for Hydrogen Production from Biomass: Gasification Process Performance—Part I. Processes 2021, 9, 1104. [Google Scholar] [CrossRef]
- Martino, M.; Ruocco, C.; Meloni, E.; Pullumbi, P.; Palma, V. Main Hydrogen Production Processes: An Over-view. Catalysts 2021, 11, 547. [Google Scholar] [CrossRef]
- Mehmeti, A.; Angelis-Dimakis, A.; Arampatzis, G.; McPhail, S.J.; Ulgiati, S. Life Cycle Assessment and Water Footprint of Hydrogen Production Methods: From Conventional to Emerging Technologies. Environments 2018, 5, 24. [Google Scholar] [CrossRef] [Green Version]
- Zaccara, A.; Petrucciani, A.; Matino, I.; Branca, T.A.; Dettori, S.; Iannino, V.; Colla, V.; Bampaou, M.; Panopoulos, K. Renewable Hydrogen Production Processes for the Off-Gas Valorization in Integrated Steelworks through Hydrogen Intensified Methane and Methanol Syntheses. Metals 2020, 10, 1535. [Google Scholar] [CrossRef]
- Zhang, H.X.; Zhang, Y.K.; Zhu, Z.P.; Lu, Q.G. Circulating fluidized bed gasification of low rank coal: Influence of O2/C molar ratio on gasification performance and sulphur transformation. J. Therm. Sci. 2016, 25, 363–371. [Google Scholar] [CrossRef]
- Poudel, J.; Choi, J.H.; Oh, S.C. Process Design Characteristics of Syngas (CO/H2) Separation Using Composite Membrane. Sustainability 2019, 11, 703. [Google Scholar] [CrossRef] [Green Version]
- Rahmad, B.; Raharjo, S.; Ediyanto, E.; Putra, G.P. Coal Gasification and Coal Microscopic Characteristics in Tanjung Baru, Lahat Regency, South Sumatera. In Seminar Nasional Teknik Kimia Kejuangan; UPN Veteran Yogyakarta: Yogyakarta, Indonesia, 2020; p. 5. [Google Scholar]
- Chmielniak, T.; Dreszer, K. Technical and economical considerations of new coal processing technologies. Chemist 2010, 64, 222–247. [Google Scholar]
- Deniz, I.; Vardar-Sukan, F.; Yüksel, M.; Saglam, M.; Ballice, L.; Yesil-Celiktas, O. Hydrogen production from marine biomass by hydrothermal gasification. Energy Convers. Manag. 2015, 96, 124–130. [Google Scholar] [CrossRef]
- Calzavara, Y.; Joussot-Dubien, C.; Boissonnet, G.; Sarrade, S. Evaluation of biomass gasification in supercritical water process for hydrogen production. Energy Convers. Manag. 2005, 46, 615–631. [Google Scholar] [CrossRef]
- Crocker, M. Thermochemical Conversion of Biomass to Liquid Fuels and Chemicals: Hydrothermal Processing of Biomass; Savage, P.E., Levine, R.B., Huelsman, C.M., Eds.; USA: Royal Society Chemistry: London, UK, 2010; pp. 192–221. [Google Scholar]
- Ge, Z.; Jin, H.; Guo, L. Hydrogen production by catalytic gasification of coal in supercritical water with alkaline catalysts: Explore the way to complete gasification of coal. Int. J. Hydrogen Energy 2014, 39, 19583–19592. [Google Scholar] [CrossRef]
- Mączka, T.; Pawlak-Kruczek, H.; Niedzwiecki, L.; Ziaja, E.; Chorążyczewski, A. Plasma Assisted Combustion as a Cost-Effective Way for Balancing of Intermittent Sources: Techno-Economic Assessment for 200 MWel Power Unit. Energies 2020, 13, 5056. [Google Scholar] [CrossRef]
- Yoon, S.J.; Lee, J.G. Hydrogen-rich syngas production through coal and charcoal gasification using microwave steam and air plasma torch. Int. J. Hydrogen Energy 2012, 37, 17093–17100. [Google Scholar] [CrossRef]
- Liu, Q.; Hu, C.; Peng, B.; Liu, C.; Li, Z.; Wu, K.; Zhang, H.; Xiao, R. High H2/CO ratio syngas production from chemical looping co-gasification of biomass and polyethylene with CaO/Fe2O3 oxygen carrier. Energy Convers. Manag. 2019, 199. [Google Scholar] [CrossRef]
- Yoon, S.J.; Yun, Y.M.; Seo, M.W.; Kim, Y.K.; Ra, H.W.; Lee, J.G. Hydrogen and syngas production from glycerol through microwave plasma gasification. Int. J. Hydrogen Energy 2013, 38, 14559–14567. [Google Scholar] [CrossRef]
- El-Shafie, M.; Kambara, S.; Hayakawa, Y. Hydrogen Production Technologies Overview. J. Power Energy Eng. 2019, 7, 107–154. [Google Scholar] [CrossRef] [Green Version]
- Hong, Y.C.; Lee, S.J.; Shin, D.H.; Kim, Y.J.; Lee, B.J.; Cho, S.Y.; Chang, H.S. Syngas production from gasification of brown coal in a microwave torch plasma. Energy 2012, 47, 36–40. [Google Scholar] [CrossRef]
- Vidaković-Koch, T. Editorial on Special Issue Electrolysis Processes. Processes 2020, 8, 578. [Google Scholar] [CrossRef]
- Brauns, J.; Turek, T. Alkaline Water Electrolysis Powered by Renewable Energy: A Review. Processes 2020, 8, 248. [Google Scholar] [CrossRef] [Green Version]
- Perez-Herranz, V.; Perez-Page, M.; Beneito, R. Monitoring and control of a hydrogen production and storage system consisting of water electrolysis and metal hydrides. Int. J. Hydrogen Energy 2010, 35, 912–919. [Google Scholar] [CrossRef]
- Chanda, D.; Hnat, J.; Paidar, M.; Schauer, J.; Bouzek, K. Synthesis and characterization of NiFe2O4 electrocatalyst for the hydrogen evolution reaction in alkaline water electrolysis using different polymer binders. J. Power Sources 2015, 285, 217–226. [Google Scholar] [CrossRef]
- Millet, P.; Grigoriev, S. Chapter 2—Water Electrolysis Technologies. In Renewable Hydrogen Technologies. Production, Purification, Storage, Applications and Safety; Gandia, L.M., Arzamendi, G., Dieguez, P.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2013; pp. 19–41. ISBN 978-0-444-56352-1. [Google Scholar]
- Fiegenbaum, F.; Martini, E.M.; de Souza, M.O.; Becker, M.R.; de Souza, R.F. Hydrogen production by water electrolysis using tetra-alkyl-ammonium-sulfonic acid ionic liquid electrolytes. J. Power Sources 2013, 243, 822–825. [Google Scholar] [CrossRef] [Green Version]
- Hug, W.; Divisek, J.; Mergel, J.; Seeger, W.; Steeb, H. Highly efficient advanced alkaline electrolyzer for solar operation. Int. J. Hydrogen Energy 1992, 17, 699–705. [Google Scholar] [CrossRef]
- Ando, Y.; Tanaka, T. Proposal for a new system for simultaneous production of hydrogen and hydrogen peroxide by water electrolysis. Int. J. Hydrogen Energy 2004, 29, 1349–1354. [Google Scholar] [CrossRef]
- Costa, M.; La Villetta, M.; Piazzullo, D.; Cirillo, D. A Phenomenological Model of a Downdraft Biomass Gasifier Flexible to the Feedstock Composition and the Reactor Design. Energies 2021, 14, 4226. [Google Scholar] [CrossRef]
- Ni, F.M.; Leung, D.Y.C.; Leung, M.K.H.; Sumathy, K. An overview of hydrogen production from biomass. Fuel Process. Technol. 2006, 87, 461–472. [Google Scholar] [CrossRef]
- Nath, K.; Das, D. Hydrogen from biomass. Curr. Sci. 2003, 85, 265–271. [Google Scholar]
- Asadullah, M. Barriers of commercial power generation using biomass gasification gas: A review. Renew. Sustain. Energy Rev. 2014, 29, 201–215. [Google Scholar] [CrossRef]
- Park, S.-W.; Lee, S.-Y.; Jeong, Y.-O.; Han, G.-H.; Seo, Y.-C. Effects of Oxygen Enrichment in Air Oxidants on Biomass Gasification Efficiency and the Reduction of Tar Emissions. Energies 2018, 11, 2664. [Google Scholar] [CrossRef] [Green Version]
- Xiao, X.; Wang, X.; Zheng, Z.; Qin, W.; Zhou, Y. Catalytic Coal Gasification Process Simulation with Alkaline Organic Wastewater in a Fluidized Bed Reactor Using Aspen Plus. Energies 2019, 12, 1367. [Google Scholar] [CrossRef] [Green Version]
- Antoniou, N.; Monlau, F.; Sambusiti, C.; Ficara, E.; Barakat, A.; Zabaniotou, A. Contribution to Circular Economy options of mixed agricultural wastes management: Coupling anaerobic digestion with gasification for enhanced energy and material recovery. J. Clean. Prod. 2019, 209, 505–514. [Google Scholar] [CrossRef]
- Duque-Acevedo, M.; Belmonte-Ureña, L.J.; Yakovleva, N.; Camacho-Ferre, F. Analysis of the Circular Economic Production Models and Their Approach in Agriculture and Agricultural Waste Biomass Management. Int. J. Environ. Res. Public Health 2020, 17, 9549. [Google Scholar] [CrossRef] [PubMed]
- Castello, D.; Pedersen, T.H.; Rosendahl, L.A. Continuous Hydrothermal Liquefaction of Biomass: A Critical Review. Energies 2018, 11, 3165. [Google Scholar] [CrossRef] [Green Version]
- Xu, C.; Hu, S.; Xiang, J.; Zhang, L.; Sun, L.; Shuai, C.; Chen, Q.; He, L.; Edreis, E.M.A. Interaction and kinetic analysis for coal and biomass co-gasification by TG–FTIR. Bioresour. Technol. 2014, 154, 313–321. [Google Scholar] [CrossRef] [PubMed]
- Marcantonio, V.; Monforti Ferrario, A.; Di Carlo, A.; Del Zotto, L.; Monarca, D.; Bocci, E. Biomass Steam Gasification: A Comparison of Syngas Composition between a 1-D MATLAB Kinetic Model and a 0-D Aspen Plus Quasi-Equilibrium Model. Computation 2020, 8, 86. [Google Scholar] [CrossRef]
- Werle, S. Obtaining gas fuel from unconventional biomass in the gasification process. Instal 2014, 1, 7–10. [Google Scholar]
- Song, T.; Wu, J.; Shen, L.; Xiao, J. Experimental investigation on hydrogen production from biomass gasification in intercon-nected fluidized beds. Biomass Bioenergy 2012, 36, 258–267. [Google Scholar] [CrossRef]
- Ni, M.; Leung, M.K.H.; Sumathy, K.; Leung, D.Y.C. Potential of renewable hydrogen production for energy supply in HongKong. Int. J. Hydrogen Energy 2006, 31, 1401–1412. [Google Scholar] [CrossRef]
- Uddin, M.N.; Daud, W.M.A.W.; Abbas, H.F. Potential hydrogen and non-condensable gases production from biomass pyrol-ysis: Insights into the process variables. Renew. Sustain. Energy Rev. 2013, 27, 204–224. [Google Scholar] [CrossRef]
- Zhao, B.; Zhang, X.; Sun, L.; Meng, G.; Chen, L.; Xiaolu, Y. Hydrogen production from biomass combining pyrolysis and the secondary decomposition. Int. J. Hydrogen Energy 2010, 35, 2606–2611. [Google Scholar] [CrossRef]
- Alvarez, J.; Kumagai, S.; Wu, C.; Yoshioka, T.; Bilbao, J.; Olazar, M.; Williams, P.T. Hydrogen production from biomass and plastic mixtures by pyrolysis-gasification. Int. J. Hydrogen Energy 2014, 39, 10883–10891. [Google Scholar] [CrossRef]
- Pan, P.; Hu, C.; Yang, W.; Li, Y.; Dong, L.; Zhu, L.; Tong, D.; Qing, R.; Fan, Y. The direct pyrolysis and catalytic pyrolysis of Nannochloropsis sp. residue for renewable bio-oils. Bioresour. Technol. 2010, 101, 4593–4599. [Google Scholar] [CrossRef] [PubMed]
- Lin, K.C.; Lin, Y.C.; Hsiao, Y.H. Microwave plasma studies of Spirulina algae pyrolysis with relevance to hydrogen produc-tion. Energy 2014, 64, 567–574. [Google Scholar] [CrossRef]
- Maddi, B.; Viamajala, S.; Varanasi, S. Comparative study of pyrolysis of algal biomass from natural lake blooms with ligno-cellulosic biomass. Bioresour. Technol. 2011, 102, 11018–11026. [Google Scholar] [CrossRef] [PubMed]
- Levin, D.B.; Chahine, R. Challenges for renewable hydrogen production from biomass. Int. J. Hydrogen Energy 2010, 35, 4962–4969. [Google Scholar] [CrossRef]
- Kersten, S.R.A.; Potic, B.; Prins, W.; van Swaaij, W.P.M. Gasification of model compounds and wood in hot compressed water. Ind. Eng. Chem. Res. 2006, 45, 4169–4177. [Google Scholar] [CrossRef]
- Reddy, S.N.; Nanda, S.; Dalai, A.K.; Kozinski, J.A. Supercritical water gasification of biomass for hydrogen production. Int. J. Hydrogen Energy 2014, 39, 6912–6926. [Google Scholar] [CrossRef]
- Okajima, I.; Sako, T. Energy conversion of biomass with supercritical and subcritical water using large-scale plants. J. Biosci. Bioeng. 2014, 117, 1–9. [Google Scholar] [CrossRef]
- Lu, Y.; Guo, L.; Zhang, X.; Yan, Q. Thermodynamic modeling and analysis of biomass gasification for hydrogen production in supercritical water. Chem. Eng. J. 2007, 131, 233–244. [Google Scholar] [CrossRef]
- Lu, Y.J.; Jin, H.; Guo, L.J.; Zhang, X.M.; Cao, C.Q.; Guo, X. Hydrogen production by biomass gasification in supercritical water with a fluidized bed reactor. Int. J. Hydrogen Energy 2008, 33, 6066–6075. [Google Scholar] [CrossRef]
- Miao, X.; Wu, Q.; Yang, C. Fast pyrolysis of microalgae to produce renewable fuels. J. Anal. Appl. Pyrolysis 2004, 71, 855–863. [Google Scholar] [CrossRef]
- Miao, X.; Wu, Q. High yield bio-oil production from fast pyrolysis by metabolic controlling of Chlorella protothecoides. J. Biotechnol. 2004, 110, 85–93. [Google Scholar] [CrossRef]
- Grierson, S.; Strezov, V.; Ellem, G.; Mcgregor, R.; Herbertson, J. Thermal characterisation of microalgae under slow pyrolysis conditions. J. Anal. Appl. Pyrolysis 2009, 85, 118–123. [Google Scholar] [CrossRef]
- Hu, Z.; Zheng, Y.; Yan, F.; Xiao, B.; Liu, S. Bio-oil production through pyrolysis of blue-green algae blooms (BGAB): Product distribution and bio-oil characterization. Energy 2013, 52, 119–125. [Google Scholar] [CrossRef]
- Giang, T.T.; Lunprom, S.; Liao, Q.; Reungsang, A.; Salakkam, A. Enhancing Hydrogen Production from Chlorella sp. Biomass by Pre-Hydrolysis with Simultaneous Saccharification and Fermentation (PSSF). Energies 2019, 12, 908. [Google Scholar] [CrossRef] [Green Version]
- Zieliński, M.; Korzeniewska, E.; Filipkowska, Z.; Dębowski, M.; Harnisz, M.; Kwiatkowski, R. Biohydrogen production at low load of organic matter by psychrophilic bacteria. Energy 2017, 134, 1132–1139. [Google Scholar] [CrossRef]
- Dębowski, M.; Korzeniewska, E.; Filipkowska, Z.; Zieliński, M.; Kwiatkowski, R. Possibility of hydrogen production during cheese whey fermentation process by different strains of psychrophilic bacteria. Int. J. Hydrogen Energy 2014, 39, 1972–1978. [Google Scholar] [CrossRef]
- Zhang, H.; Li, J.; Zhang, Q.; Zhu, S.; Yang, S.; Zhang, Z. Effect of Substrate Concentration on Photo-Fermentation Bio-Hydrogen Production Process from Starch-Rich Agricultural Leftovers under Oscillation. Sustainability 2020, 12, 2700. [Google Scholar] [CrossRef] [Green Version]
- Kapdan, I.K.; Kargi, F. Bio-hydrogen production from waste materials. Enzym. Microb. Technol. 2006, 38, 569–582. [Google Scholar] [CrossRef]
- Kumar, N.; Das, D. Enhancement of hydrogen production by Enterobacter cloacae IIT-BT 08. Process. Biochem. 2000, 35, 589–593. [Google Scholar] [CrossRef]
- Kumar, N.; Das, D. Continuous hydrogen production by immobilized Enterobacter cloacae IIT-BT 08 using lignocellulosic ma-terials as solid matrices. Enzym. Microb. Technol. 2001, 29, 280–287. [Google Scholar] [CrossRef]
- Wu, X.; Yao, W.; Zhu, J. Effect of pH on continuous biohydrogen production from liquid swine manure with glucose supple-ment using an anaerobic sequencing batch reactor. Int. J. Hydrogen Energy 2010, 35, 6592–6599. [Google Scholar] [CrossRef]
- Fang, H.H.P.; Liu, H. Effect of pH on hydrogen production from glucose by a mixed culture. Bioresour. Technol. 2002, 82, 87–93. [Google Scholar] [CrossRef]
- Kim, J.K.; Nhat, L.; Chun, Y.N.; Kim, S.W. Hydrogen production conditions from food waste by dark fermentation with Clostridium beijerinckii KCTC 1785. Biotechnol. Bioprocess Eng. 2008, 13, 499–504. [Google Scholar] [CrossRef]
- Song, Z.X.; Dai, Y.; Fan, Q.L.; Li, X.H.; Fan, Y.T.; Hou, H.W. Effects of pretreatment method of natural bacteria source on microbial community and bio-hydrogen production by dark fermentation. Int. J. Hydrogen Energy 2012, 37, 5631–5636. [Google Scholar] [CrossRef]
- Lanzilli, M.; Esercizio, N.; Vastano, M.; Xu, Z.; Nuzzo, G.; Gallo, C.; Manzo, E.; Fontana, A.; D’Ippolito, G. Effect of Cultivation Parameters on Fermentation and Hydrogen Production in the Phylum Thermotogae. Int. J. Mol. Sci. 2021, 22, 341. [Google Scholar] [CrossRef]
- Fabiano, B.; Perego, P. Thermodynamic study and optimization of hydrogen production by Enterobacter aerogenes. Int. J. Hydrogen Energy 2002, 27, 149–156. [Google Scholar] [CrossRef]
- Tenca, A.; Schievano, A.; Perazzolo, F.; Adani, F.; Oberti, R. Biohydrogen from thermophilic co-fermentation of swine manure with fruit and vegetable waste: Maximizing stable production without pH control. Bioresour. Technol. 2011, 102, 8582–8588. [Google Scholar] [CrossRef]
- Xiao, B.; Liu, J. Biological hydrogen production from sterilized sewage sludge by anaerobic self-fermentation. J. Hazard. Mater. 2009, 168, 163–167. [Google Scholar] [CrossRef]
- Chen, W.H.; Chen, S.Y.; Khanal, S.K.; Sung, S. Kinetic study of biological hydrogen production by anaerobic fermentation. Int. J. Hydrogen Energy 2006, 31, 2170–2178. [Google Scholar] [CrossRef]
- Shin, H.S.; Youn, J.H.; Kim, S.H. Hydrogen production from food waste in anaerobic mesophilic and thermophilic acidogenesis. Int. J. Hydrogen Energy 2004, 29, 1355–1363. [Google Scholar] [CrossRef]
- Kisielewska, M.; Dębowski, M.; Zieliński, M. Improvement of biohydrogen production using a reduced pressure fermentation. Bioprocess Biosyst. Eng. 2015, 38, 1925–1933. [Google Scholar] [CrossRef]
- Saye, L.M.G.; Navaratna, T.A.; Chong, J.P.J.; O’Malley, M.K.; Theodorou, M.; Reilly, M. The Anaerobic Fungi: Challenges and Opportunities for Industrial Lignocellulosic Biofuel Production. Microorganisms 2021, 9, 694. [Google Scholar] [CrossRef]
- Eroglu, E.; Gündüz, U.; Yücel, M.; Türker, L.; Eroglu, I. Photobiological hydrogen production from olive mill wastewater as sole substrate sources. Int. J. Hydrogen Energy 2004, 29, 163–171. [Google Scholar] [CrossRef]
- Fan, Q.; Neubauer, P.; Lenz, O.; Gimpel, M. Heterologous Hydrogenase Overproduction Systems for Biotechnology—An Overview. Int. J. Mol. Sci. 2020, 21, 5890. [Google Scholar] [CrossRef] [PubMed]
- Contreras-Angulo, J.R.; Mata, T.M.; Cuellar-Bermudez, S.P.; Caetano, N.S.; Chandra, R.; Garcia-Perez, J.S.; Muylaert, K.; Parra-Saldivar, R. Symbiotic Co-Culture of Scenedesmus sp. and Azospirillum brasilense on N-Deficient Media with Biomass Production for Biofuels. Sustainability 2019, 11, 707. [Google Scholar] [CrossRef] [Green Version]
- Kim, M.S.; Baek, J.S.; Lee, J.K. Comparison of H2 accumulation by Rhodobacter sphaeroides KD131 and its uptake hydrogenase and PHB synthase deficient mutant. Int. J. Hydrogen Energy 2006, 31, 121–127. [Google Scholar] [CrossRef]
- Koku, H.; Eroglu, I.; Gündüz, U.; Yücel, M.; Türker, L. Kinetics of biohydrogen production by the photosynthetic bacterium Rhodobacter spheroids O.U. 001. Int. J. Hydrogen Energy 2003, 28, 381–388. [Google Scholar] [CrossRef]
- Skjånes, K.; Andersen, U.; Heidorn, T.; Borgvang, S.A. Design and construction of a photobioreactor for hydrogen production, including status in the field. Environ. Boil. Fishes 2016, 28, 2205–2223. [Google Scholar] [CrossRef] [Green Version]
- Limongi, A.R.; Viviano, E.; De Luca, M.; Radice, R.P.; Bianco, G.; Martelli, G. Biohydrogen from Microalgae: Production and Applications. Appl. Sci. 2021, 11, 1616. [Google Scholar] [CrossRef]
- Khetkorn, W.; Rastogi, R.P.; Incharoensakdi, A.; Lindblad, P.; Madamwar, D.; Pandey, A.; Larroche, C. Microalgal hydrogen production—A review. Bioresour. Technol. 2017, 243, 1194–1206. [Google Scholar] [CrossRef] [PubMed]
- Yetis, M.; Gündüz, U.; Eroglu, I.; Yücel, M.; Türker, L. Photoproduction of hydrogen from sugar refinery wastewater by Rhodobacter sphaeroides O.U.001. Int. J. Hydrogen Energy 2000, 25, 1035–1041. [Google Scholar] [CrossRef]
- Oh, Y.K.; Scol, E.H.; Kim, M.S.; Park, S. Photoproduction of hydrogen from acetate by a chemoheterotrophic bacterium Rho-dopseudomonas palustris P4. Int. J. Hydrogen Energy 2004, 29, 1115–1121. [Google Scholar]
- Argun, H.; Kargi, F. Photo-fermentative hydrogen gas production from dark fermentation effluent of ground wheat solution: Effects of light source and light intensity. Int. J. Hydrogen Energy 2010, 35, 1595–1603. [Google Scholar] [CrossRef]
- Laocharoen, S.; Reungsang, A. Isolation, characterization and optimization of photo-hydrogen production conditions by newly isolated Rhodobacter sphaeroides KKU-PS5. Int. J. Hydrogen Energy 2014, 39, 10870–10882. [Google Scholar] [CrossRef]
- Sun, Y.; He, J.; Yang, G.; Sun, G.; Sage, V. A Review of the Enhancement of Bio-Hydrogen Generation by Chemicals Addition. Catalysts 2019, 9, 353. [Google Scholar] [CrossRef] [Green Version]
- Kumar, G.; Shobana, S.; Nagarajan, D.; Lee, D.-J.; Lee, K.-S.; Lin, C.-Y.; Chen, C.-Y.; Chang, J.-S. Biomass based hydrogen production by dark fermentation—recent trends and opportunities for greener processes. Curr. Opin. Biotechnol. 2018, 50, 136–145. [Google Scholar] [CrossRef]
- Manish, S.; Banerjee, R. Comparison of biohydrogen production processes. Int. J. Hydrogen Energy 2008, 33, 279–286. [Google Scholar] [CrossRef]
- Nath, K.; Kumar, A.; Das, D. Hydrogen production by Rhodobacter sphaeroides strain O.U.001 using spent media of Enterobacter cloacae strain DM11. Appl. Microbiol. Biotechnol. 2005, 68, 533–541. [Google Scholar] [CrossRef]
- Yokoi, H.; Mori, S.; Hirose, J.; Hayashi, S.; Takasaki, Y. H2 production from starch by mixed culture of Clostridium butyricum and Rhodobacter sp. M-19. Biotechnol. Lett. 1998, 20, 895–899. [Google Scholar] [CrossRef]
- Ghosh, D.; Sobro, I.F.; Hallenbeck, P.C. Optimization of the hydrogen yield from single-stage photofermentation of glucose by Rhodobacter capsulatus JP91 using response surface methodology. Bioresour. Technol. 2012, 123, 199–206. [Google Scholar] [CrossRef]
- Keskin, T.; Hallenbeck, P.C. Hydrogen production from sugar industry wastes using single-stage photofermentation. Bioresour. Technol. 2012, 112, 131–136. [Google Scholar] [CrossRef] [PubMed]
- Abo-Hashesh, M.; Ghosh, D.; Tourigny, A.; Taous, A.; Hallenbeck, P.C. Single stage photofermentative hydrogen production from glucose: An attractive alternative to two stage photofermentation or co-culture approaches. Int. J. Hydrogen Energy 2011, 36, 13889–13895. [Google Scholar] [CrossRef]
- Seifert, K.; Waligorska, M.; Laniecki, M. Hydrogen generation in photobiological process from dairy wastewater. Int. J. Hydrogen Energy 2010, 35, 9624–9629. [Google Scholar] [CrossRef]
- Anam, K.; Habibi, M.S.; Harwati, T.U.; Susilaningsih, D. Photofermentative hydrogen production using Rhodobium marinum from bagasse and soy sauce wastewater. Int. J. Hydrogen Energy 2012, 37, 15436–15442. [Google Scholar] [CrossRef]
- Fang, H.H.P.; Liu, H.; Zhang, T. Phototrophic hydrogen production from acetate and butyrate in wastewater. Int. J. Hydrogen Energy 2005, 30, 785–793. [Google Scholar] [CrossRef]
- Khanal, S.K.; Chen, W.H.; Li, L.; Sung, S. Biological hydrogen production: Effects of pH and intermediate products. Int. J. Hydrogen Energy 2004, 29, 1123–1131. [Google Scholar] [CrossRef]
- Yokoi, H.; Saitsu, A.S.; Uchida, H.; Hirose, J.; Hayashi, S.; Takasaki, Y. Microbial hydrogen production from sweet potato starch residue. J. Biosci. Bioeng. 2001, 91, 58–63. [Google Scholar] [CrossRef]
- Cheng, J.; Xia, A.; Liu, Y.; Lin, R.; Zhou, J.; Cen, K. Combination of dark- and photo-fermentation to improve hydrogen production from Arthrospira platensis wet biomass with ammonium removal by zeolite. Int. J. Hydrogen Energy 2012, 37, 13330–13337. [Google Scholar] [CrossRef]
- Su, H.; Cheng, J.; Zhou, J.; Song, W.; Cen, K. Combination of dark- and photo-fermentation to enhance hydrogen production and energy conversion efficiency. Int. J. Hydrogen Energy 2009, 34, 8846–8853. [Google Scholar] [CrossRef]
- Su, H.; Cheng, J.; Zhou, J.; Song, W.; Cen, K. Improving hydrogen production from cassava starch by combination of dark and photo fermentation. Int. J. Hydrogen Energy 2009, 34, 1780–1786. [Google Scholar] [CrossRef]
- Levin, D.B.; Pitt, L.; Love, M. Biohydrogen production: Prospects and limitations to practical application. Int. J. Hydrogen Energy 2004, 29, 173–185. [Google Scholar] [CrossRef]
- Zuorro, A.; García-Martínez, J.B.; Barajas-Solano, A.F. The Application of Catalytic Processes on the Production of Algae-Based Biofuels: A Review. Catalysts 2021, 11, 22. [Google Scholar] [CrossRef]
- Show, K.Y.; Yan, Y.G.; Ling, M.; Ye, G.X.; Li, T.; Lee, D.J. Hydrogen production from algal biomass—Advances, challenges and prospects. Bioresour. Technol. 2018, 257, 290–300. [Google Scholar] [CrossRef]
- Jiménez-Llanos, J.; Ramírez-Carmona, M.; Rendón-Castrillón, L.; Ocampo-López, C. Sustainable biohydrogen production by Chlorella sp. microalgae: A review. Int. J. Hydrogen Energy 2020, 45, 8310–8328. [Google Scholar] [CrossRef]
- Liu, Y.; Min, J.; Feng, X.; He, Y.; Liu, J.; Wang, Y.; He, J.; Do, H.; Sage, V.; Yang, G.; et al. A Review of Biohydrogen Productions from Lignocellulosic Precursor via Dark Fermentation: Perspective on Hydrolysate Composition and Electron-Equivalent Balance. Energies 2020, 13, 2451. [Google Scholar] [CrossRef]
- Tamburic, B.; Zemichael, F.W.; Maitland, G.C.; Hellgardt, K. Parameters affecting the growth and hydrogen production of the green alga Chlamydomonas reinhardtii. Int. J. Hydrogen Energy 2011, 36, 1–5. [Google Scholar] [CrossRef]
- Oncel, S.; Vardar-Sukan, F. Photo-bioproduction of hydrogen by Chlamydomonas reinhardtii using a semi-continuous process regime. Int. J. Hydrogen Energy 2009, 34, 7592–7602. [Google Scholar] [CrossRef]
- Rattanapoltee, P.; Dujjanutat, P.; Muanruksa, P.; Kaewkannetra, P. Biocircular platform for third generation biodiesel production: Batch/fed batch mixotrophic cultivations of microalgae using glycerol waste as a carbon source. Biochem. Eng. J. 2021, 175, 108128. [Google Scholar] [CrossRef]
- Laurinavichene, T.V.; Tolstygina, I.V.; Galiulina, R.R.; Ghirardi, M.L.; Seibert, M.; Tsygankov, A.A. Dilution methods to deprive Chlamydomonas reinhardtii cultures of sulfur for subsequent hydrogen photoproduction. Int. J. Hydrogen Energy 2002, 27, 1245–1249. [Google Scholar] [CrossRef]
- Skjanes, K.; Knutsen, G.; Kӓllqvist, T.; Lindblad, P. H2 production from marine and freshwater species of green algae during sulfur deprivation and considerations for bioreactor design. Int. J. Hydrogen Energy 2008, 33, 511–521. [Google Scholar] [CrossRef]
- Faraloni, C.; Ena, A.; Pintucci, C.; Torzillo, G. Enhanced hydrogen production by means of sulfur-deprived Chlamydomonas reinhardtii cultures grown in pretreated olive mill wastewater. Int. J. Hydrogen Energy 2011, 36, 5920–5931. [Google Scholar] [CrossRef]
- Zhang, L.; He, M.; Liu, J. The enhancement mechanism of hydrogen photoproduction in Chlorella protothecoides under nitrogen limitation and sulfur deprivation. Int. J. Hydrogen Energy 2014, 39, 8969–8976. [Google Scholar] [CrossRef]
- Liu, C.H.; Chang, C.Y.; Liao, Q.; Zhu, X.; Chang, J.S. Photoheterotrophic growth of Chlorella vulgaris ESP6 on organic acids from dark hydrogen fermentation effluents. Bioresour. Technol. 2013, 145, 331–336. [Google Scholar] [CrossRef] [PubMed]
- Chader, S.; Hacene, H.; Agathos, S.N. Study of hydrogen production by three strains of Chlorella isolated from the soil in the Algerian Sahara. Int. J. Hydrogen Energy 2009, 34, 4941–4946. [Google Scholar] [CrossRef]
- Lin, H.D.; Liu, B.H.; Kuo, T.T.; Tsai, H.C.; Feng, T.F.; Huang, C.C.; Chien, L.F. Knockdown of PsbO leads to induction of HydA and production of photobiological H2 in the green alga Chlorella sp. DT. Bioresour. Technol. 2013, 143, 154–162. [Google Scholar] [CrossRef] [Green Version]
- Amutha, K.B.; Murugesan, A.G. Biological hydrogen production by the algal biomass Chlorella vulgaris MSU 01 strain iso-lated from pond sediment. Bioresour. Technol. 2011, 102, 194–199. [Google Scholar] [CrossRef] [PubMed]
- Guan, Y.; Deng, M.; Yu, X.; Zhang, W. Two-stage photo-biological production of hydrogen by marine green alga Platymonas subcordiformis. Biochem. Eng. J. 2004, 19, 69–73. [Google Scholar] [CrossRef]
- Ji, C.F.; Yu, X.J.; Chen, Z.A.; Xue, S.; Legrand, J.; Zhang, W. Effects of nutrient deprivation on biochemical compositions and photo-hydrogen production of Tetraselmis subcordiformis. Int. J. Hydrogen Energy 2011, 36, 5817–5821. [Google Scholar] [CrossRef]
- Oncel, S.; Vardar Sukan, F. Effect of light intensity and the light: Dark cycles on the long term hydrogen production of Chlamydomonas reinhardtii by batch cultures. Biomass Bioenergy 2011, 35, 1066–1074. [Google Scholar] [CrossRef]
- Guo, Z.; Chen, Z.; Lu, H.; Fu, Y.; Yu, X.; Zhang, W. Sustained hydrogen photoproduction by marine green algae platymonas subcordiformis integrated with in situ hydrogen consumption by an alkaline fuel cell system. J. Biotechnol. 2008, 136, S573. [Google Scholar] [CrossRef]
- Kim, M.S.; Baek, J.S.; Yun, Y.S.; Sim, S.J.; Park, S.; Kim, S.C. Hydrogen production from Chlamydomonas reinhardtii biomass using a two-step conversion process: Anaerobic conversion and photosynthetic fermentation. Int. J. Hydrogen Energy 2006, 31, 812–816. [Google Scholar] [CrossRef]
- Sun, J.; Yuan, X.; Shi, X.; Chu, C.; Guo, R.; Kong, H. Fermentation of Chlorella sp. for anaerobic bio-hydrogen production: Influences of inoculumdsubstrate ratio, volatile fatty acids and NADH. Bioresour. Technol. 2011, 102, 10480–10485. [Google Scholar] [CrossRef]
- Cheng, H.H.; Whang, L.M.; Wu, C.W.; Chung, M.C. A two-stage bioprocess for hydrogen and methane production from rice straw bioethanol residues. Bioresour. Technol. 2012, 113, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Yun, Y.-M.; Jung, K.-W.; Kim, D.-H.; Oh, Y.-K.; Cho, S.-K.; Shin, H.-S. Optimization of dark fermentative H2 production from microalgal biomass by combined (acid + ultrasonic) pretreatment. Bioresour. Technol. 2013, 141, 220–226. [Google Scholar] [CrossRef]
- Liu, C.-H.; Chang, C.-Y.; Cheng, C.-L.; Lee, D.-J.; Chang, J.-S. Fermentative hydrogen production by Clostridium butyricum CGS5 using carbohydrate-rich microalgal biomass as feedstock. Int. J. Hydrogen Energy 2012, 37, 15458–15464. [Google Scholar] [CrossRef]
- Xia, A.; Cheng, J.; Lin, R.; Lu, H.; Zhou, J.; Cen, K. Comparison in dark hydrogen fermentation followed by photo hydrogen fermentation and methanogenesis between protein and carbohydrate compositions in Nannochloropsis oceanica biomass. Bioresour. Technol. 2013, 138, 204–213. [Google Scholar] [CrossRef] [PubMed]
- Batista, A.P.; Moura, P.; Marques, P.A.S.S.; Ortigueira, J.; Alves, L.; Gouveia, L. Scenedesmus obliquus as feedstock for biohydrogen production by Enterobacter aerogenes and Clostridium butyricum. Fuel 2014, 117, 537–543. [Google Scholar] [CrossRef] [Green Version]
- Cao, G.L.; Zhao, L.; Wang, A.J.; Wang, Z.Y.; Ren, N.Q. Single-step bioconversion of lignocellulose to hydrogen using novel moderately thermophilic bacteria. Biotechnol. Biofuels 2014, 7, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Guo, Y.C.; Dai, Y.; Bai, Y.X.; Li, Y.H.; Fan, Y.T.; Hou, H.W. Co-producing hydrogen and methane from higher-concentration of corn stalk by combining hydrogen fermentation and anaerobic digestion. Int. J. Hydrogen Energy 2014, 39, 14204–14211. [Google Scholar] [CrossRef]
- Wu, J.N.; Ein-Mozaffari, F.; Upreti, S. Effect of ozone pretreatment on hydrogen production from barley straw. Bioresour. Technol. 2013, 144, 344–349. [Google Scholar] [CrossRef] [PubMed]
- Levin, D.B.; Islam, R.; Cicek, N.; Sparling, R. Hydrogen production by Clostridium thermocellum 27405 from cellulosic biomass substrates. Int. J. Hydrogen Energy 2006, 31, 1496–1503. [Google Scholar] [CrossRef]
- Cui, M.J.; Shen, J.Q. Effects of acid and alkaline pretreatments on the biohydrogen production from grass by anaerobic dark fermentation. Int. J. Hydrogen Energy 2012, 37, 1120–1124. [Google Scholar] [CrossRef]
- Li, Y.C.; Nissilä, M.E.; Wu, S.Y.; Lin, C.Y.; Puhakka, J.A. Silage as source of bacteria and electrons for dark fermentative hydrogen production. Int. J. Hydrogen Energy 2012, 37, 15518–15524. [Google Scholar] [CrossRef]
- Cui, M.J.; Yuan, Z.L.; Zhi, X.H.; Wei, L.L.; Shen, J.Q. Biohydrogen production from poplar leaves pretreated by different methods using anaerobic mixed bacteria. Int. J. Hydrogen Energy 2010, 35, 4041–4047. [Google Scholar] [CrossRef]
- Chen, C.C.; Chuang, Y.S.; Lin, C.Y.; Lay, C.H.; Sen, B. Thermophilic dark fermentation of untreated rice straw using mixed cultures for hydrogen production. Int. J. Hydrogen Energy 2012, 37, 15540–15546. [Google Scholar] [CrossRef]
- Sikora, A. Hydrogen production in microbial processes. Adv. Microbiol. 2008, 47, 465–482. [Google Scholar]
- Kim, D.H.; Kim, M.S. Hydrogenases for biological hydrogen production. Bioresour. Technol. 2011, 102, 8423–8431. [Google Scholar] [CrossRef] [PubMed]
- Raj, R.; Kumari, M. Blue green algae (BGA) and its application. J. Pharmacogn. Phytochem. 2020, 9, 287–296. [Google Scholar]
- Troshina, O.; Serebryakova, L.; Sheremetieva, M.; Lindblad, P. Production of H2 by the unicellular cyanobacterium Gloe-ocapsa alpicola CALU 743 during fermentation. Int. J. Hydrogen Energy 2002, 27, 1283–1289. [Google Scholar] [CrossRef]
- Aoyama, K.; Uemura, I.; Miyake, J.; Asada, Y. Fermentative metabolism to produce hydrogen gas and organic compounds in a cyanobacterium, Spirulina platensis. J. Ferment. Bioeng. 1997, 83, 17–20. [Google Scholar] [CrossRef]
- Khetkorn, W.; Lindblad, P.; Incharoensakdi, A. Enhanced biohydrogen production by the N2-fixing cyanobacterium Anabaena siamensis strain TISTR 8012. Int. J. Hydrogen Energy 2010, 35, 12767–12776. [Google Scholar] [CrossRef]
- Khetkorn, W.; Lindblad, P.; Incharoensakdi, A. Inactivation of uptake hydrogenase leads to enhanced and sustained hydro-gen production with high nitrogenase activity under high light exposure in the cyanobacterium Anabaena siamensis TISTR 8012. J. Biol. Eng. 2012, 6, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Khatoon, H.; Rahman, N.A.; Banerjee, S.; Harun, N.; Suleiman, S.S.; Zakaria, N.H.; Lananan, F.; Hamid, S.H.A.; Endut, A. Effects of different salinities and pH on the growth and proximate composition of Nannochloropsis sp. and Tetraselmis sp. isolated from South China Sea cultured under control and natural condition. Int. Biodeterior. Biodegrad. 2014, 95, 11–18. [Google Scholar] [CrossRef]
- Yao, C.H.; Ai, J.N.; Cao, X.P.; Xue, S. Salinity manipulation as an effective method for enhanced starch production in the marine microalga Tetraselmis subcordiformis. Bioresour. Technol. 2013, 146, 663–671. [Google Scholar] [CrossRef]
- Dudek, M.; Dębowski, M.; Zieliński, M.; Nowicka, A. Use of a wastewater after anaerobic pretreatment to microalgae Platymonas subcordiformis growth. Ecol. Eng. 2017, 18, 14–20. [Google Scholar]
- Guo, Z.; Liu, Y.; Guo, H.; Yan, S.; Mu, J. Microalgae cultivation using an aquaculture wastewater as growth medium for bio-mass and biofuel production. J. Environ. Sci. 2013, 25, 85–88. [Google Scholar] [CrossRef]
- Zhang, Y.; Fan, X.; Yang, Z.; Wang, H.; Yang, D.; Guo, R. Characterization of H2 photoproduction by a new marine green alga, Platymonas helgolandica var. tsingtaoensis. Appl. Energy 2012, 92, 38–43. [Google Scholar] [CrossRef]
- Xie, J.; Zhang, Y.; Li, Y.; Wang, Y. Mixotrophic cultivation of Platymonas subcordiformis. Environ. Boil. Fishes 2001, 13, 343–347. [Google Scholar] [CrossRef]
- Ran, C.; Zhang, F.; Sun, H.; Zhao, B. Effect of culture medium on hydrogen production by sulfur-deprived marine green algae Platymonas subcordiformis. Biotechnol. Bioprocess Eng. 2009, 14, 835–841. [Google Scholar] [CrossRef]
- Chinnasamy, S.; Bhatnagar, A.; Hunt, R.W.; Das, K.C. Microalgae cultivation in a wastewater dominated by carpet mill effluents for biofuel applications. Bioresour. Technol. 2010, 101, 3097–3105. [Google Scholar] [CrossRef] [PubMed]
- Dasgupta, C.N.; Gilbert, J.J.; Lindblad, P.; Heidorn, T.; Borgvang, S.A.; Skjanes, K.; Das, D. Recent trends on the development of photobiological processes and photobioreactors for the improvement of hydrogen production. Int. J. Hydrogen Energy 2010, 35, 10218–10238. [Google Scholar] [CrossRef]
- Tamburic, B.; Zemichael, F.W.; Crudge, P.; Maitland, G.C.; Hellgardt, K. Design of a novel flat-plate photobioreactor system for green algal hydrogen production. Int. J. Hydrogen Energy 2011, 36, 6578–6591. [Google Scholar] [CrossRef] [Green Version]
- Saleem, M.; Chakrabarti, M.H.; Raman, A.A.A.; Hasan, D.B.; Daud, W.M.A.W.; Mustafa, A. Hydrogen production by Chlamydomonas reinhardtii in a two-stage process with and without illumination at alkaline pH. Int. J. Hydrogen Energy 2012, 37, 4930–4934. [Google Scholar] [CrossRef]
- Oncel, S.; Kose, A. Comparison of tubular and panel type photobioreactors for biohydrogen production utilizing Chlamydomonas reinhardtii considering mixing time and light intensity. Bioresour. Technol. 2014, 151, 265–270. [Google Scholar] [CrossRef] [PubMed]
- Oncel, S.; Sabankay, M. Microalgal biohydrogen production considering light energy and mixing time as the two key features for scale-up. Bioresour. Technol. 2012, 121, 228–234. [Google Scholar] [CrossRef]
- Day, J.G.; Tsavalos, A.J. An investigation of the heterotrophic culture of the green algaTetraselmis. Environ. Boil. Fishes 1996, 8, 73–77. [Google Scholar] [CrossRef]
- Dudek, M.; Dębowski, M.; Zieliński, M.; Nowicka, A.; Rusanowska, P. Water from the Vistula Lagoon as a medium in mixotrophic growth and hydrogen production by Platymonas subcordiformis. Int. J. Hydrogen Energy 2018, 43, 9529–9534. [Google Scholar] [CrossRef]
- Yao, C.; Ai, J.; Cao, X.; Xue, S.; Zhang, W. Enhancing starch production of a marine green microalga Tetraselmis subcordiformis through nutrient limitation. Bioresour. Technol. 2012, 118, 438–444. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Wang, J.; Jiang, P.; Bian, S.; Qin, S. Transformation of Platymonas (Tetraselmis) subcordiformis (Prasinophyceae, Chlorophyta) by agitation with glass beads. World J. Microbiol. Biotechnol. 2010, 26, 1653–1657. [Google Scholar] [CrossRef]
- Tamburic, B.; Zemichael, F.W.; Maitland, G.C.; Hellgardt, K. A novel nutrient control method to deprive green algae of sulphur and initiate spontaneous hydrogen production. Int. J. Hydrogen Energy 2012, 37, 8988–9001. [Google Scholar] [CrossRef]
- Dębowski, M.; Zieliński, M.; Dudek, M.; Grala, A. Acquisition feasibility and methane fermentation effectiveness of biomass of microalgae occurring in eutrophicated aquifers on the example of the Vistula Lagoon. Int. J. Green Energy 2014, 13, 395–407. [Google Scholar] [CrossRef]
- Ogbonna, J.C.; Tanaka, H. Night biomass loss and changes in biochemical composition of cells during light/dark cyclic culture of Chlorella pyrenoidosa. J. Ferment. Bioeng. 1996, 82, 558–564. [Google Scholar] [CrossRef]
| Microalgal Strains/ Biomass | Process | Alga Type | Reactor Type | Temp. [K] | Products [%] | Energy Yield [MJ/kg] | Ref. | ||
|---|---|---|---|---|---|---|---|---|---|
| Oil Fraction | Gas Fraction | Solid Fraction | |||||||
| Nannochlorsis sp. | Direct pyrolysis catalytic pyrolysis | Nannochlorsis sp. | Fixed-bed reactor | 573–773 | 20.0–31.1 | 18.9–33.5 | 24.2–45.3 | 24.6 | [109] |
| Nannochloropsis sp. | Nannochloropsis sp. | 10.0–19.0 | 11.0–34.0 | 56.0–19.0 | 32.7 | ||||
| Chlorella protothecoide | Rapid pyrolysis | Chlorella protothecoide | Fluidised-bed reactor | 773 | 17 | 28 | 55 | 29.0 | [118] |
| Microcystis Aeruginosa | Catalytic pyrolysis | Microcystis Aeruginosa | 23 | 56 | 21 | 30.0 | |||
| Chlorella protothecoides | Rapid pyrolysis | Chlorella protothecoides | Fluidised-bed reactor | 773 | 58 | 30.0 | 12.0 | 41.0 | [119] |
| Tetraselmis chuii | Slow pyrolysis | Tetraselmis chuii | Conventional tubular oven | 773 | 43 | 20 | 37 | 3.4 | [120] |
| Chlorella vulgaris | Chlorella vulgaris | 41 | 25 | 34 | 1.8 | ||||
| Chlorella like | Chlorella like | 41 | 22 | 37 | 4.8 | ||||
| Chaetocerous muelleri | Chaetocerous muelleri | 33 | 14 | 53 | 1.2 | ||||
| Dunaliella tertiolecta | Dunaliella tertiolecta | 24 | 13 | 63 | 2.4 | ||||
| Synechococcus | Synechococcus | 38 | 18 | 44 | 1.4 | ||||
| BGAB blue-green algae (>90% Microcystis) | Pyrolysis | BGAB blue-green algae (>90% Microcystis) | Fixed-bed reactor | 773 | 26.66–54.97 | 16.25–41.33 | 57.09–20.39 | 31.9 | [121] |
| Organism | Substrate | Light Intensity [W/m2] | Temp. [°C] | H2 Production | Ref. |
|---|---|---|---|---|---|
| Rhodobacter sphaeroides O.U.001 | olive mill wastewater | 150 | 30 | 35 dm3 H2/dm3substrate | [141] |
| Rhodobacter capsulatus JP91 | glucose | 175 | 30 | 5.5 mol H2/molglucose | [158] |
| Rhodobacter capsulatus | sucrose (sugar industry molasses) | 200 | 30 | 10.5 mol H2/molsucrose | [159] |
| Rhodobacter capsulatus | sucrose | 200 | 30 | 14 mol H2/molsucrose | [159] |
| Rhodobacter capsulatus JY91 | glucose | 200 | 30 | 3.3 mol H2/molglucose | [160] |
| Rhodobacter sphaeroides O.U. 001 | milk industry wastewater | 116 | 28 | 3.2 dm3 H2/dm3substrate | [161] |
| Rhodobium marinum | soy sauce production wastewater | 240 | 30 | 2.14 molH2/molglucose | [162] |
| Rhodobium marinum | sugar cane bagasse | 240 | 30 | 41 cm3 H2 | [162] |
| Rhodobacter capsulatus | malonate | 200 | 30 | 3.7 mol H2/molsubstrate | [163] |
| Rhodobacter capsulatus | acetate | 200 | 30 | 2.5 mol H2/molsubstrate | [163] |
| Microalgal Strains/Biomass | Condition | Performance H2 Production [cm3/dm3] | Ref. |
|---|---|---|---|
| Platymonas Subcordiformis | torus photobioreactor two-phase incubation 0–1000 μmol photon/m2·s | 157.7 | [24] |
| Chlamydomonas reinhardtii | CST-PBR (continuously stirred type photobioreactor) 140 μE/m2·s, 100 rpm | 321.0 | [175] |
| Chlamydomonas reinhardtii | cylindrical flasks, 100 μE/m2·s, 28 °C | 180.0 | [177] |
| Chlamydomonas reinhardtii | glass photobioreactors, 300 μE/m2·s, 30 ± 1.5 °C, 400–500 rpm | 120.0 | [178] |
| Chlorella vulgaris MSU 01 | illumination of 8 klux (2 nos.)—halogen lamps | 220 | [184] |
| Tetraselmis Subcordiformis | two-phase incubation, 160 μE/m2·s, 25 °C, 150 rpm | 55.8 | [186] |
| Chlamydomonas reinhardtii | flat PBRs, two-phase incubation, 70 × 2 μE/m2·s | 210.9 | [187] |
| Platymonas Subcordiformis | - | 50.0 | [188] |
| Feedstock | Condition | Performance H2 Production [cm3/gTS] | Ref. |
|---|---|---|---|
| Arthrospira platensis | batch, 35 °C | 96.6 | [166] |
| Chlamydomonas reinhardtii | batch, 37 °C | 40.0 | [189] |
| Chlorella sp. | batch, 35 °C, pH = 6.5 | 6.1 | [190] |
| Chlorella Pyrenoidosa sp. | batch, 35 °C, pH = 6.0 | 8.8 | [191] |
| Chlorella vulgaris | batch, 35 °C | 41.2 | [192] |
| Chlorella vulgaris ESP6 | batch, 35 °C | 81.0 | [193] |
| Nannochloropsis Oceanica sp. | batch, 35 °C, pH = 6.0 | 0–2.0 | [194] |
| Scenedesmus obliquus | batch, 37 °C | 90.3 | [195] |
| Corn cob | batch, 60 °C, pH = 7 | 3.23–3.27 | [196] |
| Corn stalk | batch, 60 °C, pH = 7 | 3.28–3.47 | [196] |
| Corn stalk | batch, 36 °C, pH = 7.5 | 79.8 | [197] |
| Corn Straw | batch, 35 °C, pH = 6.0 | 69.6–93.4 | [198] |
| Delignified wood | batch, pH = 7 | 2.5–7.8 | [199] |
| Grass | batch, 35 °C, pH = 7 | 72.2 | [200] |
| Grass silage | batch, 37 °C, pH = 7 | 37.8 | [201] |
| Poplar leaves | batch, 35 °C, pH = 7 | 33.45 | [202] |
| Rice Straw | batch, 55 °C, pH = 6.5 | 24.8 | [203] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dębowski, M.; Dudek, M.; Zieliński, M.; Nowicka, A.; Kazimierowicz, J. Microalgal Hydrogen Production in Relation to Other Biomass-Based Technologies—A Review. Energies 2021, 14, 6025. https://doi.org/10.3390/en14196025
Dębowski M, Dudek M, Zieliński M, Nowicka A, Kazimierowicz J. Microalgal Hydrogen Production in Relation to Other Biomass-Based Technologies—A Review. Energies. 2021; 14(19):6025. https://doi.org/10.3390/en14196025
Chicago/Turabian StyleDębowski, Marcin, Magda Dudek, Marcin Zieliński, Anna Nowicka, and Joanna Kazimierowicz. 2021. "Microalgal Hydrogen Production in Relation to Other Biomass-Based Technologies—A Review" Energies 14, no. 19: 6025. https://doi.org/10.3390/en14196025
APA StyleDębowski, M., Dudek, M., Zieliński, M., Nowicka, A., & Kazimierowicz, J. (2021). Microalgal Hydrogen Production in Relation to Other Biomass-Based Technologies—A Review. Energies, 14(19), 6025. https://doi.org/10.3390/en14196025









