An Integrated Device of a Lithium-Ion Battery Combined with Silicon Solar Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of LIB and Solar Cell
2.2. Characterizations
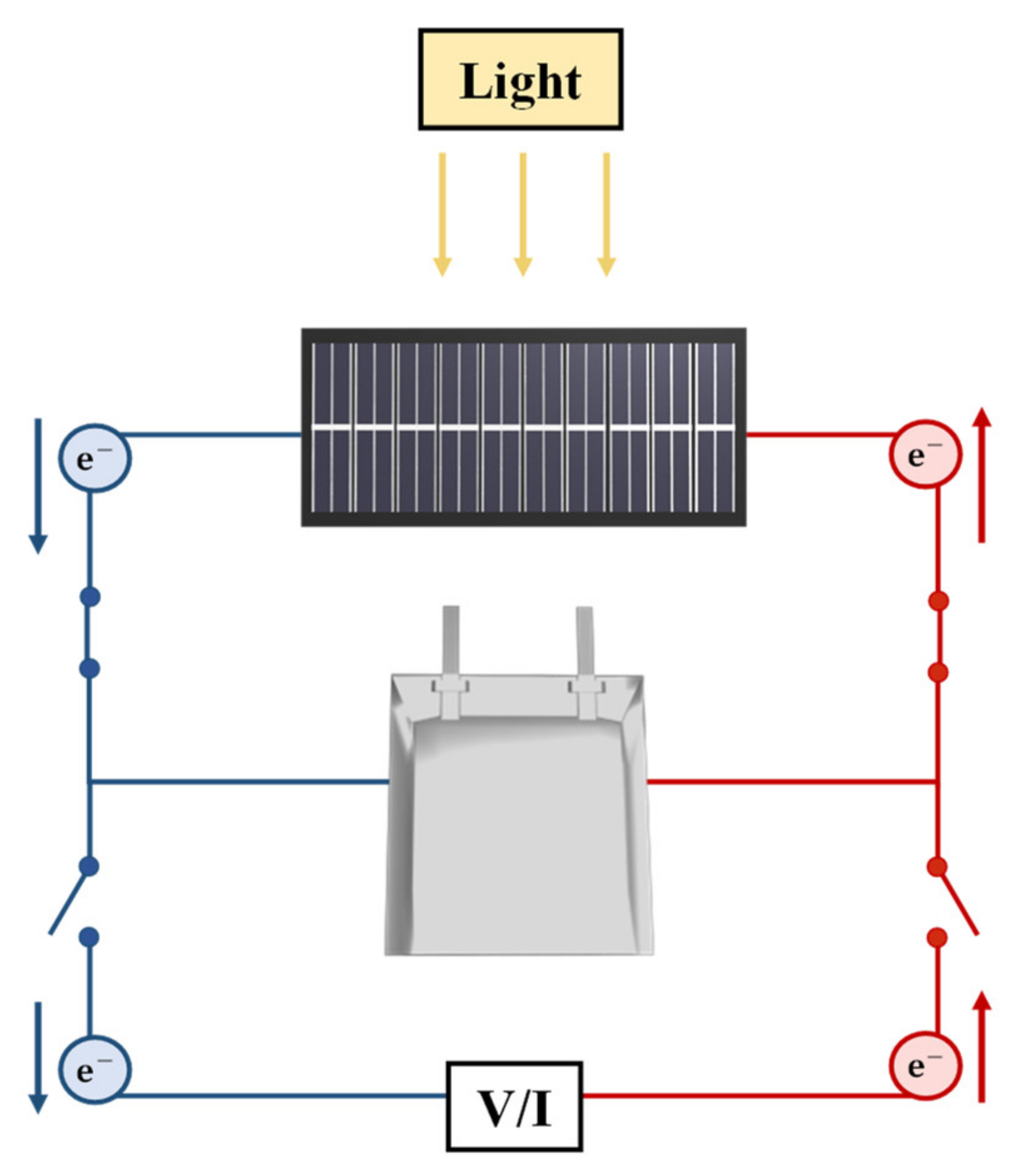
2.3. Integrated Si Solar Cells-LIB Device
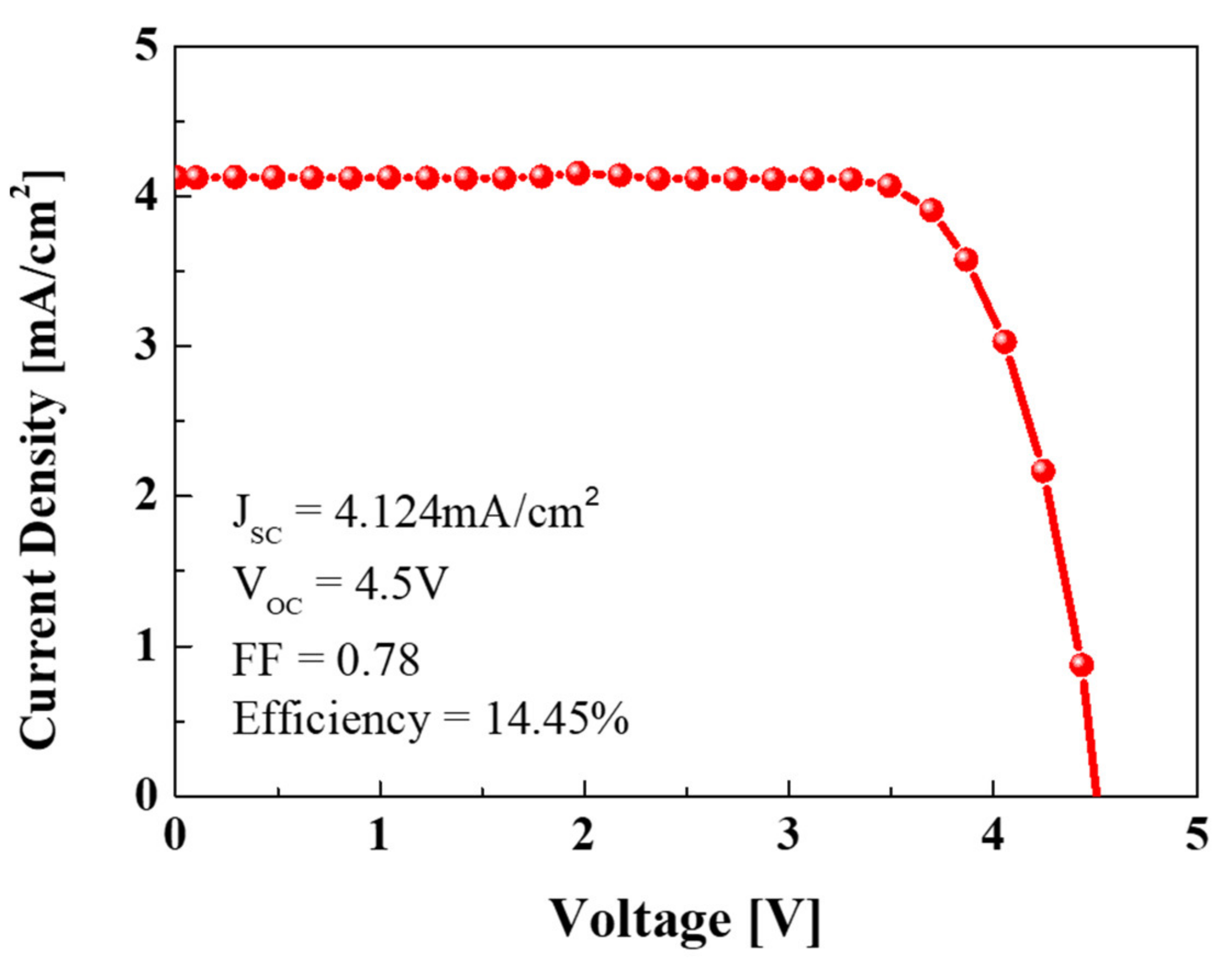
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kannan, N.; Vakeesan, D. Solar energy for future world—A review. Renew. Sustain. Energy Rev. 2016, 62, 1092–1105. [Google Scholar] [CrossRef]
- Nozik, A.J. Photoelectrochemistry: Applications to solar energy conversion. Annu. Rev. Phys. Chem. 1978, 29, 189–222. [Google Scholar] [CrossRef]
- Bo, L.; Shahidehpour, M. Short-term scheduling of battery in a grid-connected PV/battery system. IEEE Trans. Power Syst. 2005, 20, 1053–1061. [Google Scholar] [CrossRef]
- Lupangu, C.; Bansal, R.C. A review of technical issues on the development of solar photovoltaic systems. Renew. Sustain. Energy Rev. 2017, 73, 950–965. [Google Scholar] [CrossRef]
- Licht, S. A description of energy conversion in photoelectrochemical solar cells. Nature 1987, 330, 148–151. [Google Scholar] [CrossRef]
- Sharon, M.; Veluchamy, P.; Natarajan, C.; Kumar, D. Solar rechargeable battery-principle and materials. Electrochim. Acta. 1991, 36, 1107–1126. [Google Scholar] [CrossRef]
- Nomiyama, T.; Kuriyaki, H.; Hirakawa, K. Photo-rechargeable battery using new layer compound CuFeTe2. Synth. Met. 1995, 71, 2237–2238. [Google Scholar] [CrossRef]
- Liao, Z.; Ruan, X. Control strategy of bi-directional DC/DC converter for a novel stand-alone photovoltaic power system. IEEE Veh. Power Propuls. Conf. 2008, 1–6. [Google Scholar] [CrossRef]
- Shiau, J.; Ma, C. Li-Ion Battery Charging with a Buck-Boost Power Converter for a Solar Powered Battery Management System. Energies 2013, 6, 1669–1699. [Google Scholar] [CrossRef] [Green Version]
- Gibson, T.L.; Kelly, N.A. Solar photovoltaic charging of lithium-ion batteries. IEEE Veh. Power Propuls. Conf. 2009, 310–316. [Google Scholar] [CrossRef]
- Luo, B.; Ye, D.; Wang, L. Recent Progress on Integrated Energy Conversion and Storage Systems. Adv. Sci. 2017, 4, 1700104. [Google Scholar] [CrossRef] [Green Version]
- Chu, S.; Cui, Y.; Liu, N. The path towards sustainable energy. Nat. Mater. 2017, 16, 16–22. [Google Scholar] [CrossRef]
- Freitasgomes, I.S.; Perez, Y.; Suomalainen, E. Coupling small batteries and PV generation: A review. Renew. Sustain. Energy Rev. 2020, 126, 109835. [Google Scholar] [CrossRef]
- Ellis, B.; Perry, L.K.; Ryan, D.H.; Nazar, L.F. Small Polaron Hopping in LixFePO4 Solid Solutions: Coupled Lithium-Ion and Electron Mobility. J. Am. Chem. Soc. 2006, 128, 11416–11422. [Google Scholar] [CrossRef]
- Zhao, Q.; Zhang, Y.; Meng, Y.; Wang, Y.; Ou, J.; Guo, Y.; Xiao, D. Phytic acid derived LiFePO4 beyond theoretical capacity as high-energy density cathode for lithium ion battery. Nano Energy 2017, 34, 408–420. [Google Scholar] [CrossRef]
- Watanabe, S.; Kinoshita, M.; Nakura, K. Capacity fade of LiNi(1-x-y)CoxAlyO2 cathode for lithium-ion batteries during accelerated calendar and cycle life test. I. Comparison analysis between LiNi(1-x-y)CoxAlyO2 and LiCoO2 cathodes in cylindrical lithium-ion cells during long term storage test. J. Power Sources 2014, 247, 412–422. [Google Scholar] [CrossRef]
- Chen, Y.; Song, S.; Zhang, X.; Liu, Y. The challenges, solutions and development of high energy Ni-rich NCM/NCA LiB cathode materials. J. Phys. Conf. Ser. 2019, 1347, 012012. [Google Scholar] [CrossRef] [Green Version]
- Kim, K.; Park, S.; Kwon, T.; Park, J.; Ahn, H.; Lee, M. Characterization of Li-V-O nanorod phases and their effect on electrochemical properties of Li1+xV3O8 cathode materials synthesized by hydrothermal reaction and subsequent heat treatment. Electrochim. Acta 2013, 89, 708–716. [Google Scholar] [CrossRef]
- Park, C.; Lee, S.; Kim, K.; Kim, M.; Choi, S.; Shin, D. Electrochemical Properties of composite cathode using bimodal sized electrolyte for all-solid-state batteries. J. Electrochem. Soc. 2019, 166, A5318–A5322. [Google Scholar] [CrossRef]
- Feng, Z.; Peng, Z.; Huang, X.; Rajagopalan, R.; Tang, Y.; Wang, H. Enhanced electrochemical properties of Li(Ni0.8Co0.1Mn0.1)O2 at elevated temperature by simultaneous structure and interface regulating. J. Electrochem. Soc. 2019, 166, A1439–A1448. [Google Scholar] [CrossRef]
- Noh, H.; Youn, S.; Yoon, C.; Sun, Y. Comparison of the structural and electrochemical properties of layered Li[NixCoyMnz]O2 (x = 1/3, 0.5, 0.6, 0.7, 0.8 and 0.85) cathode material for lithium-ion batteries. J. Power Sources 2013, 233, 121–130. [Google Scholar] [CrossRef]
- Sharma, S.; Jain, K.K.; Sharma, A. Solar cells: In research and applications—A review. Mater. Sci. Appl. 2015, 6, 1145–1155. [Google Scholar] [CrossRef] [Green Version]
- Um, H.; Choi, K.; Hwang, I.; Kim, S.; Seo, K.; Lee, S. Monolithically integrated, photo-rechargeable portable power sources based on miniaturized Si solar cells and printed solid-state lithium-ion batteries. Energy Environ. Sci. 2017, 10, 931–940. [Google Scholar] [CrossRef]
- Wang, X.; Adelmann, P.; Reindl, T. Use of LiFePO4 batteries in stand-alone solar system. Energy Proced. 2012, 25, 135–140. [Google Scholar] [CrossRef] [Green Version]
- Zaghib, K.; Dontigny, M.; Guerfi, A.; Charest, P.; Rodrigues, I.; Mauger, A.; Julien, C.M. Safe and fast-charging Li-ion battery with long shelf life for power applications. J. Power Sources 2011, 196, 3949–3954. [Google Scholar] [CrossRef]
- Abe, Y.; Hori, N.; Kumagai, S. Electrochemical Impedance Spectroscopy on the Performance Degradation of LiFePO4/Graphite Lithium-Ion Battery Due to Charge-Discharge Cycling under Different C-Rates. Energies 2019, 12, 4507. [Google Scholar] [CrossRef] [Green Version]
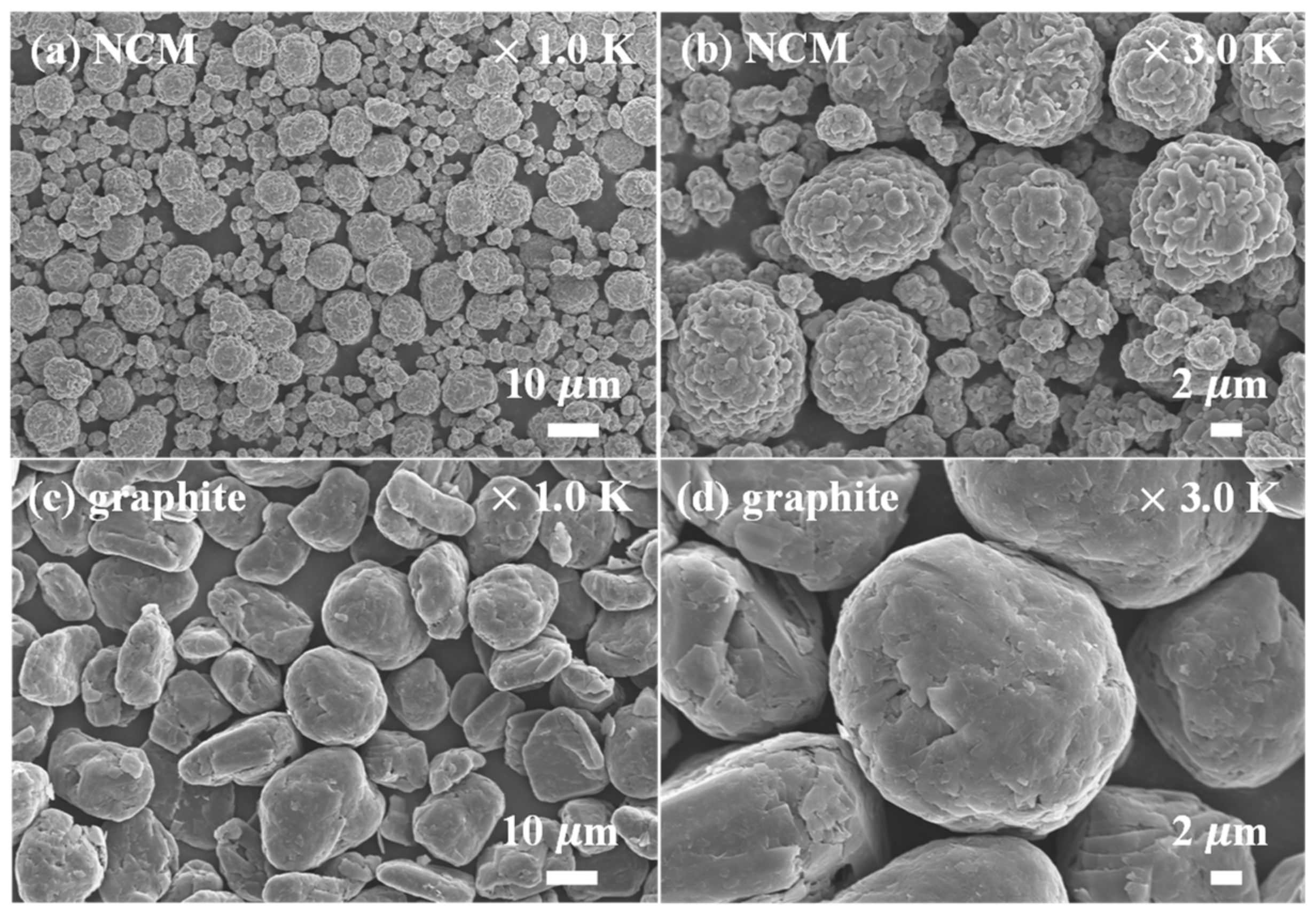

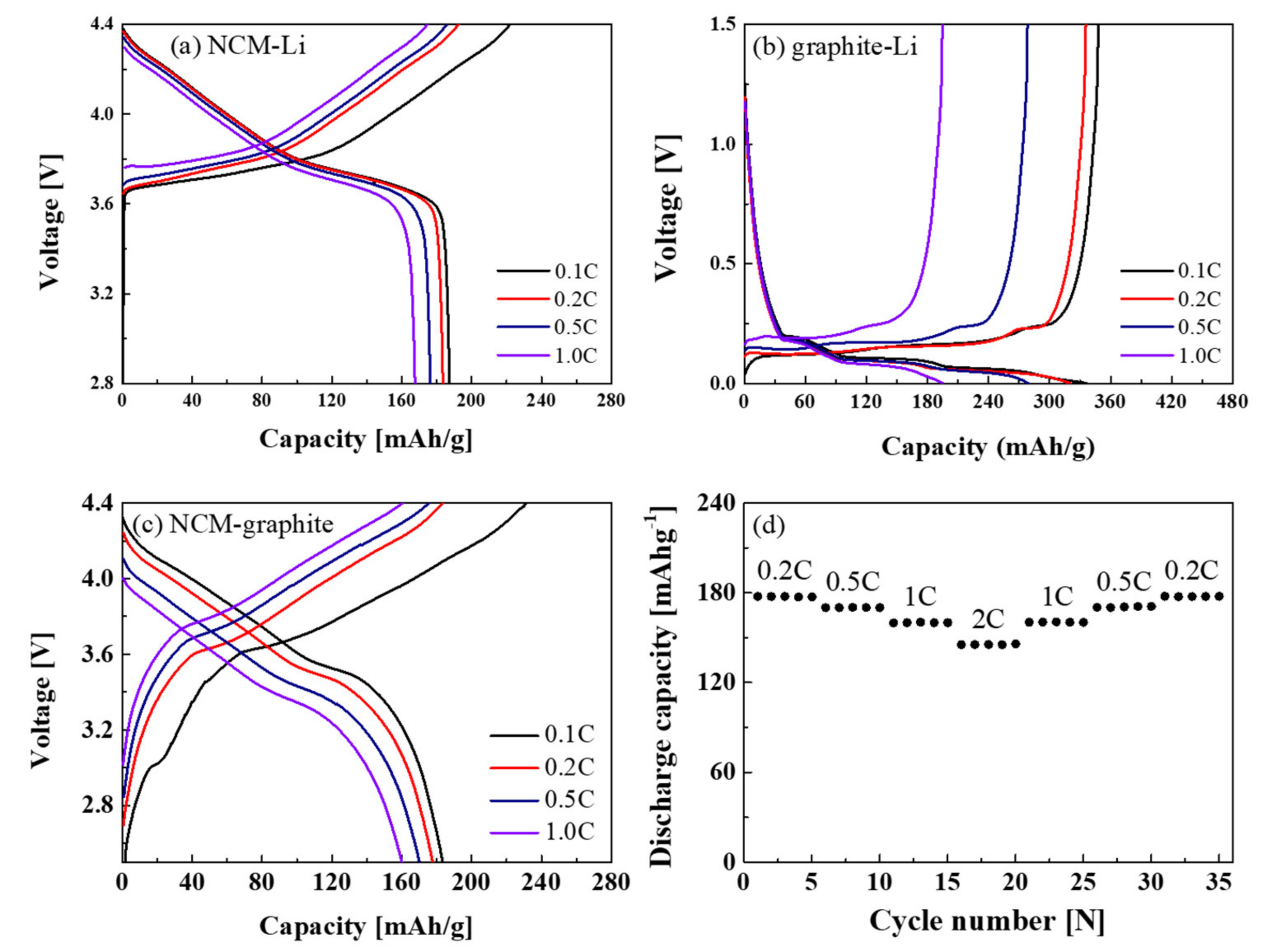
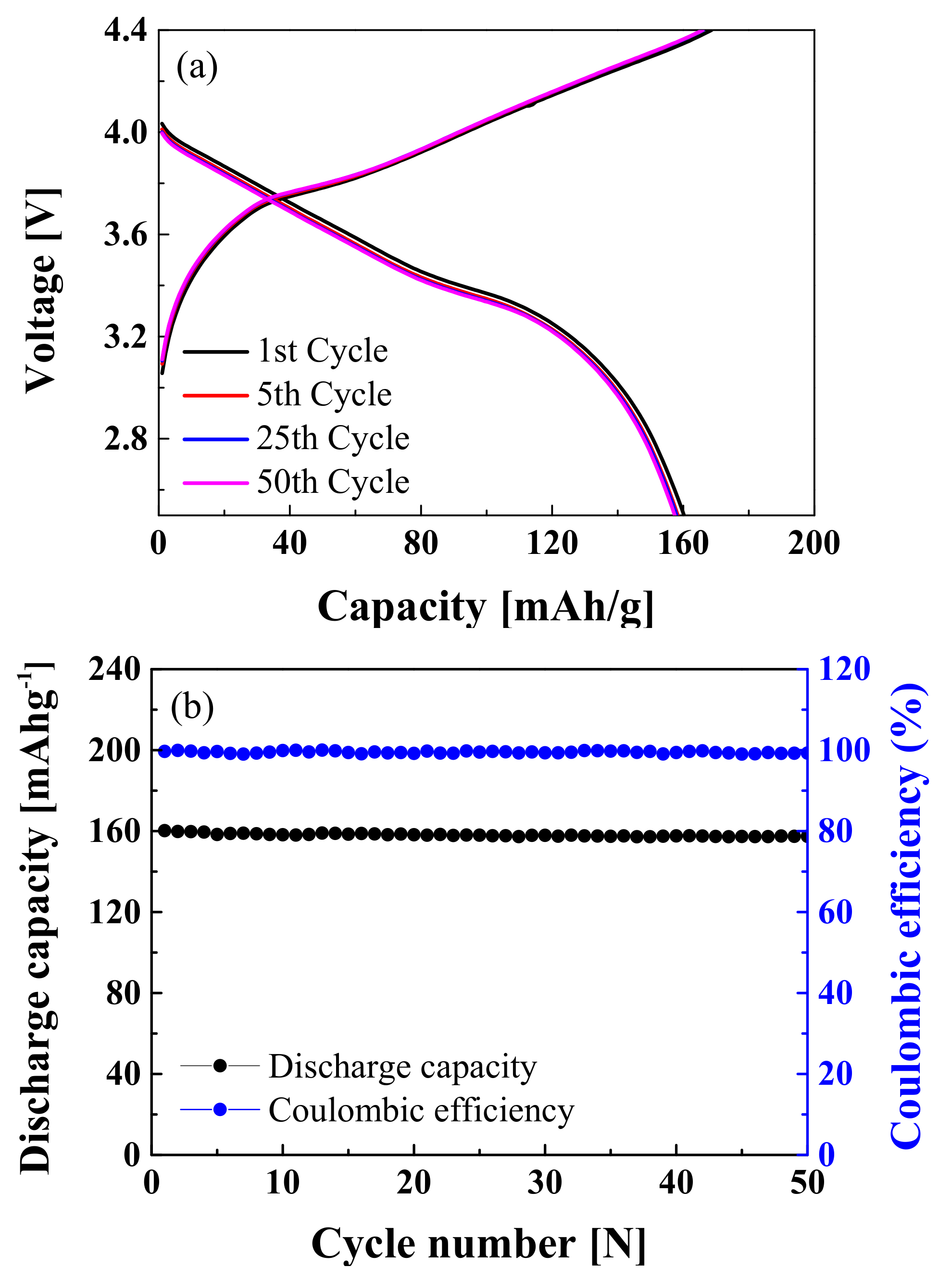


| Sample | a (Å) | c (Å) | c/a | Unit volume (Å3) | I(003)/(104) |
|---|---|---|---|---|---|
| NCM622 | 2.910 | 14.292 | 4.911 | 101.67 | 1.575 |
| Atom | X | Y | Z | Occupancy |
|---|---|---|---|---|
| Li1 | 0.00000 | 0.00000 | 0.00000 | 1.0 (fixed) |
| Ni1 | 0.00000 | 0.00000 | 0.50000 | 0.65 (fixed) |
| Co1 | 0.00000 | 0.00000 | 0.50000 | 0.15 (fixed) |
| Mn1 | 0.00000 | 0.00000 | 0.50000 | 0.20 (fixed) |
| O1 | 0.00000 | 0.00000 | 0.24224 (10) | 1.0 (fixed) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lim, H.; Na, D.; Lee, C.-R.; Seo, H.-K.; Kwon, O.-H.; Kim, J.-K.; Seo, I. An Integrated Device of a Lithium-Ion Battery Combined with Silicon Solar Cells. Energies 2021, 14, 6010. https://doi.org/10.3390/en14196010
Lim H, Na D, Lee C-R, Seo H-K, Kwon O-H, Kim J-K, Seo I. An Integrated Device of a Lithium-Ion Battery Combined with Silicon Solar Cells. Energies. 2021; 14(19):6010. https://doi.org/10.3390/en14196010
Chicago/Turabian StyleLim, Hyeonsu, Dan Na, Cheul-Ro Lee, Hyung-Kee Seo, O-Hyeon Kwon, Jae-Kwang Kim, and Inseok Seo. 2021. "An Integrated Device of a Lithium-Ion Battery Combined with Silicon Solar Cells" Energies 14, no. 19: 6010. https://doi.org/10.3390/en14196010
APA StyleLim, H., Na, D., Lee, C.-R., Seo, H.-K., Kwon, O.-H., Kim, J.-K., & Seo, I. (2021). An Integrated Device of a Lithium-Ion Battery Combined with Silicon Solar Cells. Energies, 14(19), 6010. https://doi.org/10.3390/en14196010








