Gasification of Coal by CO2: The Impact of the Heat Transfer Limitation on the Progress, Reaction Rate and Kinetics of the Process
Abstract
1. Introduction
2. Experimental Setup and Tests
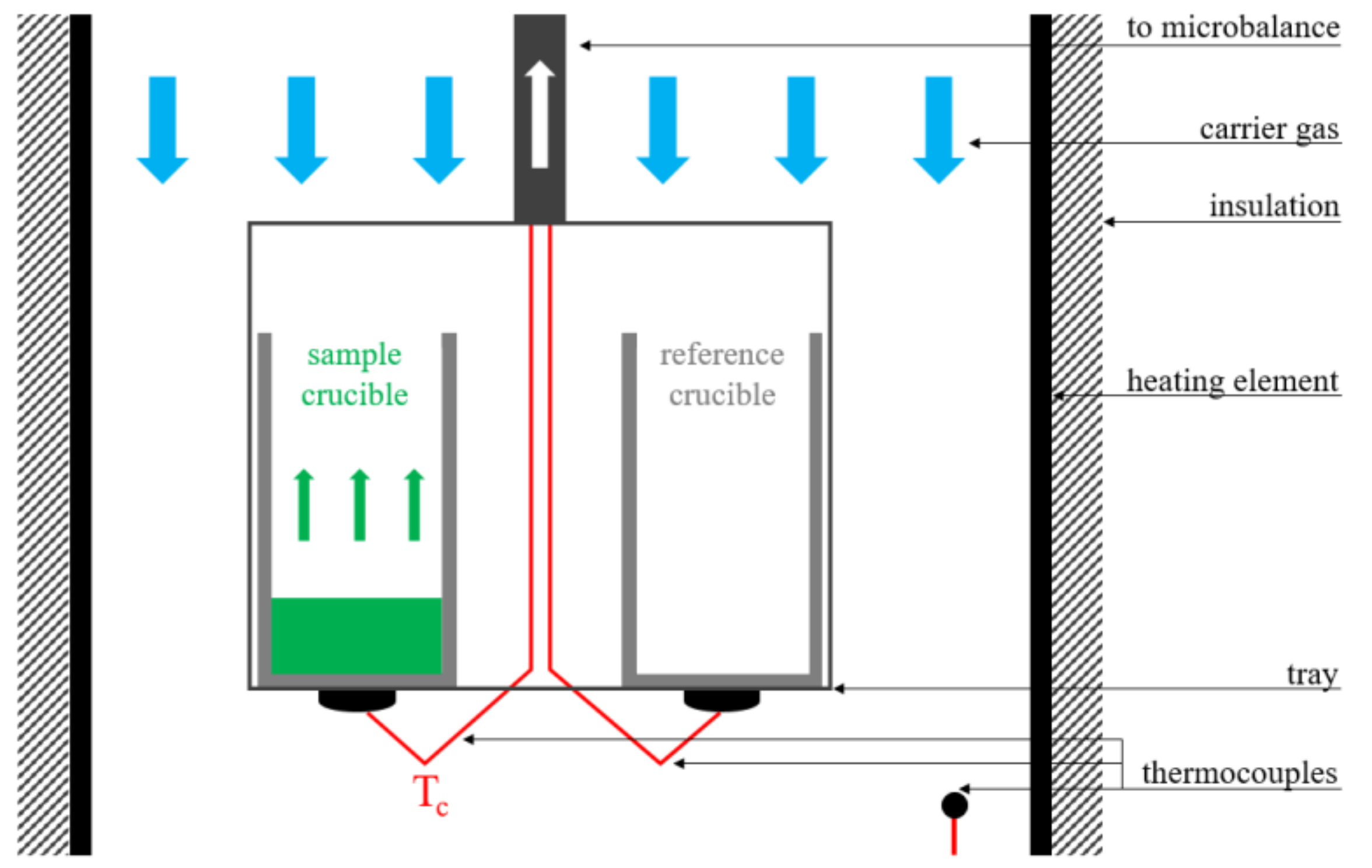
2.1. Facility and Instrumentation
2.2. Sample Properties
- -
- Lignites, LB supplied by Bełchatów Coal Mine (Poland) and LT supplied by Turów Coal Mine (Poland);
- -
- Hard coals, HJ supplied by Janina Coal Mine located in Libiąż (Poland), HS supplied by Sobieski Coal Mine located in Jaworzno (Poland) and HZ supplied by Ziemowit Coal Mine located in Lędziny (Poland);
- -
- Anthracite, AI mined in Ibbenbüren Coal Mine (Germany).
3. Modeling Study
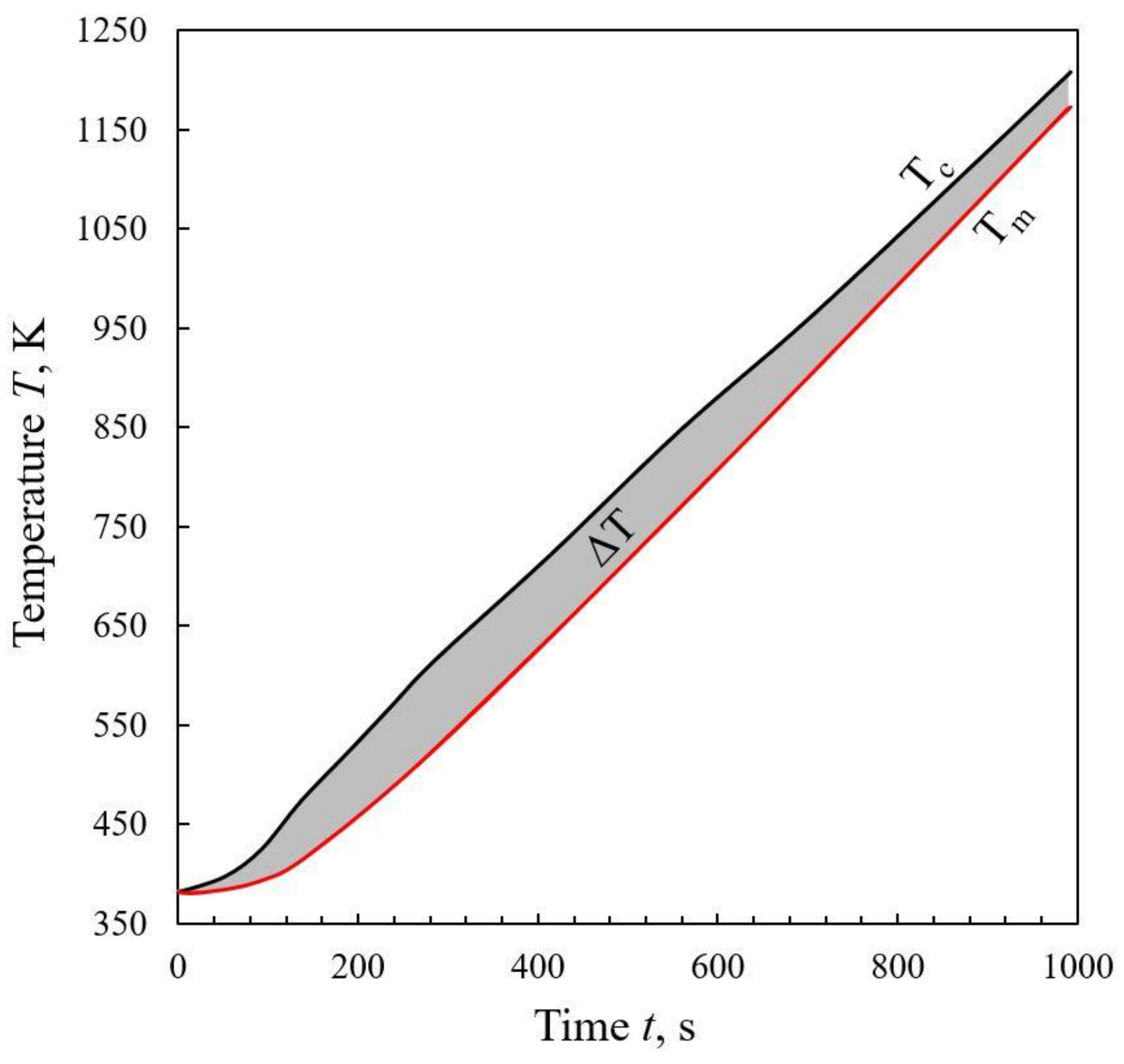
3.1. Thermal Lag Model

3.2. Kinetic Regime
3.3. Reaction Kinetics
4. Results and Discussion
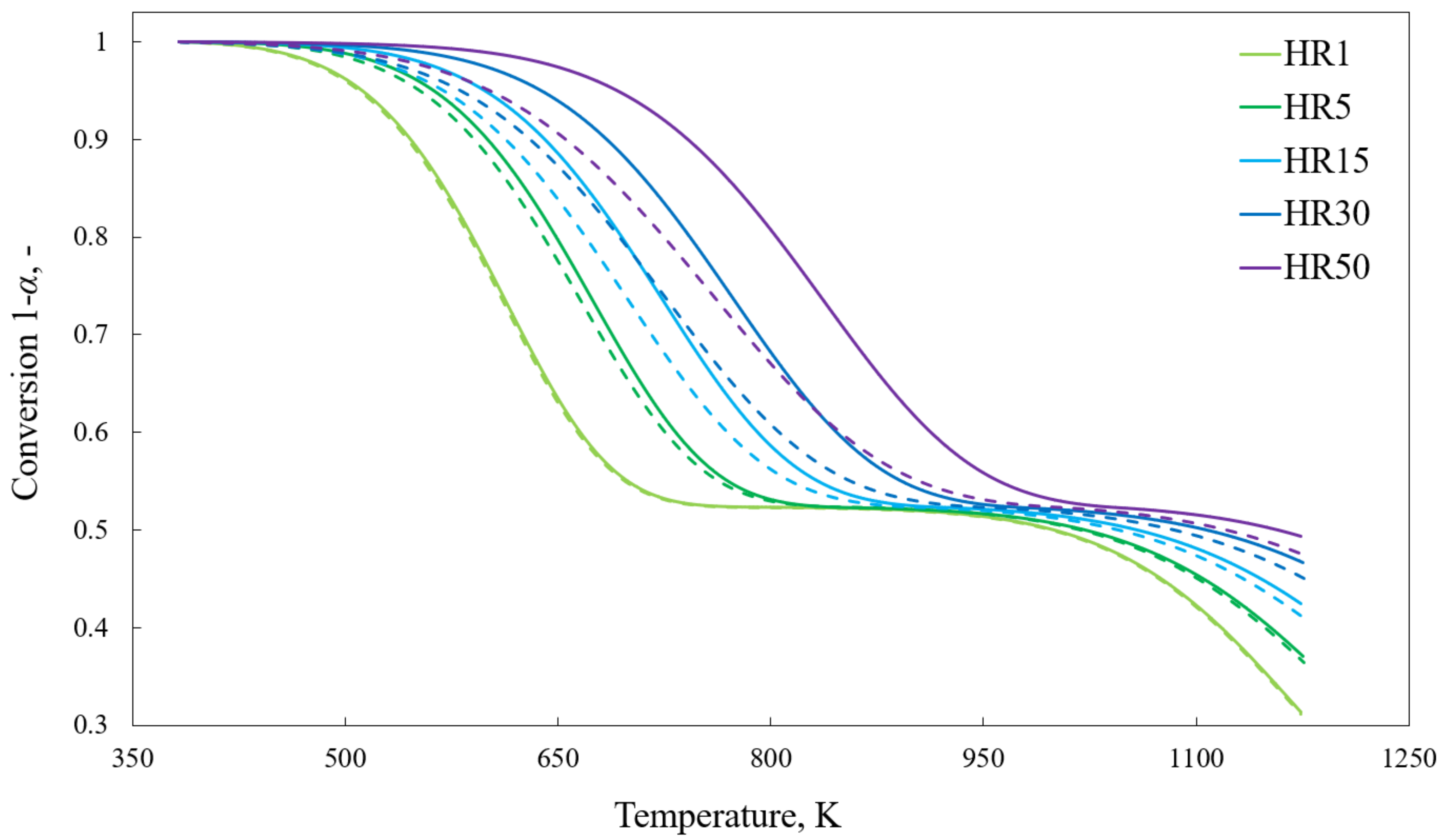
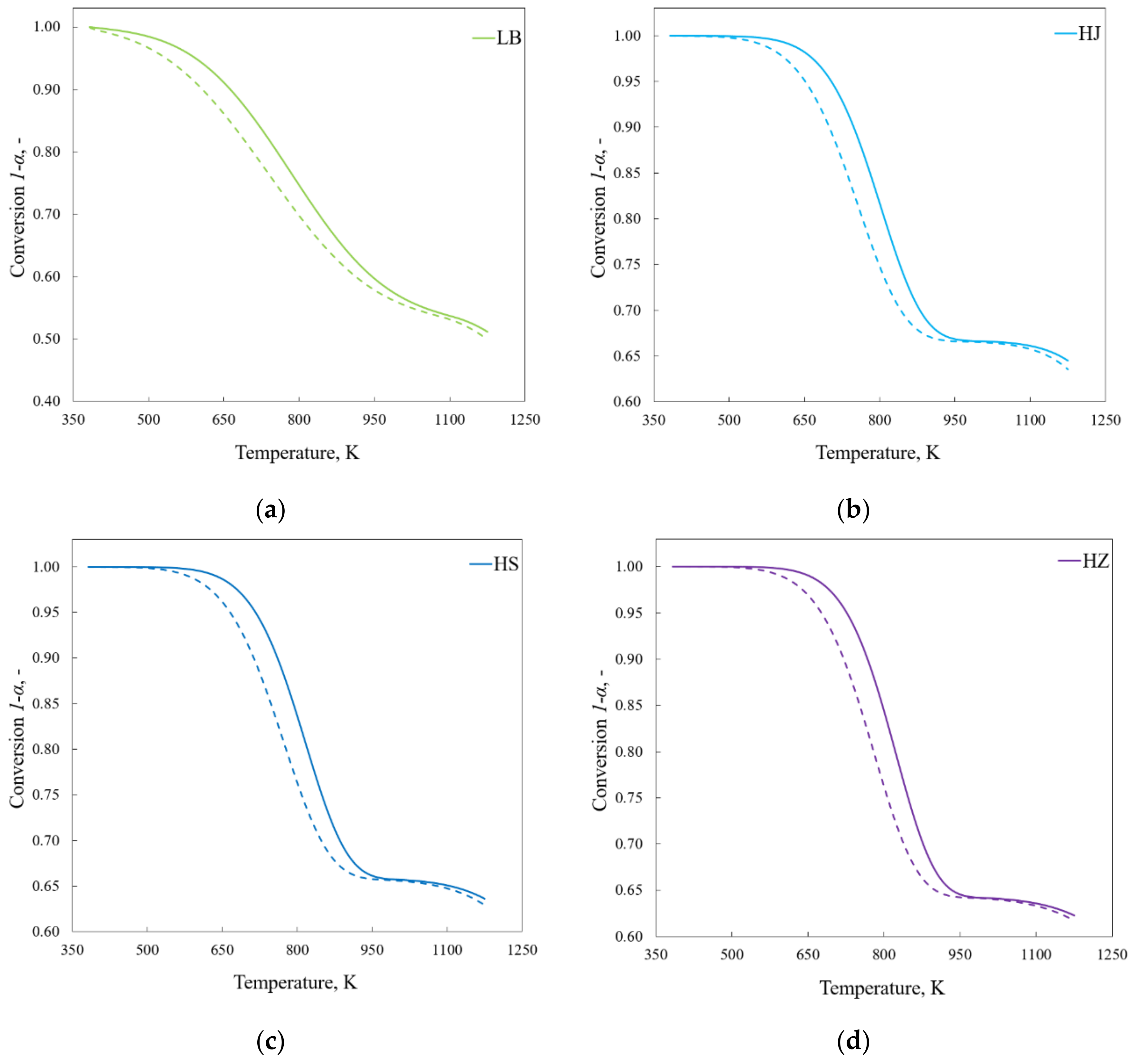
4.1. Progress and Reaction Rate
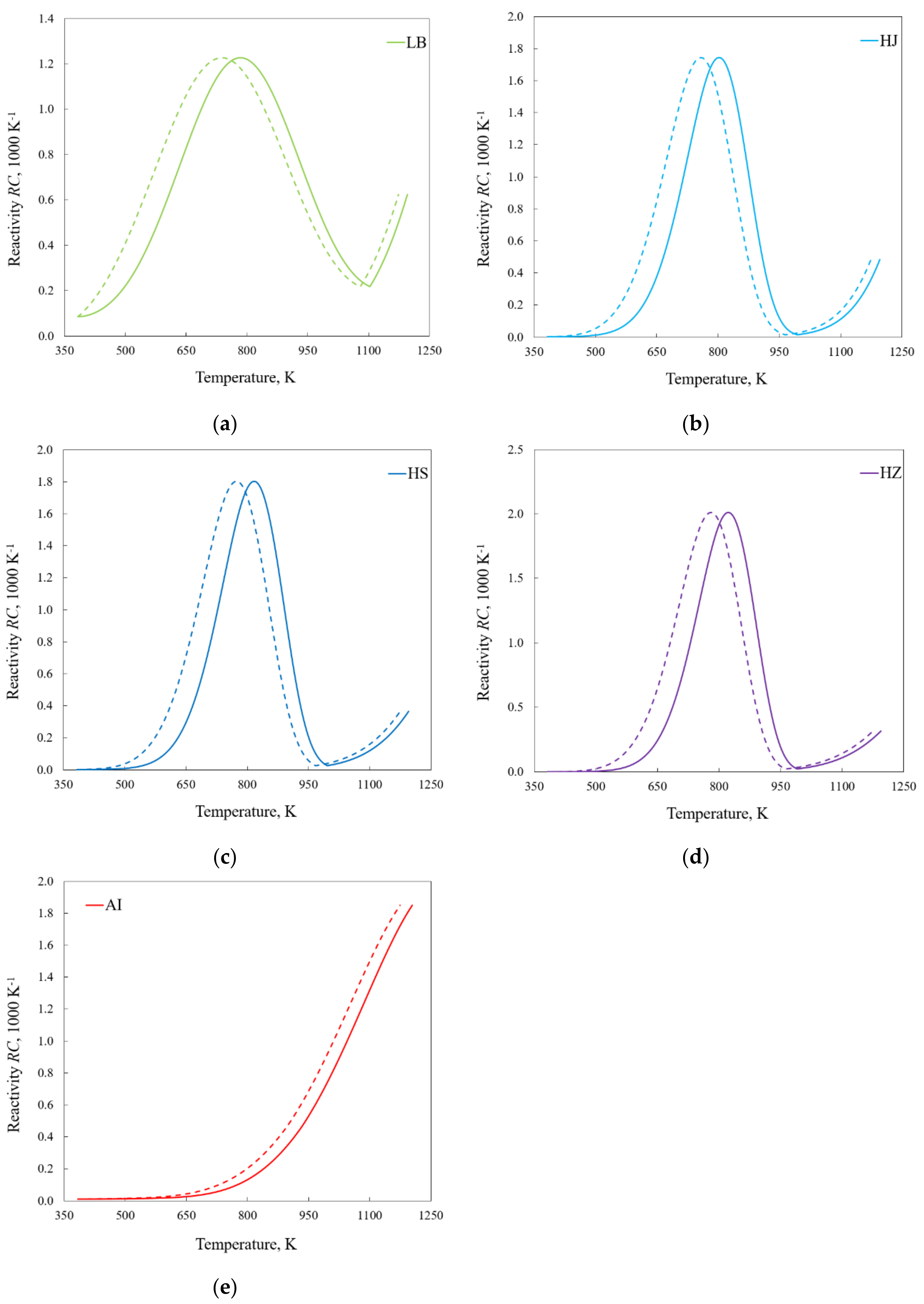
4.2. Kinetics
5. Conclusions
- -
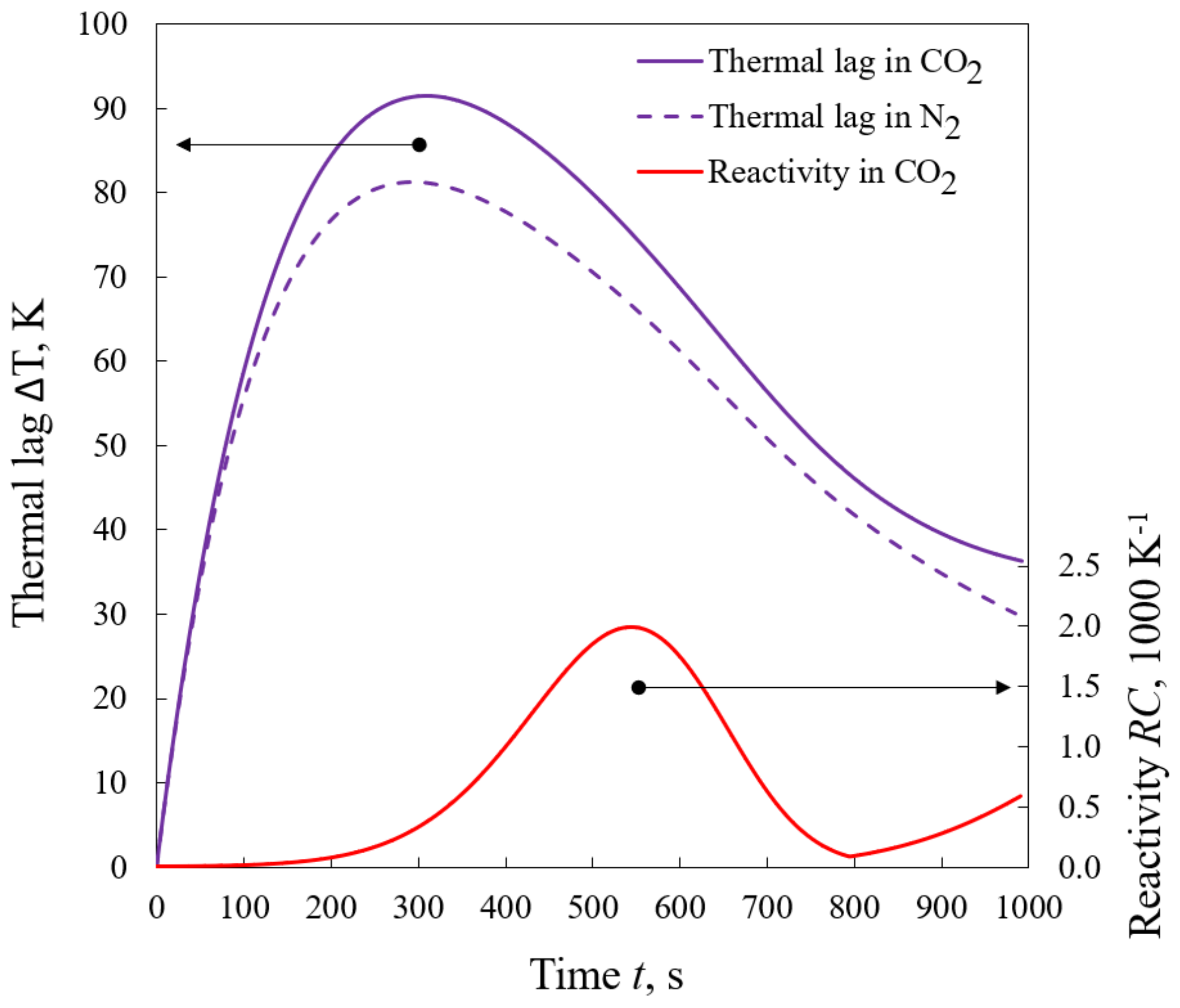
- The nature of the changes in thermal lag was complex. Unlike in the case of a nitrogen atmosphere, the endothermic devolatilization process does not significantly affect it, while the impact of the char–CO2 reaction was visible.
- -
- As it was expected, the average thermal lag in carbon dioxide was greater than that in nitrogen (64.0 K vs. 57.6 K), which is related to both the properties of CO2 itself and the occurrence of the reverse Boudouard reaction.
- -
- At low temperatures, CO2 is an inert gas and does not significantly affect the mechanism of coal pyrolysis.
- -
- The slightly higher reactivity of the fuel during devolatilization in CO2 than in N2 may be attributed to the properties of gases but not to the change in the process mechanism.
- -
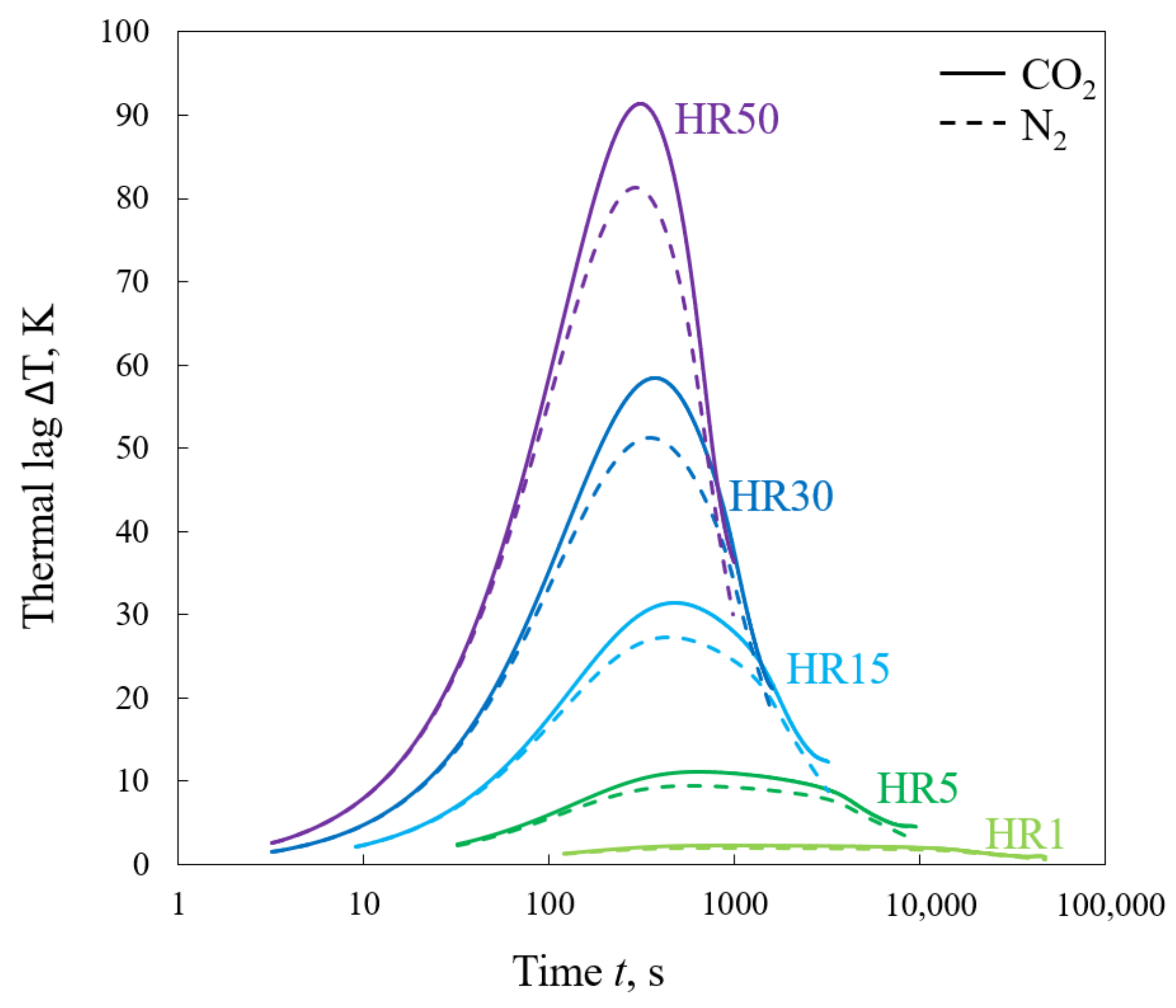
- Fuels having a higher volatiles content were characterized by a slightly higher thermal lag at the stage of devolatilization, while fuels rich in fixed carbon were characterized by this at the stage of the char–CO2 reaction.
- -
- Thermal lag influences the shape of the conversion curve, e.g., curves determined using the model show higher reactivity than those obtained experimentally.
- -
- Discrepancies between TRmax determined at different heating rates are, to some extent, affected by the finite heat transfer rate; however, the TRmax parameter cannot be treated as independent of the heating rate.
- -
- The onset temperature of the reverse Boudouard reaction depends on the type of fuel; however, no simple relationship with the basic physicochemical parameters characterizing fuels was found.
- -
- Disregarding thermal lag may significantly hinder the interpretation of the analyzed phenomena, e.g., after considering the limitations of heat transfer, the values of the activation energy describing devolatilization became more similar for different heating rates, which suggests that the mechanism of the observed reaction seems not to change as much as previously supposed.
- -
- Thermal lag has an impact on the kinetic parameter Aα0.5 describing devolatilization, up to 19.8%. In the case of the char–CO2 reaction, this influence is expected to be even greater.
- -
- There is an influence of the properties of the coal structure on the progress of the reverse Boudouard reaction; however, it does not decrease proportionally to the coal rank.
- -
- A relationship between the content of CaO and reactivity [77] was observed for hard coals but not for lignites.
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Nomenclature
| Aα0.1 | Reaction rate coefficient at the conversion of 10%, s−1 |
| Aα0.5 | Reaction rate coefficient at the conversion of 50%, s−1 |
| Ac | The outer surface area of the crucible, m2 |
| Bi | Biot number |
| Cpash | Specific heat of ash, J·kg−1·K−1 |
| Cpc | Specific heat of crucible material, J·kg−1·K−1 |
| Cpch | Specific heat of char, J·kg−1·K−1 |
| Cpg | Specific heat of gas atmosphere surrounding the crucible, J·kg−1·K−1 |
| Cps | Specific heat of specimen, J·kg−1·K−1 |
| dc | Crucible diameter, m |
| df | Furnace diameter, m |
| ds | Sample grain diameter, m |
| Ea | The activation energy of devolatilization, J⋅mol−1 |
| Eb | The activation energy of char–CO2 reaction, J⋅mol−1 |
| h | Overall heat transfer coefficient, W∙m−2∙K−1 |
| hc | Convection heat transfer coefficient, W∙m−2∙K−1 |
| hr | Radiation heat transfer coefficient, W∙m−2∙K−1 |
| k0,a | The pre-exponential factor for devolatilization, s−1 |
| k0,b | The pre-exponential factor for char–CO2 reaction, s−1 |
| lb | Height of a layer of sample in a crucible, m |
| lc | Crucible height, m |
| m0 | The initial mass of the specimen, kg |
| mc | Mass of the crucible, kg |
| Nu | Nusselt number |
| Pr | Prandtl number |
| Py′ | External pyrolysis number |
| R | Universal gas constant, J⋅K−1⋅mol−1 |
| RC | Sample reactivity, K−1 |
| Re | Reynolds number |
| t | Time, s |
| Tα | The temperature corresponding to the conversion of an exact amount of fuel, K |
| Tc | Crucible temperature measured by the temperature sensor, K |
| Tm | Sample temperature computed using model, K |
| Tr | The reference temperature, K |
| TRCmax | Maximum reactivity temperature, K |
| Ts | True sample temperature, K |
| xash | Share of ash |
| xch | Share of char |
| xvol | Share of volatiles |
| Greek symbols | |
| α | Degree of sample conversion |
| αa | Degree of sample conversion during devolatilization |
| αb | Degree of sample conversion during the char–CO2 reaction |
| β | Heating rate, K∙s−1 |
| ∆Hdev | The heat of pyrolysis, J∙kg−1 (assumes positive values when the reaction is endothermic) |
| ∆Hgas | The heat of the char–CO2 reaction, J∙kg−1 (assumes positive values when the reaction is endothermic) |
| ∆T | Thermal lag, K |
| ∆TRmax | The difference between TRmax determined at HR1 and HR50 |
| μ | Emissivity |
| λg | Thermal conductivity of gas atmosphere surrounding the crucible, W∙m−1∙K−1 |
| λs | Thermal conductivity of the sample |
| νg | Kinematic viscosity of gas atmosphere surrounding the crucible, m2∙s−1 |
| ρg | The density of gas atmosphere surrounding the crucible, kg·m−3 |
| ρs | Density of sample |
| σ | Stefan–Boltzmann constant, equal to 5.670367∙10−8 W∙m−2∙K−4 |
References
- International Energy Agency. Global Energy Review 2020: The Impacts of the Covid-19 Crisis on Global Energy Demand and CO2 Emissions. 2020. Available online: https://iea.blob.core.windows.net/assets/7e802f6a-0b30-4714-abb1-46f21a7a9530/Global_Energy_Review_2020.pdf (accessed on 8 August 2021).
- Jakob, M.; Steckel, J.C.; Jotzo, F.; Sovacool, B.K.; Cornelsen, L.; Chandra, R.; Edenhofer, O.; Holden, C.; Löschel, A.; Nace, T.; et al. The future of coal in a carbon-constrained climate. Nat. Clim. Chang. 2020, 10, 704–707. [Google Scholar] [CrossRef]
- Massachusetts Institute of Technology. The Future of Coal: Options in a Carbon-Constrained World. 2007. Available online: https://energy.mit.edu/wp-content/uploads/2007/03/MITEI-The-Future-of-Coal.pdf (accessed on 8 August 2021).
- Greig, C.; Uden, S. The value of CCUS in transitions to net-zero emissions. Electr. J. 2021, 34, 107004. [Google Scholar] [CrossRef]
- Miller, B.G. Clean Coal Engineering Technology; Elsevier: Amsterdam, The Netherlands, 2011. [Google Scholar]
- Mauerhofer, A.; Fuchs, J.; Müller, S.; Benedikt, F.; Schmid, J.; Hofbauer, H. CO2 gasification in a dual fluidized bed reactor system: Impact on the product gas composition. Fuel 2019, 253, 1605–1616. [Google Scholar] [CrossRef]
- Chan, Y.H.; Rahman, S.N.F.S.A.; Lahuri, H.M.; Khalid, A. Recent progress on CO-rich syngas production via CO2 gasification of various wastes: A critical review on efficiency, challenges and outlook. Environ. Pollut. 2021, 278, 116843. [Google Scholar] [CrossRef] [PubMed]
- Roncancio, R.; Gore, J.P. CO2 char gasification: A systematic review from 2014 to 2020. Energy Convers. Manag. X 2021, 10, 100060. [Google Scholar] [CrossRef]
- Irfan, M.F.; Usman, M.R.; Kusakabe, K. Coal gasification in CO2 atmosphere and its kinetics since 1948: A brief review. Energy 2011, 36, 12–40. [Google Scholar] [CrossRef]
- Czajka, K.M. Proximate analysis of coal by micro-TG method. J. Anal. Appl. Pyrolysis 2018, 133, 82–90. [Google Scholar] [CrossRef]
- Gonzalo-Tirado, C.; Jiménez, S.; Ballester, J. Kinetics of CO2 gasification for coals of different ranks under oxy-combustion conditions. Combust. Flame 2013, 160, 411–416. [Google Scholar] [CrossRef]
- Czajka, K.; Modliński, N.; Kisiela-Czajka, A.M.; Naidoo, R.; Peta, S.; Nyangwa, B. Volatile matter release from coal at different heating rates –experimental study and kinetic modelling. J. Anal. Appl. Pyrolysis 2019, 139, 282–290. [Google Scholar] [CrossRef]
- Illanes, A. Immobilized Biocatalysts. Compr. Biotech. 2011, 2, 25–39. [Google Scholar]
- Vyazovkin, S.; Chrissafis, K.; Di Lorenzo, M.L.; Koga, N.; Pijolat, M.; Roduit, B.; Sbirrazzuoli, N.; Suñol, J.J. ICTAC Kinetics Committee recommendations for collecting experimental thermal analysis data for kinetic computations. Thermochim. Acta 2014, 590, 1–23. [Google Scholar] [CrossRef]
- Czajka, K.M. The impact of the thermal lag on the interpretation of cellulose pyrolysis. Energy 2021, 236, 121497. [Google Scholar] [CrossRef]
- Stenseng, M.; Jensen, A.; Dam-Johansen, K. Investigation of biomass pyrolysis by thermogravimetric analysis and differential scanning calorimetry. J. Anal. Appl. Pyrolysis 2001, 58–59, 765–780. [Google Scholar] [CrossRef]
- Narayan, N.; Antal, M.J. Thermal Lag, Fusion, and the Compensation Effect during Biomass Pyrolysis. Ind. Eng. Chem. Res. 1996, 35, 1711–1721. [Google Scholar] [CrossRef]
- Burnham, A.; Braun, R.L.; Gregg, H.R.; Samoun, A.M. Comparison of methods for measuring kerogen pyrolysis rates and fitting kinetic parameters. Energy Fuels 1987, 1, 452–458. [Google Scholar] [CrossRef]
- Wall, T.; Liu, Y.; Spero, C.; Elliott, L.; Khare, S.; Rathnam, R.; Zeenathal, F.; Moghtaderi, B.; Buhre, B.; Sheng, C.; et al. An overview on oxyfuel coal combustion—State of the art research and technology development. Chem. Eng. Res. Des. 2009, 87, 1003–1016. [Google Scholar] [CrossRef]
- Mianowski, A.; Robak, Z.; Tomaszewicz, M.; Stelmach, S. The Boudouard–Bell reaction analysis under high pressure conditions. J. Therm. Anal. Calorim. 2012, 110, 93–102. [Google Scholar] [CrossRef][Green Version]
- Gomez Quevedo, R.A. Fundamental Kinetic Studies of CO2 and Steam Gasification (Doctoral Dissertation); University of Calgary: Calgary, AB, Canada, 2015. [Google Scholar]
- Dupont, C.; Boissonnet, G.; Seiler, J.-M.; Gauthier-Maradei, P.; Schweich, D. Study about the kinetic processes of biomass steam gasification. Fuel 2007, 86, 32–40. [Google Scholar] [CrossRef]
- International Organisation for Standardisation. Solid Mineral Fuels–Hard Coal: Determination of Moisture in the General Analysis Test Sample by Drying in Nitrogen; ISO 11722:2013; ISO: Geneva, Switzerland, 2013. [Google Scholar]
- International Organisation for Standardisation. Hard Coal and Coke: Determination of Volatile Matter; ISO 562:2010; ISO: Geneva, Switzerland, 2010. [Google Scholar]
- International Organisation for Standardisation. Solid Mineral Fuels: Determination of Ash; ISO 171:2010; ISO: Geneva, Switzerland, 2010. [Google Scholar]
- Polish Committee for Standardization. Paliwa Stałe: Oznaczanie Zawartości Siarki Całkowitej i Popiołowej Automatycznymi Analizatorami; PN-G-04571:1998; Polish Committee for Standardization: Warsaw, Poland, 1998. (In Polish) [Google Scholar]
- Polish Committee for Standardization. Paliwa Stałe: Oznaczanie Zawartości Siarki Popiołowej Metodą Spalania w Wysokiej Temperaturze; PN-G-04581:1999/Az1:2002; Polish Committee for Standardization: Warsaw, Poland, 1999. (In Polish) [Google Scholar]
- International Organisation for Standardisation. Solid Biofuels: Determination of Calorific Value; ISO 18125:2017; ISO: Geneva, Switzerland, 2017. [Google Scholar]
- Mularski, J.; Pawlak-Kruczek, H.; Modlinski, N. A review of recent studies of the CFD modelling of coal gasification in entrained flow gasifiers, covering devolatilization, gas-phase reactions, surface reactions, models and kinetics. Fuel 2020, 271, 117620. [Google Scholar] [CrossRef]
- Mularski, J.; Modliński, N. Entrained-Flow Coal Gasification Process Simulation with the Emphasis on Empirical Char Conversion Models Optimization Procedure. Energies 2021, 14, 1729. [Google Scholar] [CrossRef]
- Mularski, J.; Modliński, N. Impact of Chemistry–Turbulence Interaction Modeling Approach on the CFD Simulations of Entrained Flow Coal Gasification. Energies 2020, 13, 6467. [Google Scholar] [CrossRef]
- Hadad, Y.; Jafarpur, K. Laminar forced convection heat transfer from isothermal cylinders with active ends and different aspect ratios in axial air flows. Heat Mass Transf. 2011, 47, 59–68. [Google Scholar] [CrossRef]
- Ginnings, D.C.; Corruccini, R.J. Enthalpy, Specific Heat, and Entropy of Aluminum Oxide from 0° to 900° C. J. Res. Nat. Bur. Stand. 1947, 38, 593–600. [Google Scholar] [CrossRef]
- Leśniak, B.; Słupik, Ł.; Jakubina, G. The determination of the specific heat capacity of coal based on literature data. Chemik 2013, 67, 560–571. [Google Scholar]
- Eisermann, W.; Johnson, P.; Conger, W. Estimating thermodynamic properties of coal, char, tar and ash. Fuel Process. Technol. 1980, 3, 39–53. [Google Scholar] [CrossRef]
- Richardson, M. The specific heats of coals, cokes and their ashes. Fuel 1993, 72, 1047–1053. [Google Scholar] [CrossRef]
- Lemmon, E.W.; Huber, M.L.; McLinden, M.O. NIST Standard Reference Database 23: Reference Fluid Thermodynamic and Transport Properties-REFPROP; National Institute of Standards and Technology: Gaithersburg, MD, USA, 2013. [Google Scholar]
- Bell, I.H.; Wronski, J.; Quoilin, S.; Lemort, V. Pure and Pseudo-pure Fluid Thermophysical Property Evaluation and the Open-Source Thermophysical Property Library CoolProp. Ind. Eng. Chem. Res. 2014, 53, 2498–2508. [Google Scholar] [CrossRef] [PubMed]
- Farag, I.H.; Allam, T. Carbon dioxide standard emissivity by mixed gray-gases model. Chem. Eng. Commun. 1982, 14, 123–131. [Google Scholar] [CrossRef]
- Milosavljevic, I.; Oja, A.V.; Suuberg, E.M. Thermal Effects in Cellulose Pyrolysis: Relationship to Char Formation Processes. Ind. Eng. Chem. Res. 1996, 35, 653–662. [Google Scholar] [CrossRef]
- Uhrig, A. Determination of Pyrolysis Reaction Kinetics of Raw and Torrefied Biomass. Bachelor’s Thesis, University of Twente, Enschede, The Netherlands, 2014. [Google Scholar]
- Patisson, F.; LeBas, E.; Hanrot, F.; Ablitzer, D.; Houzelot, J.-L. Coal pyrolysis in a rotary kiln: Part I. Model of the pyrolysis of a single grain. Met. Mater. Trans. A 2000, 31, 381–390. [Google Scholar] [CrossRef]
- Prins, M.J.; Ptasinski, K.J.; Janssen, F.J. Torrefaction of wood: Part 1. Weight loss kinetics. J. Anal. Appl. Pyrolysis 2006, 77, 28–34. [Google Scholar] [CrossRef]
- Vyazovkin, S.; Burnham, A.K.; Favergeon, L.; Koga, N.; Moukhina, E.; Pérez-Maqueda, L.A.; Sbirrazzuoli, N. ICTAC Kinetics Committee recommendations for analysis of multi-step kinetics. Thermochim. Acta 2020, 689, 178597. [Google Scholar] [CrossRef]
- Weerachanchai, P.; Tangsathitkulchai, C.; Tangsathitkulchai, M. Comparison of pyrolysis kinetic models for thermogravimetric analysis of biomass. J. Sci. Technol. 2010, 17, 387–400. [Google Scholar]
- Chen, H.; Liu, N.; Fan, W. Two-step Consecutive Reaction Model of Biomass Thermal Decomposition by DSC. Acta Physico-Chim. Sin. 2006, 22, 786–790. [Google Scholar] [CrossRef]
- Guo, J.; Lua, A. Kinetic study on pyrolytic process of oil-palm solid waste using two-step consecutive reaction model. Biomass Bioenergy 2001, 20, 223–233. [Google Scholar] [CrossRef]
- Czajka, K.; Kisiela, A.; Moroń, W.; Ferens, W.; Rybak, W. Pyrolysis of solid fuels: Thermochemical behaviour, kinetics and compensation effect. Fuel Process. Technol. 2016, 142, 42–53. [Google Scholar] [CrossRef]
- Doyle, C.D. Estimating isothermal life from thermogravimetric data. J. Appl. Polym. Sci. 1962, 6, 639–642. [Google Scholar] [CrossRef]
- Garnier, B.; Lanzetta, F.; Lemasson, P.H.; Virgone, J. Lecture 5A: Measurements with contact in heat. Metti 5 Spring School, Roscoff, 13–18 June 2011. Available online: https://www.sft.asso.fr/Local/sft/dir/user-3775/documents/actes/Metti5_School/Lectures&Tutorials-Texts/Text-L5A-Garnier-Lanzetta.pdf (accessed on 8 August 2021).
- Falsetti, C.; Kapulla, R.; Paranjape, S.; Paladino, D. Thermal radiation, its effect on thermocouple measurements in the PANDA facility and how to compensate it. Nucl. Eng. Des. 2021, 375, 111077. [Google Scholar] [CrossRef]
- Jalalabadi, T.; Moghtaderi, B.; Allen, J. Thermochemical Conversion of Biomass in the Presence of Molten Alkali-Metal Carbonates under Reducing Environments of N2 and CO2. Energies 2020, 13, 5395. [Google Scholar] [CrossRef]
- Yang, M.; Luo, B.; Shao, J.; Zeng, K.; Zhang, X.; Yang, H.; Chen, H. The influence of CO2 on biomass fast pyrolysis at medium temperatures. J. Renew. Sustain. Energy 2018, 10, 013108. [Google Scholar] [CrossRef]
- Jamil, K.; Hayashi, J.-I.; Li, C.-Z. Pyrolysis of a Victorian brown coal and gasification of nascent char in CO2 atmosphere in a wire-mesh reactor. Fuel 2004, 83, 833–843. [Google Scholar] [CrossRef]
- Duan, L.; Zhao, C.; Zhou, W.; Qu, C.; Chen, X. Investigation on Coal Pyrolysis in CO2 Atmosphere. Energy Fuels 2009, 23, 3826–3830. [Google Scholar] [CrossRef]
- Jindarom, C.; Meeyoo, V.; Rirksomboon, T.; Rangsunvigit, P. Thermochemical decomposition of sewage sludge in CO2 and N2 atmosphere. Chemosphere 2007, 67, 1477–1484. [Google Scholar] [CrossRef]
- Kim, Y.J.; Kim, M.I.; Yun, C.H.; Chang, J.Y.; Park, C.R.; Inagaki, M. Comparative study of carbon dioxide and nitrogen atmospheric effects on the chemical structure changes during pyrolysis of phenol–formaldehyde spheres. J. Colloid Interface Sci. 2004, 274, 555–562. [Google Scholar] [CrossRef] [PubMed]
- Bilkic, B.; Haykiri-Acma, H.; Yaman, S. Combustion reactivity estimation parameters of biomass compared with lignite based on thermogravimetric analysis. Energy Sources Part A 2020, 1–14. [Google Scholar] [CrossRef]
- Pilatau, A.; Czajka, K.; Filho, G.P.; Medeiros, H.S.; Kisiela, A.M. Evaluation Criteria for the Assessment of the Influence of Additives (AlCl3 and ZnCl2) on Pyrolysis of Sunflower Oil Cake. Waste Biomass-Valoriz. 2017, 8, 2595–2607. [Google Scholar] [CrossRef]
- Rybak, W.; Moroń, W.; Czajka, K.M.; Kisiela, A.M.; Ferens, W.; Jodkowski, W.; Andryjowicz, C. Co-combustion of unburned carbon separated from lignite fly ash. Energy Procedia 2017, 120, 197–205. [Google Scholar] [CrossRef]
- Speight, J.G. Heavy Oil Recovery and Upgrading; Gulf Professional Publishing: Laramie, WY, USA, 2019; p. 576. [Google Scholar]
- Wang, T.; Stiegel, G. Integrated Gasification Combined Cycle (IGCC) Technologies; Woodhead Publishing: Amsterdam, The Netherlands, 2017; p. 10. [Google Scholar]
- Tangstad, M.; Baukes, J.P.; Steenkamp, J.D.; Ringdalen, E. Coal-based reducing agents in ferroalloys and silicon production. In New Trends in Coal Conversion; Suarez-Ruiz, I., Rubiera, F., Diez, M.A., Eds.; Woodhead Publishing: Amsterdam, The Netherlands, 2019; pp. 405–438. [Google Scholar]
- Riley, J.; Siriwardane, R.; Tian, H.; Benincosa, W.; Poston, J. Experimental and kinetic analysis for particle scale modeling of a CuO-Fe2O3-Al2O3 oxygen carrier during reduction with H2 in chemical looping combustion applications. Appl. Energy 2018, 228, 1515–1530. [Google Scholar] [CrossRef]
- Kisiela, A.M.; Czajka, K.M.; Moron, W.; Rybak, W.; Andryjowicz, C. Unburned carbon from lignite fly ash as an adsorbent for SO2 removal. Energy 2016, 116, 1454–1463. [Google Scholar] [CrossRef]
- Guldogan, Y.; Evren, V.; Durusoy, T.; Bozdemir, T. Effects of Heating Rate and Particle Size on Pyrolysis Kinetics of Mengen Lignite. Energy Sources 2001, 23, 337–344. [Google Scholar]
- Guo, Z.; Zhang, L.; Wang, P.; Liu, H.; Jia, J.; Fu, X.; Li, S.; Wang, X.; Li, Z.; Shu, X. Study on kinetics of coal pyrolysis at different heating rates to produce hydrogen. Fuel Process. Technol. 2013, 107, 23–26. [Google Scholar] [CrossRef]
- Ozbas, K.E.; Kok, M.V. Effect of Heating Rate on Thermal Properties and Kinetics of Raw and Cleaned Coal Samples. Energy Sources 2003, 25, 33–42. [Google Scholar] [CrossRef]
- Urych, B. Determination of Kinetic Parameters of Coal Pyrolysis to Simulate the Process of Underground Coal Gasification (UCG). J. Sustain. Min. 2014, 13, 3–9. [Google Scholar] [CrossRef]
- Dwivedi, K.K.; Chatterjee, P.K.; Karmakar, M.K.; Pramanick, A.K. Pyrolysis characteristics and kinetics of Indian low rank coal using thermogravimetric analysis. Int. J. Coal Sci. Technol. 2019, 6, 102–112. [Google Scholar] [CrossRef]
- Dingcheng, L.; Qiang, X.; Guangsheng, L.; Junya, C.; Jun, Z. Influence of heating rate on reactivity and surface chemistry of chars derived from pyrolysis of two Chinese low rank coals. Int. J. Min. Sci. Technol. 2018, 28, 613–619. [Google Scholar] [CrossRef]
- Song, Y.H.; She, J.M.; Lan, X.Z.; Zhou, J. Pyrolysis Characteristics and Kinetics of Low Rank Coal. Mater. Sci. Forum 2011, 695, 493–496. [Google Scholar] [CrossRef]
- Xu, Y.; Zhang, Y.; Wang, Y.; Zhang, G.; Chen, L. Thermogravimetric study of the kinetics and characteristics of the pyrolysis of lignite. React. Kinet. Mech. Catal. 2013, 110, 225–235. [Google Scholar] [CrossRef]
- Yakar Elbeyli, I.; Piskin, S.; Sutcu, H. Pyrolysis Kinetics of Turkish Bituminous Coals by Thermal Analysis. Turk. J. Eng. Environ. Sci. 2004, 28, 233–239. [Google Scholar]
- Barrio, M.; Hustad, J.E. CO2 Gasification of Birch Char and the Effect of CO Inhibition on the Calculation of Chemical Kinetics. In Progress in Thermochemical Biomass Conversion; Wiley: Hoboken, NJ, USA, 2008; pp. 47–60. [Google Scholar]
- Risnes, H.; Srensen, L.H.; Hustad, J.E.; Sørensen, L.H. CO2 Reactivity of Chars from Wheat, Spruce and Coal. In Progress in Thermochemical Biomass Conversion; Wiley: Hoboken, NJ, USA, 2008; pp. 61–72. [Google Scholar]
- Liu, G.-S.; Tate, A.; Bryant, G.; Wall, T. Mathematical modeling of coal char reactivity with CO2 at high pressures and temperatures. Fuel 2000, 79, 1145–1154. [Google Scholar] [CrossRef]
- Rybak, W.; Nowak-Woźny, D.; Moroń, W.; Urbanek, B.; Hrycaj, G. Transformacja substancji mineralnej w warunkach spalania tlenowego. In Spalanie Tlenowe Dla Kotłów Pyłowych i Fluidalnych Zintegrowanych z Wychwytem CO2: Kinetyka i Mechanizm Spalania Tlenowego Oraz Wychwytu CO2; Nowak, W., Rybak, W., Czakiert, T., Eds.; Wydawnictwo Politechniki Częstochowskiej: Częstochowa, Poland, 2013; pp. 176–194. (In Polish) [Google Scholar]
- Dahou, T.; Defoort, F.; Khiari, B.; Labaki, M.; Dupont, C.; Jeguirim, M. Role of inorganics on the biomass char gasification reactivity: A review involving reaction mechanisms and kinetics models. Renew. Sustain. Energy Rev. 2021, 135, 110136. [Google Scholar] [CrossRef]
- Bennici, S.; Jeguirim, M.; Limousy, L.; Haddad, K.; Vaulot, C.; Michelin, L.; Josien, L.; Zorpas, A.A. Influence of CO2 Concentration and Inorganic Species on the Gasification of Lignocellulosic Biomass Derived Chars. Waste Biomass-Valoriz. 2019, 10, 3745–3752. [Google Scholar] [CrossRef]
- Vázquez, M.D.P.G.; García, R.; Gil, M.; Pevida, C.; Rubiera, F. Unconventional biomass fuels for steam gasification: Kinetic analysis and effect of ash composition on reactivity. Energy 2018, 155, 426–437. [Google Scholar] [CrossRef]
- Delannay, F.; Tysoe, W.; Heinemann, H.; Somorjai, G. The role of KOH in the steam gasification of graphite: Identification of the reaction steps. Carbon 1984, 22, 401–407. [Google Scholar] [CrossRef]
- Bouraoui, Z.; Dupont, C.; Jeguirim, M.; Limousy, L.; Gadiou, R. CO2 gasification of woody biomass chars: The influence of K and Si on char reactivity. Comptes Rendus Chim. 2016, 19, 457–465. [Google Scholar] [CrossRef]



| Smp. | M | V | FC | A | FR | C | H | N | S | O * | HHV |
|---|---|---|---|---|---|---|---|---|---|---|---|
| wt% ar | - | wt% ar | MJ∙kg−1 | ||||||||
| LB | 4.4 | 44.4 | 35.0 | 16.2 | 0.79 | 55.2 | 4.5 | 0.7 | 1.8 | 17.2 | 18.9 |
| LT | 2.0 | 46.7 | 33.8 | 17.5 | 0.72 | 59.0 | 4.8 | 0.5 | 1.3 | 14.9 | 21.7 |
| HJ | 2.1 | 32.7 | 55.6 | 9.6 | 1.70 | 75.7 | 4.3 | 1.2 | 1.2 | 5.9 | 24.7 |
| HS | 3.7 | 33.0 | 52.9 | 10.4 | 1.60 | 76.0 | 4.1 | 1.3 | 1.6 | 2.9 | 25.8 |
| HZ | 2.4 | 34.9 | 55.0 | 7.7 | 1.58 | 77.1 | 4.6 | 1.2 | 1.1 | 5.9 | 27.8 |
| AI | 1.7 | 4.9 | 90.3 | 3.1 | 18.4 | 89.0 | 3.5 | 1.2 | 0.5 | 1.0 | 33.2 |
| lc, m | dc, m | df, m | m0, kg | mc, kg | μ | σ, Wm−2K−4 | ΔHdev, Jkg−1 | ΔHgas, Jkg−1 | h d, Wm−2K−1 |
|---|---|---|---|---|---|---|---|---|---|
| 10−2 | 5·10−3 | 5·10−2 | 10−6 | 1.8·10−4 | 0.15 a | 5.67·10−8 | 400·103 b | 604·104 c | 17.2–79.0 |
| Sample | HR | Approach | Devolatilization | Char–CO2 Reaction | ||||
|---|---|---|---|---|---|---|---|---|
| kJ mol−1 | s−1 | s−1 | kJ mol−1 | s−1 | s−1 | |||
| LT | 1 | Mod. | 41.42 | 6.23 × 10−1 | 1.57 × 10−4 | 126.4 | 73.6 | 2.56 × 10−5 |
| Exp. | 41.86 | 6.67 × 10−1 | 1.57 × 10−4 | 126.9 | 76.5 | 2.56 × 10−5 | ||
| 5 | Mod. | 43.43 | 2.03 | 7.02 × 10−4 | 117.4 | 85.9 | 1.18 × 10−4 | |
| Exp. | 45.53 | 2.72 | 7.17 × 10−4 | 119.2 | 99.8 | 1.19 × 10−4 | ||
| 15 | Mod. | 39.94 | 1.90 | 1.76 × 10−3 | 112.2 | 92.6 | 3.23 × 10−4 | |
| Exp. | 45.66 | 4.16 | 1.87 × 10−3 | 116.9 | 1.35 × 102 | 3.25 × 10−4 | ||
| 30 | Mod. | 36.88 | 1.50 | 2.98 × 10−3 | 112.3 | 1.12 × 102 | 6.00 × 10−4 | |
| Exp. | 48.03 | 6.74 | 3.41 × 10−3 | 122.4 | 2.35 × 102 | 6.09 × 10−4 | ||
| 50 | Mod. | 36.46 | 1.61 | 4.51 × 10−3 | 117.3 | 2.17 × 102 | 9.77 × 10−4 | |
| Exp. | 53.30 | 13.1 | 5.41 × 10−3 | 130.7 | 5.91 × 102 | 1.00 × 10−3 | ||
| Sample | HR | Approach | Devolatilization | Char–CO2 Reaction | ||||
|---|---|---|---|---|---|---|---|---|
| kJ mol−1 | s−1 | s−1 | kJ mol−1 | s−1 | s−1 | |||
| LB | HR5 | Mod. | 26.18 | 5.93 × 10−2 | 4.37 × 10−4 | 135.6 | 5.32 × 102 | 1.23 × 10−4 |
| Exp. | 27.58 | 7.35 × 10−2 | 4.47 × 10−4 | 137.7 | 6.24 × 102 | 1.24 × 10−4 | ||
| HJ | Mod. | 53.08 | 12.4 | 8.36 × 10−4 | 184.2 | 5.81 × 104 | 1.59 × 10−4 | |
| Exp. | 55.49 | 17.3 | 8.52 × 10−4 | 186.7 | 6.88 × 104 | 1.61 × 10−4 | ||
| HS | Mod. | 58.59 | 23.6 | 8.57 × 10−4 | 124.9 | 96.8 | 1.13 × 10−4 | |
| Exp. | 61.10 | 32.8 | 8.73 × 10−4 | 126.7 | 1.11 × 102 | 1.14 × 10−4 | ||
| HZ | Mod. | 66.23 | 57.5 | 8.90 × 10−4 | 117.8 | 42.9 | 1.08 × 10−4 | |
| Exp. | 68.84 | 80.1 | 9.06 × 10−4 | 119.5 | 48.9 | 1.08 × 10−4 | ||
| AI | Mod. | nd | nd | nd | 61.58 | 1.26 × 10−1 | 6.95 × 10−5 | |
| Exp. | nd | nd | nd | 62.95 | 1.44 × 10−1 | 7.04 × 10−5 | ||
| Sample | K2O | CaO | Al2O3 | SiO2 | P2O5 |
|---|---|---|---|---|---|
| wt% | |||||
| LB | 0.11 | 23.0 | 17.2 | 29.1 | 0.13 |
| LT | 0.48 | 19.1 | 22.0 | 32.4 | 0.17 |
| HJ | 1.92 | 4.81 | 26.9 | 40.6 | 0.71 |
| HS | 1.47 | 5.31 | 23.0 | 44.6 | 0.93 |
| HZ | 0.88 | 6.97 | 27.2 | 36.3 | 0.55 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Czajka, K.M. Gasification of Coal by CO2: The Impact of the Heat Transfer Limitation on the Progress, Reaction Rate and Kinetics of the Process. Energies 2021, 14, 5569. https://doi.org/10.3390/en14175569
Czajka KM. Gasification of Coal by CO2: The Impact of the Heat Transfer Limitation on the Progress, Reaction Rate and Kinetics of the Process. Energies. 2021; 14(17):5569. https://doi.org/10.3390/en14175569
Chicago/Turabian StyleCzajka, Krzysztof M. 2021. "Gasification of Coal by CO2: The Impact of the Heat Transfer Limitation on the Progress, Reaction Rate and Kinetics of the Process" Energies 14, no. 17: 5569. https://doi.org/10.3390/en14175569
APA StyleCzajka, K. M. (2021). Gasification of Coal by CO2: The Impact of the Heat Transfer Limitation on the Progress, Reaction Rate and Kinetics of the Process. Energies, 14(17), 5569. https://doi.org/10.3390/en14175569





