Biodiesel Production over Niobium-Containing Catalysts: A Review
Abstract
1. Introduction
Biodiesel: Main Catalysts
2. Niobium Catalysts
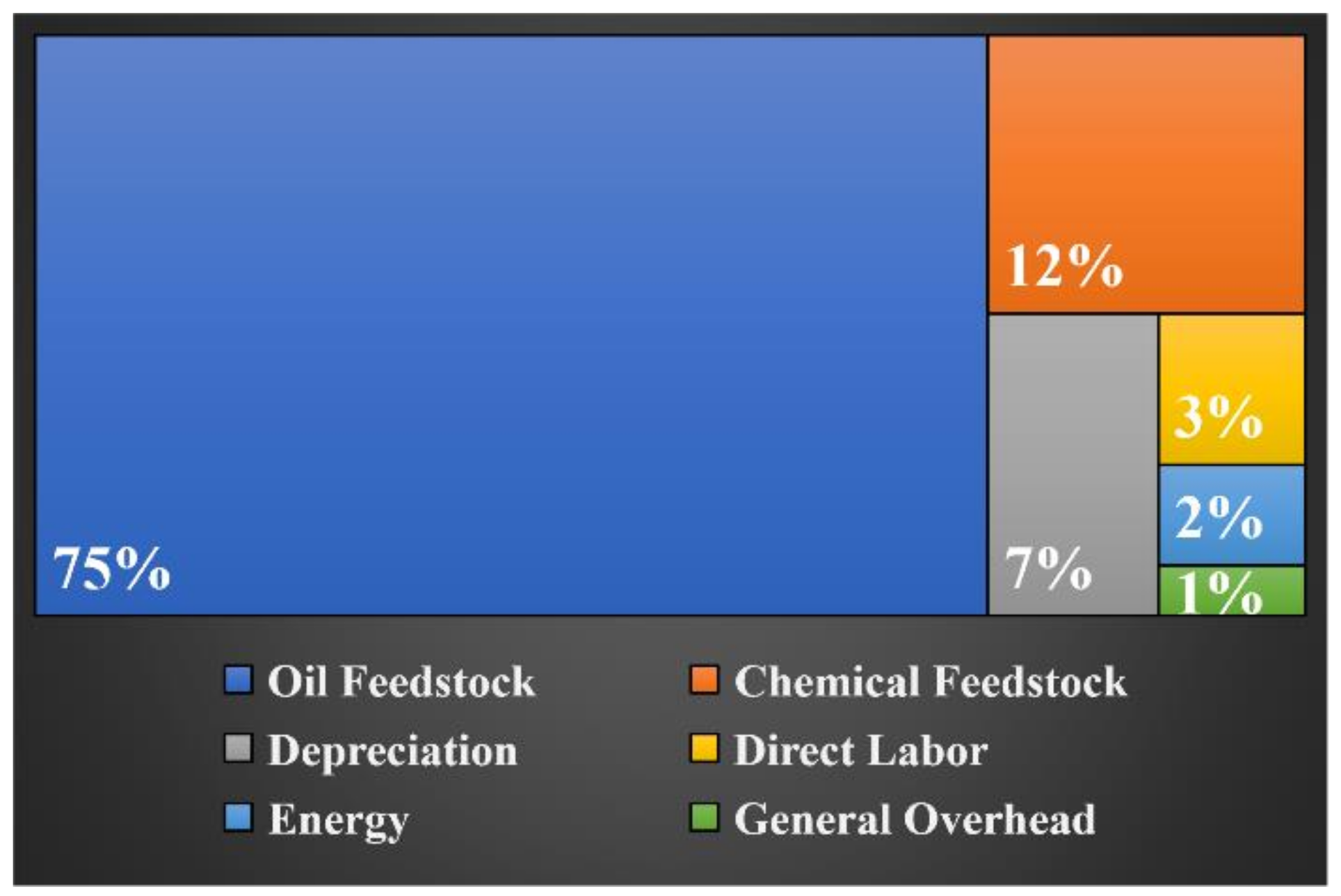
2.1. Economic and Sustainability Evaluation by Using the Niobium-Containing Catalysts
2.2. Characteristic of the Niobium Compounds
3. Niobium-Catalyzed Processes of Biofuel Production
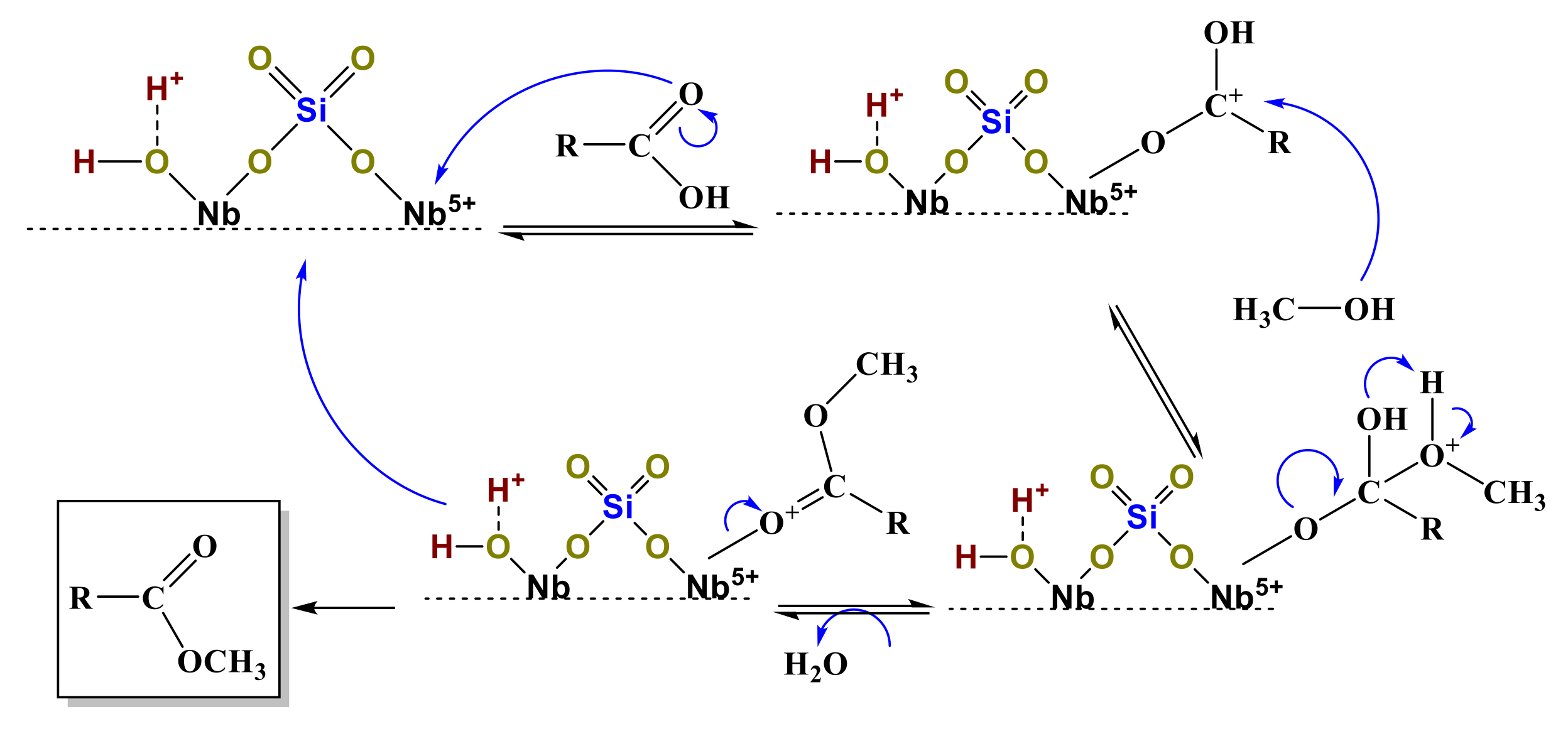
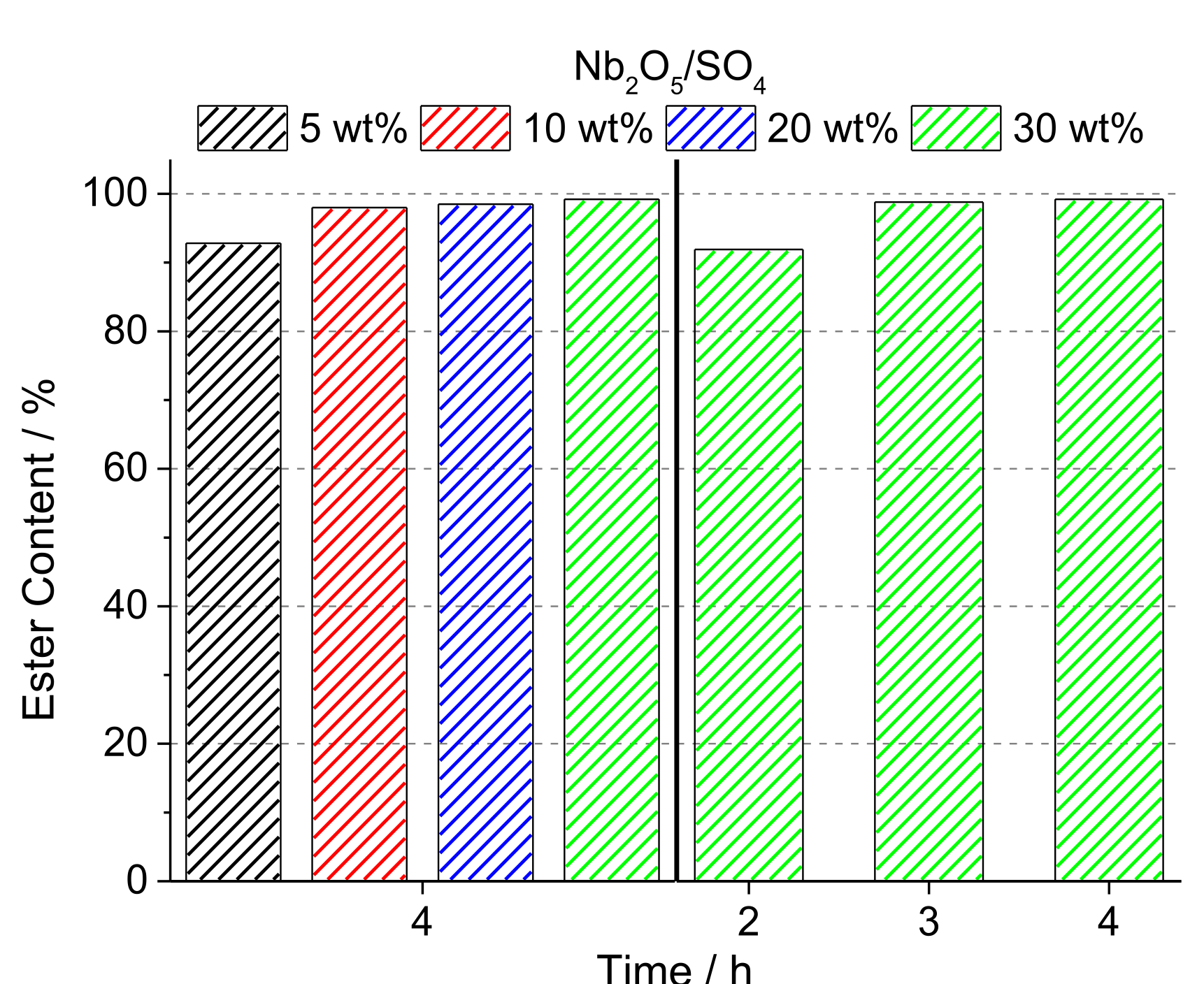
3.1. Sulfated Niobium Oxide
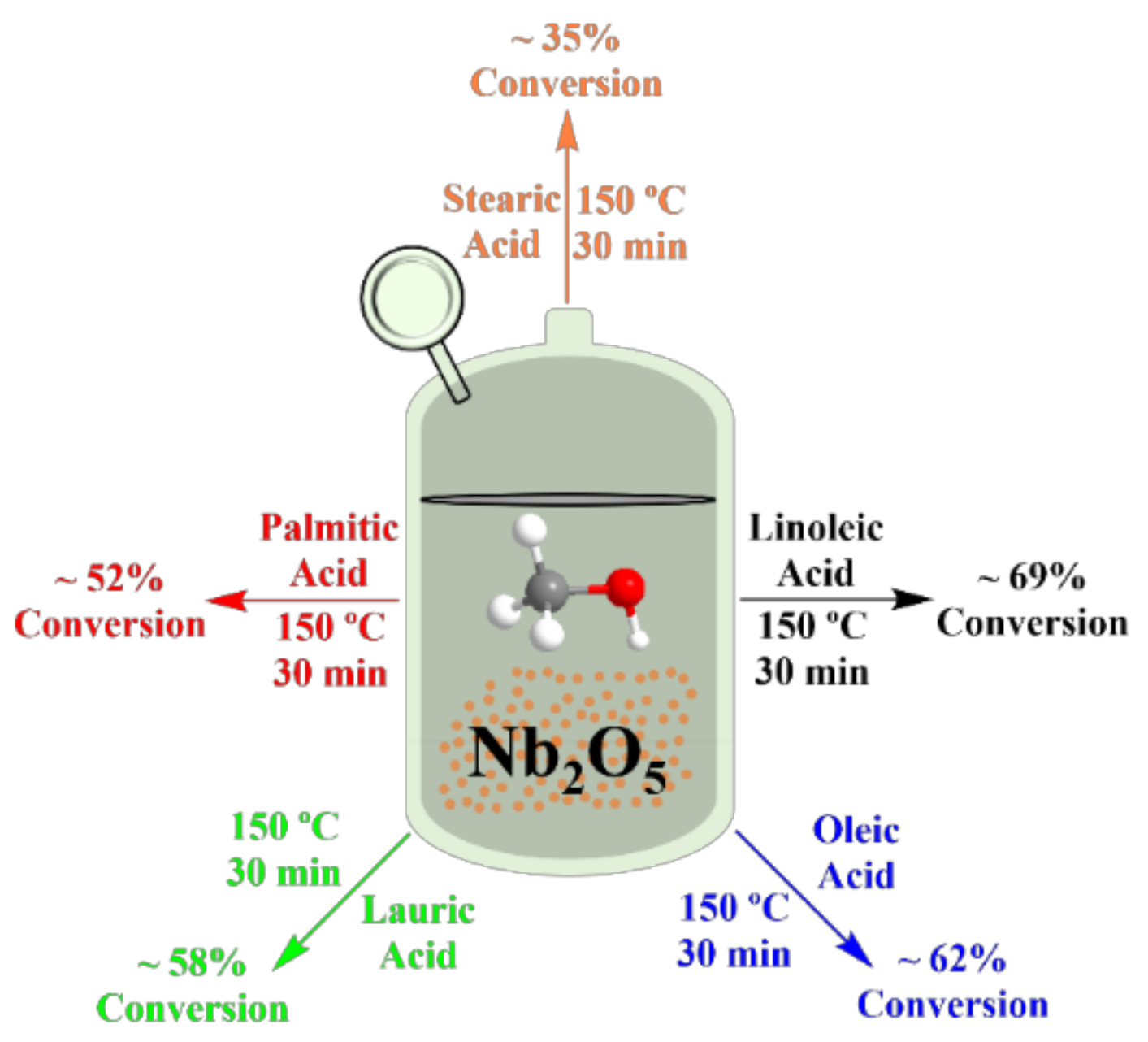
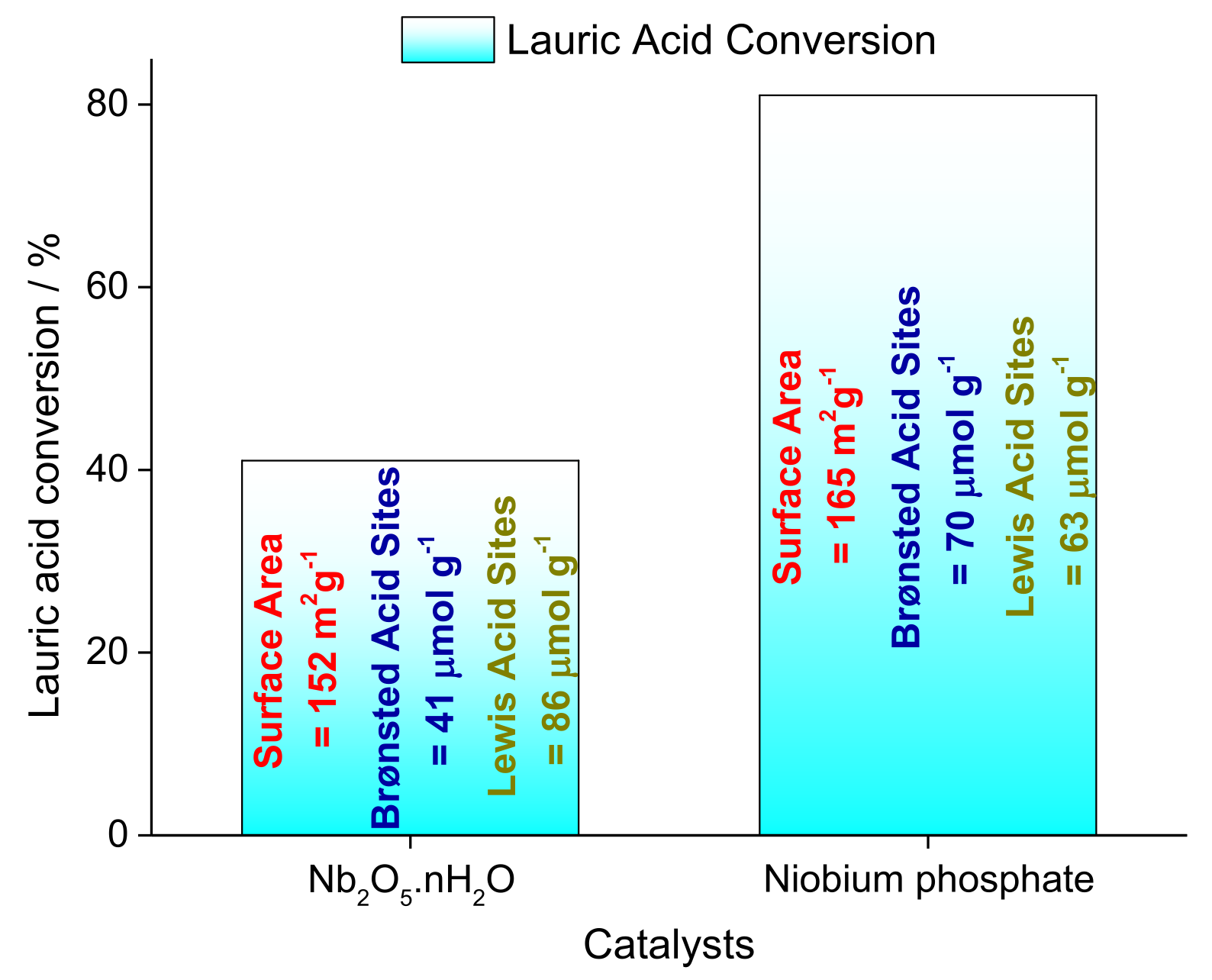
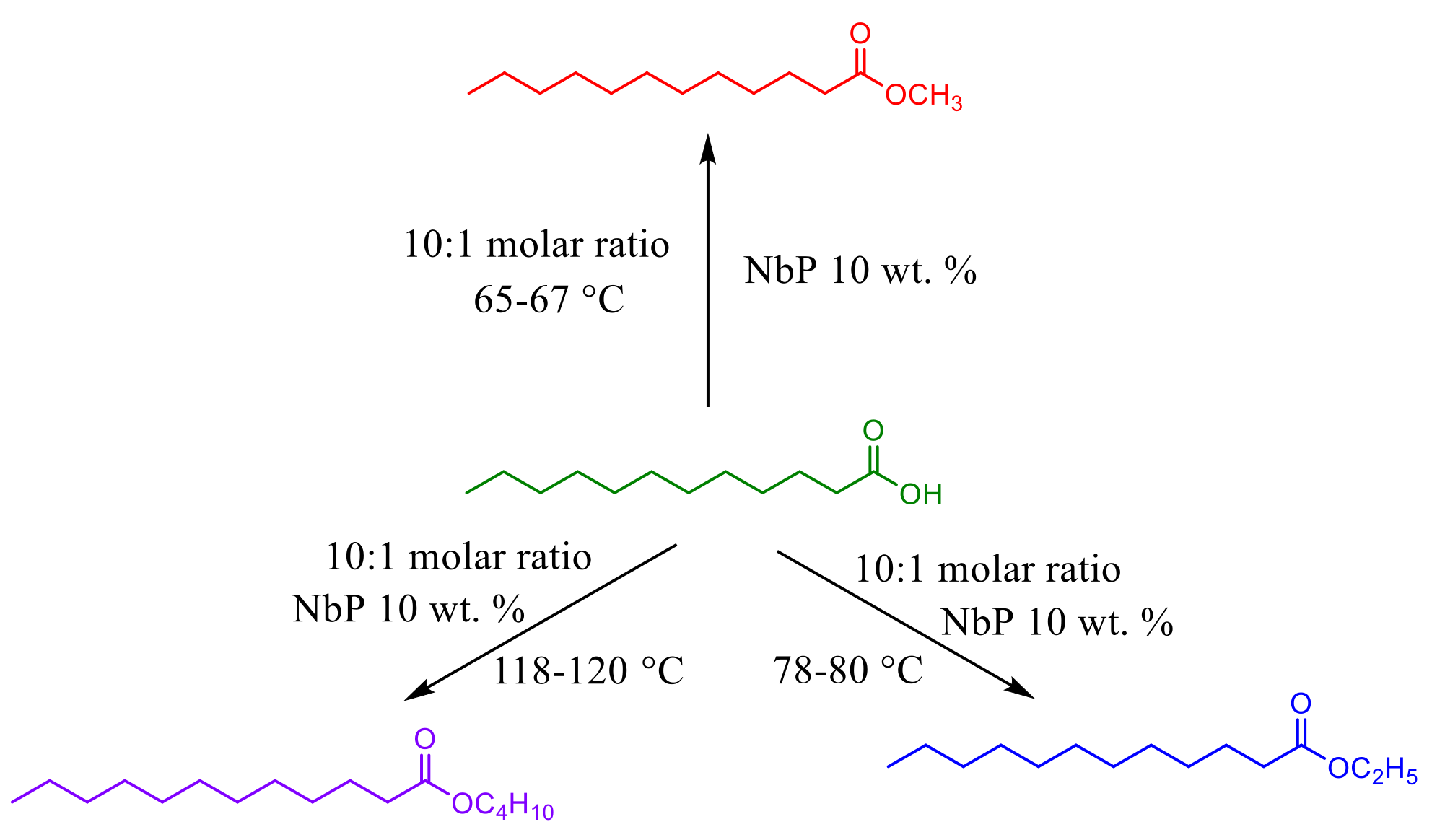
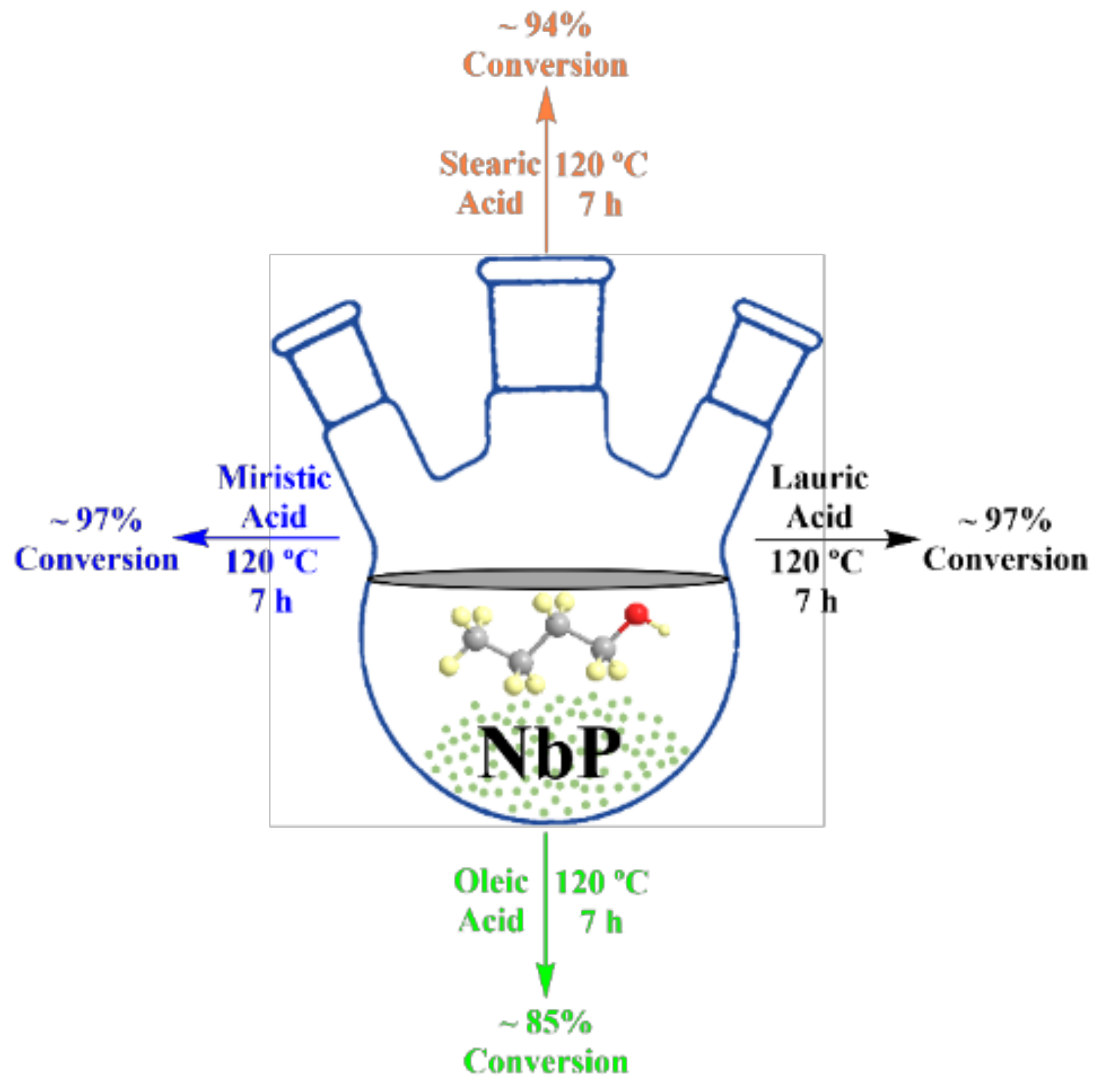
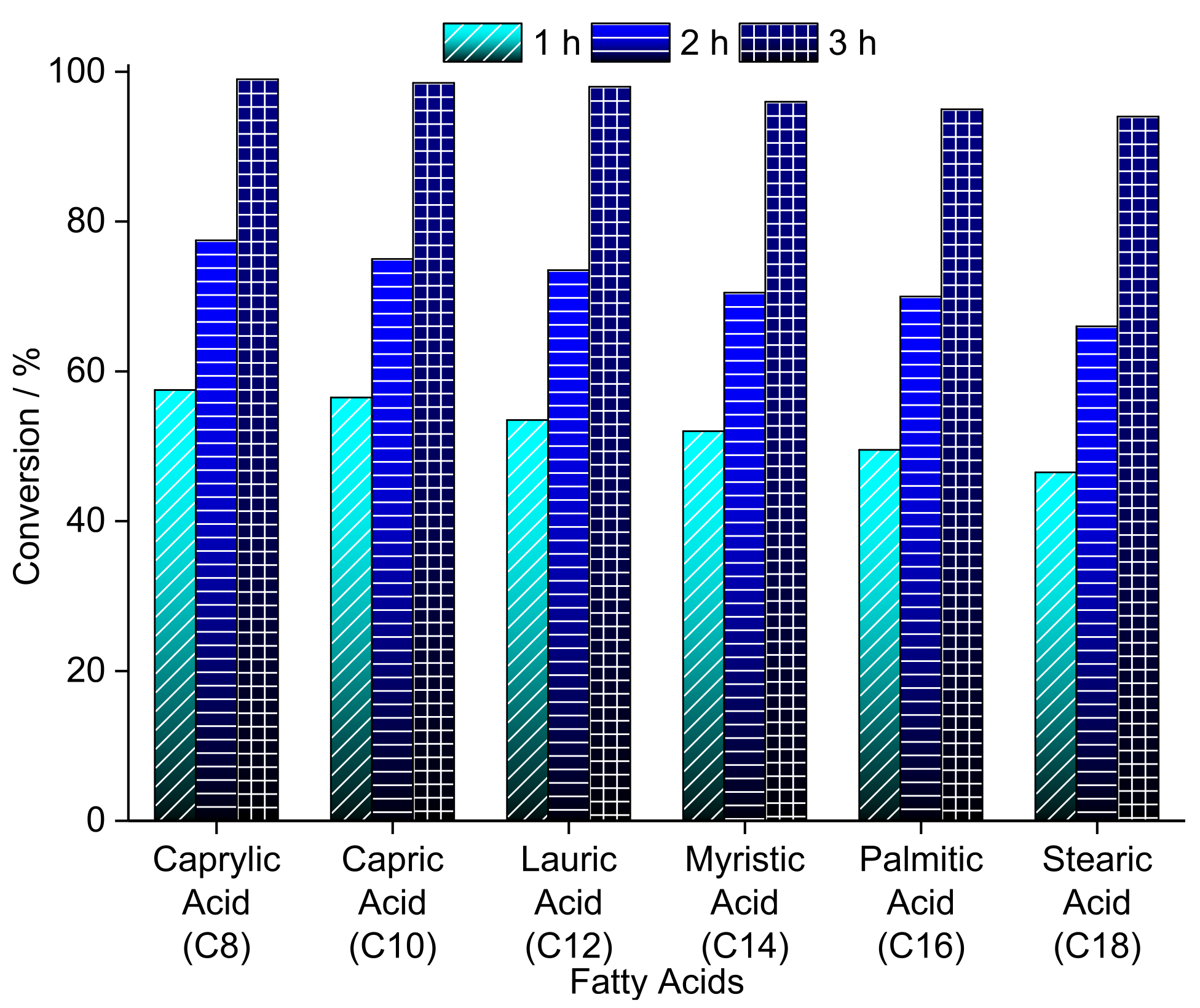
3.2. Niobium Oxide and Niobium Phosphate
3.3. Niobium as Catalytic Support and as a Dopant
3.4. Others
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Mortensen, P.M.; Grunwaldt, J.-D.; Jensen, P.A.; Knudsen, K.G.; Jensen, A.D. A review of catalytic upgrading of bio-oil to engine fuels. Appl. Catal. A Gen. 2011, 407, 1–19. [Google Scholar] [CrossRef]
- Saravanan, A.P.; Pugazhendhi, A.; Mathimani, T. A comprehensive assessment of biofuel policies in the BRICS nations: Implementation, blending target and gaps. Fuel 2020, 272, 117635. [Google Scholar] [CrossRef]
- Kang, S.; Fu, J.; Zhang, G. From lignocellulosic biomass to levulinic acid: A review on acid-catalyzed hydrolysis. Renew. Sustain. Energy Rev. 2018, 94, 340–362. [Google Scholar] [CrossRef]
- Kang, S.; Miao, R.; Guo, J.; Fu, J. Sustainable production of fuels and chemicals from biomass over niobium based catalysts: A review. Catal. Today 2020, 15, 61–76. [Google Scholar] [CrossRef]
- Nunes, L.J.R.; Causer, T.P.; Ciolkosz, D. Biomass for energy: A review on supply chain management models. Renew. Sustain. Energy Rev. 2020, 120, 109658. [Google Scholar] [CrossRef]
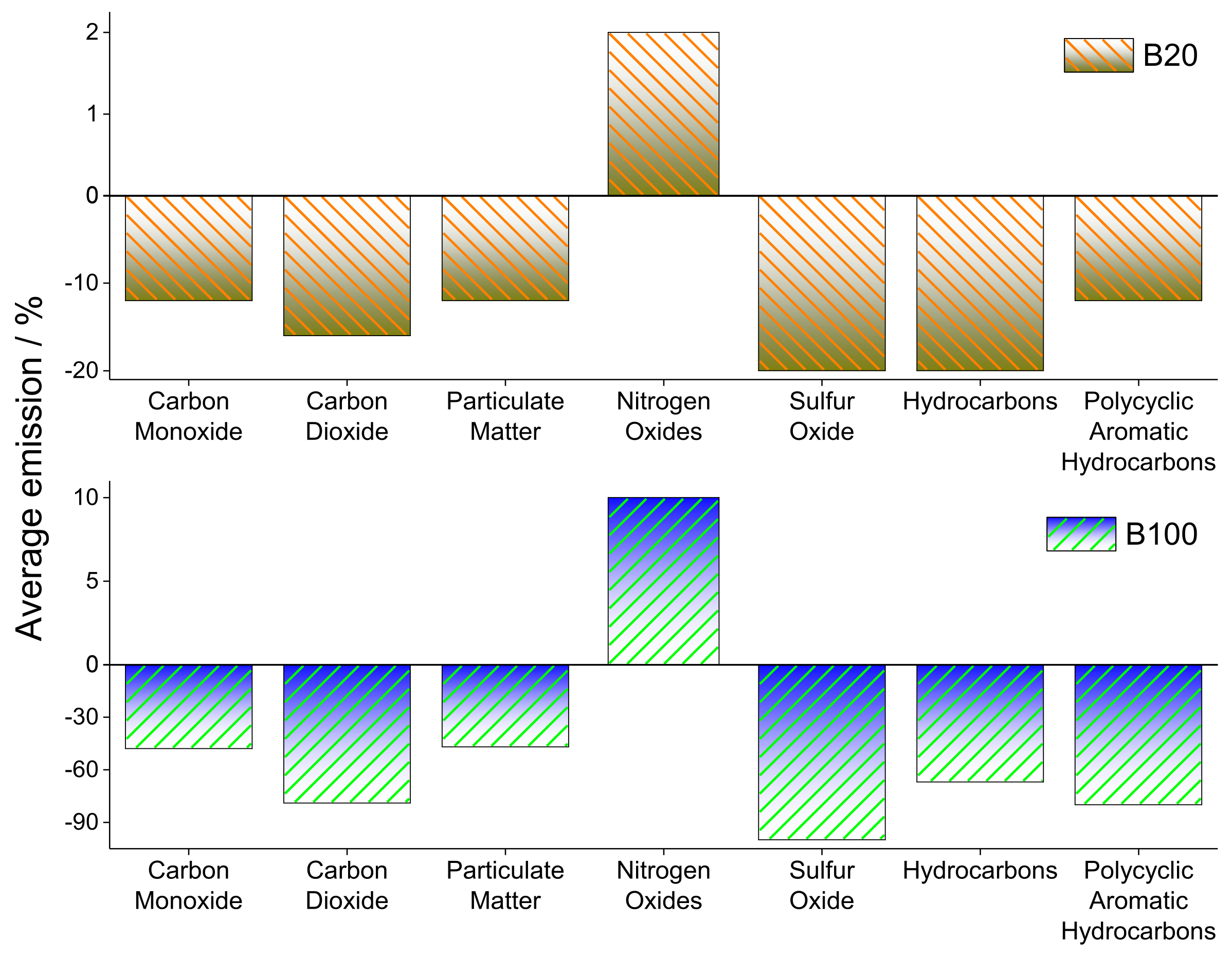
- Omidvarborna, H.; Kumar, A.; Kim, D.-S. Characterization of particulate matter emitted from transit buses fueled with B20 in idle modes. J. Environ. Chem. Eng. 2014, 2, 2335–2342. [Google Scholar] [CrossRef]
- De Lima, A.L.; Mota, C.J.A. Biodiesel: A survey on production methods and catalysts. In Jatropha, Challenges for a New Energy Crop; Springer: Singapore, 2019; Volume 3, pp. 475–491. ISBN 9789811331046. [Google Scholar]
- Da Silva, M.J.; Liberto, N.A. Soluble and Solid Supported Keggin Heteropolyacids as Catalysts in Reactions for Biodiesel Production: Challenges and Recent Advances. Curr. Org. Chem. 2016, 20, 1263–1283. [Google Scholar] [CrossRef]
- Bassan, I.A.L.; Nascimento, D.R.; San Gil, R.A.S.; Da Silva, M.I.P.; Moreira, C.R.; Gonzalez, W.A.; Faro, A.C.; Onfroy, T.; Lachter, E.R. Esterification of fatty acids with alcohols over niobium phosphate. Fuel Process. Technol. 2013, 106, 619–624. [Google Scholar] [CrossRef]
- Scaldaferri, C.A.; Pasa, V.M.D. Production of jet fuel and green diesel range biohydrocarbons by hydroprocessing of soybean oil over niobium phosphate catalyst. Fuel 2019, 245, 458–466. [Google Scholar] [CrossRef]
- Wong, Y.C.; Tan, Y.P.; Taufiq-Yap, Y.H.; Ramli, I. Effect of calcination temperatures of CaO/Nb2O5 Mixed Oxides Catalysts on Biodiesel Production. Sains Malays. 2014, 43, 783–790. [Google Scholar]
- Kiss, A.A.; Dimian, A.C.; Rothenberg, G. Biodiesel by catalytic reactive distillation powered by metal oxides. Energy Fuels 2008, 22, 598–604. [Google Scholar] [CrossRef]
- Maddikeri, G.L.; Pandit, A.B.; Gogate, P.R. Intensification approaches for biodiesel synthesis from waste cooking oil: A review. Ind. Eng. Chem. Res. 2012, 51, 14610–14628. [Google Scholar] [CrossRef]
- Singh, D.; Sharma, D.; Soni, S.L.; Sharma, S.; Kumar Sharma, P.; Jhalani, A. A review on feedstocks, production processes, and yield for different generations of biodiesel. Fuel 2020, 262, 116553. [Google Scholar] [CrossRef]
- Kumar, A.; Kumar, K.; Kaushik, N.; Sharma, S.; Mishra, S. Renewable energy in India: Current status and future potentials. Renew. Sustain. Energy Rev. 2010, 14, 2434–2442. [Google Scholar] [CrossRef]
- Xie, W.; Wang, H. Grafting copolymerization of dual acidic ionic liquid on core-shell structured magnetic silica: A magnetically recyclable Brönsted acid catalyst for biodiesel production by one-pot transformation of low-quality oils. Fuel 2021, 283, 118893. [Google Scholar] [CrossRef]
- Xie, W.; Wang, H. Immobilized polymeric sulfonated ionic liquid on core-shell structured Fe3O4/SiO2 composites: A magnetically recyclable catalyst for simultaneous transesterification and esterifications of low-cost oils to biodiesel. Renew. Energy 2020, 145, 1709–1719. [Google Scholar] [CrossRef]
- Xie, W.; Wan, F. Immobilization of polyoxometalate-based sulfonated ionic liquids on UiO-66-2COOH metal-organic frameworks for biodiesel production via one-pot transesterification-esterification of acidic vegetable oils. Chem. Eng. J. 2019, 365, 40–50. [Google Scholar] [CrossRef]
- Xie, W.; Wan, F. Basic ionic liquid functionalized magnetically responsive Fe3O4@HKUST-1 composites used for biodiesel production. Fuel 2018, 220, 248–256. [Google Scholar] [CrossRef]
- Kryszak, D.; Trejda, M.; Benedyczak, N.; Ziolek, M. Ca/MCF catalysts—The impact of niobium and material structure on basicity. Catal. Today 2019, 325, 11–17. [Google Scholar] [CrossRef]
- Melo Júnior, C.A.R.; Albuquerque, C.E.R.; Carneiro, J.S.A.; Dariva, C.; Fortuny, M.; Santos, A.F.; Egues, S.M.S.; Ramos, A.L.D. Solid-acid-catalyzed esterification of oleic acid assisted by microwave heating. Ind. Eng. Chem. Res. 2010, 49, 12135–12139. [Google Scholar] [CrossRef]
- Vieira, J.L.; Paul, G.; Iga, G.D.; Cabral, N.M.; Bueno, J.M.C.; Bisio, C.; Gallo, J.M.R. Niobium phosphates as bifunctional catalysts for the conversion of biomass-derived monosaccharides. Appl. Catal. A 2021, 617, 118099–118110. [Google Scholar] [CrossRef]
- De Jong, K.P. Synthesis of Solid Catalysts; Wiley-VCH: Weinheim, Germany, 2009; ISBN 9783527320400. [Google Scholar]
- Batalha, D.C.; Luz, S.C.; Taylor, J.G.; Fajardo, H.V.; Noremberg, B.S.; Cherubin, I.J.S.; Silva, R.M.; Gonçalves, M.R.F.; Bergmann, C.P.; Valentini, A.; et al. Application of Al2O3/AlNbO4 in the oxidation of aniline to azoxybenzene. Chem. Pap. 2020, 74, 543–553. [Google Scholar] [CrossRef]
- Batalha, D.C.; Marins, N.H.; e Silva, R.M.; Carreño, N.L.V.; Fajardo, H.V.; da Silva, M.J. Oxidation of terpenic alcohols with hydrogen peroxide promoted by Nb2O5 obtained by microwave-assisted hydrothermal method. Mol. Catal. 2020, 489, 110941. [Google Scholar] [CrossRef]
- Pessoa Junior, W.A.G.; Takeno, M.L.; Nobre, F.X.; Barros, S.D.S.; Sá, I.S.C.; Silva, E.P.; Manzato, L.; Iglauer, S.; de Freitas, F.A. Application of water treatment sludge as a low-cost and eco-friendly catalyst in the biodiesel production via fatty acids esterification: Process optimization. Energy 2020, 213, 118824. [Google Scholar] [CrossRef]
- Vasić, K.; Hojnik Podrepšek, G.; Knez, Ž.; Leitgeb, M. Biodiesel Production Using Solid Acid Catalysts Based on Metal Oxides. Catalysts 2020, 10, 237. [Google Scholar] [CrossRef]
- García-Sancho, C.; Moreno-Tost, R.; Mérida-Robles, J.M.; Santamaría-González, J.; Jiménez-López, A.; Maireles-Torres, P. Niobium-containing MCM-41 silica catalysts for biodiesel production. Appl. Catal. B Environ. 2011, 108–109, 161–167. [Google Scholar] [CrossRef]
- Rezende, M.; de Lima, A.L.; Silva, B.; Mota, C.; Torres, E.; da Rocha, G.; Cardozo, I.; Costa, K.; Guarieiro, L.; Pereira, P.; et al. Biodiesel: An Overview II. J. Braz. Chem. Soc. 2021, 32, 1301–1344. [Google Scholar] [CrossRef]
- Gardy, J.; Rehan, M.; Hassanpour, A.; Lai, X.; Nizami, A.-S. Advances in nano-catalysts based biodiesel production from non-food feedstocks. J. Environ. Manag. 2019, 249, 109316. [Google Scholar] [CrossRef]
- Gonçalves, J.D.A.; Ramos, A.L.D.; Rocha, L.L.L.; Domingos, A.K.; Monteiro, R.S.; Peres, J.S.; Furtado, N.C.; Taft, C.A.; Aranda, D.A.G. Niobium oxide solid catalyst: Esterification of fatty acids and modeling for biodiesel production. J. Phys. Org. Chem. 2011, 24, 54–64. [Google Scholar] [CrossRef]
- Nico, C.; Monteiro, T.; Graça, M.P.F. Niobium oxides and niobates physical properties: Review and prospects. Prog. Mater. Sci. 2016, 80, 1–37. [Google Scholar] [CrossRef]
- Ziolek, M.; Sobczak, I. The role of niobium component in heterogeneous catalysts. Catal. Today 2017, 285, 211–225. [Google Scholar] [CrossRef]
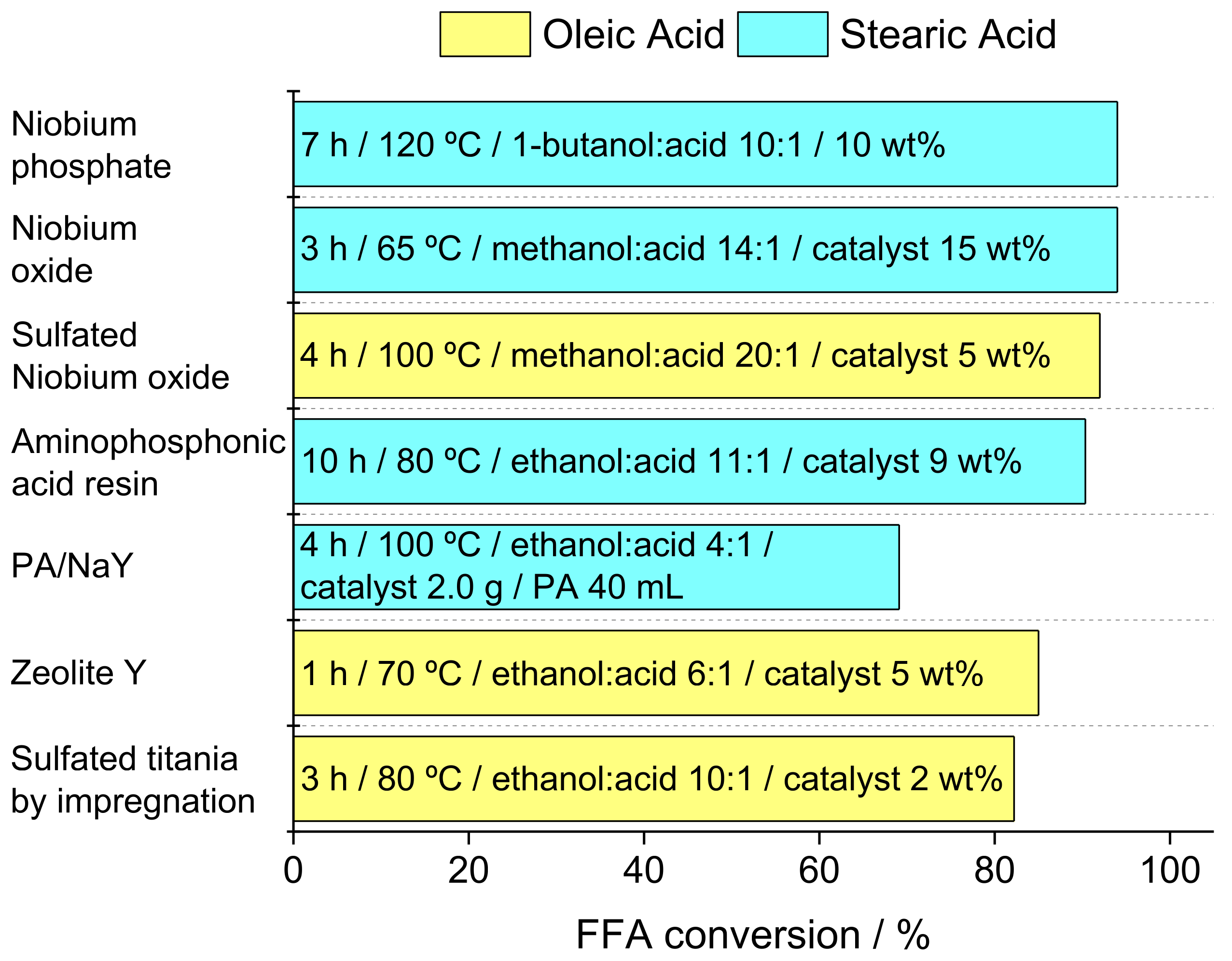
- Srilatha, K.; Lingaiah, N.; Sai Prasad, P.S.; Prabhavathi Devi, B.L.A.; Prasad, R.B.N.; Venkateswar, S. Influence of carbon chain length and unsaturation on the esterification activity of fatty acids on Nb2O5 catalyst. Ind. Eng. Chem. Res. 2009, 48, 10816–10819. [Google Scholar] [CrossRef]
- Sturt, N.R.M.; Vieira, S.S.; Moura, F.C.C. Catalytic activity of sulfated niobium oxide for oleic acid esterification. J. Environ. Chem. Eng. 2019, 7. [Google Scholar] [CrossRef]
- Liu, W.; Yin, P.; Liu, X.; Chen, W.; Chen, H.; Liu, C.; Qu, R.; Xu, Q. Microwave assisted esterification of free fatty acid over a heterogeneous catalyst for biodiesel production. Energy Convers. Manag. 2013, 76, 1009–1014. [Google Scholar] [CrossRef]
- Liu, W.; Yin, P.; Liu, X.; Zhang, S.; Qu, R. Biodiesel production from the esterification of fatty acid over organophosphonic acid. J. Ind. Eng. Chem. 2015, 21, 893–899. [Google Scholar] [CrossRef]
- Doyle, A.M.; Albayati, T.M.; Abbas, A.S.; Alismaeel, Z.T. Biodiesel production by esterification of oleic acid over zeolite Y prepared from kaolin. Renew. Energy 2016, 97, 19–23. [Google Scholar] [CrossRef]
- Ropero-Vega, J.L.; Aldana-Pérez, A.; Gómez, R.; Niño-Gómez, M.E. Sulfated titania [TiO2/SO42−]: A very active solid acid catalyst for the esterification of free fatty acids with ethanol. Appl. Catal. A Gen. 2010, 379, 24–29. [Google Scholar] [CrossRef]
- Agarwal, B.; Kailasam, K.; Sangwan, R.S.; Elumalai, S. Traversing the history of solid catalysts for heterogeneous synthesis of 5-hydroxymethylfurfural from carbohydrate sugars: A. review. Renew. Sustain. Energy Rev. 2018, 82, 2408–2425. [Google Scholar] [CrossRef]
- Mejía, C.H.; Verbart, D.M.A.; de Jong, K.P. Niobium-based solid acids in combination with a methanol synthesis catalyst for the direct production of dimethyl ether from synthesis gas. Catal. Today 2021, 369, 77–87. [Google Scholar] [CrossRef]
- Lopes, O.F.; de Mendonça, V.R.; Silva, F.B.F.; Paris, E.C.; Ribeiro, C. Niobium oxides: An overview of the synthesis of Nb2O5 and its application in heterogeneous photocatalysis. Quím. Nova 2014, 38, 106–117. [Google Scholar] [CrossRef]
- DNPM (Departamento Nacional de Produção Mineral). Sumário Mineral. Available online: https://www.gov.br/anm/pt-br/centrais-de-conteudo/publicacoes/serie-estatisticas-e-economia-mineral/sumario-mineral/sumariomineral_2017 (accessed on 20 April 2021).
- Enhag, P. Encyclopedia of the Elements; Wiley-VCH: Weinheim, Germany, 2004; ISBN 9783527306664. [Google Scholar]
- Schlewitz, J.H. Niobium and Niobium Compounds. In Kirk-Othmer Encyclopedia of Chemical Technology; John Wiley & Sons Inc.: Hoboken, NJ, USA, 2000. [Google Scholar]
- Zhao, Y.; Zhou, X.; Ye, L.; Chi Edman Tsang, S. Nanostructured Nb2O5 catalysts. Nano Rev. 2012, 3, 17631. [Google Scholar] [CrossRef]
- Moreno, E.L.; Rajagopal, K. Desafios da acidez na catálise em estado sólido. Quim. Nova 2009, 32, 538–542. [Google Scholar] [CrossRef]
- Rodrigues, R.; Mandelli, D.; Gonçalves, N.S.; Pescarmona, P.P.; Carvalho, W.A. Acetalization of acetone with glycerol catalyzed by niobium-aluminum mixed oxides synthesized by a sol–gel process. J. Mol. Catal. A Chem. 2016, 422, 122–130. [Google Scholar] [CrossRef]
- Abdel-Rehim, M.A.; dos Santos, A.C.B.; Camorim, V.L.L.L.; Faro, A.D.C., Jr. Acid–base reactions on alumina-supported niobia. Appl. Catal. A Gen. 2006, 305, 211–218. [Google Scholar] [CrossRef]
- De Carvalho, G.S.G.; de Siqueira, M.M.; do Nascimento, M.P.; de Oliveira, M.A.L.; Amarante, G.W. Nb2O5 supported in mixed oxides catalyzed mineralization process of methylene blue. Heliyon 2020, 6, e04128. [Google Scholar] [CrossRef]
- Braga, V.S.; Dias, J.A.; Dias, S.C.L.; de Macedo, J.L. Catalyst Materials Based on Nb2O5 Supported on SiO2−Al2O3: Preparation and Structural Characterization. Chem. Mater. 2005, 17, 690–695. [Google Scholar] [CrossRef]
- Chagas, P.; Oliveira, H.S.; Mambrini, R.; Le Hyaric, M.; de Almeida, M.V.; Oliveira, L.C.A. A novel hydrofobic niobium oxyhydroxide as catalyst: Selective cyclohexene oxidation to epoxide. Appl. Catal. A Gen. 2013, 454, 88–92. [Google Scholar] [CrossRef]
- Souza, T.E.; Padula, I.D.; Teodoro, M.M.G.; Chagas, P.; Resende, J.M.; Souza, P.P.; Oliveira, L.C.A. Amphiphilic property of niobium oxyhydroxide for waste glycerol conversion to produce solketal. Catal. Today 2015, 254, 83–89. [Google Scholar] [CrossRef]
- Heracleous, E.; Lemonidou, A. Ni–Nb–O mixed oxides as highly active and selective catalysts for ethene production via ethane oxidative dehydrogenation. Part II: Mechanistic aspects and kinetic modeling. J. Catal. 2006, 237, 175–189. [Google Scholar] [CrossRef]
- Catrinck, M.N.; Barbosa, P.S.; Filho, H.R.O.; Monteiro, R.S.; Barbosa, M.H.P.; Ribas, R.M.; Teófilo, R.F. One-step process to produce furfural from sugarcane bagasse over niobium-based solid acid catalysts in a water medium. Fuel Process. Technol. 2020, 207, 106482. [Google Scholar] [CrossRef]
- Skrodczky, K.; Antunes, M.M.; Han, X.; Santangelo, S.; Scholz, G.; Valente, A.A.; Pinna, N.; Russo, P.A. Niobium pentoxide nanomaterials with distorted structures as efficient acid catalysts. Commun. Chem. 2019, 2, 129. [Google Scholar] [CrossRef]
- Da Silva, I.A.; Mota, C.J.A. Conversion of CO2 to Light Olefins Over Iron-Based Catalysts Supported on Niobium Oxide. Front. Energy Res. 2019, 7, 49. [Google Scholar] [CrossRef]
- Gao, D.-M.; Zhao, B.; Liu, H.; Morisato, K.; Kanamori, K.; He, Z.; Zeng, M.; Wu, H.; Chen, J.; Nakanishi, K. Synthesis of a hierarchically porous niobium phosphate monolith by a sol–gel method for fructose dehydration to 5-hydroxymethylfurfural. Catal. Sci. Technol. 2018, 8, 3675–3685. [Google Scholar] [CrossRef]
- Batista, L.M.B.; Oliveira, J.L.F.; Bezerra, F.A.; Araújo, A.M.D.M.; Fernandes, V.J., Jr.; Araujo, A.S.; Alves, A.P.M.; Gondim, A.D. Synthesis, characterization and evaluation of niobium catalysts in the flash pyrolysis of glycerol. Solid State Sci. 2019, 97, 105977. [Google Scholar] [CrossRef]
- Kumari, P.K.; Rao, B.S.; Dhana Lakshmi, D.; Sai Paramesh, N.R.; Sumana, C.; Lingaiah, N. Tungstophosphoric acid supported on mesoporouus niobiumoxophosphate: An efficient solid acid catalyst for etherification of 5-hydroxymethylfurfural to 5-ethoxymethylfurfural. Catal. Today 2019, 325, 53–60. [Google Scholar] [CrossRef]
- Xia, Q.; Xia, Y.; Xi, J.; Liu, X.; Wang, Y. Energy-efficient production of 1-octanol from biomass-derived furfural-acetone in water. Green Chem. 2015, 17, 4411–4417. [Google Scholar] [CrossRef]
- Sobczak, I.; Jagodzinska, K.; Ziolek, M. Glycerol oxidation on gold catalysts supported on group five metal oxides—A comparative study with other metal oxides and carbon based catalysts. Catal. Today 2010, 158, 121–129. [Google Scholar] [CrossRef]
- Borges, M.E.; Díaz, L. Recent developments on heterogeneous catalysts for biodiesel production by oil esterification and transesterification reactions: A review. Renew. Sustain. Energy Rev. 2012, 16, 2839–2849. [Google Scholar] [CrossRef]
- Patel, A.; Narkhede, N. Biodiesel synthesis via esterification and transesterification over a new heterogeneous catalyst comprising lacunary silicotungstate and MCM-41. Catal. Sci. Technol. 2013, 3, 3317. [Google Scholar] [CrossRef]
- Rao, Y.; Trudeau, M.; Antonelli, D. Sulfated and Phosphated Mesoporous Nb Oxide in the Benzylation of Anisole and Toluene by Benzyl Alcohol. J. Am. Chem. Soc. 2006, 128, 13996–13997. [Google Scholar] [CrossRef]
- Da Conceição, L.R.V.; Carneiro, L.M.; Rivaldi, J.D.; de Castro, H.F. Solid acid as catalyst for biodiesel production via simultaneous esterification and transesterification of macaw palm oil. Ind. Crops Prod. 2016, 89, 416–424. [Google Scholar] [CrossRef]
- Carniti, P.; Gervasini, A.; Biella, S.; Auroux, A. Niobic acid and niobium phosphate as highly acidic viable catalysts in aqueous medium: Fructose dehydration reaction. Catal. Today 2006, 118, 373–378. [Google Scholar] [CrossRef]
- Câmara, L.D.T.; Aranda, D.A.G. Reaction kinetic study of biodiesel production from fatty acids esterification with ethanol. Ind. Eng. Chem. Res. 2011, 50, 2544–2547. [Google Scholar] [CrossRef]
- Sun, Q.; Fu, Y.; Yang, H.; Auroux, A.; Shen, J. Dehydration of methanol to dimethyl ether over Nb2O5 and NbOPO4 catalysts: Microcalorimetric and FT-IR studies. J. Mol. Catal. A Chem. 2007, 275, 183–193. [Google Scholar] [CrossRef]
- Natalino, R.; Varejão, E.V.V.; da Silva, M.J.; Cardoso, A.L.; Fernandes, S.A. p-Sulfonic acid calix[n]arenes: The most active and water tolerant organocatalysts in esterification reactions. Catal. Sci. Technol. 2014, 4, 1369–1375. [Google Scholar] [CrossRef]
- Rade, L.L.; Lemos, C.O.T.; Barrozo, M.A.D.S.; Ribas, R.M.; Monteiro, R.D.S.; Hori, C.E. Optimization of esterification reaction over niobium phosphate in a packed bed tubular reactor. Renew. Energy 2019, 131, 348–355. [Google Scholar] [CrossRef]
- Arpini, B.H.; Cubides-Román, D.C.; Javarini, C.L.; De Araújo, M.C.; David, G.F.; Dos Santos, R.B.; Romão, W.; Neto, A.C.; Lacerda, V. Simple niobium catalysts applied in reflux and ultrasound-assisted systems for biofuel synthesis. J. Braz. Chem. Soc. 2019, 30, 1897–1905. [Google Scholar] [CrossRef]
- Reguera, F.M.; Araujo, L.R.R.D.; Picardo, M.C.; Bello, F.D.O.; Scofield, C.F.; Pastura, N.M.R.; Gonzalez, W.D.A. The use of niobium based catalysts for liquid fuel production. Mater. Res. 2004, 7, 343–348. [Google Scholar] [CrossRef]
- Yan, S.; Kim, M.; Salley, S.O.; Ng, K.Y.S. Oil transesterification over calcium oxides modified with lanthanum. Appl. Catal. A Gen. 2009, 360, 163–170. [Google Scholar] [CrossRef]
- Kulkarni, M.G.; Gopinath, R.; Meher, L.C.; Dalai, A.K. Solid acid catalyzed biodiesel production by simultaneous esterification and transesterification. Green Chem. 2006, 8, 1056. [Google Scholar] [CrossRef]
- Ahn, S.; Thornburg, N.E.; Li, Z.; Wang, T.C.; Gallington, L.C.; Chapman, K.W.; Notestein, J.M.; Hupp, J.T.; Farha, O.K. Stable Metal–Organic Framework-Supported Niobium Catalysts. Inorg. Chem. 2016, 55, 11954–11961. [Google Scholar] [CrossRef]
- Da Silva, M.J.; Lopes, N.P.G.; Ferreira, S.O.; da Silva, R.C.; Natalino, R.; Chaves, D.M.; Texeira, M.G. Monoterpenes etherification reactions with alkyl alcohols over cesium partially exchanged Keggin heteropoly salts: Effects of catalyst composition. Chem. Pap. 2021, 75, 153–168. [Google Scholar] [CrossRef]
- Da Silva, M.J.; Vilanculo, C.B.; Teixeira, M.G.; Julio, A.A. Catalysis of vegetable oil transesterification by Sn(II)-exchanged Keggin heteropolyacids: Bifunctional solid acid catalysts. React. Kinet. Mech. Catal. 2017, 122, 1011–1030. [Google Scholar] [CrossRef]
- Da Silva, M.J.; Chaves, D.M.; Teixeira, M.G.; Oliveira Bruziquesi, C.G. Esterification of levulinic acid over Sn(II) exchanged Keggin heteropolyacid salts: An efficient route to obtain bioaditives. Mol. Catal. 2021, 504, 111495. [Google Scholar] [CrossRef]
- Da Silva, M.J.; Chaves, D.M.; Ferreira, S.O.; da Silva, R.C.; Gabriel Filho, J.B.; Bruziquesi, C.G.O.; Al-Rabiah, A.A. Impacts of Sn(II) doping on the Keggin heteropolyacid-catalyzed etherification of glycerol with tert-butyl alcohol. Chem. Eng. Sci. 2022, 247, 116913. [Google Scholar] [CrossRef]
- Da Silva, M.J.; de Oliveira, C.M. Catalysis by Keggin Heteropolyacid Salts. Curr. Catal. 2018, 7, 26–34. [Google Scholar] [CrossRef]
- Srilatha, K.; Issariyakul, T.; Lingaiah, N.; Sai Prasad, P.S.; Kozinski, J.; Dalai, A.K. Efficient esterification and transesterification of used cooking oil using 12-tungstophosphoric acid (TPA)/Nb2O5 catalyst. Energy Fuels 2010, 24, 4748–4755. [Google Scholar] [CrossRef]
- Baroi, C.; Dalai, A.K. Review on Biodiesel Production from Various Feedstocks Using 12-Tungstophosphoric Acid (TPA) as a Solid Acid Catalyst Precursor. Ind. Eng. Chem. Res. 2014, 53, 18611–18624. [Google Scholar] [CrossRef]
- Chaves, D.M.; Ferreira, S.O.; Chagas da Silva, R.; Natalino, R.; José da Silva, M. Glycerol Esterification over Sn (II)-Exchanged Keggin Heteropoly Salt Catalysts: Effect of Thermal Treatment Temperature. Energy Fuels 2019, 33, 7705–7716. [Google Scholar] [CrossRef]
- Okoye, P.U.; Abdullah, A.Z.; Hameed, B.H. Synthesis of oxygenated fuel additives via glycerol esterification with acetic acid over bio-derived carbon catalyst. Fuel 2017, 209, 538–544. [Google Scholar] [CrossRef]
- Popova, M.; Lazarova, H.; Kalvachev, Y.; Todorova, T.; Szegedi, Á.; Shestakova, P.; Mali, G.; Dasireddy, V.D.B.C.; Likozar, B. Zr-modified hierarchical mordenite as heterogeneous catalyst for glycerol esterification. Catal. Commun. 2017, 100, 10–14. [Google Scholar] [CrossRef]
- Da Silva, M.J.; Liberto, N.A.; De Andrade Leles, L.C.; Pereira, U.A. Fe4(SiW12O40)3-catalyzed glycerol acetylation: Synthesis of bioadditives by using highly active Lewis acid catalyst. J. Mol. Catal. A Chem. 2016, 422, 69–83. [Google Scholar] [CrossRef]
- Gonçalves, C.E.; Laier, L.O.; Cardoso, A.L.; da Silva, M.J. Bioadditive synthesis from H3PW12O40-catalyzed glycerol esterification with HOAc under mild reaction conditions. Fuel Process. Technol. 2012, 102, 46–52. [Google Scholar] [CrossRef]
- Surasit, C.; Yoosuk, B.; Pohmakotr, M.; Tantirungrotechai, J. Biodiesel Synthesis from Palm Fatty Acid Distillate Using Tungstophosphoric Acid Supported on Cesium-Containing Niobia. J. Am. Oil Chem. Soc. 2017, 94, 465–474. [Google Scholar] [CrossRef]
- Subramaniyan, K.; Arumugam, P. Sulfated niobia supported on KIT-6 as a catalyst for transesterification of groundnut oil. J. Porous Mater. 2016, 23, 639–646. [Google Scholar] [CrossRef]
- Tesser, R.; Vitiello, R.; Carotenuto, G.; Garcia Sancho, C.; Vergara, A.; Maireles Torres, P.J.; Li, C.; Di Serio, M. Niobia supported on silica as a catalyst for Biodiesel production from waste oil. Catal. Sustain. Energy 2015, 2, 33–42. [Google Scholar] [CrossRef][Green Version]
- Silva, Â.; Wilson, K.; Lee, A.F.; Cristina, V.; Carolina, A.; Bacilla, C.; Mary, K.; Nakagaki, S. Applied Catalysis B: Environmental Nb2O5/SBA-15 catalyzed propanoic acid esterification. Appl. Catal. B Environ. 2017, 205, 498–504. [Google Scholar] [CrossRef]
- Ziolek, M. Niobium-containing catalysts—The state of the art. Catal. Today 2003, 78, 47–64. [Google Scholar] [CrossRef]
- Trejda, M.; Stawicka, K.; Dubinska, A.; Ziolek, M. Development of niobium containing acidic catalysts for glycerol esterification. Catal. Today 2012, 187, 129–134. [Google Scholar] [CrossRef]
- Katada, N.; Hatanaka, T.; Ota, M.; Yamada, K.; Okumura, K.; Niwa, M. Biodiesel production using heteropoly acid-derived solid acid catalyst H4PNbW11O40/WO3-Nb2O5. Appl. Catal. A Gen. 2009, 363, 164–168. [Google Scholar] [CrossRef]
- Pietre, M.K.; Almeida, L.C.P.; Landers, R.; Vinhas, R.C.G.; Luna, F.J. H3PO4- and H2SO4-treated niobic acid as heterogeneous catalyst for methyl ester production. React. Kinet. Mech. Catal. 2010, 99, 269–280. [Google Scholar] [CrossRef]
- Alessio, C.; Ribeiro, J.S.; Celante, D.; Brondani, L.; Castilhos, F. Kinetics of methyl esters production with dimethyl carbonate over niobium phosphate. Energy Convers. Manag. 2017, 151, 670–680. [Google Scholar] [CrossRef]
- De Sairre, M.I.; Bronze-Uhle, É.S.; Donate, P.M. Niobium(V) oxide: A new and efficient catalyst for the transesterification of β-keto esters. Tetrahedron Lett. 2005, 46, 2705–2708. [Google Scholar] [CrossRef]
- Aranda, D.A.G.; De Goncalves, J.A.; Peres, J.S.; Ramos, A.L.D.; De Melo, C.A.R.; Antunes, O.A.C.; Furtado, N.C.; Taft, C.A. The use of acids, niobium oxide, and zeolite catalysts for esterification reactions. J. Phys. Org. Chem. 2009, 22, 709–716. [Google Scholar] [CrossRef]
- Anjali, K.; Vijayan, A.; Venkatesha, N.J.; Sakthivel, A. Niobium based macromolecule preparation and its potential application in biomass derived levulinic acid esterification. Inorg. Chem. Commun. 2021, 123, 108302. [Google Scholar] [CrossRef]
- Sy, Y.; Nurhazanah, N.; Maulana, A.; Mahidin, M.; Husin, H. Study of pH influences on the performance of Na-loaded NbOPO4 solid acid catalyst for biofuel production. J. Phys. Conf. Ser. 2019, 1402, 055006. [Google Scholar] [CrossRef]
- Alves, M.A.L.; Pinheiro, N.S.; Brondani, L.N.; Celante, D.; Ketzer, F.; Castilhos, F. Assessment of niobium phosphate as heterogeneous catalyst in esterification with methyl acetate. J. Chem. Technol. Biotechnol. 2019, 94, 3172–3179. [Google Scholar] [CrossRef]
- Ribeiro, J.D.S.; Celante, D.; Simões, S.S.; Bassaco, M.M.; da Silva, C.; de Castilhos, F. Efficiency of heterogeneous catalysts in interesterification reaction from macaw oil (Acrocomia aculeata) and methyl acetate. Fuel 2017, 200, 499–505. [Google Scholar] [CrossRef]
- Policano, M.D.; Rivaldi, J.D.; De Castro, H.F.; Carneiro, L.M. Simultaneous esterification and transesterification of andiroba oil using niobium oxide-sulfate as catalyst. Int. J. Eng. Res. Sci. 2016, 2, 2395–6992. [Google Scholar]
- Brandão, R.F.; Quirino, R.L.; Mello, V.M.; Tavares, A.P.; Peres, A.C.; Guinhos, F.; Rubim, J.C.; Suarez, P.A.Z. Synthesis, characterization and use of Nb2O5 based catalysts in producing biofuels by transesterification, esterification and pyrolysis. J. Braz. Chem. Soc. 2009, 20, 954–966. [Google Scholar] [CrossRef]
- Takagaki, A.; Sasaki, R.; Tagusagawa, C.; Domen, K. Intercalation-induced esterification over a layered transition metal oxide. Top. Catal. 2009, 52, 592–596. [Google Scholar] [CrossRef]




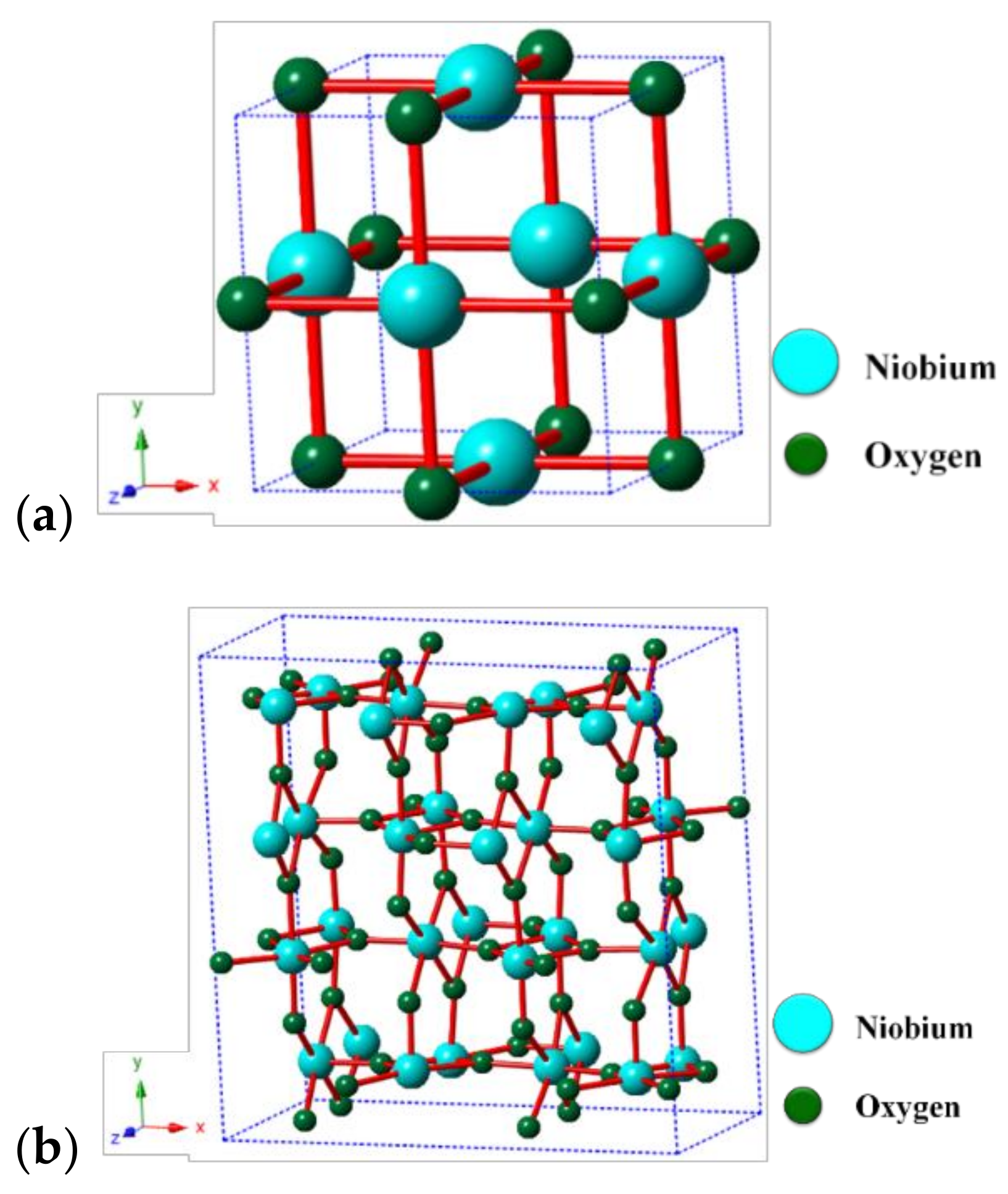
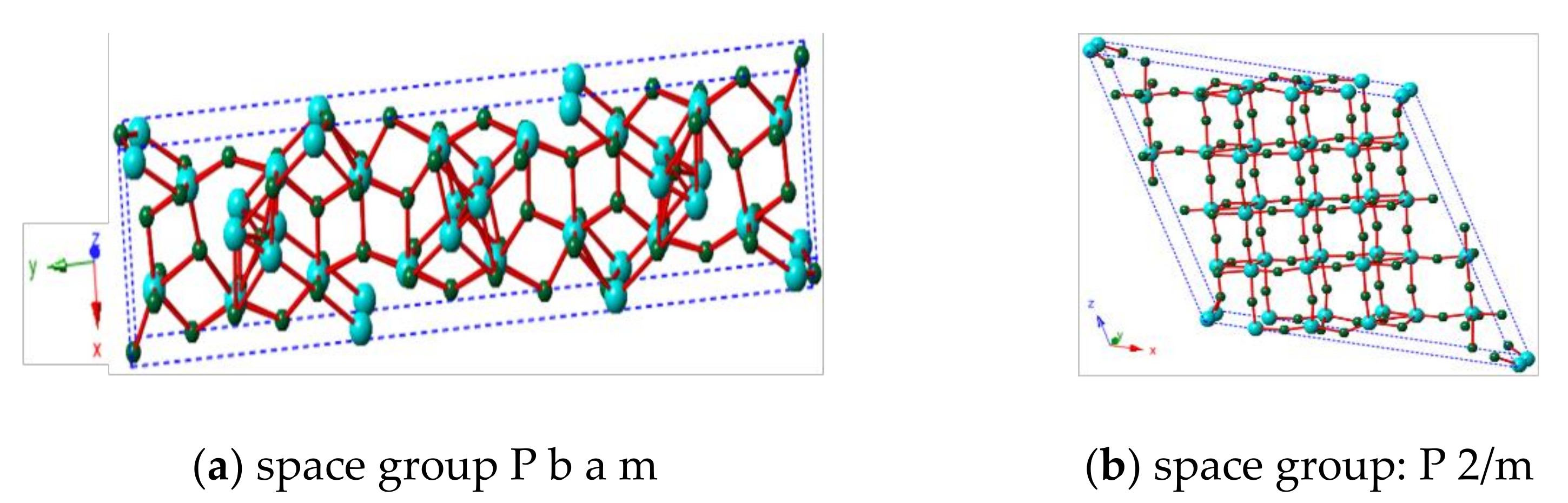

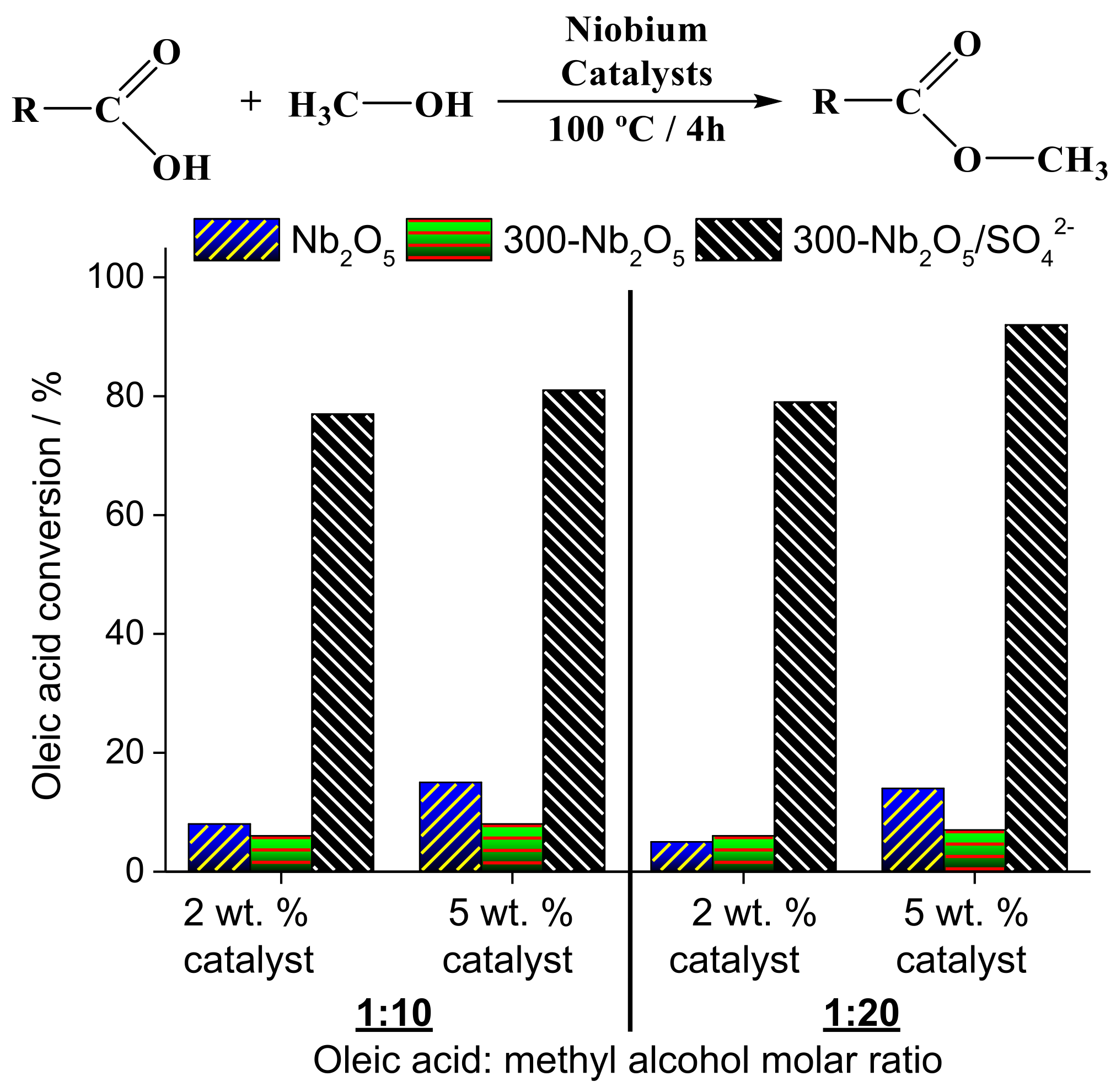
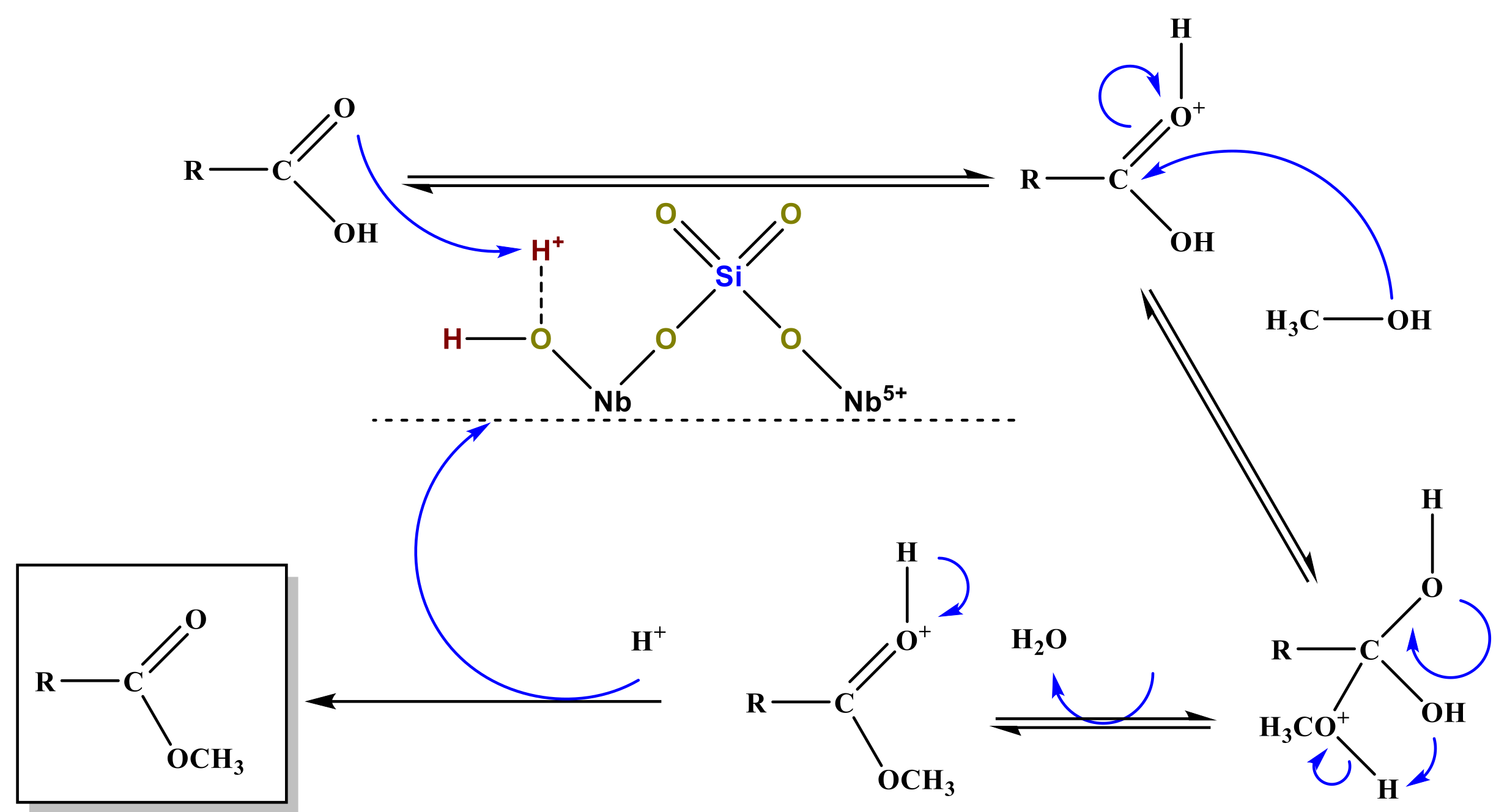
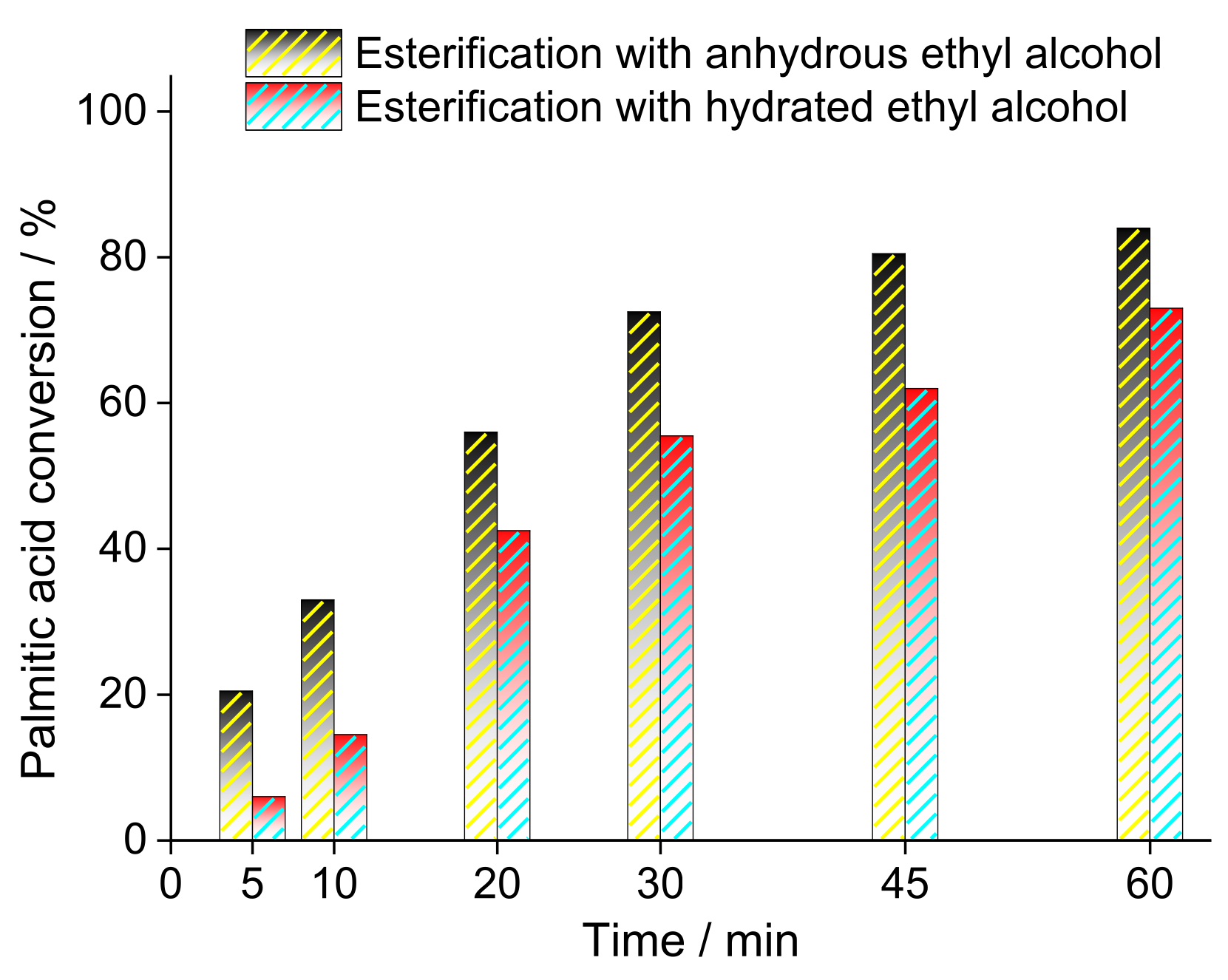
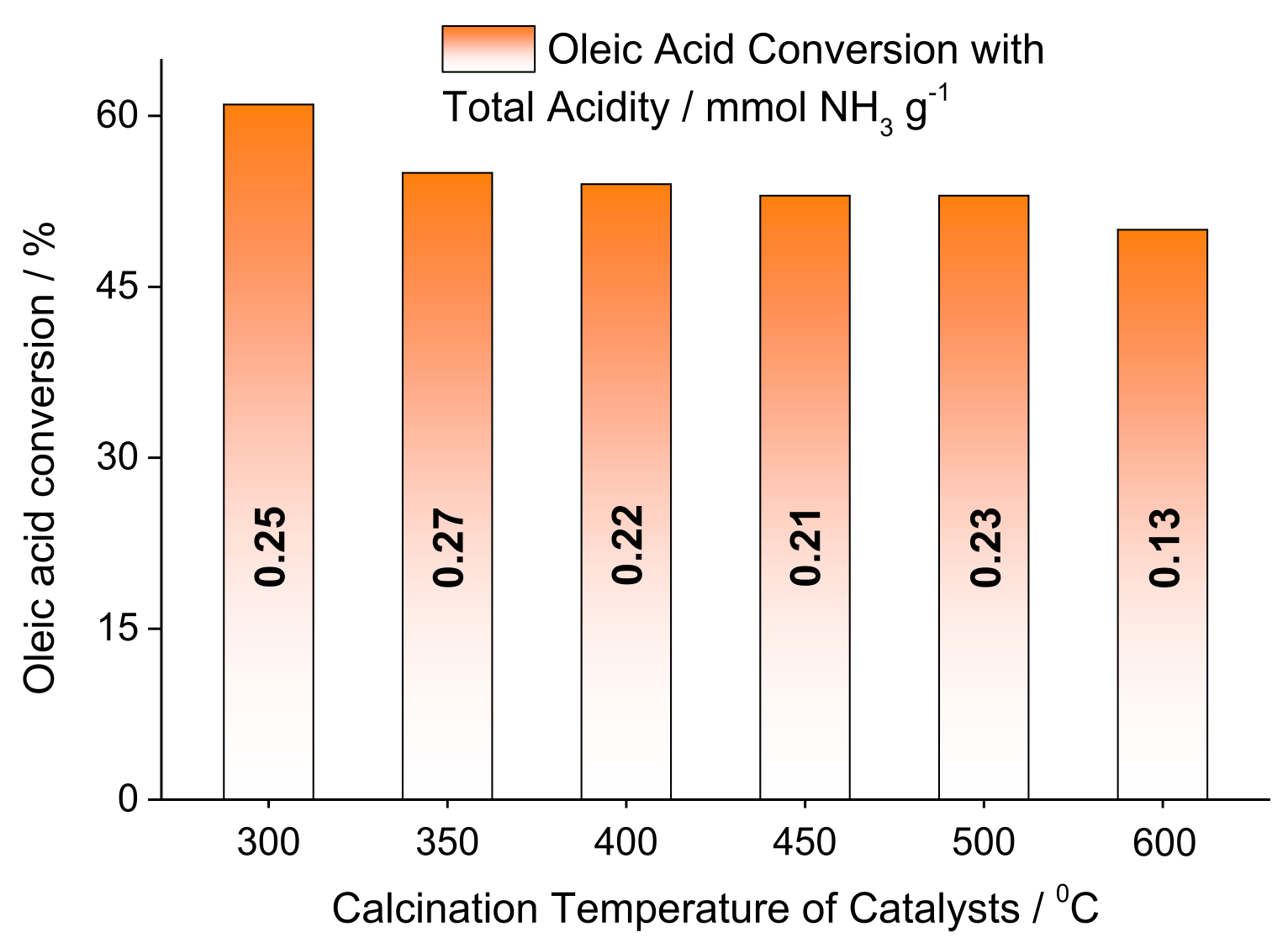
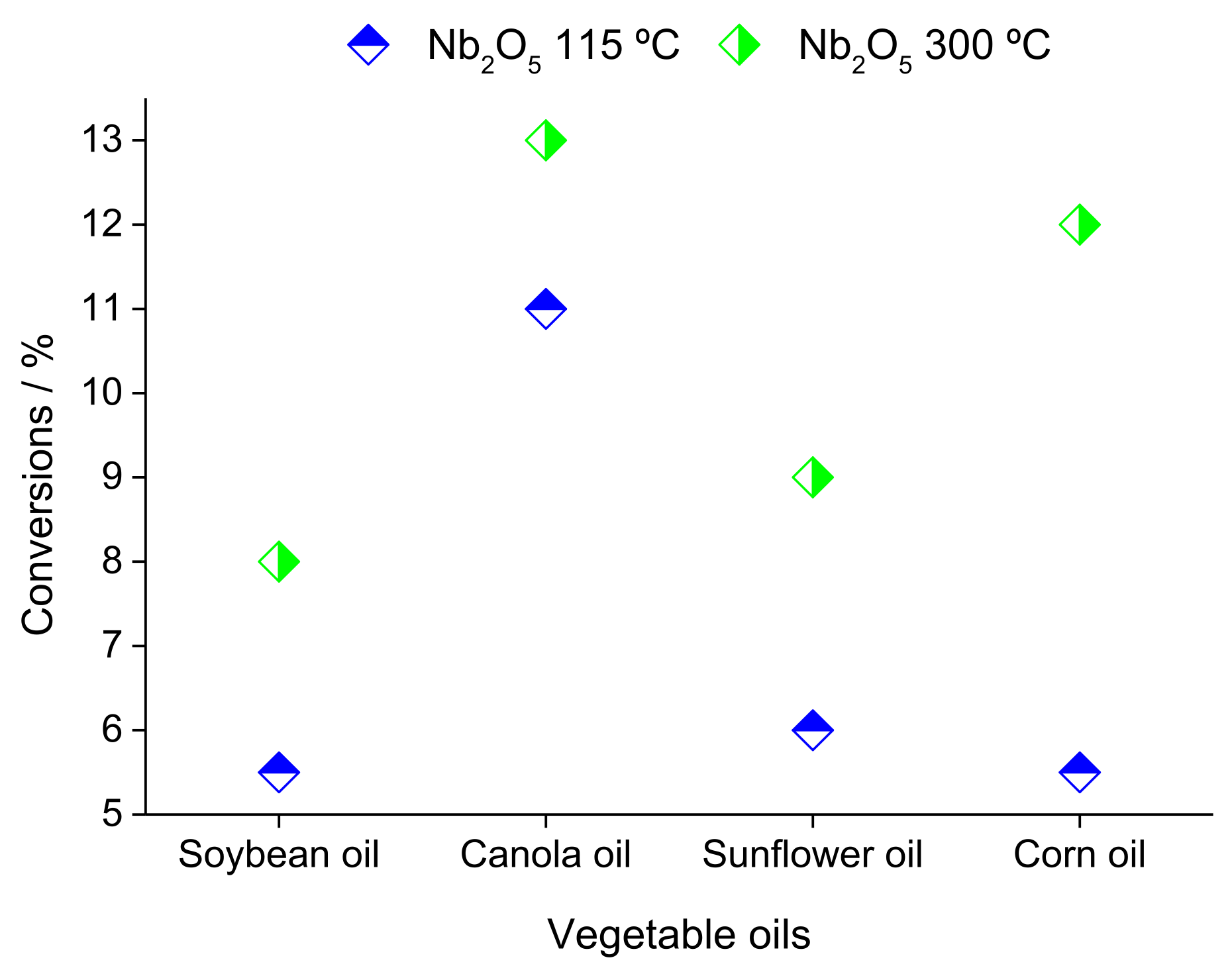


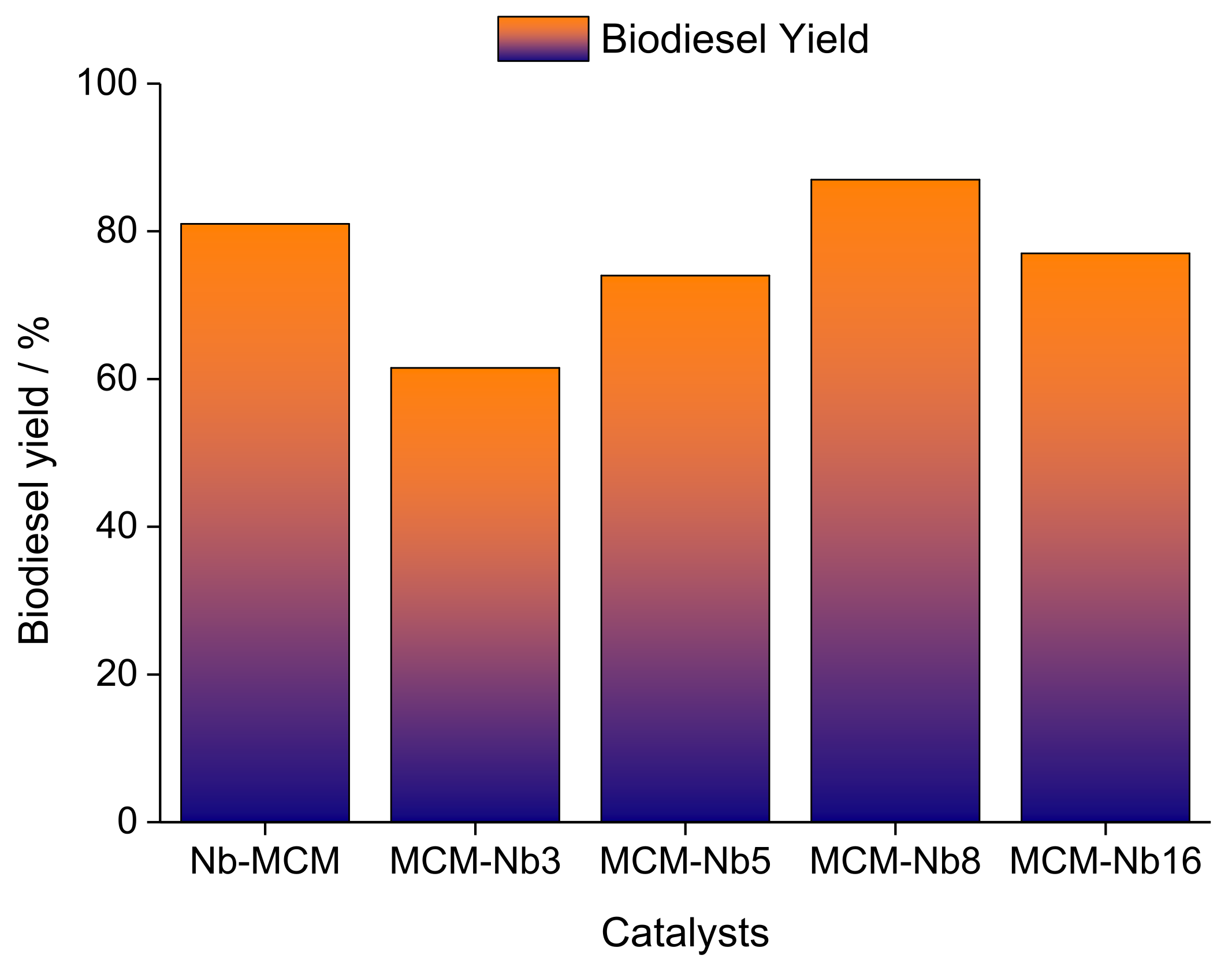

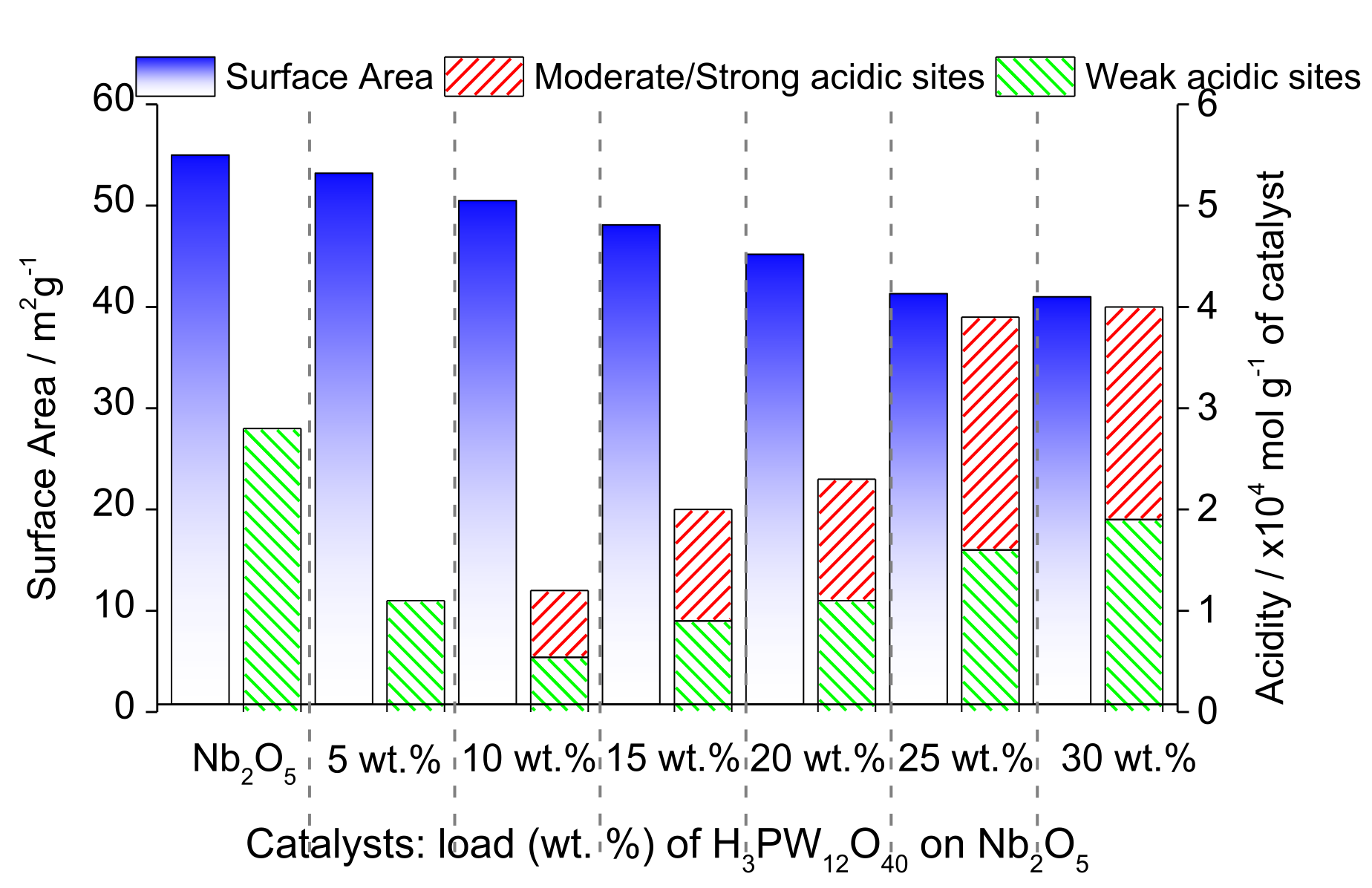

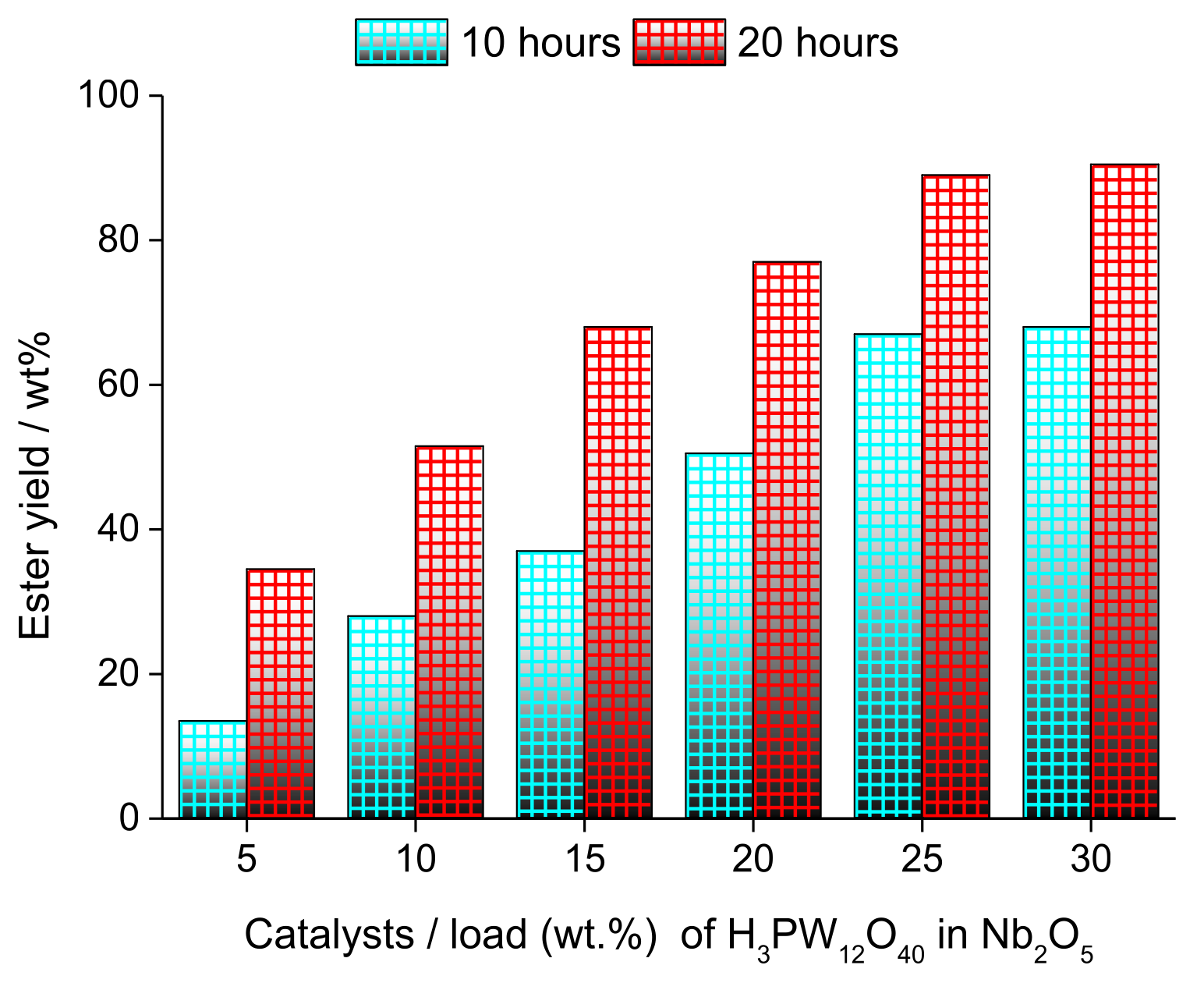
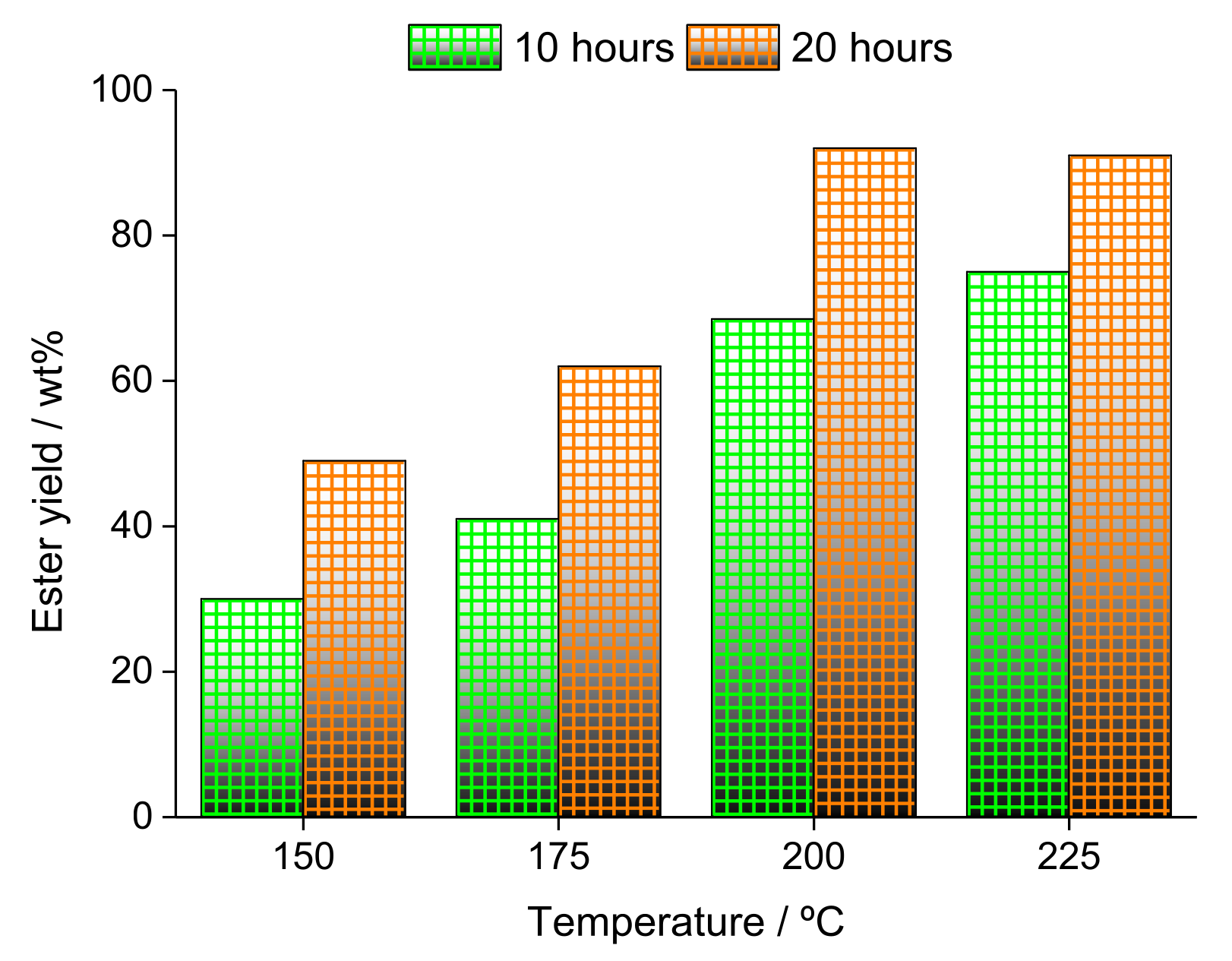
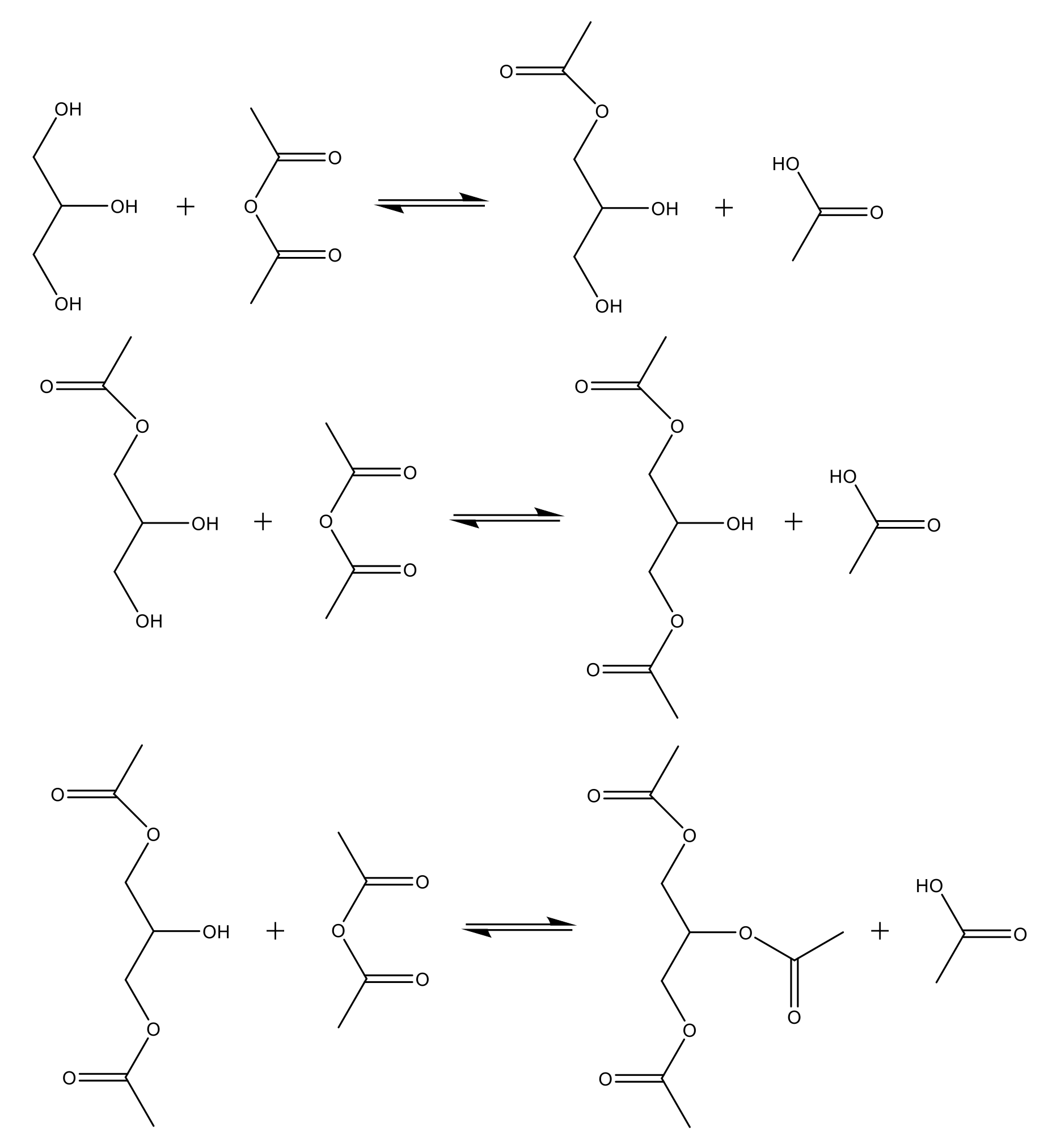
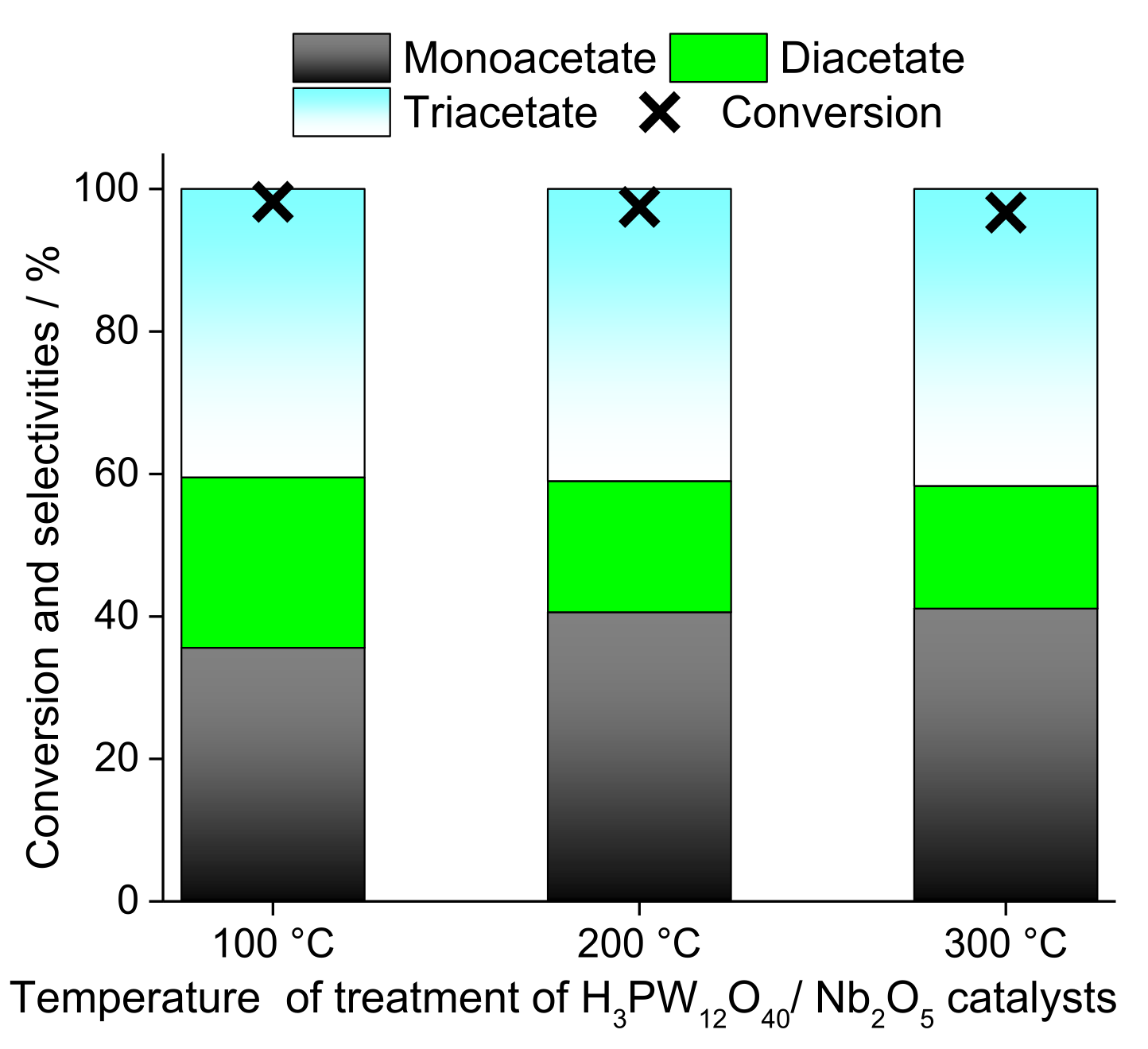

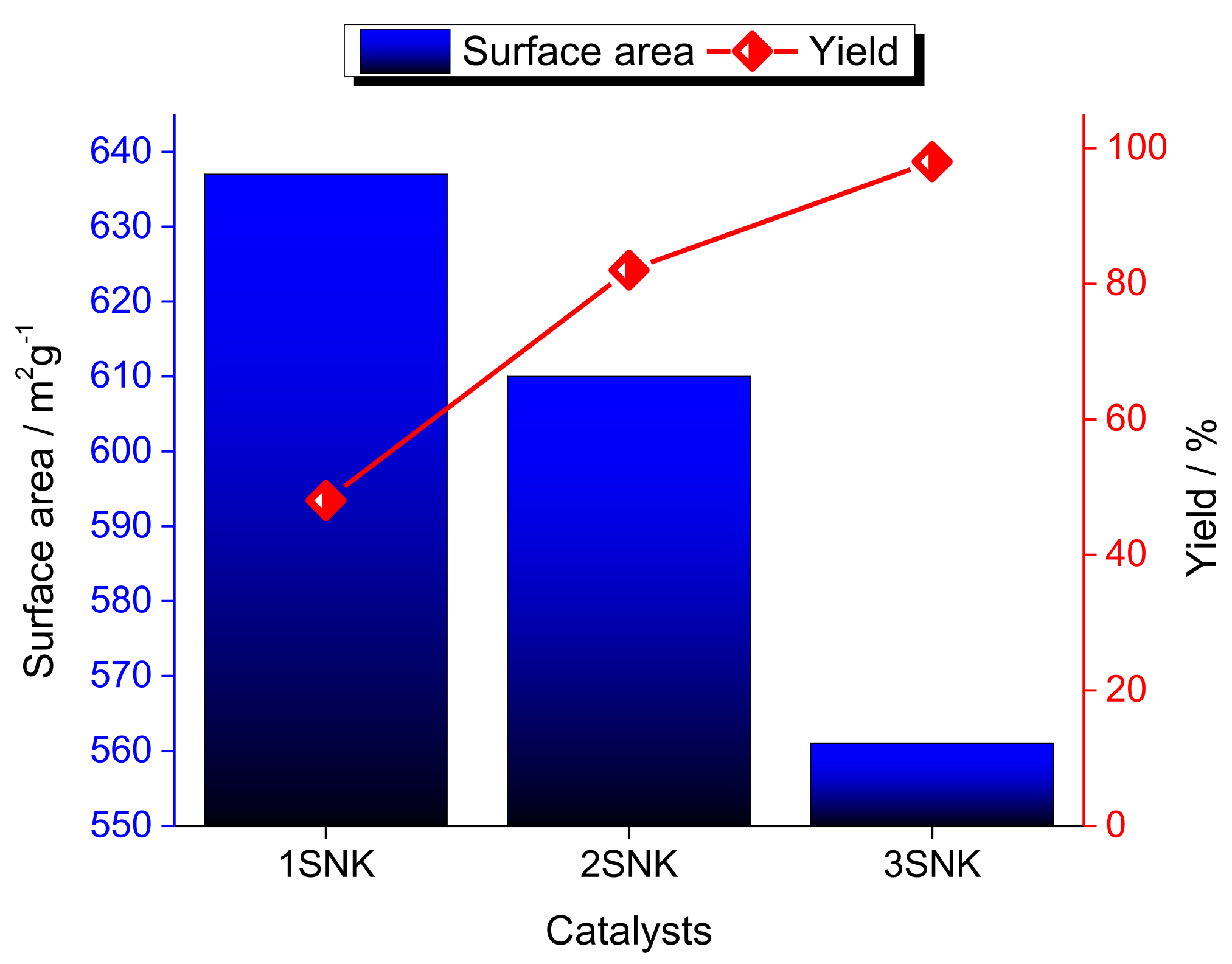








| Alcohol | ΔH (kJ.mol−1) | ΔS (J.(mol·K−1) |
|---|---|---|
| Hydrated ethyl alcohol | 151.3 | 319.3 |
| Anhydrous ethyl alcohol | 111.7 | 249.8 |
| Catalyst | Substrate | Reaction Conditions | Conversion a/Yield b (%) | Ref. |
|---|---|---|---|---|
| NbOPO4 (5% based on FFA mass) | Oleic Acid | Oleic acid: dimethyl carbonate molar ratio (1:5)/250 °C/3 h | 83 a | [97] |
| Nb2O5 (10% weight of β-ketoester) | 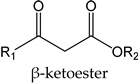 | 1- t-butyl alcohol/8 h R1 = R2 = CH3 2- Glycidol/5.5 h R1 = R2 = CH3 3- n-butyl alcohol/5.5 h R1 = R2 = CH3 * β-ketoester (1 equiv) and alcohol (2 equiv) | 1—93 a/60 b 2—80 a/76 b 3—100 a/98 b | [98] |
| Nb2O5 small pellets (2% w/w in relation to fatty acid) | Fatty acid mixture (real vacuum distillated palm oil) | Anhydrous methanol: fatty acid mixture molar ratio (3)/130 °C/1 h | 82 a | [99] |
| Nb-TCPP-SBA-AM c (0.01 g) | Levulinic acid | Acid: methanol molar ratio (1:5)/60 °C/6 h | 74 a/59 b | [100] |
| Na/NbOPO4 d (2% of oil mass) | Vegetable oil | Oil: methanol mass ratio (1:12)/65 °C/3 h | 98.5 b | [101] |
| NbOPO4 (10% w/w) | Oleic acid | Methyl acetate:oleic acid molar ratio (10:1)/240 °C/2 h | 79.05% w/w | [102] |
| 1- NbOPO4 (5% w/w) 2- Nb2O5 (5% w/w) | Macaw oil | Methyl acetate: macaw oil (30:1)/250 °C/2 h | 1—86.93 a 2—74.64 a | [103] |
| SO42−/Nb2O5 (5% w/w) | Andiroba oil | Anhydrous ethanol: oil molar ratio (120)/260 °C/7 h | 66.7 a | [104] |
| 1- Nb2O5/HNO3 2- Nb2O5/H3PO4 3- Nb2O5/H2SO4 | Soybean fatty acids | Soybean fatty acids (10 g)/methanol (4 g)/160 °C/1 h | 1—40 a 2—57 a 3—57 a | [105] |
| HNbMoO6 (0.1 g) | Lactic acid | Lactic acid (0.05 mol)/n-butanol (0.25 mol)/343 K/5 h | 35.1 b | [106] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Batalha, D.C.; da Silva, M.J. Biodiesel Production over Niobium-Containing Catalysts: A Review. Energies 2021, 14, 5506. https://doi.org/10.3390/en14175506
Batalha DC, da Silva MJ. Biodiesel Production over Niobium-Containing Catalysts: A Review. Energies. 2021; 14(17):5506. https://doi.org/10.3390/en14175506
Chicago/Turabian StyleBatalha, Daniel Carreira, and Márcio José da Silva. 2021. "Biodiesel Production over Niobium-Containing Catalysts: A Review" Energies 14, no. 17: 5506. https://doi.org/10.3390/en14175506
APA StyleBatalha, D. C., & da Silva, M. J. (2021). Biodiesel Production over Niobium-Containing Catalysts: A Review. Energies, 14(17), 5506. https://doi.org/10.3390/en14175506