Production of Acrylic Acid from Biomass-Derived Fumaric Acid under Hydrothermal Conditions
Abstract
:1. Introduction
2. Material and Methods
2.1. Materials
2.2. Experimental Procedure
2.3. Product Analysis
2.4. Definition
3. Results and Discussion
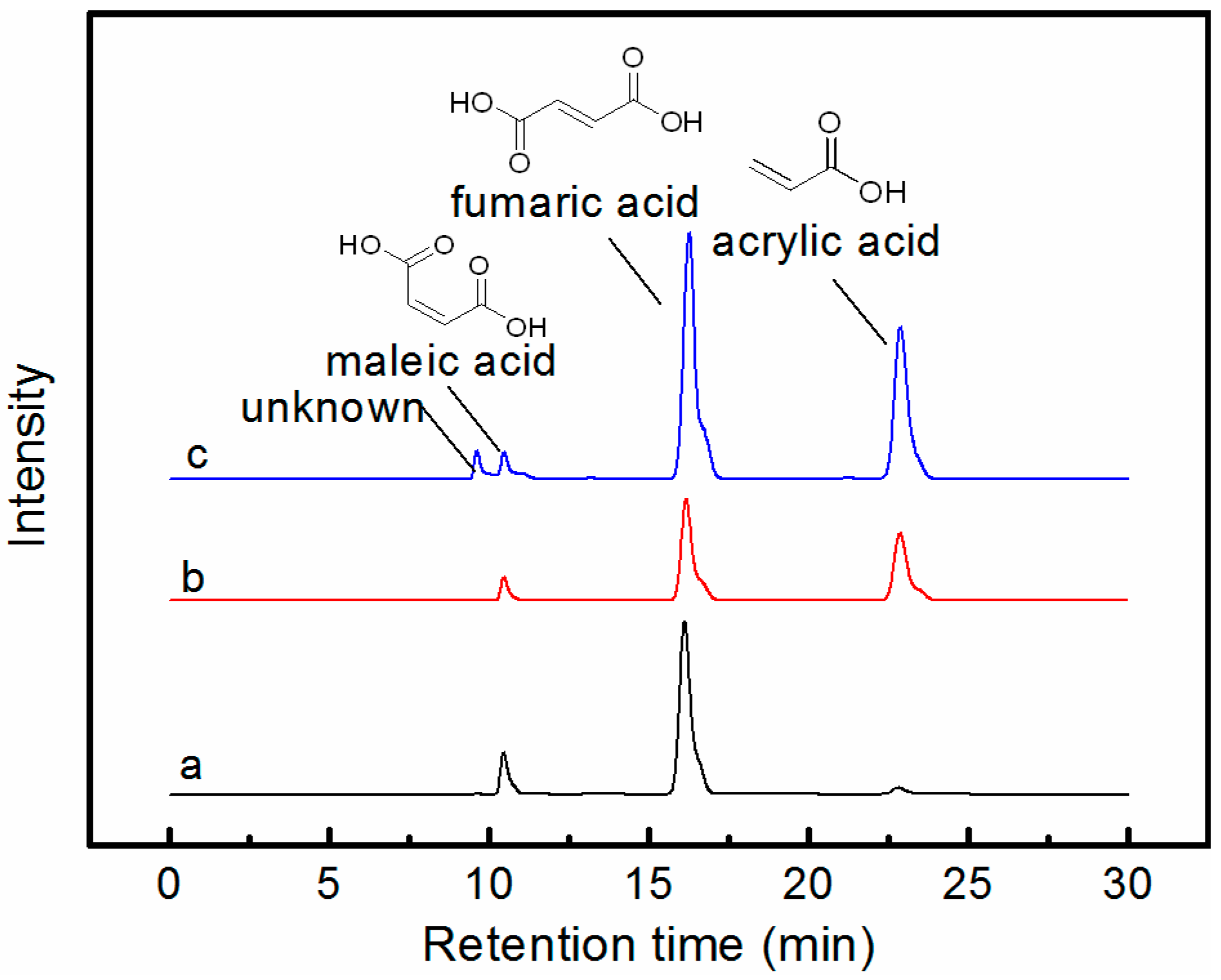
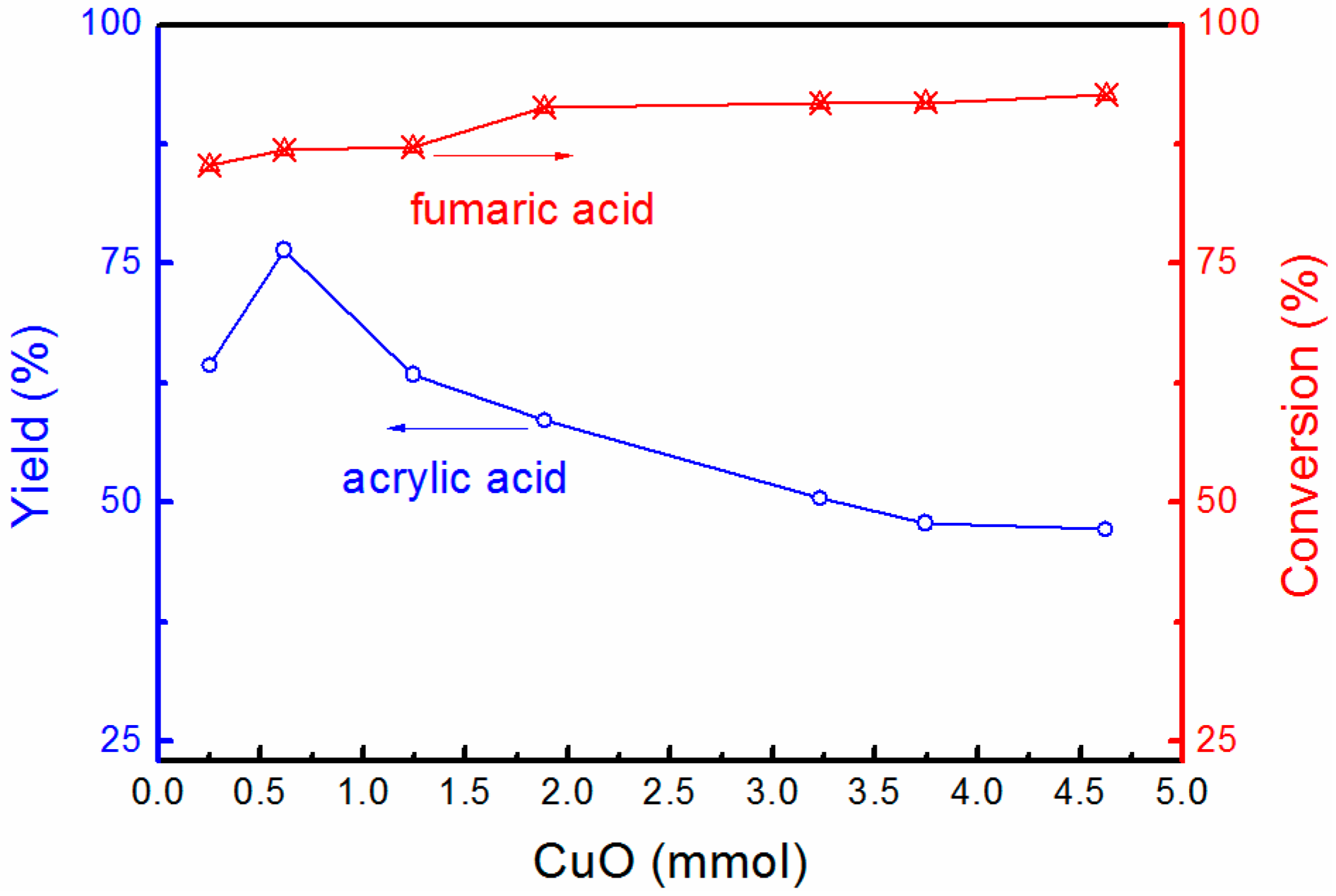
3.1. Effects of Different Copper Oxides on the Yield of Acrylic Acid
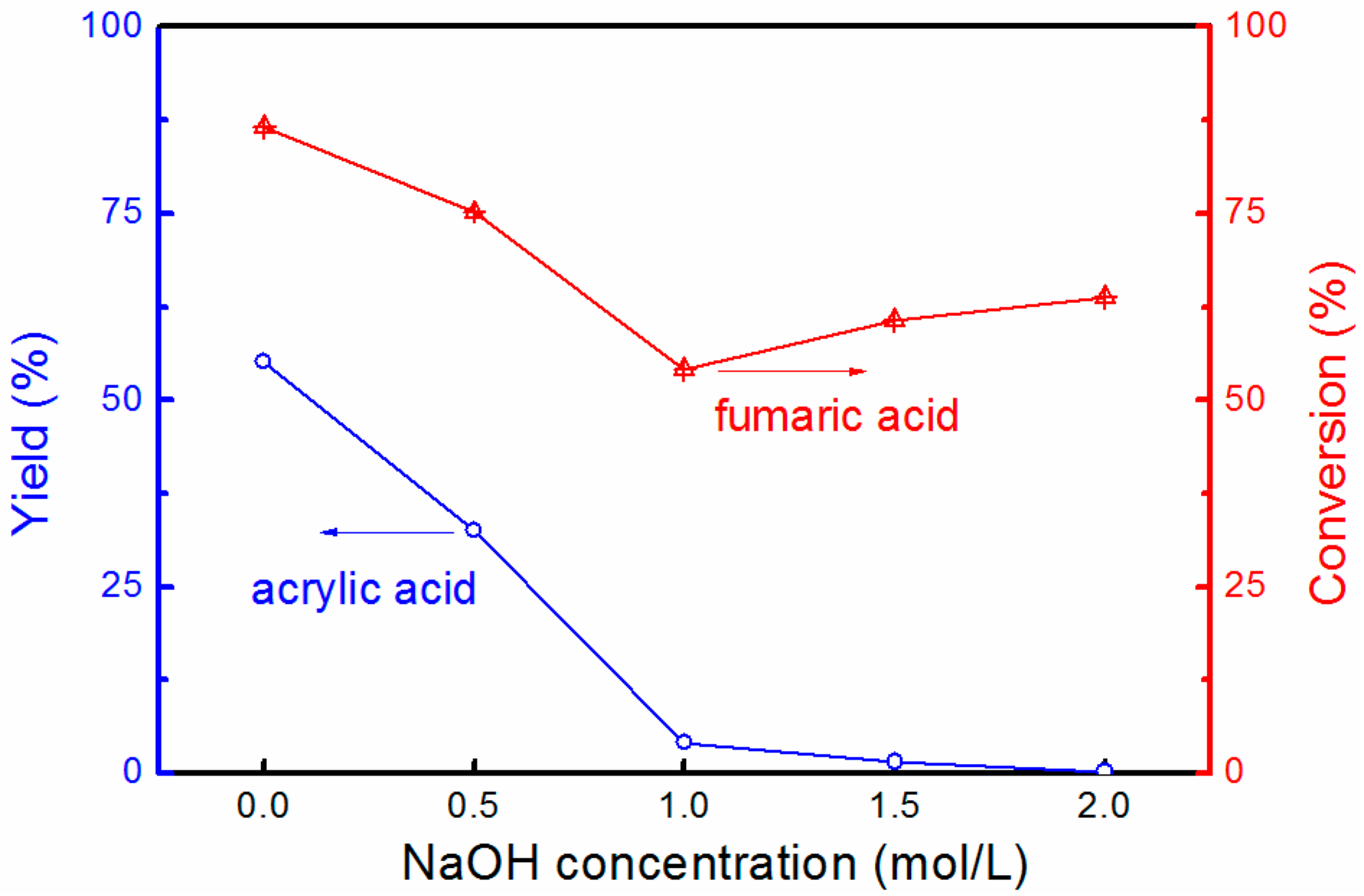
3.2. Reaction Characteristics and Optimization of the Acrylic Acid Yield
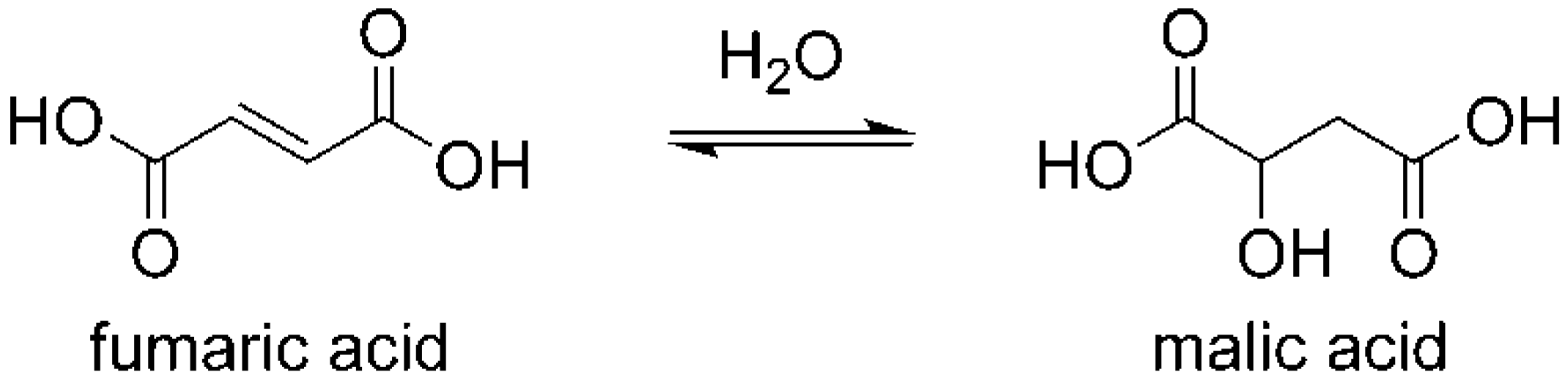
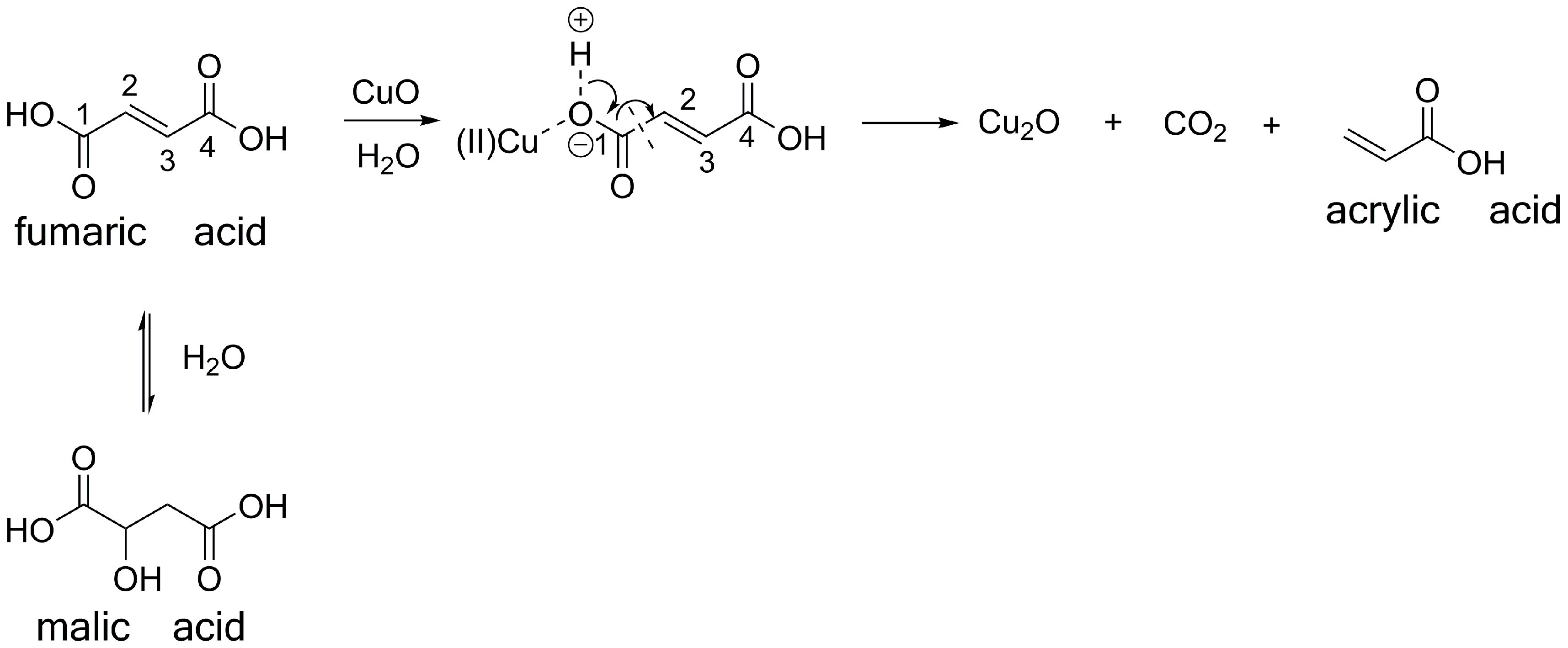
3.3. Possible Reaction Pathways of the Fumaric Acid Oxidation to Acrylic Acid
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Beerthuis, R.; Rothenberg, G.; Shiju, N.R. Catalytic routes towards acrylic acid, adipic acid and epsilon-caprolactam starting from biorenewables. Green Chem. 2015, 17, 1341–1361. [Google Scholar] [CrossRef] [Green Version]
- Bitar, R.; Cools, P.; De Geyter, N.; Morent, R. Acrylic acid plasma polymerization for biomedical use. Appl. Surf. Sci. 2018, 448, 168–185. [Google Scholar] [CrossRef]
- Nagarajan, V.; Mohanty, A.K.; Misra, M. Perspective on polylactic acid (PLA) based sustainable materials for durable applications: Focus on toughness and heat resistance. ACS Sustain. Chem. Eng. 2016, 4, 2899–2916. [Google Scholar] [CrossRef]
- Melchiorre, M.; Esposito, R.; Di Serio, M.; Abbate, G.; Lampasi, A.; Balducci, A.; Ruffo, F. Lactic acid-based solvents for sustainable EDLC electrolytes. Energies 2021, 14, 4250. [Google Scholar] [CrossRef]
- Ai, M. Oxidation of propane to acrylic acid. Catal. Today 1992, 13, 679–684. [Google Scholar] [CrossRef]
- Sun, D.; Yamada, Y.; Sato, S.; Ueda, W. Glycerol as a potential renewable raw material for acrylic acid production. Green Chem. 2017, 19, 3186–3213. [Google Scholar] [CrossRef]
- Mazloom, G.; Alavi, S.M. Different catalytic reactor technologies in selective oxidation of propane to acrylic acid and acrolein. Part. Sci. Technol. 2018, 36, 61–71. [Google Scholar] [CrossRef]
- Kadar, J.; Heene-Würl, N.; Hahn, S.; Nagengast, J.; Kehrer, M.; Taccardi, N.; Collias, D.; Dziezok, P.; Wasserscheid, P.; Albert, J. Acrylic acid synthesis from lactide in a continuous liquid-phase process. ACS Sustain. Chem. Eng. 2019, 7, 7140–7147. [Google Scholar] [CrossRef]
- Mok, W.S.L.; Antal, M.J., Jr.; Jones, M., Jr. Formation of acrylic acid from lactic acid in supercritical water. J. Org. Chem. 1989, 54, 4596–4602. [Google Scholar] [CrossRef]
- Lira, C.T.; McCrackin, P.J. Conversion of lactic acid to acrylic acid in near-critical water. Ind. Eng. Chem. Res. 1993, 32, 2608–2613. [Google Scholar] [CrossRef]
- Aida, T.M.; Ikarashi, A.; Saito, Y.; Watanabe, M.; Smith, R.L.; Arai, K. Dehydration of lactic acid to acrylic acid in high temperature water at high pressures. J. Supercrit. Fluid. 2009, 50, 257–264. [Google Scholar] [CrossRef]
- Hua, D.; Zhuang, X.; Tong, D.; Yu, W.; Zhou, C. Catalytic oxidehydration of glycerol to acrylic acid. Prog. Chem. 2016, 28, 375–390. [Google Scholar] [CrossRef]
- Vavasori, A.; Calgaro, L.; Pietrobon, L.; Ronchin, L. The coupling of carbon dioxide with ethene to produce acrylic acid sodium salt in one pot by using Ni(II) and Pd(II)-phosphine complexes as precatalysts. Pure Appl. Chem. 2018, 90, 315–326. [Google Scholar] [CrossRef]
- Nakason, K.; Panyapinyopol, B.; Kanokkantapong, V.; Viriya-empikul, N.; Kraithong, W.; Pavasant, P. Hydrothermal carbonization of unwanted biomass materials: Effect of process temperature and retention time on hydrochar and liquid fraction. J. Energy Inst. 2018, 91, 786–796. [Google Scholar] [CrossRef]
- Kumar, M.; Oyedun, A.O.; Kumar, A. A review on the current status of various hydrothermal technologies on biomass feedstock. Renew. Sust. Energ. Rev. 2018, 81, 1742–1770. [Google Scholar] [CrossRef]
- Gollakota, A.R.K.; Kawale, H.D.; Kishore, N.; Gu, S. A review on hydrothermal liquefaction of biomass. Renew. Sust. Energy Rev. 2018, 98, 515–517. [Google Scholar] [CrossRef]
- Akiya, N.; Savage, P.E. Roles of water for chemical reactions in high-temperature water. Chem. Rev. 2002, 102, 2725–2750. [Google Scholar] [CrossRef] [PubMed]
- Harry-O’kuru, R.E.; Gordon, S.H.; Klokkenga, M. Bio-generation of succinic acid by fermentation of Physaria fendleri seed polysaccharides. Ind. Crop. Prod. 2015, 77, 116–122. [Google Scholar] [CrossRef]
- Isikgor, F.H.; Becer, C.R. Lignocellulosic biomass: A sustainable platform for the production of bio-based chemicals and polymers. Polym. Chem. 2015, 6, 4497–4559. [Google Scholar] [CrossRef] [Green Version]
- Guo, F.; Wu, M.; Dai, Z.; Zhang, S.; Zhang, W.; Dong, W.; Zhou, J.; Jiang, M.; Xin, F. Current advances on biological production of fumaric acid. Biochem. Eng. J. 2020, 153, 107397–107407. [Google Scholar] [CrossRef]
- Woźniak, M.J.; Prochaska, K. Fumaric acid separation from fermentation broth using nanofiltration (NF) and bipolar electrodialysis (EDBM). Sep. Purif. Technol. 2014, 125, 179–186. [Google Scholar] [CrossRef]
- Xu, Q.; Li, S.; Fu, Y.; Tai, C.; Huang, H. Two-stage utilization of corn straw by Rhizopus oryzae for fumaric acid production. Bioresour. Technol. 2010, 101, 6262–6264. [Google Scholar] [CrossRef]
- Deng, F.; Aita, G.M. Fumaric Acid Production by Rhizopus oryzae ATCC® 20344™ from Lignocellulosic Syrup. BioEnergy Res. 2018, 11, 330–340. [Google Scholar] [CrossRef]
- Yao, G.D.; Huo, Z.B.; Jin, F.M. Direct reduction of copper oxide into copper under hydrothermal conditions. Res. Chem. Intermediat. 2011, 37, 351–358. [Google Scholar] [CrossRef]
- Wang, F.; Wang, Y.; Jin, F.; Yao, G.; Huo, Z.; Zeng, X.; Jing, Z. One-Pot Hydrothermal Conversion of Cellulose into Organic Acids with CuO as an Oxidant. Ind. Eng. Chem. Res. 2014, 53, 7939–7946. [Google Scholar] [CrossRef]
- Lu, M.; Zeng, X.; Cao, J.L.; Huo, Z.B.; Jin, F.M. Production of formic and acetic acids from phenol by hydrothermal oxidation. Res. Chem. Intermediat. 2011, 37, 201–209. [Google Scholar] [CrossRef]
- Jin, F.M.; Enomoto, H. Rapid and highly selective conversion of biomass into value-added products in hydrothermal conditions: Chemistry of acid/base-catalysed and oxidation reactions. Energy Environ. Sci. 2011, 4, 382–397. [Google Scholar] [CrossRef]
- Yin, G.; Jin, F.; Yao, G.; Jing, Z. Hydrothermal Conversion of Catechol into Four-Carbon Dicarboxylic Acids. Ind. Eng. Chem. Res. 2015, 54, 68–75. [Google Scholar] [CrossRef]
- Yin, G.; Huo, Z.; Zeng, X.; Yao, G.; Jing, Z.; Jin, F. Reduction of CuO into Cu with Guaiacol as a Model Compound of Lignin with a Homogeneous Catalyst of NaOH. Ind. Eng. Chem. Res. 2014, 53, 7856–7865. [Google Scholar] [CrossRef]
- Cronin, D.J.; Zhang, X.; Bartley, J.; Doherty, W.O.S. Lignin Depolymerization to Dicarboxylic Acids with Sodium Percarbonate. ACS Sustain. Chem. Eng. 2017, 5, 6253–6260. [Google Scholar] [CrossRef] [Green Version]
- Kloetzer, L.; Ilica, R.-A.; Cascaval, D.; Galaction, A.-I. Separation of fumaric acid by amine extraction without and with 1-octanol as phase modifier. Sep. Purif. Technol. 2019, 227, 115724. [Google Scholar] [CrossRef]
- Franch, M.I.; Ayllón, J.A.; Peral, J.; Domènech, X. Photocatalytic degradation of short-chain organic diacids. Catal. Today 2002, 76, 221–233. [Google Scholar] [CrossRef]
- Oliviero, L.; Barbier, J.; Duprez, D.; Wahyu, H.; Ponton, J.W.; Metcalfe, I.S.; Mantzavinos, D. Wet air oxidation of aqueous solutions of maleic acid over Ru/CeO2 catalysts. Appl. Catal. B Environ. 2001, 35, 1–12. [Google Scholar] [CrossRef]
- Jin, F.M.; Cao, J.X.; Kishida, H.; Moriya, T.; Enomoto, H. Impact of phenolic compounds on hydrothermal oxidation of cellulose. Carbohyd. Res. 2007, 342, 1129–1132. [Google Scholar] [CrossRef]
- Shen, Z.; Zhou, J.F.; Zhou, X.F.; Zhang, Y.L. The production of acetic acid from microalgae under hydrothermal conditions. Appl. Energy 2011, 88, 3444–3447. [Google Scholar] [CrossRef]
- Jin, F.M.; Zhou, Z.Y.; Moriya, T.; Kishida, H.; Higashijima, H.; Enomoto, H. Controlling hydrothermal reaction pathways to improve acetic acid production from carbohydrate biomass. Environ. Sci. Technol. 2005, 39, 1893–1902. [Google Scholar] [CrossRef]
- Jin, F.; Zhang, G.; Jin, Y.; Watanabe, Y.; Kishita, A.; Enomoto, H. A new process for producing calcium acetate from vegetable wastes for use as an environmentally friendly deicer. Bioresour. Technol. 2010, 101, 7299–7306. [Google Scholar] [CrossRef]
- Shen, Z.; Jin, F.; Zhang, Y.; Wu, B.; Kishita, A.; Tohji, K.; Kishida, H. Effect of Alkaline Catalysts on Hydrothermal Conversion of Glycerin into Lactic Acid. Ind. Eng. Chem. Res. 2009, 48, 8920–8925. [Google Scholar] [CrossRef]
- Wang, Y.; Jin, F.; Sasaki, M.; Wahyudiono; Wang, F.; Jing, Z.; Goto, M. Selective conversion of glucose into lactic acid and acetic acid with copper oxide under hydrothermal conditions. AIChE J. 2013, 59, 2096–2104. [Google Scholar] [CrossRef]
- Zhong, H.; Yao, G.D.; Cui, X.; Yan, P.; Wang, X.G.; Jin, F.M. Selective conversion of carbon dioxide into methane with a 98% yield on an in situ formed Ni nanoparticle catalyst in water. Chem. Eng. J. 2019, 357, 421–427. [Google Scholar] [CrossRef]
- Erickson, L.E.; Alberty, R.A. Kinetics and mechanism of the base-catalyzed hydration of fumarate to malate. J. Phys. Chem. 1959, 63, 705–709. [Google Scholar] [CrossRef]
- Li, Q.J.; Yao, G.D.; Zeng, X.; Jing, Z.Z.; Huo, Z.B.; Jin, F.M. Facile and Green Production of Cu from CuO Using Cellulose under Hydrothermal Conditions. Ind. Eng. Chem. Res. 2012, 51, 3129–3136. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yin, G.; Zhong, H.; Yao, G.; Jin, F.; Zhao, J. Production of Acrylic Acid from Biomass-Derived Fumaric Acid under Hydrothermal Conditions. Energies 2021, 14, 5456. https://doi.org/10.3390/en14175456
Yin G, Zhong H, Yao G, Jin F, Zhao J. Production of Acrylic Acid from Biomass-Derived Fumaric Acid under Hydrothermal Conditions. Energies. 2021; 14(17):5456. https://doi.org/10.3390/en14175456
Chicago/Turabian StyleYin, Guodong, Heng Zhong, Guodong Yao, Fangming Jin, and Jianfu Zhao. 2021. "Production of Acrylic Acid from Biomass-Derived Fumaric Acid under Hydrothermal Conditions" Energies 14, no. 17: 5456. https://doi.org/10.3390/en14175456
APA StyleYin, G., Zhong, H., Yao, G., Jin, F., & Zhao, J. (2021). Production of Acrylic Acid from Biomass-Derived Fumaric Acid under Hydrothermal Conditions. Energies, 14(17), 5456. https://doi.org/10.3390/en14175456





