Inductive Power Transfer Link at 13.56 MHz for Leadless Cardiac Pacemakers
Abstract
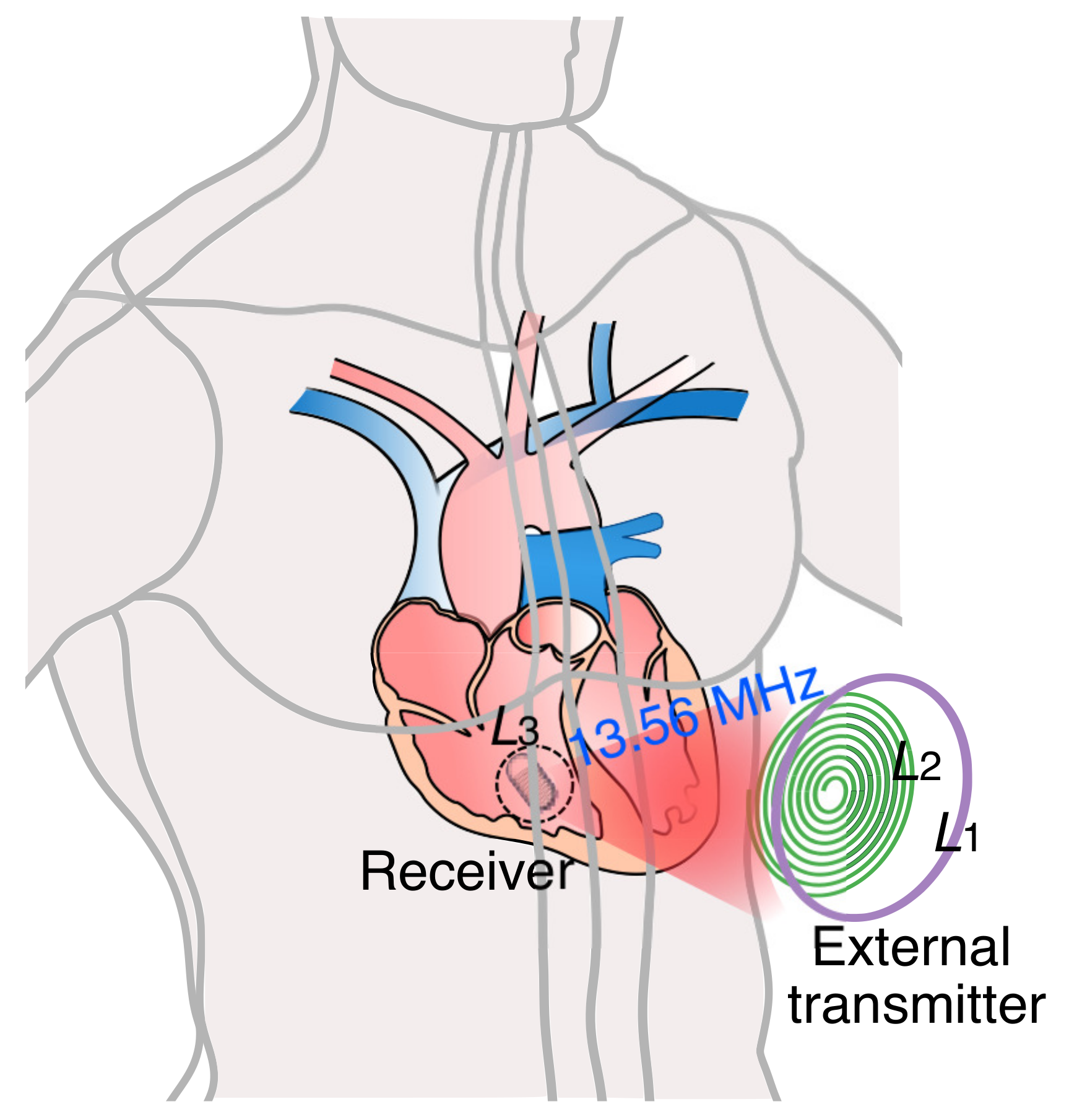
:1. Introduction
2. 3-Coil Inductive Power Link Methodology and Optimization Procedure
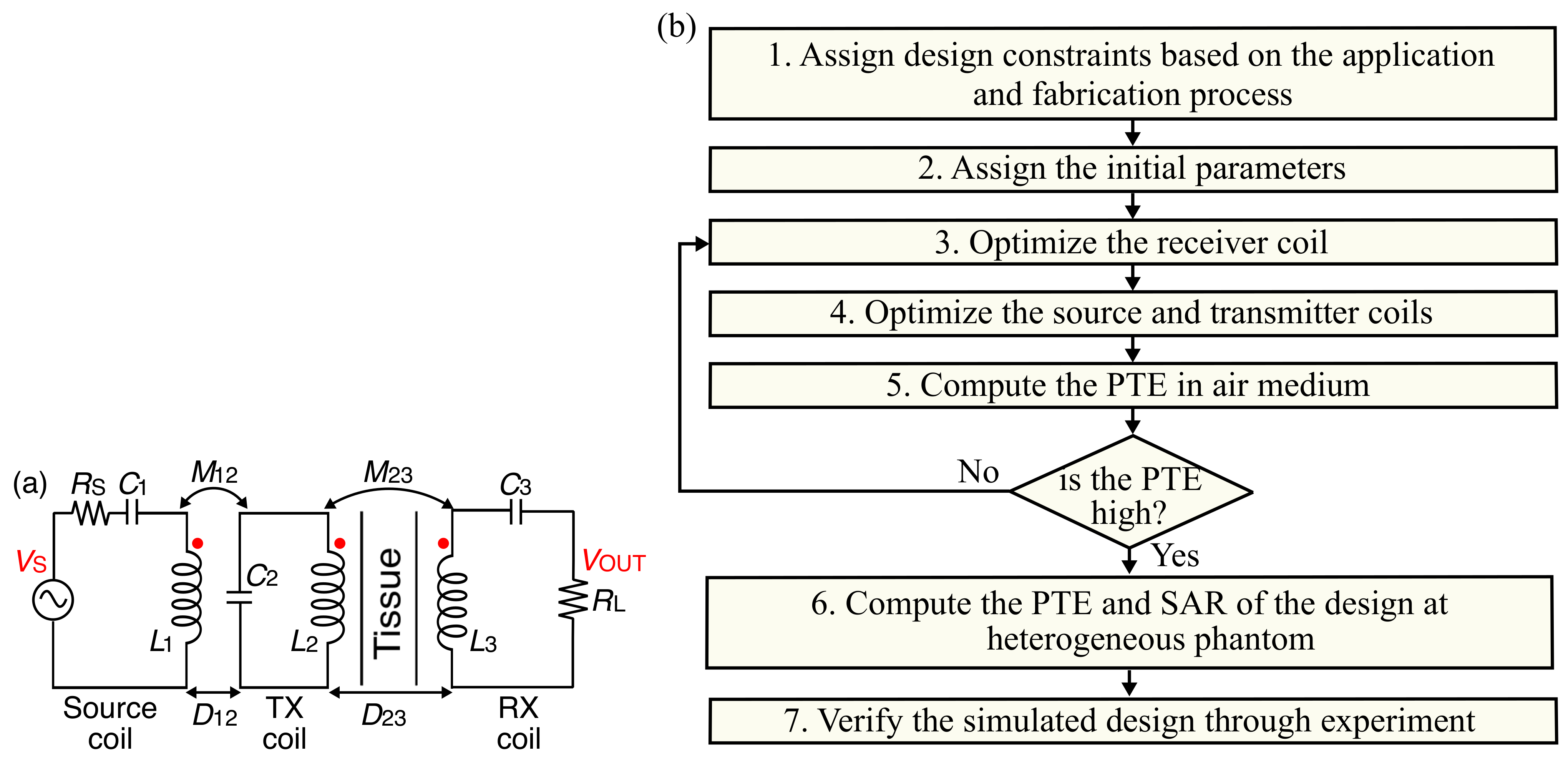
2.1. Equivalent Circuit Model of a 3-Coil Inductive Power Link
2.2. Optimization Procedure of the Proposed 3-Coil Inductive Power Link
- Step-1:
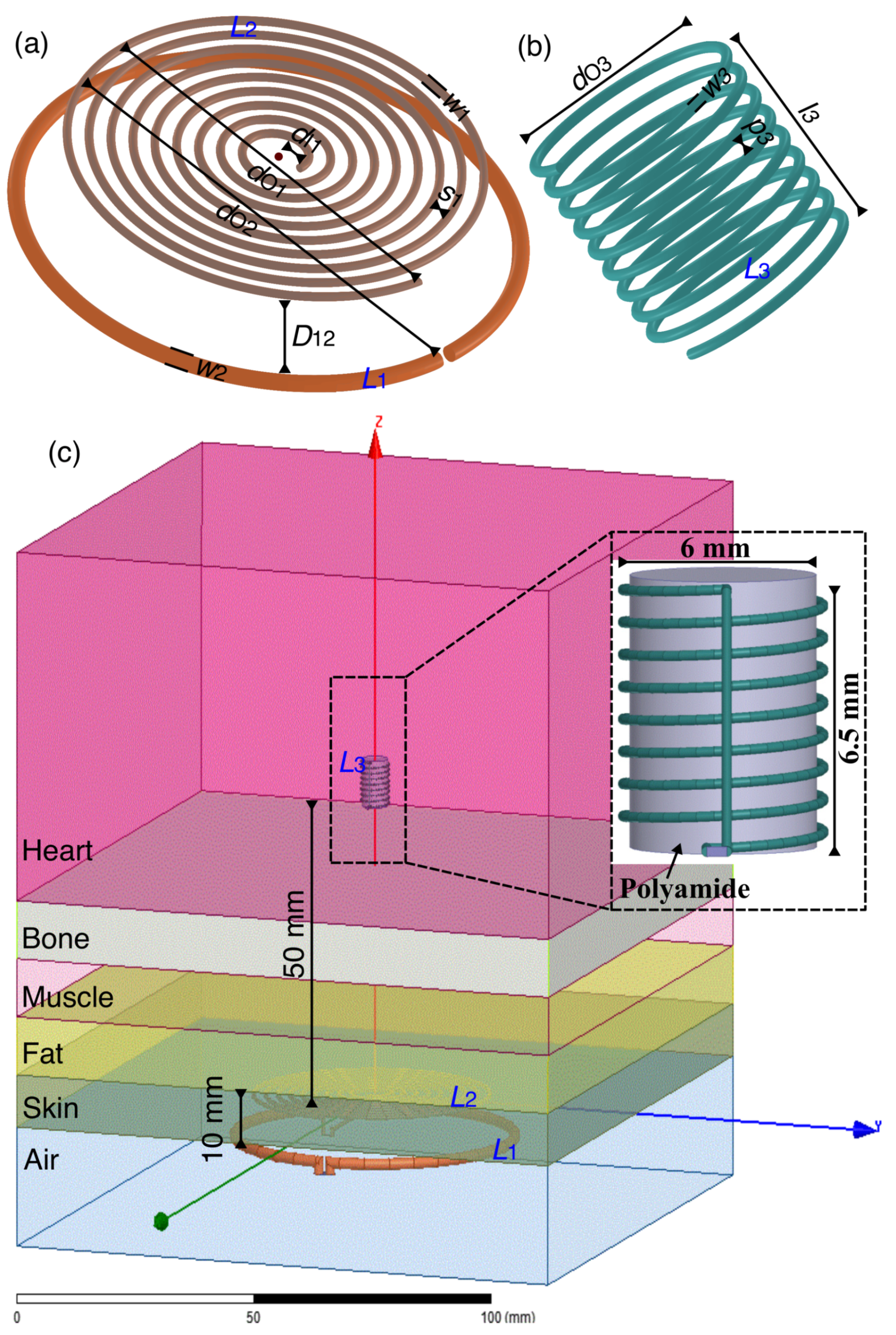
- Define the design constraints based on the LCP application. A decision has to be made on the shape and size of the coils. Considering the shape and size of modern LCPs, we have chosen a solenoidal shape receiver coil with a maximum diameter of 6 mm. As and lie on the chest, We have considered planar circular shapes to ensure comfort. , , and use copper for winding, and the operating frequency is chosen to be 13.56 MHz.
- Step-2:
- The parameters of , , and are initialized in this step. The parameters are number of turns (n), wire width (w), the inner diameter of the coil (), pitch (p), and spacing between the turns (s).
- Step-3:
- optimization is carried out in this step. Parametric sweep is performed with respect to , , and to find optimal geometry for . This way a high and self-inductance of are achieved. The can be calculated as in Equation (2).
- Step-4:
- This step carries out and optimization. The parametric sweep of and is performed with respect to , , , and , , , and . This step finds optimal geometries for and . It is important to achieve a strong as increase in eventually increases .The higher the value of , the higher the value of efficiency. , , and can be calculated as in Equations (1) and (2).
- Step-5:
- Evaluate the efficiency between and in air medium. is set to 50 mm. and are defined in this step. For a low efficiency, the process is iterated from Step-3 till a maximum efficiency is achieved. As mentioned above, maximum efficiency occurs at the resonance frequency, and therefore, the tuning capacitances , , and tune the resonance frequency.
- Step-6:
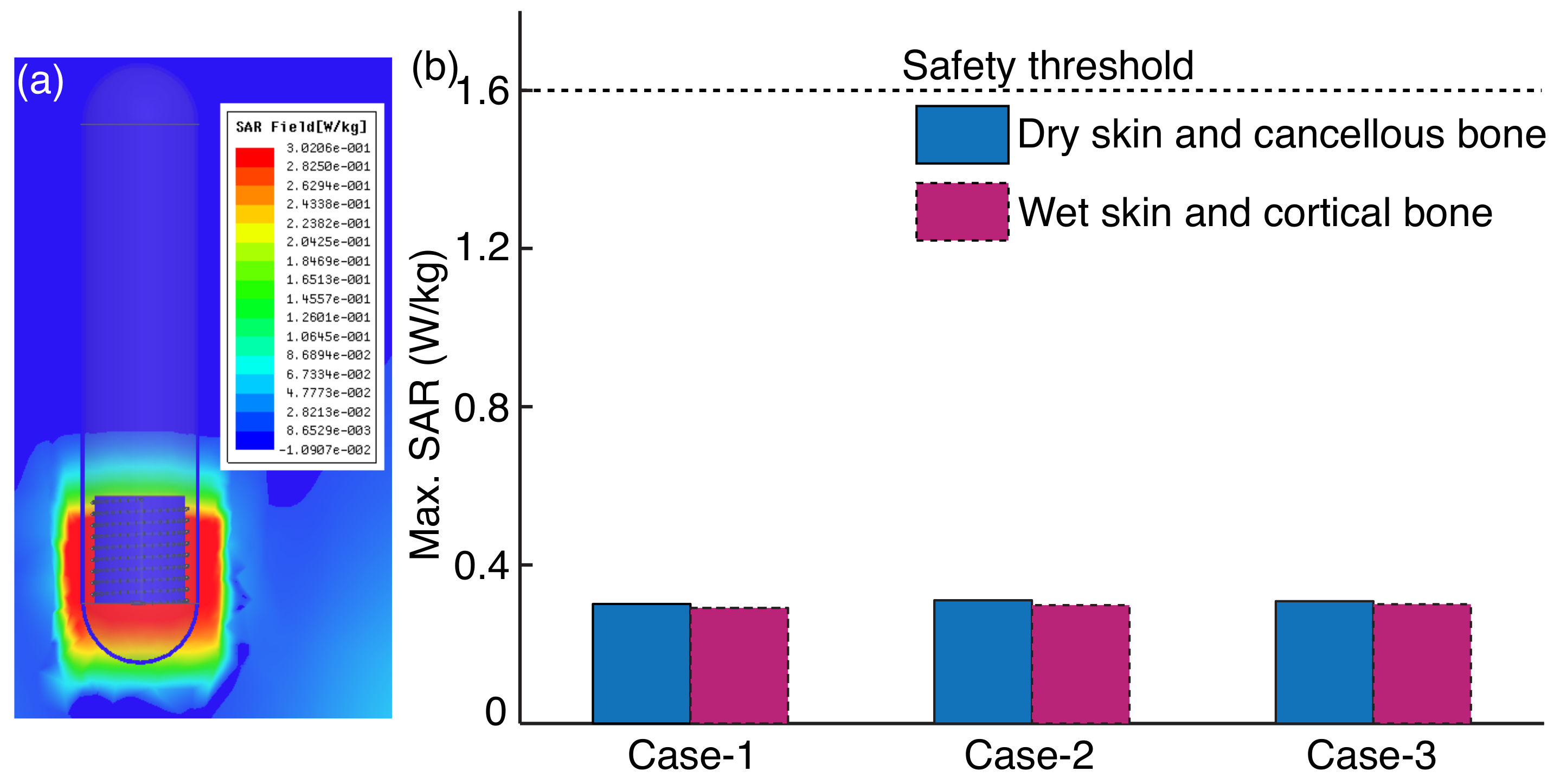
- To evaluate the efficiency of the inductive power link in a real environment, the air medium is replaced with a heterogeneous phantom between and . The presence of tissue in between the coils shifts the resonance frequency, therefore , , and have to be finely tuned. This step also evaluates the safety aspect of the inductive power link by computing the average SAR.
- Step-7:
- Finally, the optimal simulated design is experimentally evaluated.
3. Proposed Coil Geometries and Performance Analysis
3.1. External Transmitter Coil and Receiver Coil Geometries
3.2. Power Transfer Efficiency in Heterogeneous Phantom
4. Experimental Measurements
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cingolani, E.; Goldhaber, J.I.; Marban, E. Next-generation pacemakers: From small devices to biological pacemakers. Nat. Rev. Cardiol. 2018, 15, 139–150. [Google Scholar] [CrossRef]
- Miller, M.A.; Neuzil, P.; Dukkipati, S.R.; Reddy, V.Y. Leadless cardiac pacemakers: Back to the future. J. Am. Coll. Cardiol. 2015, 66, 1179–1189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reddy, V.Y.; Exner, D.V.; Cantillon, D.J.; Doshi, R.; Bunch, T.J.; Tomassoni, G.F.; Friedman, P.A.; Estes, N.M., III; Ip, J.; Niazi, I.; et al. Percutaneous implantation of an entirely intracardiac leadless pacemaker. N. Engl. J. Med. 2015, 373, 1125–1135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bernard, M.L. Pacing without wires: Leadless cardiac pacing. Ochsner J. 2016, 16, 238–242. [Google Scholar]
- Campi, T.; Cruciani, S.; Palandrani, F.; Santis, V.D.; Hirata, A.; Feliziani, M. Wireless power transfer charging system for aimds and pacemakers. IEEE Trans. Microw. Theory Tech. 2016, 64, 633–642. [Google Scholar] [CrossRef]
- Xiao, C.; Cheng, D.; Wei, K. An lcc-c compensated wireless charging system for implantable cardiac pacemakers: Theory, experiment, and safety evaluation. IEEE Trans. Power Electron. 2017, 33, 4894–4905. [Google Scholar] [CrossRef]
- Liu, C.; Jiang, C.; Song, J.; Chau, K. An effective sandwiched wireless power transfer system for charging implantable cardiac pacemaker. IEEE Trans. Ind. Electron. 2018, 66, 4108–4117. [Google Scholar] [CrossRef]
- Fu, Y.; Hu, L.; Ruan, X.; Fu, X. A transcutaneous energy transmission system for artificial heart adapting to changing impedance. Artif. Organs 2015, 39, 378–387. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Wong, S.C.; Chi, K.T.; Ruan, X. Analysis, design, and control of a transcutaneous power regulator for artificial hearts. IEEE Trans. Biomed. Circuits Syst. 2009, 3, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Das, R.; Yoo, H. Biotelemetry and wireless powering for leadless pacemaker systems. IEEE Microw. Wirel. Compon. Lett. 2015, 25, 262–264. [Google Scholar] [CrossRef]
- Das, R.; Yoo, H. A multiband antenna associating wireless monitoring and nonleaky wireless power transfer system for biomedical implants. IEEE Trans. Microw. Theoryand Tech. 2017, 65, 2485–2495. [Google Scholar] [CrossRef]
- Mao, S.; Wang, H.; Mao, Z.; Sun, M. A double-helix and cross-patterned solenoid used as a wirelessly powered receiver for medical implants. AIP Adv. 2018, 8, 056603. [Google Scholar] [CrossRef]
- Jow, U.-M.; Ghovanloo, M. Design and optimization of printed spiral coils for efficient transcutaneous inductive power transmission. IEEE Trans. Biomed. Circuits Syst. 2007, 1, 193–202. [Google Scholar] [CrossRef]
- Mutashar, S.; Hannan, M.A.; Samad, S.A.; Hussain, A. Design of spiral circular coils in wet and dry tissue for bio-implanted micro-system applications. Prog. Electromagn. Res. 2013, 32, 181–200. [Google Scholar] [CrossRef] [Green Version]
- Mutashar, S.; Hannan, M.A.; Samad, S.A.; Hussain, A. Analysis and optimization of spiral circular inductive coupling link for bio-implanted applications on air and within human tissue. Sensors 2014, 14, 11522–11541. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- RamRakhyani, A.K.; Mirabbasi, S.; Chiao, M. Design and optimization of resonance-based efficient wireless power delivery systems for biomedical implants. IEEE Trans. Biomed. Circuits Syst. 2010, 5, 48–63. [Google Scholar] [CrossRef] [PubMed]
- Kiani, M.; Jow, U.-M.; Ghovanloo, M. Design and optimization of a 3-coil inductive link for efficient wireless power transmission. IEEE Trans. Biomed. Circuits Syst. 2011, 5, 579–591. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sample, A.P.; Meyer, D.T.; Smith, J.R. Analysis, experimental results, and range adaptation of magnetically coupled resonators for wireless power transfer. IEEE Trans. Ind. Electron. 2010, 58, 544–554. [Google Scholar] [CrossRef]
- Khan, S.R.; Choi, G. Analysis and optimization of four coil planar magnetically coupled printed spiral resonators. Sensors 2016, 16, 1219. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhong, W.X.; Zhang, C.; Liu, X.; Hui, S.Y.R. A methodology for making a three-coil wireless power transfer system more energy efficient than a two-coil counterpart for extended transfer distance. IEEE Trans. Power Electron. 2014, 30, 933–942. [Google Scholar] [CrossRef]
- Mirbozorgi, S.A.; Gosselin, B.; Sawan, M. A transcutaneous power transfer interface based on a multicoil inductive link. IEEE EMBS 2012, 1659–1662. [Google Scholar]
- Bagheri, A.; Erfanian, A.; Abrishamifar, A. A Systematic Methodology for Optimal Design of Wireless Power Transfer System Using Genetic Algorithm. Energies 2020, 13, 383. [Google Scholar] [CrossRef] [Green Version]
- Ohira, T. What in the World Is Q? IEEE Microw. Mag. 2016, 17, 42–49. [Google Scholar] [CrossRef]
- Nair, V.V.; Choi, J.R. An efficiency enhancement technique for a wireless power transmission system based on a multiple coil switching technique. Energies 2016, 9, 156. [Google Scholar] [CrossRef] [Green Version]
- Betts, J.G.; Young, K.A.; Wise, J.A.; Desaix, P.; Johnson, J.E.; Korol, O.; Kruse, D.; Poe, B.; Womble, M.; DeSaix, P.; et al. Anatomy & Physiology: Openstax College; Rice University: Houston, TX, USA, 2013. [Google Scholar]
- Available online: http://niremf.ifac.cnr.it/tissprop/htmlclie/htmlclie.php (accessed on 15 March 2021).
- Palagani, Y.; Mohanarangam, K.; Shim, J.H.; Choi, J.R. Wireless power transfer analysis of circular and spherical coils under misalignment conditions for biomedical implants. Biosens. Bioelectron. 2019, 141, 111283. [Google Scholar] [CrossRef] [PubMed]
- Gabriel, C.; Gabriel, S.; Corthout, Y.E. The dielectric properties of biological tissues: I. Literature survey. Phys. Med. Biol. 1996, 41, 2231–2249. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Christ, A.; Douglas, M.G.; Roman, J.M.; Cooper, E.B.; Sample, A.P.; Waters, B.H.; Smith, J.R.; Kuster, N. Evaluation of wireless resonant power transfer systems with human electromagnetic exposure limits. IEEE Trans. One Compat. 2012, 55, 265–274. [Google Scholar] [CrossRef]
- Jia, Y.; Mirbozorgi, S.A.; Wang, Z.; Hsu, C.-C.; Madsen, T.E.; Rainnie, D.; Ghovanloo, M. Position and orientation insensitive wireless power transmission for enercage-homecage system. IEEE Trans. Biomed. Eng. 2017, 64, 2439–2449. [Google Scholar] [CrossRef]
- PDutta, K.; Jayasree, P.V.Y.; Baba, V.S.S.N.S. Sar reduction in the modelled human head for the mobile phone using different material shields. Hum. Centric Comput. Inf. Sci. 2016, 6, 3. [Google Scholar]
- Available online: https://www.fcc.gov/general/specific-absorption-rate-sar-cellulartelephones (accessed on 5 January 2021).
- Abiri, P.; Abiri, A.; Packard, R.R.S.; Ding, Y.; Yousefi, A.; Ma, J.; Bersohn, M.; Nguyen, K.-L.; Markovic, D.; Moloudi, S.; et al. Inductively powered wireless pacing via a miniature pacemaker and remote stimulation control system. Sci. Rep. 2017, 7, 6180. [Google Scholar] [CrossRef]





| Parameter | Symbol | |||
|---|---|---|---|---|
| Inductance (nH) | L | 131.2 | 1867.8 | 268.4 |
| Q-Factor | Q | 377 | 610 | 102 |
| Outer diameter (mm) | 62 | 53 | 6 | |
| Inner diameter (mm) | 58 | 4.2 | 5.6 | |
| Length (mm) | l | - | - | 6.5 |
| Number of turns | n | 1 | 9 | 9 |
| Width (mm) | w | 2 | 1 | 0.2 |
| Spacing (mm) | s | - | 1.6 | - |
| Pitch (mm) | p | - | - | 0.5 |
| Weight (g) | - | - | - | 0.05 |
| Distance (mm) | = 10 | = 50 | ||
| Type of wire | Copper | |||
| Frequency (MHz) | 13.56 | |||
| Mutual Inductance (nH) | = 141.2 = 68.5 | |||
| (dB) | −30.9 |
| Tissue Type | Relative Permittivity () | Loss Tangent | Conductivity () |
|---|---|---|---|
| Air | 1.0 | 0.0 | 0.0 |
| Skin (Dry) | 285 | 1.10 | 0.23 |
| Skin (Wet) | 177 | 2.87 | 0.38 |
| Fat | 11.8 | 3.40 | 0.03 |
| Muscle | 138 | 6.01 | 0.62 |
| Bone | 59.3 | 2.87 | 0.12 |
| (cancellous) | |||
| Bone | 30.5 | 1.97 | 0.04 |
| (cortical) | |||
| Heart | 239 | 2.91 | 0.52 |
| Publication | 2016 [5] | 2018 [6] | 2017 [33] | 2015 [10] | 2017 [11] | This Work |
|---|---|---|---|---|---|---|
| Type of device | CCP | CCP | LPS | LCP | LCP | LCP |
| Receiver coil | Circular | Circular | Half | Solenoid | Conformal | Solenoid |
| shape | cylindrical | spiral | ||||
| Receiver coil | 35.6 | 44.5 × 30.5 | 3 × 15 | 9 × 20 | 6 × 40 | 6 × 6.5 |
| size (mm) | ||||||
| Operating | 300 kHz & | 300 kHz | 13.56 MHz | 1.5 GHz | 1.5 GHz | 13.56 MHz |
| frequency | 13.56 MHz | |||||
| Distance (mm) | 10 & 10 | 10 | 40 | 55 | 55 | 50 |
| Medium | Saline | Pork | Pig | Pork heart | Pork heart | Pork heart |
| Efficiency (%) | 78 & 89 | 78.4 | ≈0.2 | 0.58 | 0.3 | ≈0.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohanarangam, K.; Palagani, Y.; Cho, K.; Choi, J.-R. Inductive Power Transfer Link at 13.56 MHz for Leadless Cardiac Pacemakers. Energies 2021, 14, 5436. https://doi.org/10.3390/en14175436
Mohanarangam K, Palagani Y, Cho K, Choi J-R. Inductive Power Transfer Link at 13.56 MHz for Leadless Cardiac Pacemakers. Energies. 2021; 14(17):5436. https://doi.org/10.3390/en14175436
Chicago/Turabian StyleMohanarangam, Krithikaa, Yellappa Palagani, Kunhee Cho, and Jun-Rim Choi. 2021. "Inductive Power Transfer Link at 13.56 MHz for Leadless Cardiac Pacemakers" Energies 14, no. 17: 5436. https://doi.org/10.3390/en14175436
APA StyleMohanarangam, K., Palagani, Y., Cho, K., & Choi, J.-R. (2021). Inductive Power Transfer Link at 13.56 MHz for Leadless Cardiac Pacemakers. Energies, 14(17), 5436. https://doi.org/10.3390/en14175436







