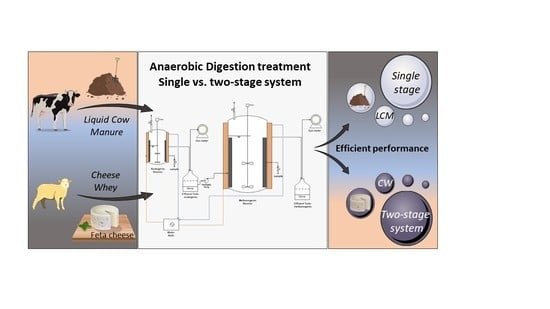
Assessment of Single- vs. Two-Stage Process for the Anaerobic Digestion of Liquid Cow Manure and Cheese Whey
Abstract
:1. Introduction
2. Materials and Methods
2.1. Substrates
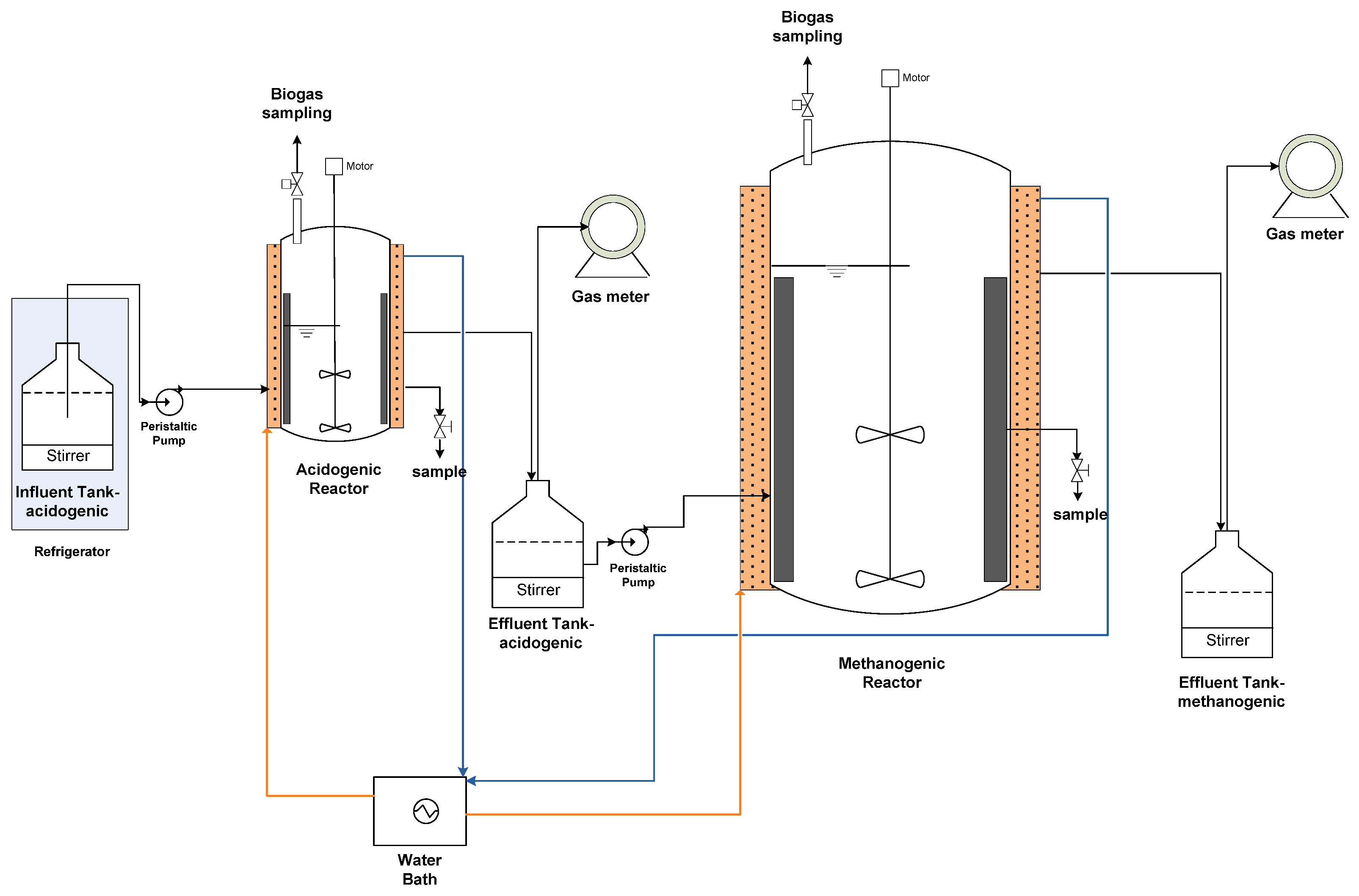
2.2. Experimental Setup
2.3. Reactors Operation
2.4. Analytical Determinations
3. Results and Discussion
3.1. Chemical Composition of the Tested Wastewaters
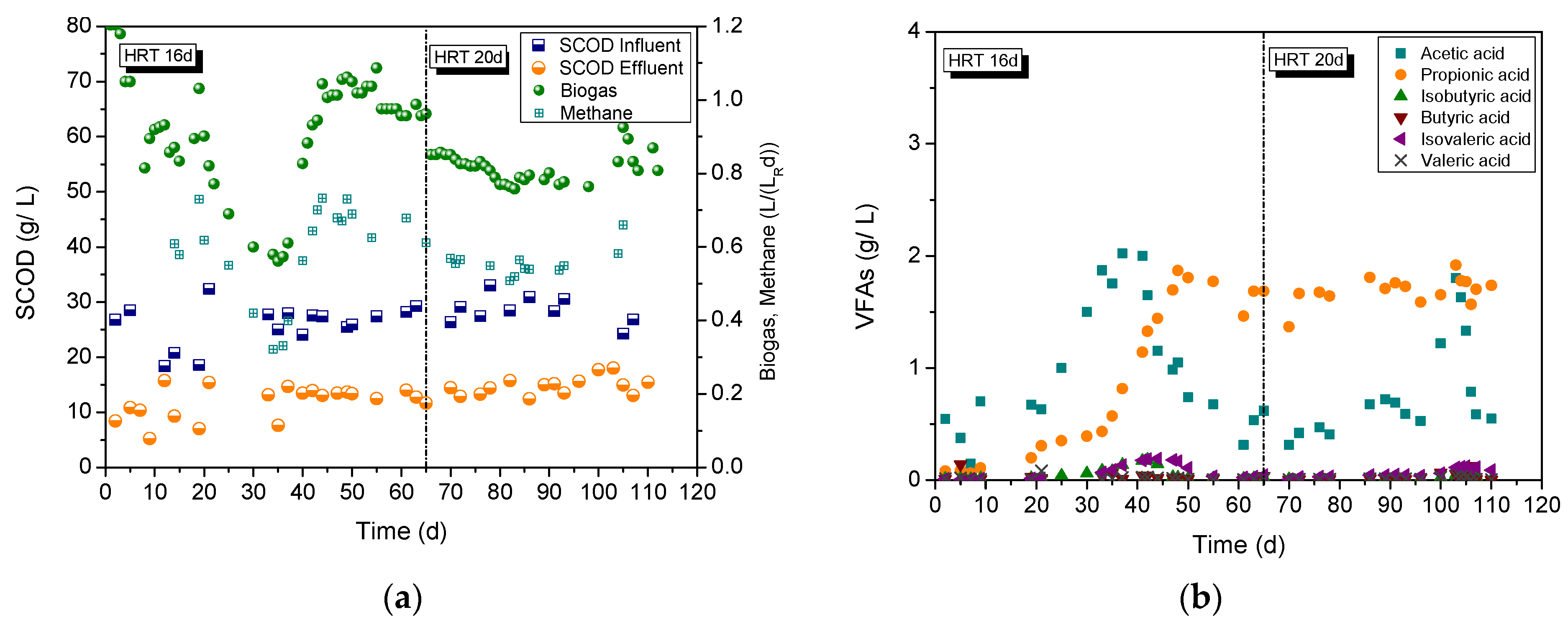
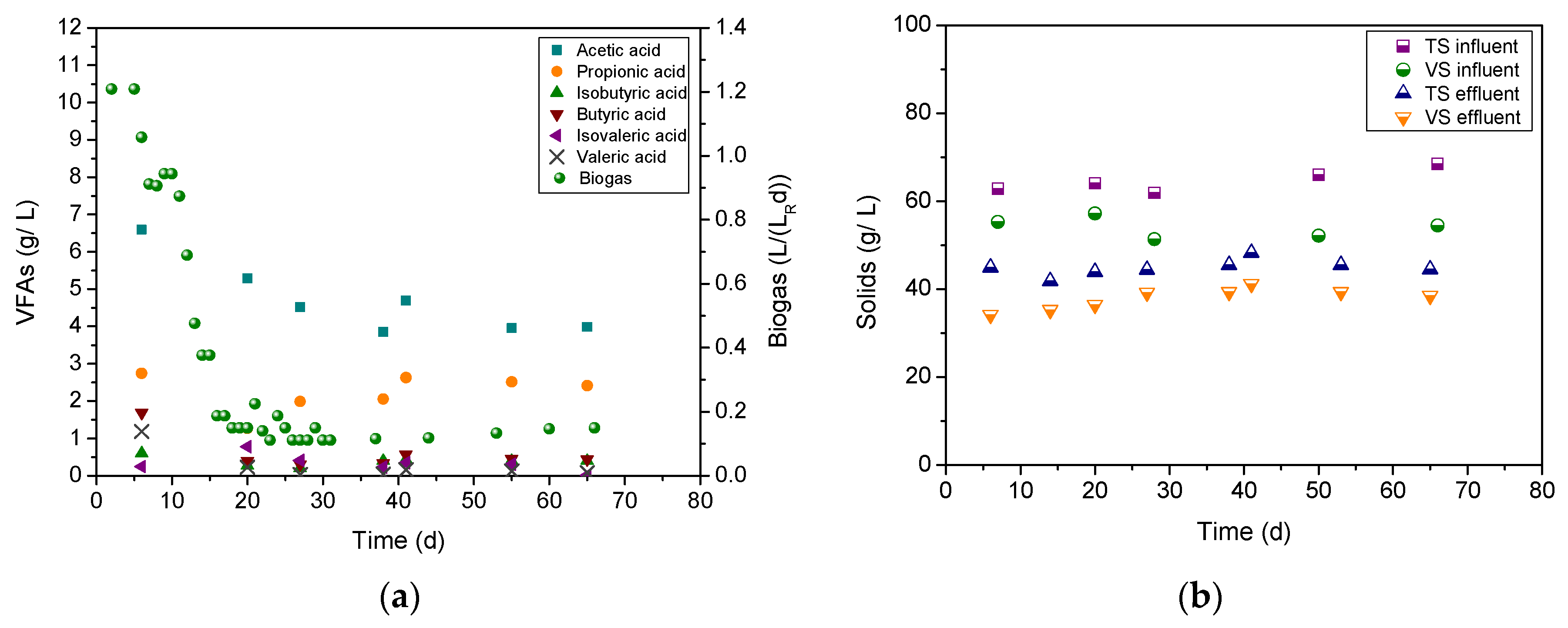
3.2. Liquid Cow Manure Treatment
3.3. Cheese Whey Treatment
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kolev Slavov, A. General characteristics and treatment possibilities of dairy wastewater–a review. Food Technol. Biotechnol. 2017, 55, 14–28. [Google Scholar] [CrossRef]
- Dareioti, M.A.; Dokianakis, S.N.; Stamatelatou, K.; Zafiri, C.; Kornaros, M. Biogas production from anaerobic co-digestion of agroindustrial wastewaters under mesophilic conditions in a two-stage process. Desalination 2009, 248, 891–906. [Google Scholar] [CrossRef]
- Coyette, C.; Schenk, H. Agriculture, Forestry and Fishery Statistics; Publications Office of the European Union: Luxembourg, 2013; ISBN 9279330055. [Google Scholar]
- Asunis, F.; De Gioannis, G.; Dessì, P.; Isipato, M.; Lens, P.N.L.; Muntoni, A.; Polettini, A.; Pomi, R.; Rossi, A.; Spiga, D. The dairy biorefinery: Integrating treatment processes for cheese whey valorisation. J. Environ. Manag. 2020, 276, 111240. [Google Scholar] [CrossRef]
- Andrio, D.; Asmura, J.; Yenie, E.; Putri, K. Enhancing BOD5/COD ratio co-substrate tofu wastewater and cow dung during ozone pretreatment. In Proceedings of the MATEC Web of Conferences, Online, 15 March 2019; EDP Sciences: Paris, France, 2019; Volume 276, p. 6027. [Google Scholar]
- Chatzipaschali, A.A.; Stamatis, A.G. Biotechnological utilization with a focus on anaerobic treatment of cheese whey: Current status and prospects. Energies 2012, 5, 3492–3525. [Google Scholar] [CrossRef] [Green Version]
- Venetsaneas, N.; Antonopoulou, G.; Stamatelatou, K.; Kornaros, M.; Lyberatos, G. Using cheese whey for hydrogen and methane generation in a two-stage continuous process with alternative pH controlling approaches. Bioresour. Technol. 2009, 100, 3713–3717. [Google Scholar] [CrossRef]
- Berendes, D.M.; Yang, P.J.; Lai, A.; Hu, D.; Brown, J. Estimation of global recoverable human and animal faecal biomass. Nat. Sustain. 2018, 1, 679–685. [Google Scholar] [CrossRef]
- Page, L.H.; Ni, J.-Q.; Heber, A.J.; Mosier, N.S.; Liu, X.; Joo, H.-S.; Ndegwa, P.M.; Harrison, J.H. Characteristics of volatile fatty acids in stored dairy manure before and after anaerobic digestion. Biosyst. Eng. 2014, 118, 16–28. [Google Scholar] [CrossRef]
- Martínez-Dalmau, J.; Berbel, J.; Ordóñez-Fernández, R. Nitrogen fertilization. A review of the risks associated with the inefficiency of its use and policy responses. Sustainability 2021, 13, 5625. [Google Scholar] [CrossRef]
- Sahoo, P.K.; Kim, K.; Powell, M.A. Managing groundwater nitrate contamination from livestock farms: Implication for nitrate management guidelines. Curr. Pollut. Rep. 2016, 2, 178–187. [Google Scholar] [CrossRef] [Green Version]
- Aneja, V.P.; Schlesinger, W.H.; Erisman, J.W. Effects of agriculture upon the air quality and climate: Research, policy, and regulations. Environ. Sci. Technol. 2009, 43, 4234–4240. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rayne, N.; Aula, L. Livestock manure and the impacts on soil health: A review. Soil Syst. 2020, 4, 64. [Google Scholar] [CrossRef]
- EBA EBA, European Biogas Association. Available online: https://www.europeanbiogas.eu/european-commission-gives-green-light-for-biogas-on-access-to-sustainable-finance (accessed on 1 August 2021).
- Cremonez, P.A.; Teleken, J.G.; Meier, T.R.W.; Alves, H.J. Two-Stage anaerobic digestion in agroindustrial waste treatment: A review. J. Environ. Manag. 2021, 281, 111854. [Google Scholar] [CrossRef]
- Van, D.P.; Fujiwara, T.; Tho, B.L.; Toan, P.P.S.; Minh, G.H. A review of anaerobic digestion systems for biodegradable waste: Configurations, operating parameters, and current trends. Environ. Eng. Res. 2020, 25, 1–17. [Google Scholar] [CrossRef] [Green Version]
- Srisowmeya, G.; Chakravarthy, M.; Devi, G.N. Critical considerations in two-stage anaerobic digestion of food waste–A review. Renew. Sustain. Energy Rev. 2019, 109587. [Google Scholar] [CrossRef]
- Dareioti, M.A.; Kornaros, M. Anaerobic mesophilic co-digestion of ensiled sorghum, cheese whey and liquid cow manure in a two-stage CSTR system: Effect of hydraulic retention time. Bioresour. Technol. 2015, 175, 553–562. [Google Scholar] [CrossRef]
- Tsigkou, K.; Tsafrakidou, P.; Kopsahelis, A.; Zagklis, D.; Zafiri, C.; Kornaros, M. Used disposable nappies and expired food products valorisation through one- & two-stage anaerobic co-digestion. Renew. Energy 2020, 147. [Google Scholar] [CrossRef]
- Negri, C.; Ricci, M.; Zilio, M.; D’Imporzano, G.; Qiao, W.; Dong, R.; Adani, F. Anaerobic digestion of food waste for bio-energy production in China and Southeast Asia: A review. Renew. Sustain. Energy Rev. 2020, 133, 110138. [Google Scholar] [CrossRef]
- Kavacik, B.; Topaloglu, B. Biogas production from co-digestion of a mixture of cheese whey and dairy manure. Biomass Bioenergy 2010, 34, 1321–1329. [Google Scholar] [CrossRef]
- Kopsahelis, A.; Stavropoulos, K.; Zafiri, C.; Kornaros, M. Anaerobic co-digestion of End-of-Life dairy products with agroindustrial wastes in a mesophilic pilot-scale two-stage system: Assessment of system’s performance. Energy Convers. Manag. 2018, 165, 851–860. [Google Scholar] [CrossRef]
- Tsigkou, K.; Tsafrakidou, P.; Zagklis, D.; Panagiotouros, A.; Sionakidis, D.; Zontos, D.M.; Zafiri, C.; Kornaros, M. Used disposable nappies and expired food products co-digestion: A pilot-scale system assessment. Renew. Energy 2021, 165, 109–117. [Google Scholar] [CrossRef]
- Micolucci, F.; Gottardo, M.; Pavan, P.; Cavinato, C.; Bolzonella, D. Pilot scale comparison of single and double-stage thermophilic anaerobic digestion of food waste. J. Clean. Prod. 2018, 171, 1376–1385. [Google Scholar] [CrossRef]
- Rabii, A.; Aldin, S.; Dahman, Y.; Elbeshbishy, E. A review on anaerobic co-digestion with a focus on the microbial populations and the effect of multi-stage digester configuration. Energies 2019, 12, 1106. [Google Scholar] [CrossRef] [Green Version]
- Dareioti, M.A.; Dokianakis, S.N.; Stamatelatou, K.; Zafiri, C.; Kornaros, M. Exploitation of olive mill wastewater and liquid cow manure for biogas production. Waste Manag. 2010, 30, 1841–1848. [Google Scholar] [CrossRef]
- Mariakakis, I.; Bischoff, P.; Krampe, J.; Meyer, C.; Steinmetz, H. Effect of organic loading rate and solids retention time on microbial population during bio-hydrogen production by dark fermentation in large lab-scale. Int. J. Hydrog. Energy 2011, 36, 10690–10700. [Google Scholar] [CrossRef]
- Eaton, A.D.; Clesceri, L.S.; Greenberg, A.E.; Franson, M.A.H. Standard Methods for the Examination of Water and Wastewater, 22nd ed.; American Water Works Association, Water Environment Federation: Washington, DC, USA, 2012. [Google Scholar]
- Joseffson, B. Rapid spectrophotometric determination of total carbohydrates. In Methods of Seawater Analysis; Grasshoff, K., Ehrhardt, M., Kremling, K., Eds.; WILEY-VCH: Weinheim, Germany, 1983; pp. 340–342. [Google Scholar]
- Dareioti, M.A.; Vavouraki, A.I.; Tsigkou, K.; Zafiri, C.; Kornaros, M. Dark Fermentation of sweet sorghum stalks, cheese whey and cow manure mixture: Effect of pH, pretreatment and organic load. Processes 2021, 9, 1017. [Google Scholar] [CrossRef]
- Wang, K.; Yun, S.; Xing, T.; Li, B.; Abbas, Y.; Liu, X. Binary and ternary trace elements to enhance anaerobic digestion of cattle manure: Focusing on kinetic models for biogas production and digestate utilization. Bioresour. Technol. 2021, 323, 124571. [Google Scholar] [CrossRef]
- Flores-Mendoza, A.P.; Hernández-García, H.; Cocotle-Ronzón, Y.; Hernandez-Martinez, E. Methanogenesis of raw cheese whey: pH and substrate–inoculum ratio evaluation at mesophyll temperature range. J. Chem. Technol. Biotechnol. 2020, 95, 1946–1952. [Google Scholar] [CrossRef]
- Nasir, I.M.; Mohd. Ghazi, T.I.; Omar, R.; Idris, A. Bioreactor performance in the anaerobic digestion of cattle manure: A review. Energy Sources Part A Recover. Util. Environ. Eff. 2014, 36, 1476–1483. [Google Scholar] [CrossRef]
- Nielsen, H.B.; Mladenovska, Z.; Westermann, P.; Ahring, B.K. Comparison of two-stage thermophilic (68 C/55 C) anaerobic digestion with one-stage thermophilic (55 C) digestion of cattle manure. Biotechnol. Bioeng. 2004, 86, 291–300. [Google Scholar] [CrossRef]
- Labatut, R.A.; Angenent, L.T.; Scott, N.R. Biochemical methane potential and biodegradability of complex organic substrates. Bioresour. Technol. 2011, 102, 2255–2264. [Google Scholar] [CrossRef] [PubMed]
- Aravani, V.P.; Tsigkou, K.; Kornaros, M.; Papadakis, V.G. Laboratory analyses for assessing the potential for biogas production of various agricultural residues in Greece. Agron. Res. 2021, 19. [Google Scholar] [CrossRef]
- Ahring, B.K.; Ibrahim, A.A.; Mladenovska, Z. Effect of temperature increase from 55 to 65 C on performance and microbial population dynamics of an anaerobic reactor treating cattle manure. Water Res. 2001, 35, 2446–2452. [Google Scholar] [CrossRef]
- Han, Y.; Green, H.; Tao, W. Reversibility of propionic acid inhibition to anaerobic digestion: Inhibition kinetics and microbial mechanism. Chemosphere 2020, 255, 126840. [Google Scholar] [CrossRef]
- Chen, Y.; Cheng, J.J.; Creamer, K.S. Inhibition of anaerobic digestion process: A review. Bioresour. Technol. 2008, 99, 4044–4064. [Google Scholar] [CrossRef]
- Dereli, R.K.; van der Zee, F.P.; Ozturk, I.; van Lier, J.B. Treatment of cheese whey by a cross-flow anaerobic membrane bioreactor: Biological and filtration performance. Environ. Res. 2019, 168, 109–117. [Google Scholar] [CrossRef] [Green Version]
- Yuan, H.; Zhu, N. Progress in inhibition mechanisms and process control of intermediates and by-products in sewage sludge anaerobic digestion. Renew. Sustain. Energy Rev. 2016, 58, 429–438. [Google Scholar] [CrossRef]
- Weiland, P. Biogas production: Current state and perspectives. Appl. Microbiol. Biotechnol. 2010, 85, 849–860. [Google Scholar] [CrossRef]
- Bertin, L.; Grilli, S.; Spagni, A.; Fava, F. Innovative two-stage anaerobic process for effective codigestion of cheese whey and cattle manure. Bioresour. Technol. 2013, 128, 779–783. [Google Scholar] [CrossRef]
- Vivekanand, V.; Mulat, D.G.; Eijsink, V.G.H.; Horn, S.J. Synergistic effects of anaerobic co-digestion of whey, manure and fish ensilage. Bioresour. Technol. 2018, 249, 35–41. [Google Scholar] [CrossRef]
- Lagoa-Costa, B.; Kennes, C.; Veiga, M.C. Cheese whey fermentation into volatile fatty acids in an anaerobic sequencing batch reactor. Bioresour. Technol. 2020, 308, 123226. [Google Scholar] [CrossRef]
- Elbeshbishy, E.; Dhar, B.R.; Nakhla, G.; Lee, H.S. A critical review on inhibition of dark biohydrogen fermentation. Renew. Sustain. Energy Rev. 2017, 79, 656–668. [Google Scholar] [CrossRef]
- Tsigkou, K.; Tsafrakidou, P.; Athanasopoulou, S.; Zafiri, C.; Kornaros, M. Effect of pH on the anaerobic fermentation of fruit/vegetables and disposable nappies hydrolysate for bio-hydrogen production. Waste Biomass Valorization 2020, 11, 539–551. [Google Scholar] [CrossRef]
- Zagklis, D.; Papadionysiou, M.; Tsigkou, K.; Tsafrakidou, P.; Zafiri, C.; Kornaros, M. Effect of pH on the economic potential of dark fermentation products from used disposable nappies and expired food products. Appl. Sci. 2021, 11, 4099. [Google Scholar] [CrossRef]
- Dareioti, M.A.; Vavouraki, A.I.; Kornaros, M. Effect of pH on the anaerobic acidogenesis of agroindustrial wastewaters for maximization of bio-hydrogen production: A lab-scale evaluation using batch tests. Bioresour. Technol. 2014, 162, 218–227. [Google Scholar] [CrossRef]
- Wang, K.; Yin, J.; Shen, D.; Li, N. Anaerobic digestion of food waste for volatile fatty acids (VFAs) production with different types of inoculum: Effect of pH. Bioresour. Technol. 2014, 161, 395–401. [Google Scholar] [CrossRef]
- Ghaly, A.E.; Ramkumar, D.R. Controlling the pH of acid cheese whey in a two-stage anaerobic digester with sodium hydroxide. Energy Sources 1999, 21, 475–502. [Google Scholar]
- Saddoud, A.; Hassaïri, I.; Sayadi, S. Anaerobic membrane reactor with phase separation for the treatment of cheese whey. Bioresour. Technol. 2007, 98, 2102–2108. [Google Scholar] [CrossRef]
- Sakarika, M.; Stavropoulos, K.; Kopsahelis, A.; Koutra, E.; Zafiri, C.; Kornaros, M. Two-stage anaerobic digestion harnesses more energy from the co-digestion of end-of-life dairy products with agro-industrial waste compared to the single-stage process. Biochem. Eng. J. 2020, 153, 107404. [Google Scholar] [CrossRef]
- Yang, K.; Yu, Y.; Hwang, S. Selective optimization in thermophilic acidogenesis of cheese-whey wastewater to acetic and butyric acids: Partial acidification and methanation. Water Res. 2003, 37, 2467–2477. [Google Scholar] [CrossRef]


| Operating Conditions | Experiments | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| E1 | E2 | E3 | E4 | ||||||
| System type | Two-stage | Single-stage | Single-stage | Two-stage | |||||
| Phase | Ac. | Met. | Met. | Met. | Ac. | Met. | |||
| Substrate | LCM | CW | |||||||
| HRT (d) | 3 | 16 | 16 | 20 | 20 | 30 | 40 | 3 | 20 |
| Flow rate (mL/d) | 250 | 250 | 250 | 200 | 200 | 150 | 100 | 250 | 200 |
| OLR (kg VS/(m3·d)) | 16.27 | 3.05 | 3.05 | 2.31 | 2.36 | 1.57 | 1.18 | 15.73 | 2.36 |
| OLR (kg COD/(m3·d)) | 20.33 | 3.81 | 3.81 | 3.05 | 3.83 | 2.55 | 1.91 | 19.12 | 3.83 |
| Parameters | Units | CW | LCM |
|---|---|---|---|
| pH | - | 6.10 ± 0.03 | 7.70 ± 0.06 |
| TS | g/L | 64.67 ± 2.62 | 69.29 ± 1.31 |
| VS | g/L | 54.09 ± 2.37 | 47.05 ± 0.69 |
| tCOD | g O2/L | 76.46 ± 1.99 | 60.09 ± 1.06 |
| sCOD | g O2/L | 58.46 ± 2.52 | 26.65 ± 0.19 |
| BOD5 | g O2/L | 36.00 ± 0.53 | 19.72 ± 0.24 |
| Total carbohydrates | g equiv.glucose/L | 54.80 ± 1.84 | 13.72 ± 0.95 |
| Soluble carbohydrates | g equiv.glucose/L | 29.20 ± 0.92 | 0.96 ± 0.03 |
| Total Kjeldahl Nitrogen, TKN | g/L | 0.73 ± 0.02 | 3.36 ± 0.00 |
| Ammonium Nitrogen | g/L | 0.10 ± 0.01 | 1.54 ± 0.02 |
| Proteins | g/L | 4.56 ± 0.13 | 21.00 ± 0.00 |
| Oil and fats | g/L | 0.09 ± 0.00 | 3.24 ± 0.04 |
| Total phosphorus | g/L | 0.32 ± 0.00 | 0.66 ± 0.01 |
| Soluble phosphorus | g/L | 0.20 ± 0.00 | 0.02 ± 0.00 |
| Alkalinity | g CaCO3/L | 0.80 ± 0.00 | 12.38 ± 0.32 |
| tVFAs | g/L | 0.00 ± 0.00 | 10.41 ± 1.63 |
| Substrate | LCM | CW | |
|---|---|---|---|
| HRT (d) | 16 | 20 | 20 |
| pH | 7.85 ± 0.10 | 7.85 ± 0.10 | 7.31 ± 0.03 |
| Biogas (L/(LR·d)) | 1.01 ± 0.04 | 0.81 ± 0.04 | 1.47 ± 0.05 |
| CH4 (L/(LR·d)) | 0.67 ± 0.04 | 0.56 ± 0.04 | 0.68 ± 0.07 |
| CH4 (%) | 66.60 ± 2.67 | 68.96 ± 3.01 | 49.53 ± 1.24 |
| Yield CH4 (mL CH4/g VSadded) | 219.63 | 242.16 | 290.36 |
| Yield CH4 (mL CH4/g CODadded) | 175.74 | 183.61 | 180.35 |
| Alkalinity (g CaCO3/L) | 22.77 ± 1.08 | 25.12 ± 0.19 | 235.72 |
| tCOD removal (%) | 20.10 ± 1.45 | 20.44 ± 2.68 | 76 ± 4.11 |
| sCOD removal (%) | 49.89 ± 0.79 | 48.16 ± 1.12 | 70.68 ± 2.23 |
| TS removal (%) | 19.92 ± 0.58 | 11.32 ± 0.36 | 32.64 ± 0.92 |
| VS removal (%) | 30.30 ± 1.01 | 19.57 ± 0.66 | 59.55 ± 1.13 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dareioti, M.A.; Vavouraki, A.I.; Tsigkou, K.; Kornaros, M. Assessment of Single- vs. Two-Stage Process for the Anaerobic Digestion of Liquid Cow Manure and Cheese Whey. Energies 2021, 14, 5423. https://doi.org/10.3390/en14175423
Dareioti MA, Vavouraki AI, Tsigkou K, Kornaros M. Assessment of Single- vs. Two-Stage Process for the Anaerobic Digestion of Liquid Cow Manure and Cheese Whey. Energies. 2021; 14(17):5423. https://doi.org/10.3390/en14175423
Chicago/Turabian StyleDareioti, Margarita Andreas, Aikaterini Ioannis Vavouraki, Konstantina Tsigkou, and Michael Kornaros. 2021. "Assessment of Single- vs. Two-Stage Process for the Anaerobic Digestion of Liquid Cow Manure and Cheese Whey" Energies 14, no. 17: 5423. https://doi.org/10.3390/en14175423
APA StyleDareioti, M. A., Vavouraki, A. I., Tsigkou, K., & Kornaros, M. (2021). Assessment of Single- vs. Two-Stage Process for the Anaerobic Digestion of Liquid Cow Manure and Cheese Whey. Energies, 14(17), 5423. https://doi.org/10.3390/en14175423