A Theoretical Study on Reversible Solid Oxide Cells as Key Enablers of Cyclic Conversion between Electrical Energy and Fuel
Abstract
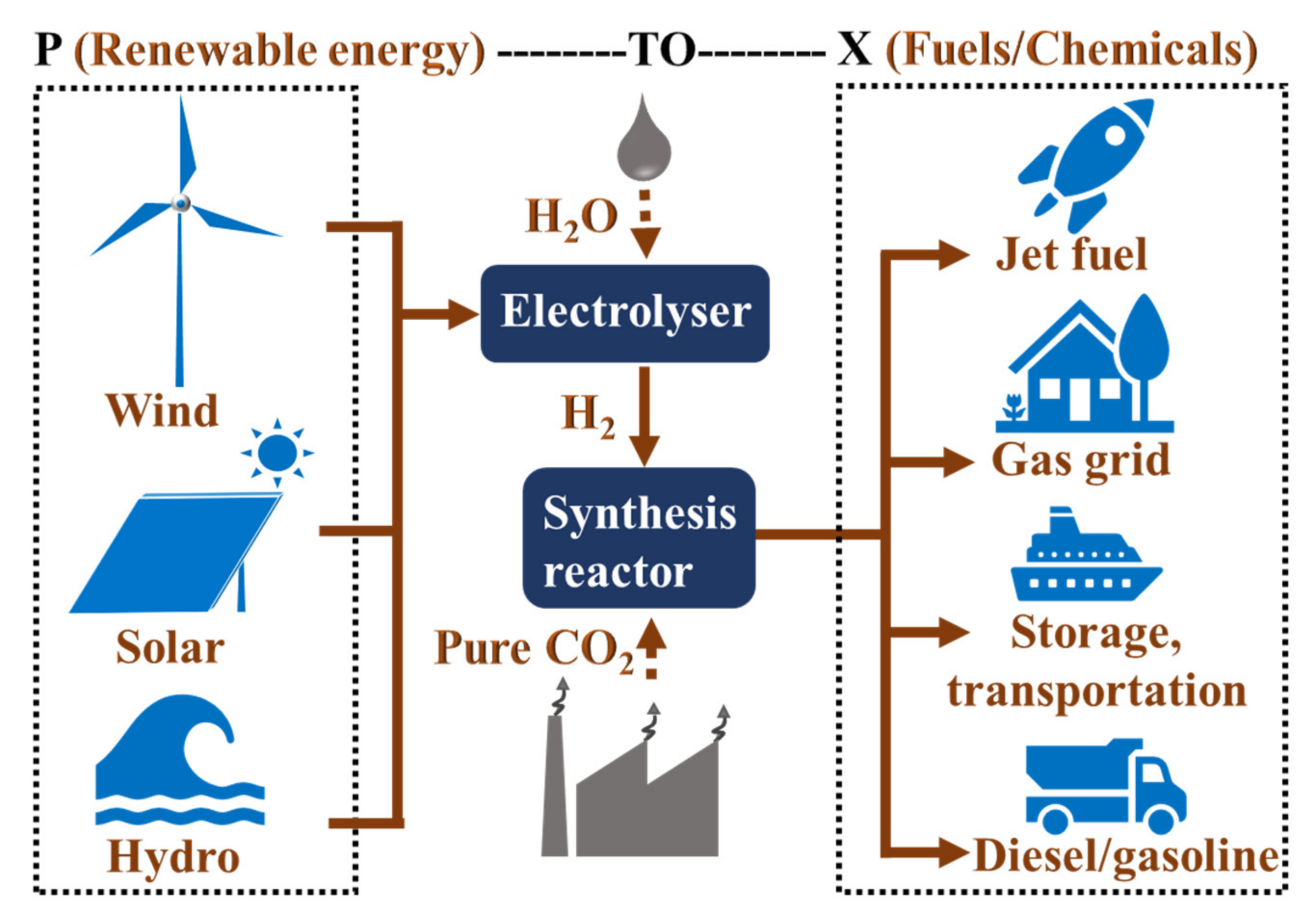
1. Introduction
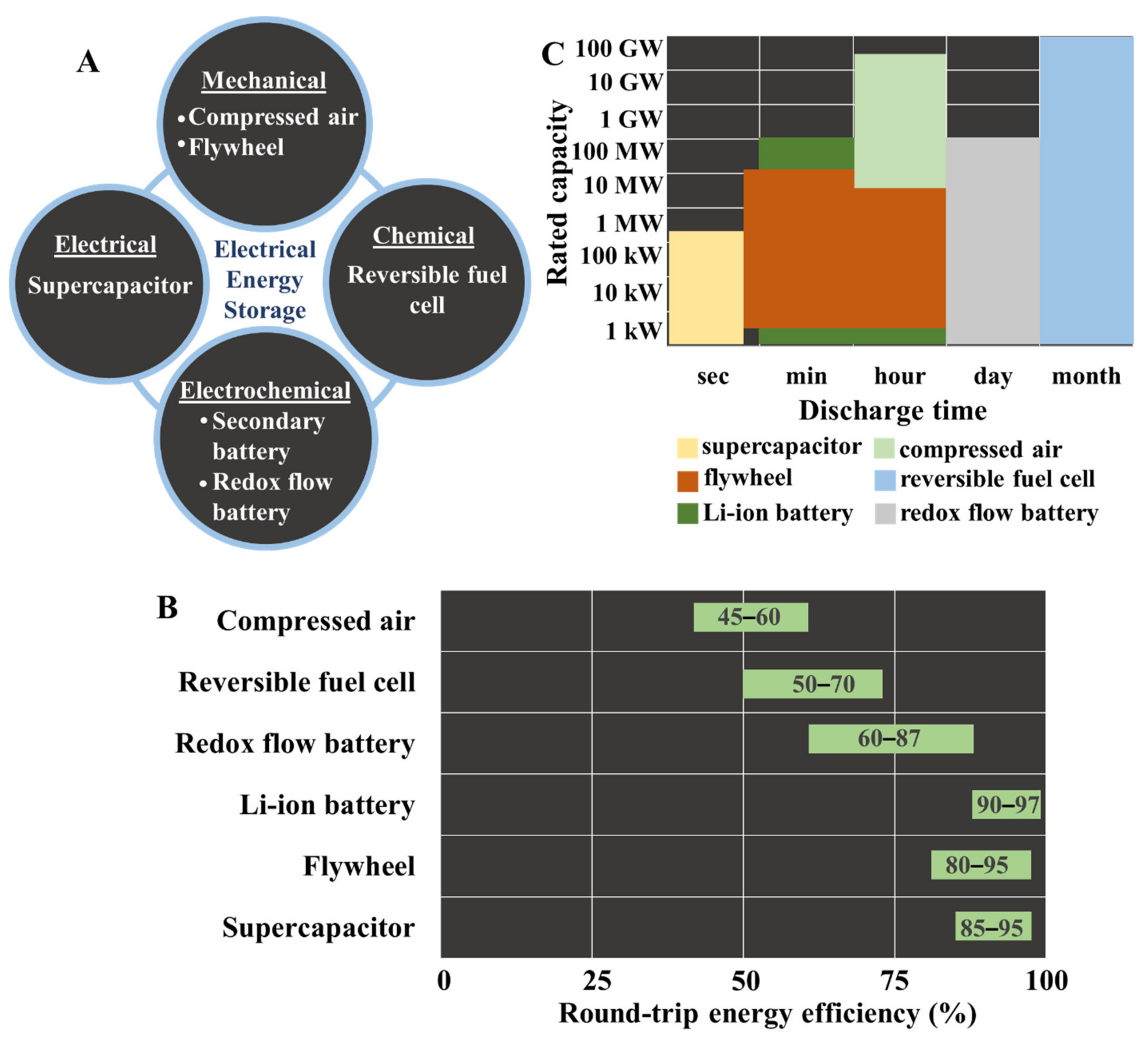
2. Current Electrical Energy Storage Technologies
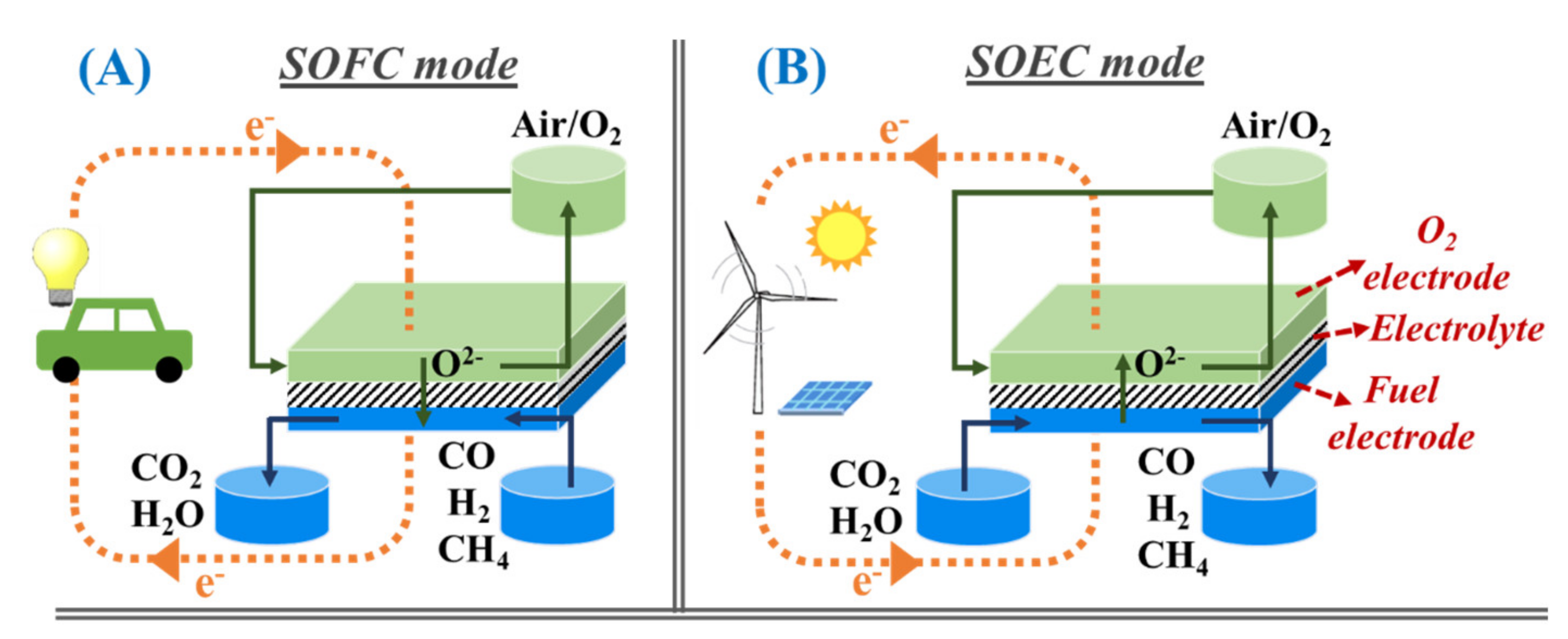
3. Reversible Solid Oxide Cells as a Key Enabler of Cyclic Energy Conversion
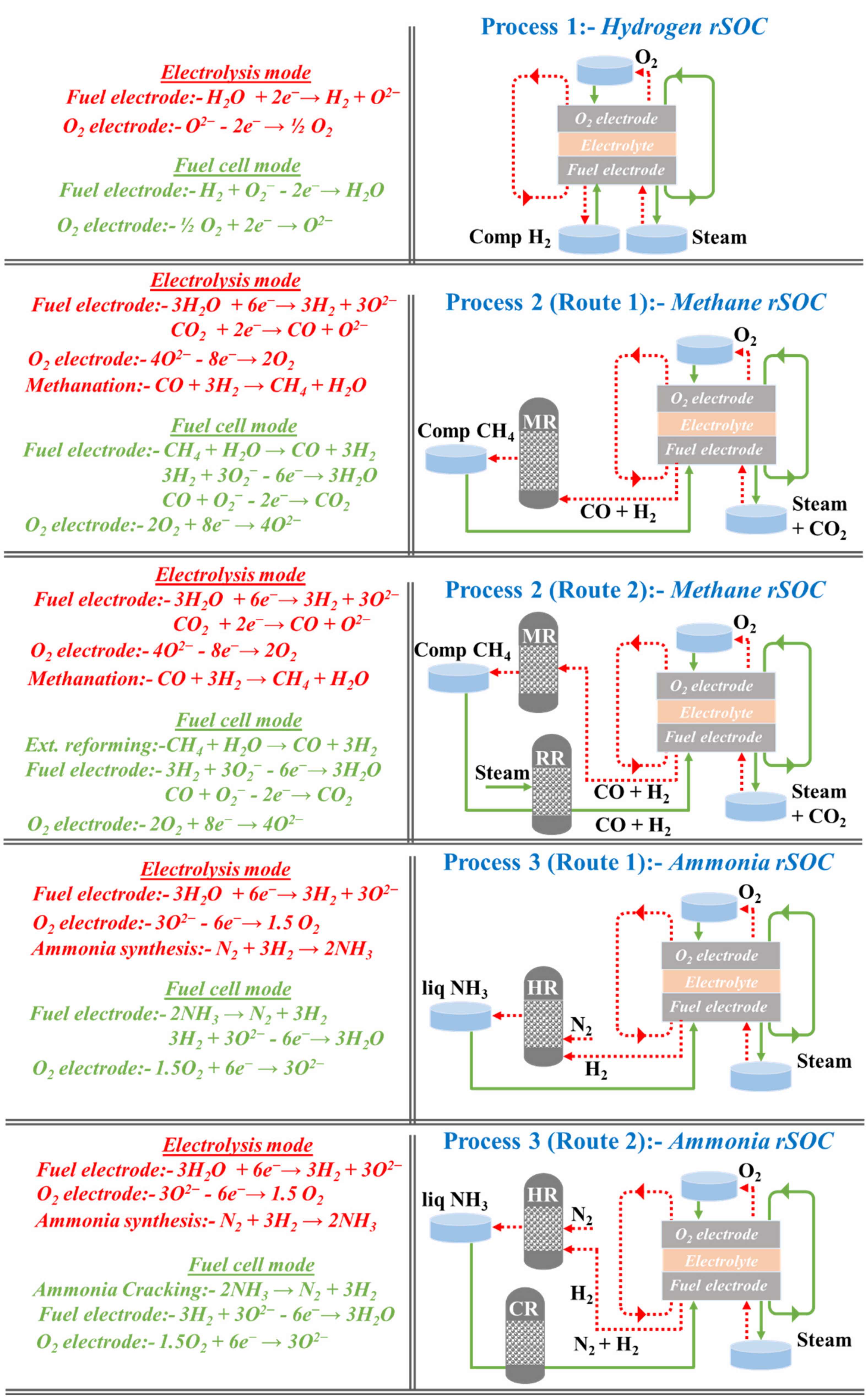
4. Theoretical Round-Trip Energy Efficiency of rSOC with Hydrogen, Methane, and Ammonia
4.1. Methodology
- SOC is in thermal equilibrium with the feed (steam or steam/CO2 mixture) and is thermally insulated so as to avoid any heat losses to the ambient.
- Energy efficiencies are based on heat recycled back from the HR or MR to the SOC.
- LHV has been used for methane (50.0 KJ/g), ammonia (18.6 KJ/g), and hydrogen (120.0 KJ/g).
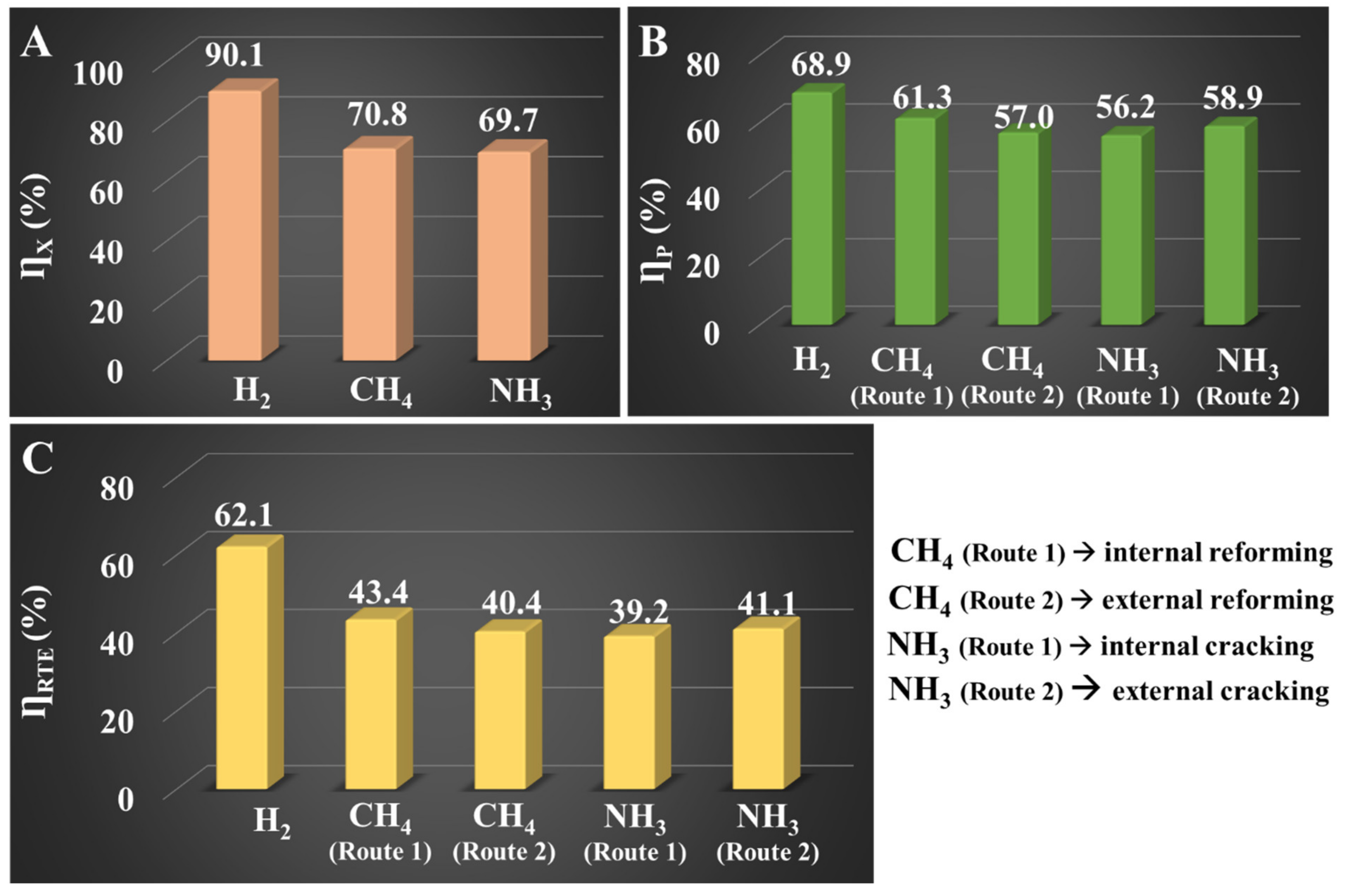
4.2. Results
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shafiee, S.; Topal, E. When will fossil fuel reserves be diminished? Energy Policy 2009, 37, 181–189. [Google Scholar] [CrossRef]
- Newell, R.; Iler, S.; Raimi, D. Global Energy Outlook Comparison Methods: 2019 Update; Resources for the Future: Washington, DC, USA, 2018. [Google Scholar]
- Murdock, H.E.; Gibb, D.; André, T. Renewables 2019 Global Status Report; Ren21: Paris, France, 2019. [Google Scholar]
- Shaughnessy, E.J.; Heeter, J.S.; Gattaciecca, J.; Sauer, J.; Trumbull, K.; Chen, E.I. Community Choice Aggregation: Challenges, Opportunities, and Impacts on Renewable Energy Markets; National Renewable Energy Lab.: Golden, CO, USA, 2019. [Google Scholar]
- International Energy Agency. Global Energy Review 2020; OECD Publishing: Paris, France, 2020. [Google Scholar]
- Rego de Vasconcelos, B.; Lavoie, J.-M. Recent advances in Power-to-X technology for the production of fuels and chemicals. Front. Chem. 2019, 7, 392. [Google Scholar] [CrossRef]
- Foit, S.R.; Vinke, I.C.; de Haart, L.G.; Eichel, R.A. Power-to-Syngas: An Enabling Technology for the Transition of the Energy System? Angew. Chem. Int. Ed. 2017, 56, 5402–5411. [Google Scholar] [CrossRef]
- Fasihi, M.; Efimova, O.; Breyer, C.J. Techno-economic assessment of CO2 direct air capture plants. J. Clean. Prod. 2019, 224, 957–980. [Google Scholar] [CrossRef]
- Choi, Y.H.; Jang, Y.J.; Park, H.; Kim, W.Y.; Lee, Y.H.; Choi, S.H.; Lee, J.S. Carbon dioxide Fischer-Tropsch synthesis: A new path to carbon-neutral fuels. Appl. Catal. B Environ. 2017, 202, 605–610. [Google Scholar] [CrossRef]
- Wei, J.; Ge, Q.; Yao, R.; Wen, Z.; Fang, C.; Guo, L.; Xu, H.; Sun, J. Directly converting CO2 into a gasoline fuel. Nat. Commun. 2017, 8, 1–9. [Google Scholar] [CrossRef]
- Schmidt, P.; Batteiger, V.; Roth, A.; Weindorf, W.; Raksha, T. Power-to-Liquids as Renewable Fuel Option for Aviation: A Review. Chem. Ing. Tech. 2018, 90, 127–140. [Google Scholar] [CrossRef]
- IRENA International Renewable Energy Agency. Renewable Energy Target Setting; The International Renewable Energy Agency: Abu Dhabi, United Arab Emirates, 2018. [Google Scholar]
- Burke, K.A. Unitized Regenerative Fuel Cell System Development. In Proceedings of the 1st International Energy Conversion Engineering Conference (IECEC), Portsmouth, VA, USA, 17–21 August 2003. [Google Scholar]
- Mitlitsky, F.; Myers, B.; Weisberg, A.H.; Molter, T.M.; Smith, W.F. Reversible (unitised) PEM fuel cell devices. Fuel Cells Bull. 1999, 2, 6–11. [Google Scholar] [CrossRef]
- Gabbasa, M.; Sopian, K.; Fudholi, A.; Asim, N. A review of unitized regenerative fuel cell stack: Material, design and research achievements. Int. J. Hydrogen Energy 2014, 39, 17765–17778. [Google Scholar] [CrossRef]
- Park, S.; Shao, Y.; Liu, J.; Wang, Y. Oxygen electrocatalysts for water electrolyzers and reversible fuel cells: Status and perspective. Energy Environ. Sci. 2012, 5, 9331–9344. [Google Scholar] [CrossRef]
- Fang, Q.; Packbier, U.; Blum, L. Long-term tests of a Jülich planar short stack with reversible solid oxide cells in both fuel cell and electrolysis modes. Int. J. Hydrogen Energy 2013, 38, 4281–4290. [Google Scholar]
- Guan, J.; Ramamurthi, B.; Ruud, J.; Hong, J.; Riley, P.; Minh, N. High Performance Flexible Reversible Solid Oxide Fuel Cell; Final Report for DOE Cooperative Agreement DE-FC36-04GO-14351; GE Global Research Center: Niskayuna, NY, USA, 2006. [Google Scholar]
- Tang, E.; Wood, T.; Benhaddad, S.; Brown, C.; He, H.; Nelson, J.; Grande, O.; Nuttall, B.; Richards, M.; Petri, R. Advanced Materials for RSOFC Dual Operation with Low Degradation; Report for United States Department of Energy; Versa Power Systems: Littleton, CO, USA, 2012. [Google Scholar]
- Erdle, E.; Dönitz, W.; Schamm, R.; Koch, A. Reversibility and polarization behaviour of high temperature solid oxide electrochemical cells. Int. J. Hydrogen Energy 1992, 17, 817–819. [Google Scholar] [CrossRef]
- Shimaki, R.; Okamoto, M.; Yanagi, C.; Kikuoka, Y.; Ueda, S.; Nakamori, N.; Kugimiya, K.; Yoshino, M.; Tokura, M.; Suda, S. Feasibility study on hydrogen-utilized electric power storage systems. In Proceedings of the World Hydrogen Energy Conference, Paris, France, 22–25 June 1992; Societe des Ingenieurs et Scientifiques de France: Paris, France, 1993; Volume 3, pp. 1927–1935. [Google Scholar]
- Kusunoki, D.; Kikuoka, Y.; Yanagi, V.; Kugimiya, K.; Yoshino, M.; Tokura, M.; Watanabe, K.; Miyamoto, H.; Ueda, S.; Tokunaga, S.; et al. Development of Mitsubishi-planar reversible cell—Fundamental test on hydrogen-utilized electric power storage system. Int. J. Hydrogen Energy 1995, 20, 831–834. [Google Scholar] [CrossRef]
- Biswas, S.; Kulkarni, A.; Giddey, S.; Bhattacharya, S.J.E.R. A Review on Synthesis of Methane as a Pathway for Renewable Energy Storage with a Focus on Solid Oxide Electrolytic Cell-Based Processes. Front. Front. Energy Res. 2020, 8, 570112. [Google Scholar] [CrossRef]
- Wendel, C.H.; Braun, R.J. Design and techno-economic analysis of high efficiency reversible solid oxide cell systems for distributed energy storage. Appl. Energy 2016, 172, 118–131. [Google Scholar] [CrossRef]
- Luo, X.; Wang, J.; Dooner, M.; Clarke, J. Overview of current development in electrical energy storage technologies and the application potential in power system operation. Appl. Energy. 2015, 137, 511–536. [Google Scholar] [CrossRef]
- Hadjipaschalis, I.; Poullikkas, A.; Efthimiou, V. Overview of current and future energy storage technologies for electric power applications. Renew. Sustain. Energy Rev. 2009, 13, 1513–1522. [Google Scholar] [CrossRef]
- Liu, S.; Wei, L.; Wang, H. Review on reliability of supercapacitors in energy storage applications. Appl. Energy 2020, 278, 115436. [Google Scholar] [CrossRef]
- Olabi, A.; Wilberforce, T.; Ramadan, M.; Abdelkareem, M.A.; Alami, A.H. Compressed air energy storage systems: Components and operating parameters–A review. J. Energy Storage 2020, 34, 102000. [Google Scholar] [CrossRef]
- Li, X.; Palazzolo, A. A review of flywheel energy storage systems: State of the art and opportunities. arXiv 2021, arXiv:210305224. preprint. [Google Scholar]
- Valøen, L.O.; Shoesmith, M.I. The effect of PHEV and HEV duty cycles on battery and battery pack performance. In Proceedings of the Plug-In Hybrid Electric Vehicle Conference: Where the Grid Meets the Road, Winnipeg, MB, Canada, 1–2 November 2007. [Google Scholar]
- El Kharbachi, A.; Zavorotynska, O.; Latroche, M.; Cuevas, F.; Yartys, V.; Fichtner, M. Exploits, advances and challenges benefiting beyond Li-ion battery technologies. J. Alloys Compd. 2020, 817, 153261. [Google Scholar] [CrossRef]
- Weber, A.Z.; Mench, M.M.; Meyers, J.P.; Ross, P.N.; Gostick, J.T.; Liu, Q. Redox flow batteries: A review. J. Appl. Electrochem. 2011, 41, 137. [Google Scholar] [CrossRef]
- Sánchez-Díez, E.; Ventosa, E.; Guarnieri, M.; Trovò, A.; Flox, C.; Marcilla, R.; Soavi, F.; Mazur, P.; Aranzabe, E.; Ferret, R. Redox flow batteries: Status and perspective towards sustainable stationary energy storage. J. Power Source 2021, 481, 228804. [Google Scholar] [CrossRef]
- Martinez-Bolanos, J.R.; Udaeta, M.E.M.; Gimenes, A.L.V.; da Silva, V.O. Economic feasibility of battery energy storage systems for replacing peak power plants for commercial consumers under energy time of use tariffs. J. Energy Storage 2020, 29, 101373. [Google Scholar] [CrossRef]
- Song, S.; Zhang, H.; Ma, X.; Shao, Z.-G.; Zhang, Y.; Yi, B. Bifunctional oxygen electrode with corrosion-resistive gas diffusion layer for unitized regenerative fuel cell. Electrochem. Commun. 2006, 8, 399–405. [Google Scholar] [CrossRef]
- Yim, S.D.; Lee, W.Y.; Yoon, Y.G.; Sohn, Y.J.; Park, G.G.; Yang, T.H.; Kim, C.S. Optimization of bifunctional electrocatalyst for PEM unitized regenerative fuel cell. Electrochim. Acta 2004, 50, 713–718. [Google Scholar] [CrossRef]
- Carmo, M.; Fritz, D.L.; Mergel, J.; Stolten, D. A comprehensive review on PEM water electrolysis. Int. J. Hydrogen Energy 2013, 38, 4901–4934. [Google Scholar] [CrossRef]
- Yao, W.; Yang, J.; Wang, J.; Nuli, Y. Chemical deposition of platinum nanoparticles on iridium oxide for oxygen electrode of unitized regenerative fuel cell. Electrochem. Commun. 2007, 9, 1029–1034. [Google Scholar] [CrossRef]
- Wu, J.; Yuan, X.Z.; Martin, J.J.; Wang, H.; Zhang, J.; Shen, J.; Merida, W.; Wu, S. A review of PEM fuel cell durability: Degradation mechanisms and mitigation strategies. J. Power Source 2008, 184, 104–119. [Google Scholar] [CrossRef]
- Doenitz, W.; Schmidberger, R. Concepts and design for scaling up high temperature water vapour electrolysis. Int. J. Hydrogen Energy 1982, 7, 321–330. [Google Scholar] [CrossRef]
- Hauch, A.; Jensen, S.H.; Ramousse, S.; Mogensen, M. Performance and durability of solid oxide electrolysis cells. J. Electrochem. Soc. 2006, 153, A1741–A1747. [Google Scholar] [CrossRef]
- Wang, Y.; Leung, D.Y.; Xuan, J.; Wang, H. A review on unitized regenerative fuel cell technologies, part-A: Unitized regenerative proton exchange membrane fuel cells. Renew. Sustain. Energy Rev. 2016, 65, 961–977. [Google Scholar] [CrossRef]
- Ebbesen, S.D.; Mogensen, M. Electrolysis of carbon dioxide in solid oxide electrolysis cells. J. Power Sources 2009, 193, 349–358. [Google Scholar] [CrossRef]
- Xu, S.; Li, S.; Yao, W.; Dong, D.; Xie, K. Direct electrolysis of CO2 using an oxygen-ion conducting solid oxide electrolyzer based on La0. 75Sr0. 25Cr0. 5Mn0. 5O3−δ electrode. J. Power Sources 2013, 230, 115–121. [Google Scholar] [CrossRef]
- Laguna-Bercero, M. Recent advances in high temperature electrolysis using solid oxide fuel cells: A review. J. Power Sources 2012, 203, 4–16. [Google Scholar] [CrossRef]
- Graves, C.; Ebbesen, S.D.; Mogensen, M. Co-electrolysis of CO2 and H2O in solid oxide cells: Performance and durability. Solid State Ionic. 2011, 192, 398–403. [Google Scholar] [CrossRef]
- Smart, W.; Weissbart, J. Study of Electrolytic Dissociation of Co2-H2o Using a Solid Oxide Electrolyte; NASA: Washington, DC, USA, 1967. [Google Scholar]
- Ilbas, M.; Kumuk, B.; Alemu, M.A.; Arslan, B. Numerical investigation of a direct ammonia tubular solid oxide fuel cell in comparison with hydrogen. Int. J. Hydrogen Energy 2020, 45, 35108–35117. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, Y.; Pérez-Fortes, M.; Aubin, P.; Lin, T.E.; Yang, Y.; Maréchal, F. Reversible solid-oxide cell stack based power-to-x-to-power systems: Comparison of thermodynamic performance. Appl. Energy 2020, 275, 115330. [Google Scholar] [CrossRef]
- Lan, R.; Irvine, J.T.; Tao, S. Ammonia and related chemicals as potential indirect hydrogen storage materials. Int. J. Hydrogen Energy 2012, 37, 1482–1494. [Google Scholar] [CrossRef]
- Aziz, M.; Wijayanta, A.T.; Nandiyanto, A.B.D. Ammonia as effective hydrogen storage: A review on production, storage and utilization. Energies 2020, 13, 3062. [Google Scholar] [CrossRef]
- Blum, L.; Deja, R.; Peters, R.; Stolten, D. Comparison of efficiencies of low, mean and high temperature fuel cell systems. Int. J. Hydrogen Energy 2011, 36, 11056–11067. [Google Scholar] [CrossRef]
- Rönsch, S.; Schneider, J.; Matthischke, S.; Schlüter, M.; Götz, M.; Lefebvre, J.; Prabhakaran, P.; Bajohr, S. Review on methanation–From fundamentals to current projects. Fuel 2016, 166, 276–296. [Google Scholar] [CrossRef]
- Meloni, E.; Martino, M.; Palma, V. A short review on Ni based catalysts and related engineering issues for methane steam reforming. Catalysts 2020, 10, 352. [Google Scholar] [CrossRef]
- Gür, T.M. Comprehensive review of methane conversion in solid oxide fuel cells: Prospects for efficient electricity generation from natural gas. Prog. Energy Combust. Sci. 2016, 54, 1–64. [Google Scholar] [CrossRef]
- Hacker, V.; Kordesch, K. Ammonia Crackers. In Handbook of Fuel Cells; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2010. [Google Scholar] [CrossRef]
- Fournier, G.G.M.; Cumming, I.W.; Hellgardt, K. High performance direct ammonia solid oxide fuel cell. J. Power Sources 2006, 162, 198–206. [Google Scholar] [CrossRef]
- Ruhnau, O.; Hirth, L.; Praktiknjo, A. Time series of heat demand and heat pump efficiency for energy system modeling. Sci. Data 2019, 6, 1–10. [Google Scholar] [CrossRef]
- Dokiya, M. SOFC system and technology. Solid State Ionic. 2002, 152, 383–392. [Google Scholar] [CrossRef]
- Hauch, A.; Küngas, R.; Blennow, P.; Hansen, A.B.; Hansen, J.B.; Mathiesen, B.V.; Mogensen, M.B. Recent advances in solid oxide cell technology for electrolysis. Science 2020, 370, eaba6118. [Google Scholar] [CrossRef]
- Wang, Z.; Mori, M.; Araki, T. Steam electrolysis performance of intermediate-temperature solid oxide electrolysis cell and efficiency of hydrogen production system at 300 Nm3 h−1. Int. J. Hydrogen Energy 2010, 35, 4451–4458. [Google Scholar] [CrossRef]
- Diethelm, S.; Herle, J.V.; Montinaro, D.; Bucheli, O. Electrolysis and Co-electrolysis performance of SOE short stacks. Fuel Cells 2013, 13, 631–637. [Google Scholar] [CrossRef]
- Giglio, E.; Lanzini, A.; Santarelli, M.; Leone, P. Synthetic natural gas via integrated high-temperature electrolysis and methanation: Part I—Energy performance. J. Energy Storage 2015, 1, 22–37. [Google Scholar] [CrossRef]
- Santhanam, S.; Heddrich, M.P.; Riedel, M.; Friedrich, K.A. Theoretical and experimental study of Reversible Solid Oxide Cell (r-SOC) systems for energy storage. Energy 2017, 141, 202–214. [Google Scholar] [CrossRef]
- Peters, R.; Deja, R.; Engelbracht, M.; Frank, M.; Blum, L.; Stolten, D. Efficiency analysis of a hydrogen-fueled solid oxide fuel cell system with anode off-gas recirculation. J. Power Source 2016, 328, 105–113. [Google Scholar] [CrossRef]
- Peters, R.; Blum, L.; Deja, R.; Hoven, I.; Tiedemann, W.; Küpper, S.; Stolten, D. Operation experience with a 20 kW SOFC system. Fuel Cells 2014, 14, 489–499. [Google Scholar] [CrossRef]
- Liso, V.; Olesen, A.C.; Nielsen, M.P.; Kær, S.K. Performance comparison between partial oxidation and methane steam reforming processes for solid oxide fuel cell (SOFC) micro combined heat and power (CHP) system. Energy 2011, 36, 4216–4226. [Google Scholar] [CrossRef]
- Baniasadi, E.; Dincer, I. Energy and exergy analyses of a combined ammonia-fed solid oxide fuel cell system for vehicular applications. Int. J. Hydrogen Energy 2011, 36, 11128–11136. [Google Scholar] [CrossRef]
- Kishimoto, M.; Muroyama, H.; Suzuki, S.; Saito, M.; Koide, T.; Takahashi, Y.; Okabe, A.; Ueguchi, S.; Jun, M.; Eguchi, K.; et al. Development of 1 kW-class Ammonia-fueled Solid Oxide Fuel Cell Stack. Fuel Cells 2020, 20, 80–88. [Google Scholar] [CrossRef]
- Giddey, S.; Badwal, S.; Munnings, C.; Dolan, M. Ammonia as a renewable energy transportation media. ACS Sustain. Chem. Eng. 2017, 5, 10231–10239. [Google Scholar] [CrossRef]
- Perna, A.; Minutillo, M.; Jannelli, E. Designing and analyzing an electric energy storage system based on reversible solid oxide cells. Energy Convers. Manag. 2018, 159, 381–395. [Google Scholar] [CrossRef]
- Clausen, L.R. Energy efficient thermochemical conversion of very wet biomass to biofuels by integration of steam drying, steam electrolysis and gasification. Energy 2017, 125, 327–336. [Google Scholar] [CrossRef]
- Laosiripojana, N.; Assabumrungrat, S. Catalytic steam reforming of methane, methanol, and ethanol over Ni/YSZ: The possible use of these fuels in internal reforming SOFC. J. Power Source 2007, 163, 943–951. [Google Scholar] [CrossRef]
- Andersson, M.; Paradis, H.; Yuan, J.; Sundén, B. Review of catalyst materials and catalytic steam reforming reactions in SOFC anodes. Int. J. Energy Res. 2011, 35, 1340–1350. [Google Scholar] [CrossRef]
- Guerra, C.F.; Reyes-Bozo, L.; Vyhmeister, E.; Caparrós, M.J.; Salazar, J.L.; Clemente-Jul, C. Technical-economic analysis for a green ammonia production plant in Chile and its subsequent transport to Japan. Renew. Energy 2020, 157, 404–414. [Google Scholar] [CrossRef]
- Yang, J.; Molouk, A.F.S.; Okanishi, T.; Muroyama, H.; Matsui, T.; Eguchi, K. A stability study of Ni/Yttria-stabilized zirconia anode for direct ammonia solid oxide fuel cells. ACS Appl. Mater. Interfaces 2015, 7, 28701–28707. [Google Scholar] [CrossRef]
- Hashinokuchi, M.; Zhang, M.; Doi, T.; Inaba, M. Enhancement of anode activity and stability by Cr addition at Ni/Sm-doped CeO2 cermet anodes in NH3-fueled solid oxide fuel cells. Solid State Ion. 2018, 319, 180–185. [Google Scholar] [CrossRef]
- Brown, T. Green Ammonia: Haldor Topsoe’s Solid Oxide Electrolyzer; Ammonia Industry: Brooklyn, NY, USA, 2019. [Google Scholar]
- MacFarlane, D.R.; Cherepanov, P.V.; Choi, J.; Suryanto, B.H.; Hodgetts, R.Y.; Bakker, J.M.; Ferrero Vallana, F.M.; Simonov, A.N. A roadmap to the ammonia economy. Joule 2020, 4, 1186–1205. [Google Scholar] [CrossRef]
- Cormos, A.-M.; Szima, S.; Fogarasi, S.; Cormos, C.-C. Economic assessments of hydrogen production processes based on natural gas reforming with carbon capture. Chem. Eng. Trans. 2018, 70, 1231–1236. [Google Scholar]
- Keipi, T.; Tolvanen, H.; Konttinen, J. Economic analysis of hydrogen production by methane thermal decomposition: Comparison to competing technologies. Energy Convers. Manag. 2018, 159, 264–273. [Google Scholar] [CrossRef]
- Iskov, H.; Kvist, T.; Bruun, J. Biogas, Biomethane and Electro-Methane Cost Comparison. Master’s Thesis, University of Gävle, Gävle, Sweden, 2019. [Google Scholar]
- Mogensen, M.; Chen, M.; Frandsen, H.; Graves, C.; Hansen, J.; Hansen, K.; Hauch, A.; Jacobsen, T.; Jensen, S.H.; Skafte, T.L.; et al. Reversible solid-oxide cells for clean and sustainable energy. Clean Energy 2019, 3, 175–201. [Google Scholar] [CrossRef]
- Eguchi, K.; Hatagishi, T.; Arai, H. Power generation and steam electrolysis characteristics of an electrochemical cell with a zirconia-or ceria-based electrolyte. Solid State Ion. 1996, 86, 1245–1249. [Google Scholar] [CrossRef]
- Momma, A.; Kaga, Y.; Takano, K.; Nozaki, K.; Negishi, A.; Kato, K.; Inagaki, T.; Yoshida, H.; Hoshino, K.; Akikusa, J.; et al. Experimental investigation of anodic gaseous concentration of a practical seal-less solid oxide fuel cell. J. Power Source 2005, 145, 169–177. [Google Scholar] [CrossRef]
- Schiller, G.; Ansar, A.; Lang, M.; Patz, O. High temperature water electrolysis using metal supported solid oxide electrolyser cells (SOEC). J. Appl. Electrochem. 2009, 39, 293–301. [Google Scholar] [CrossRef]
- Menzler, N.H.; Tietz, F.; Uhlenbruck, S.; Buchkremer, H.P.; Stöver, D. Materials and manufacturing technologies for solid oxide fuel cells. J. Mater. Sci. 2010, 45, 3109–3135. [Google Scholar] [CrossRef]
- Sohal, M.S.; O’Brien, J.E.; Stoots, C.M.; Sharma, V.I.; Yildiz, B.; Virkar, A. Degradation issues in solid oxide cells during high temperature electrolysis. J. Fuel Cell Sci. Technol. 2012, 9, 011017. [Google Scholar] [CrossRef]
- Green, R.D.; Liu, C.-C.; Adler, S.B. Carbon dioxide reduction on gadolinia-doped ceria cathodes. Solid State Ion. 2008, 179, 647–660. [Google Scholar] [CrossRef]
- Addo, P.K.; Molero-Sanchez, B.; Buyukaksoy, A.S.; Birss, V. Sulfur Tolerance of La0. 3M0. 7Fe0. 7Cr0. 3O3-δ (M = Sr, Ca) Solid Oxide Fuel Cell Anodes. ECS Trans. 2015, 66, 219–228. [Google Scholar] [CrossRef]
- Lee, S.; Kim, G.; Vohs, J.M.; Gorte, R.J. SOFC anodes based on infiltration of La0. 3Sr0. 7TiO3. J. Electrochem. Soc. 2008, 155, B1179–B1183. [Google Scholar] [CrossRef]
- Kim, G.; Corre, G.; Irvine, J.; Vohs, J.M.; Gorte, R.J. Engineering composite oxide SOFC anodes for efficient oxidation of methane. Electrochem. Solid State Lett. 2008, 11, B16–B19. [Google Scholar] [CrossRef][Green Version]
- Molero-Sánchez, B.; Addo, P.; Buyukaksoy, A.; Paulson, S.; Birss, V. Electrochemistry of La0. 3Sr0. 7Fe0. 7Cr0. 3O3− δ as an oxygen and fuel electrode for RSOFCs. Faraday Discuss. 2015, 182, 159–175. [Google Scholar] [CrossRef]
- Chen, M.; Paulson, S.; Thangadurai, V.; Birss, V. Sr-rich chromium ferrites as symmetrical solid oxide fuel cell electrodes. J. Power Source 2013, 236, 68–79. [Google Scholar] [CrossRef]
- Haag, J.M.; Bierschenk, D.M.; Barnett, S.A.; Poeppelmeier, K.R. Structural, chemical, and electrochemical characteristics of LaSr2Fe2CrO9-δ-based solid oxide fuel cell anodes. Solid State Ion. 2012, 212, 1–5. [Google Scholar] [CrossRef]
- Myung, J.-H.; Ko, H.J.; Im, C.H.; Moon, J.; Hyun, S.-H. Development of solid oxide fuel cells (SOFCs) by tape-casting and single-step co-firing of monolithic laminates. Int. J. Hydrogen Energy. 2014, 39, 2313–2319. [Google Scholar] [CrossRef]
- Yoon, K.J.; Ye, G.; Gopalan, S.; Pal, U.B. Cost-effective single step cofiring process for manufacturing solid oxide fuel cells using HSC™ anode. J. Fuel Cell Sci. Technol. 2010, 7, 021010. [Google Scholar] [CrossRef]
- Liu, M.; Dong, D.; Zhao, F.; Gao, J.; Ding, D.; Liu, X.; Meng, G. High-performance cathode-supported SOFCs prepared by a single-step co-firing process. J. Power Source 2008, 182, 585–588. [Google Scholar] [CrossRef]

| Variable or Parameter | Value |
|---|---|
| SOC temperature, °C | 800 |
| SOEC electrical efficiency, % | 90 |
| SOFC electrical efficiency, % | 50 |
| Ambient temperature, °C | 298 |
| Oxygen storage temperature, °C | 298 |
| Methanation reactor temperature, °C | 250 [49] |
| Methanation reactor pressure, bars | 25 [49] |
| Methanation process efficiency, % | 75 [53] |
| Methane reformer temperature, °C | 800 [54] |
| Methane reformer pressure, bars | 25 [54] |
| Methane reforming process efficiency, % | 78 [54] |
| Internal reforming conversion, % | 90 [55] |
| Haber Bosch reactor temperature, °C | 400 [50] |
| Haber Bosch reactor pressure, bars | 200 [50] |
| Haber Bosch process efficiency, % | 66 [50] |
| Ammonia cracker temperature, °C | 800 [55] |
| Ammonia cracker pressure, bar | 1 [56] |
| Ammonia cracking efficiency, % | 90 [57] |
| Ammonia internal cracking conversion, % | 85 [57] |
| Heat pump efficiency, % | 90 [58] |
| Parameters for SOEC Mode | Value |
|---|---|
| H2 production rate, tons per year | 4400.00 |
| Electrolyser lifetime (projected), years | 3.42 |
| H2 produced over lifetime, tons | 15,068.49 |
| Energy content of H2, kWh/kg | 33.33 |
| Energy content of H2 produced over lifetime, GWh | 502.23 |
| SOEC conversion efficiency, % | 90.00 |
| SOEC system efficiency (with heat integration), % | 90.00 |
| Electrical input required for SOEC over lifetime, GWh | 620.04 |
| Capacity factor, % [82] | 69.00 |
| Hours of operation (Electrolyser ON), hours | 20,700 |
| SOC size, MW | 43.41 |
| SOC capex, $ per kW [83] | 450.00 |
| Cost of SOC for target, M$ | 19.53 |
| Electricity cost, cents per kWh | 1.50 |
| Total electricity cost, M$ | 9.30 |
| Total cost of H2, M$ | 28.83 |
| Cost of H2 produced, $ per kg | 1.91 |
| Parameters for SOFC Mode | Value |
| Electricity produced over life, GWh | 500.00 |
| SOFC conversion efficiency, % | 90.00 |
| SOFC system efficiency (with heat integration), % | 69.00 |
| Hydrogen input energy required for SOFC over lifetime, GWh | 805.15 |
| Capacity factor, % [82] | 69.00 |
| Hours of operation (Electrolyser ON), hours | 20,700 |
| SOC size, MW | 56.37 |
| SOC capex, $ per kW [82] | 450.00 |
| Cost of SOC for target, M$ | 25.36 |
| Total cost of electricity, M$ | 26.17 |
| Cost of electricity generated, $ per MWh | 52.34 |
| Parameters for SOEC Mode | Value |
|---|---|
| H2 production rate, tons per year | 4400.00 |
| Syngas production rate, tons per year | 29,333.00 |
| Methane produced from syngas at 85% conversion, tons per year | 9035.00 |
| Electrolyser lifetime (projected), years | 3.42 |
| syngas produced over lifetime, tons | 100,456.62 |
| CO2 removed over lifetime, tons | 110,502.28 |
| Methane produced over lifetime, tons | 30,940.64 |
| Energy content of H2, kWh per kg | 33.33 |
| Energy content of CO, kWh per kg | 2.80 |
| Energy content of syngas, kWh per kg | 7.99 |
| Energy content of syngas produced over lifetime, GWh | 802.66 |
| SOEC system efficiency (with heat integration), % | 90.00 |
| Electrical Input required for SOEC over lifetime, MWh | 891.84 |
| Capacity factor, % [82] | 69.00 |
| Hours of operation (Electrolyser ON), hours | 20,700.00 |
| SOC size, MW | 62.44 |
| SOC capex, $ per kW [82] | 450.00 |
| Cost of SOC for target, M$ | 28.10 |
| Cost of methanation plant, per kW [82] | 1056.00 |
| Cost of methanation, M$ | 65.96 |
| Electricity cost, cents per kWh | 1.50 |
| Total electricity cost, M$ | 13.37 |
| CO2 cost at 43.09$ per ton, M$ [82] | 4.76 |
| Total cost of methane produced, M$ | 112.20 |
| Cost of methane produced, $ per kg | 3.63 |
| Parameters for SOFC Mode | Value |
| Electrolyser lifetime (projected), hours | 30,000.00 |
| Electrical energy produced over lifetime, GWh | 500.00 |
| SOFC system efficiency (with heat integration and internal reforming), % | 70.20 |
| Syngas energy Input required for SOFC over lifetime, GWh | 793.65 |
| Capacity factor, % [82] | 69.00 |
| Hours of operation (Electrolyser ON), hours | 20,700 |
| SOC size, MW | 49.87 |
| SOC capex, $ per kW [82] | 450.00 |
| Cost of electrolyser for target, M$ | 22.44 |
| Total cost of electricity, M$ | 23.15 |
| Cost of electricity generated, $ per MWh | 46.30 |
| Parameters for SOEC Mode | Value |
|---|---|
| H2 production rate, tons per year | 4400.00 |
| Ammonia produced from H2, tons per year | 24,185.00 |
| Electrolyser lifetime (projected), years | 3.42 |
| Ammonia produced over lifetime, tons | 80,341.69 |
| Energy content of ammonia, kWh per kg | 5.21 |
| Energy content of ammonia produced over life, GWh | 418.42 |
| SOEC system efficiency (with heat integration), % | 90.00 |
| Electrical input required for SOEC over lifetime, GWh | 464.91 |
| Capacity factor, % [82] | 69.00 |
| Hours of operation (Electrolyser ON), hours | 20,700.00 |
| Electrolyser size, MW | 32.55 |
| SOC capex, $ per kW [82] | 450.00 |
| Cost of SOC for target, M$ | 14.65 |
| Cost of Haber Bosch unit, $ per kW [76] | 512 |
| Cost of ammonia synthesis in Haber Bosch unit, $ | 16.65 |
| Electricity cost, cents per kWh | 1.50 |
| Total electricity cost, M$ | 6.97 |
| Total cost of ammonia, M$ | 38.27 |
| Cost of ammonia, $ per kg | 0.48 |
| Parameters for SOFC Mode | Value |
| Electrolyser lifetime (projected), hours | 30,000 |
| Electrical energy produced over lifetime, GWh | 500.00 |
| SOFC system efficiency (with heat integration and internal cracking), % | 69.00 |
| Ammonia energy Input required for SOFC over lifetime, GWh | 724.63 |
| Capacity factor, % [82] | 69.00 |
| Hours of operation (Electrolyser ON), hours | 20,700 |
| SOC size, MW | 50.73 |
| SOC capex, per kW [82] | 450.00 |
| Cost of SOC for target, M$ | 22.83 |
| Total cost of electricity, M$ | 23.55 |
| Cost of electricity, $ per MWh | 47.11 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Biswas, S.; Rathore, S.S.; Kulkarni, A.P.; Giddey, S.; Bhattacharya, S. A Theoretical Study on Reversible Solid Oxide Cells as Key Enablers of Cyclic Conversion between Electrical Energy and Fuel. Energies 2021, 14, 4517. https://doi.org/10.3390/en14154517
Biswas S, Rathore SS, Kulkarni AP, Giddey S, Bhattacharya S. A Theoretical Study on Reversible Solid Oxide Cells as Key Enablers of Cyclic Conversion between Electrical Energy and Fuel. Energies. 2021; 14(15):4517. https://doi.org/10.3390/en14154517
Chicago/Turabian StyleBiswas, Saheli, Shambhu Singh Rathore, Aniruddha Pramod Kulkarni, Sarbjit Giddey, and Sankar Bhattacharya. 2021. "A Theoretical Study on Reversible Solid Oxide Cells as Key Enablers of Cyclic Conversion between Electrical Energy and Fuel" Energies 14, no. 15: 4517. https://doi.org/10.3390/en14154517
APA StyleBiswas, S., Rathore, S. S., Kulkarni, A. P., Giddey, S., & Bhattacharya, S. (2021). A Theoretical Study on Reversible Solid Oxide Cells as Key Enablers of Cyclic Conversion between Electrical Energy and Fuel. Energies, 14(15), 4517. https://doi.org/10.3390/en14154517







