Modification of Canola Oil Physicochemical Properties by Hexane and Ethanol with Regards of Its Application in Diesel Engine
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Properties of Fuel Mixtures
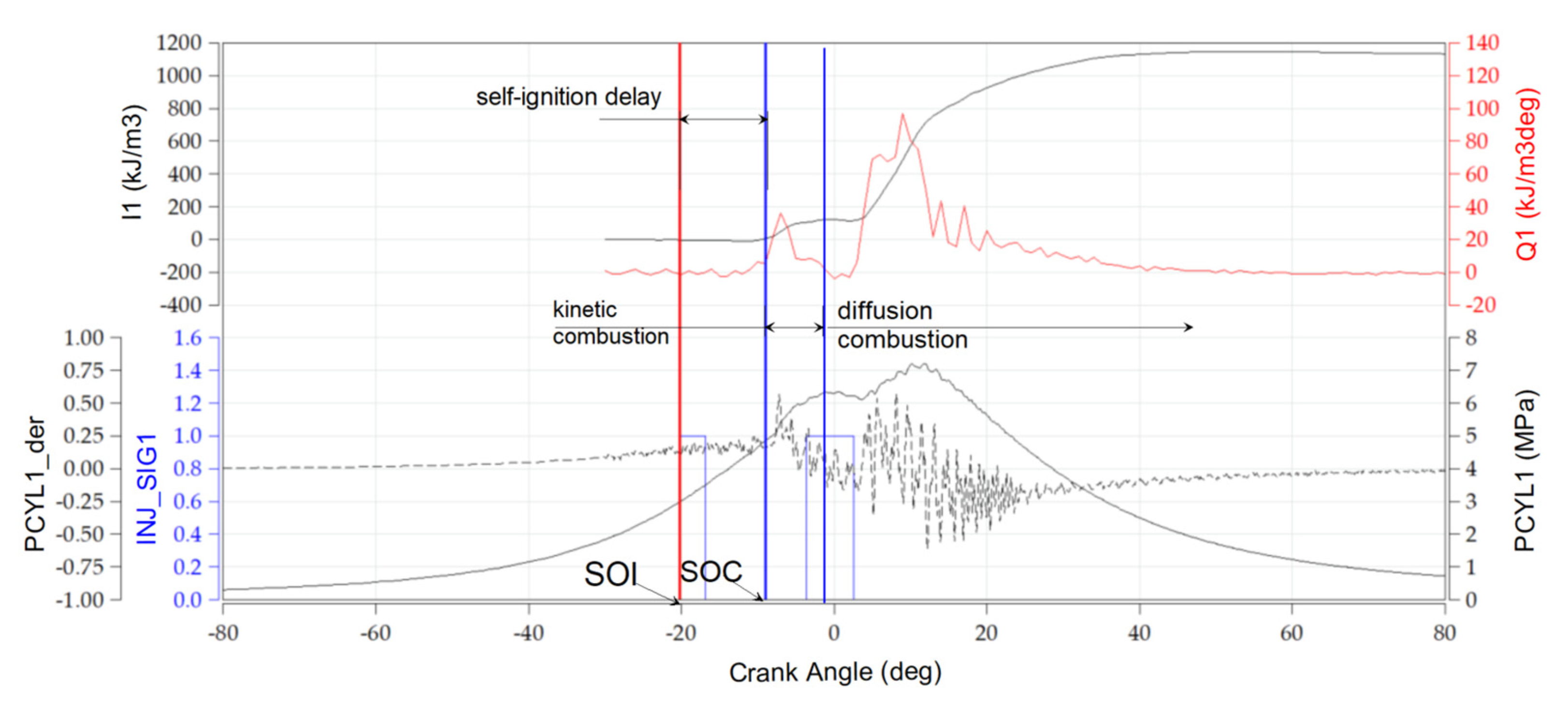
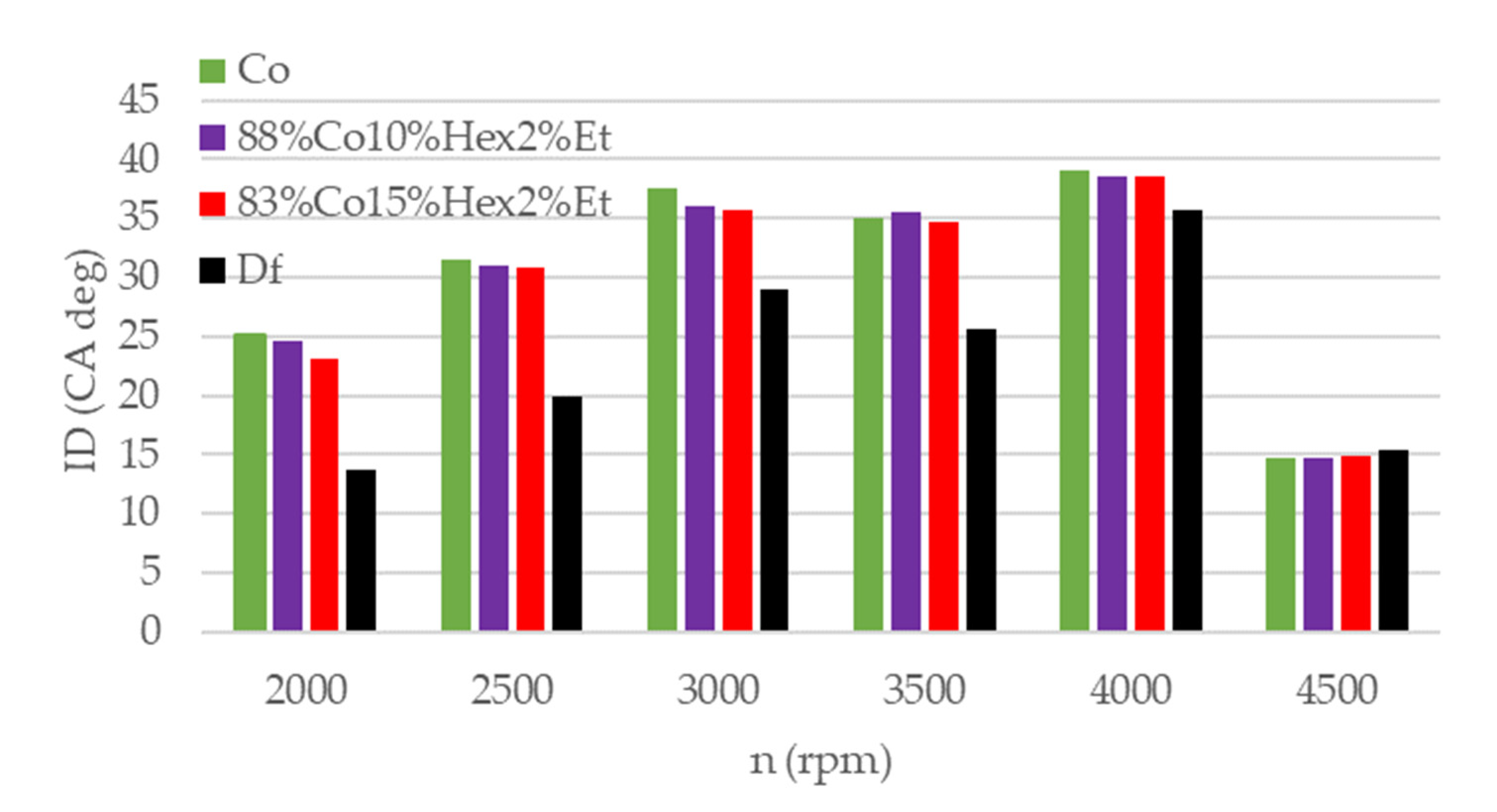
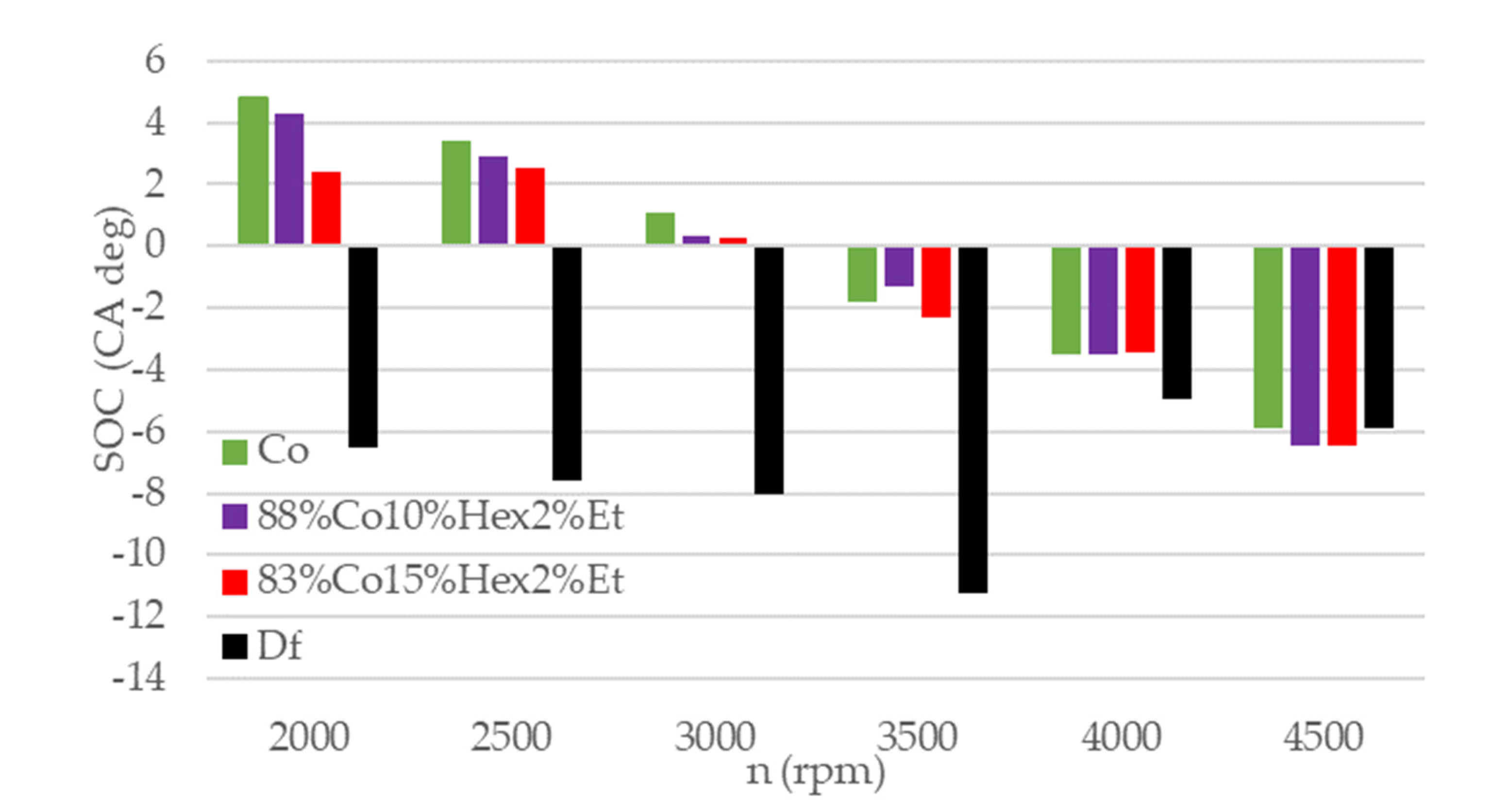
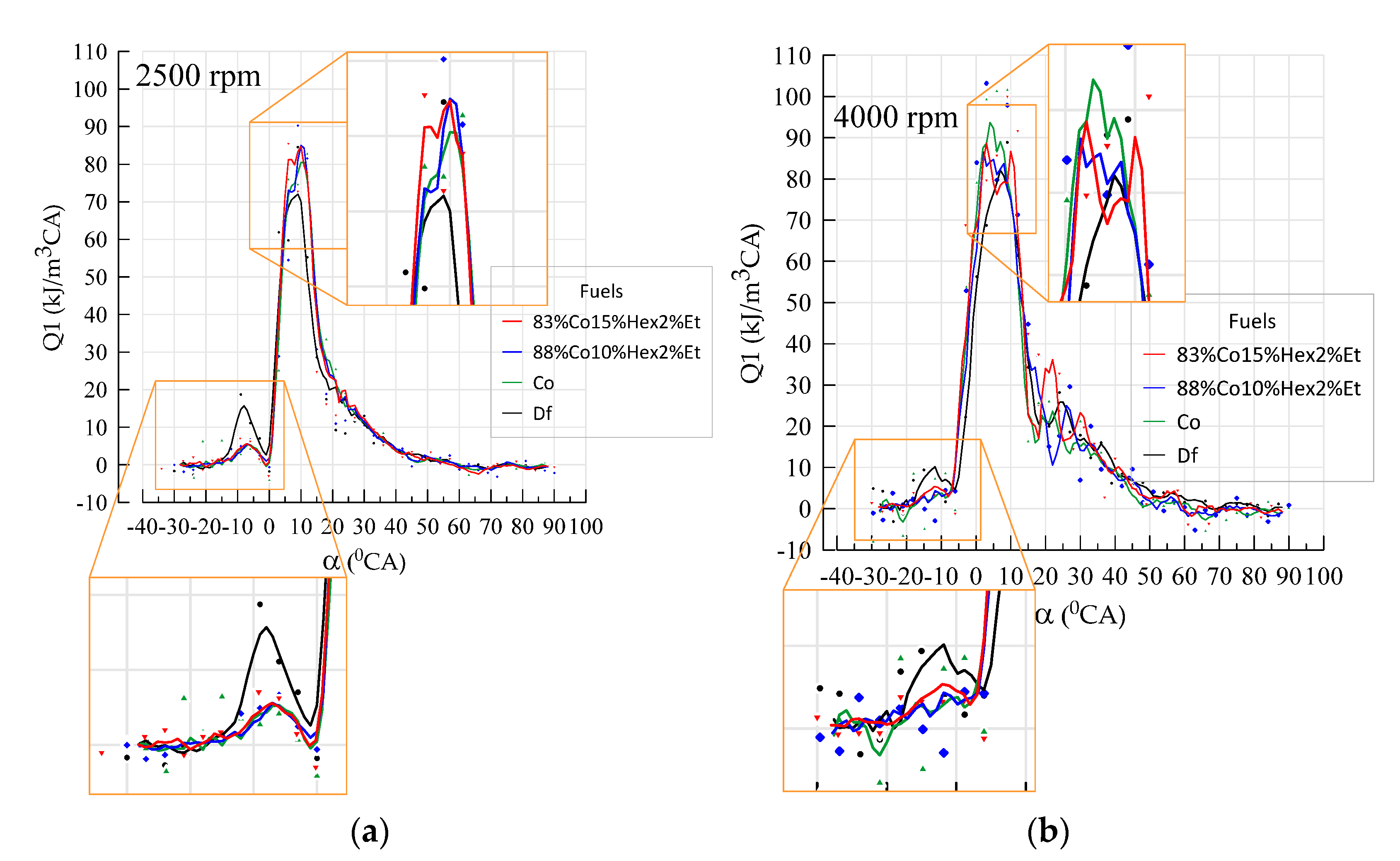
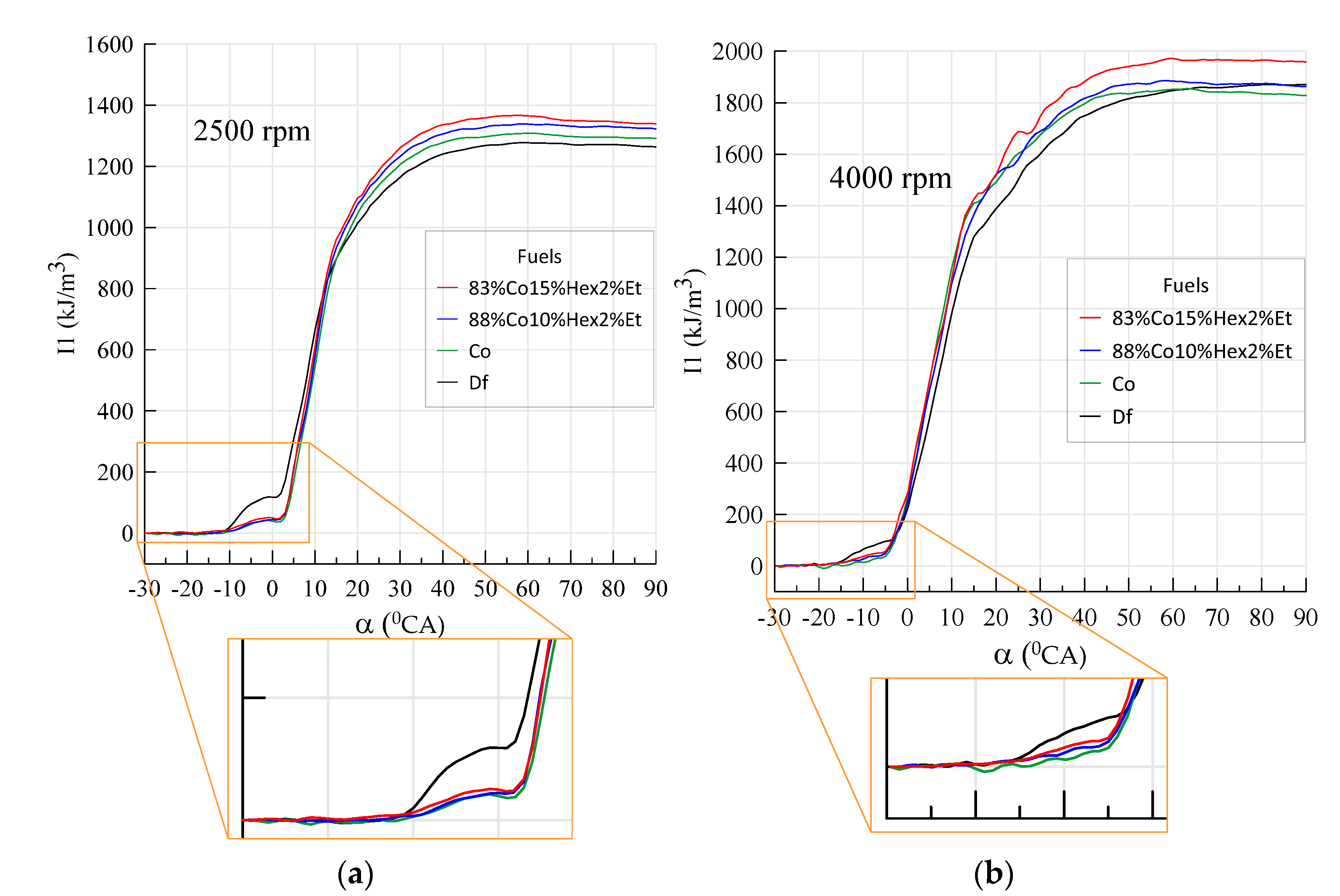
3.2. Engine Test Results
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, J.; Sun, H.; Dai, C.; Li, S. GPIO-based rail pressure control for diesel high pressure common rail injection system. J. Eng. 2019, 2019, 8293–8298. [Google Scholar] [CrossRef]
- Schöppe, D.; Atzler, F.; Kastner, O.; Kapphan, F. High Performance Diesel Direct Driven Piezo Common Rail Injection System. In Proceedings of the 23rd Aachen Colloquium: Automobile and Engine Technology, Aachen, Germany, 7–8 October 2014. [Google Scholar]
- Chen, W.; Pan, J.; Fan, B.; Liu, Y.; Peter, O. Effect of injection strategy on fuel-air mixing and combustion process in a direct injection diesel rotary engine (DI-DRE). Energy Convers. Manag. 2017, 154, 68–80. [Google Scholar] [CrossRef]
- Zimont, V.L. Theory of turbulent combustion of a homogeneous fuel mixture at high reynolds numbers. Combust. Explos. Shock. Waves 1979, 15, 305–311. [Google Scholar] [CrossRef]
- Longwic, R.; Sander, P.; Nieoczym, A.; Lotko, W.; Krzysiak, Z.; Samociuk, W.; Baąkowsk, H. Effect of some properties of hydrocarbon fuels on self-ignition delay. Przem. Chem. 2017, 96, 1123–1127. [Google Scholar] [CrossRef]
- Hasan, M.M.; Rahman, M.; Kadirgama, K. A review on homogeneous charge compression ignition engine performance using biodiesel–diesel blend as a fuel. Int. J. Automot. Mech. Eng. 2015, 11, 2199–2211. [Google Scholar] [CrossRef]
- Babu, A.K.; Devaradjane, G. Vegetable oils as fuel for diesel engines: An overview. In Proceedings of the ASME International Mechanical Engineering Congress and Exposition, Proceedings; American Society of Mechanical Engineers (ASME): New York, NY, USA; 2002; Volume 3, pp. 313–318. [Google Scholar] [CrossRef]
- Lotko, W.; Longwic, R.; Swat, M. The Effect of Rape Oil Diesel Oil Mixture Composition on Particulate Matter Emission Level in Diesel Engine. SAE Tech. Pap. Ser. 2001. [Google Scholar] [CrossRef]
- Rakopoulos, C.D.; Rakopoulos, D.C.; Kosmadakis, G.M.; Papagiannakis, R.G. Experimental comparative assessment of butanol or ethanol diesel-fuel extenders impact on combustion features, cyclic irregularity, and regulated emissions balance in heavy-duty diesel engine. Energy 2019, 174, 1145–1157. [Google Scholar] [CrossRef]
- Górski, K.; Sander, P.; Longwic, R. The assessment of ecological parameters of diesel engine supplied with mixtures of canola oil with n-hexane. In Proceedings of the IOP Conference Series: Materials Science and Engineering; IOP Publishing: Bristol, UK, 2018; Volume 421, p. 042025. [Google Scholar]
- Vrabie, V.; Scarpete, D.; Zbarcea, O. Vegetable Oils as Alternative Fuel for New Generation of Diesel Engines: A Review. Trans Motauto World 2016, 1, 18–22. [Google Scholar]
- Martins, J.; Brito, F.P. Alternative Fuels for Internal Combustion Engines. Energies 2020, 13, 4086. [Google Scholar] [CrossRef]
- Bergthorson, J.M.; Thomson, M.J. A review of the combustion and emissions properties of advanced transportation biofuels and their impact on existing and future engines. Renew. Sustain. Energy Rev. 2015, 42, 1393–1417. [Google Scholar] [CrossRef]
- Agarwal, D.; Agarwal, A. Performance and emissions characteristics of Jatropha oil (preheated and blends) in a direct injection compression ignition engine. Appl. Therm. Eng. 2007, 27, 2314–2323. [Google Scholar] [CrossRef]
- Souza, S.P.; Pacca, S.; de Ávila, M.T.; Borges, J.L.B. Greenhouse gas emissions and energy balance of palm oil biofuel. Renew. Energy 2010, 35, 2552–2561. [Google Scholar] [CrossRef]
- Ge, J.C.; Yoon, S.K.; Kim, M.S.; Choi, N.J. Application of Canola Oil Biodiesel/Diesel Blends in a Common Rail Diesel Engine. Appl. Sci. 2016, 7, 34. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.; Przybylski, R.; Dawson, K.; Eskin, N.A.M.; Biliaderis, C.G. Comparison of the composition and properties of canola and sunflower oil sediments with canola seed hull lipids. J. Am. Oil Chem. Soc. 1996, 73, 493–498. [Google Scholar] [CrossRef]
- McGrath, J.; Hickner, M.; Hofer, R. Introduction. In Polymer Science: A Comprehensive Reference; Elsevier: Amsterdam, The Netherlands, 2012; Volume 10, pp. 1–3. [Google Scholar]
- Shahid, E.M.; Jamal, Y. A review of biodiesel as vehicular fuel. Renew. Sustain. Energy Rev. 2008, 12, 2484–2494. [Google Scholar] [CrossRef]
- Von Wielligh, A.; Burger, N.; Wilcocks, T. Diesel engine failures due to combustion disturbances, caused by fuel with insufficient lubricity. Ind. Lubr. Tribol. 2003, 55, 65–75. [Google Scholar] [CrossRef]
- Longwic, R.; Sander, P. The course of combustion process under real conditions of work of a traction diesel engine supplied by mixtures of canola oil containing n-hexane. In Proceedings of the IOP Conference Series: Materials Science and Engineering; IOP Publishing: Bristol, UK, 2018; Volume 421, p. 042050. [Google Scholar]
- Longwic, R.; Sander, P.; Zdziennicka, A.; Szymczyk, K.; Jańczuk, B. Combustion Process of Canola Oil and n-Hexane Mixtures in Dynamic Diesel Engine Operating Conditions. Appl. Sci. 2019, 10, 80. [Google Scholar] [CrossRef] [Green Version]
- Longwic, R.; Sander, P.; Lotko, W.; Gorski, K.; Janczuk, B.; Zdziennicka, A.; Szymczyk, K. Self-ignition of rapeseed and n-hexane mixtures in diesel engine. Przem. Chem. 2020, 99, 206–210. [Google Scholar] [CrossRef]
- Zdziennicka, A.; Szymczyk, K.; Jańczuk, B.; Longwic, R.; Sander, P. Surface, Volumetric, and Wetting Properties of Oleic, Linoleic, and Linolenic Acids with Regards to Application of Canola Oil in Diesel Engines. Appl. Sci. 2019, 9, 3445. [Google Scholar] [CrossRef] [Green Version]
- Zdziennicka, A.; Szymczyk, K.; Jańczuk, B.; Longwic, R.; Sander, P. Adhesion of canola and diesel oils to some parts of diesel engine in the light of surface tension components and parameters of these substrates. Int. J. Adhes. Adhes. 2015, 60, 23–30. [Google Scholar] [CrossRef]
- Longwic, R.; Sander, P. The characteristics of the combustion process occurring under real operating conditions of traction. In Proceedings of the IOP Conference Series: Materials Science and Engineering; IOP Publishing: Bristol, UK, 2016; Volume 148, p. 012071. [Google Scholar]
- Trost, D.; Polcar, A.; Boldor, D.; Nde, D.; Wolak, A.; Kumbár, V. Temperature Dependence of Density and Viscosity of Biobutanol-Gasoline Blends. Appl. Sci. 2021, 11, 3172. [Google Scholar] [CrossRef]
- Kumbár, V.; Polcar, A.; Votava, J. Fyzikální a mechanické vlastnosti směsí bioetanolu a benzinu. Listy Cukrov. Řepař 2015, 131, 3. [Google Scholar]
- Fowkes, F.M. Attractive forces at interfaces. Ind. Eng. Chem. 1964, 56, 40–52. [Google Scholar] [CrossRef]
- Van Oss, C.; Costanzo, P. Adhesion of anionic surfactants to polymer surfaces and low-energy materials. J. Adhes. Sci. Technol. 1992, 6, 477–487. [Google Scholar] [CrossRef]
- Gros, A.T.; Feuge, R.O. Surface and interfacial tensions, viscosities, and other physical properties of some n-aliphatic acids and their methyl and ethyl esters. J. Am. Oil Chem. Soc. 1952, 29, 313–317. [Google Scholar] [CrossRef]

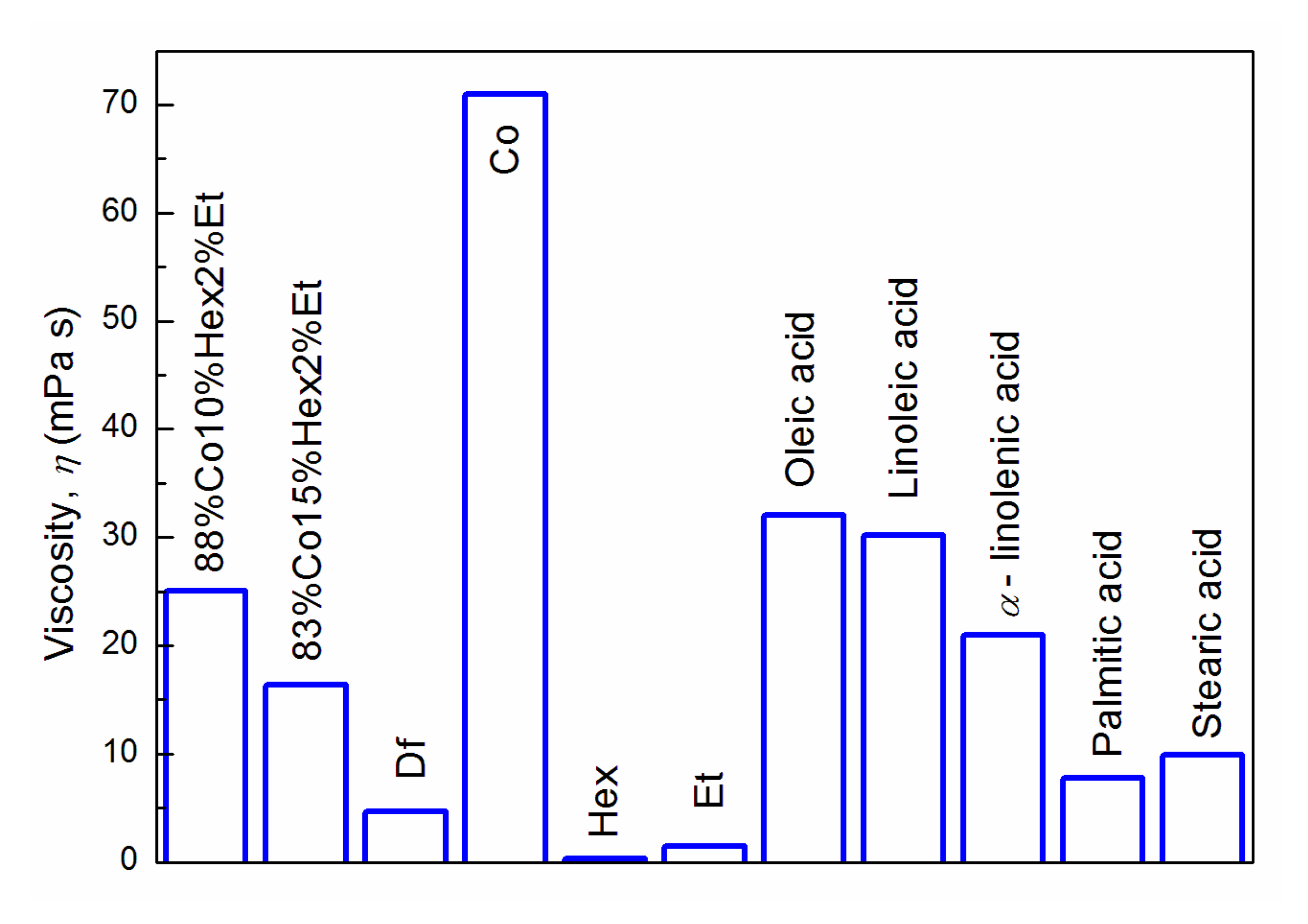

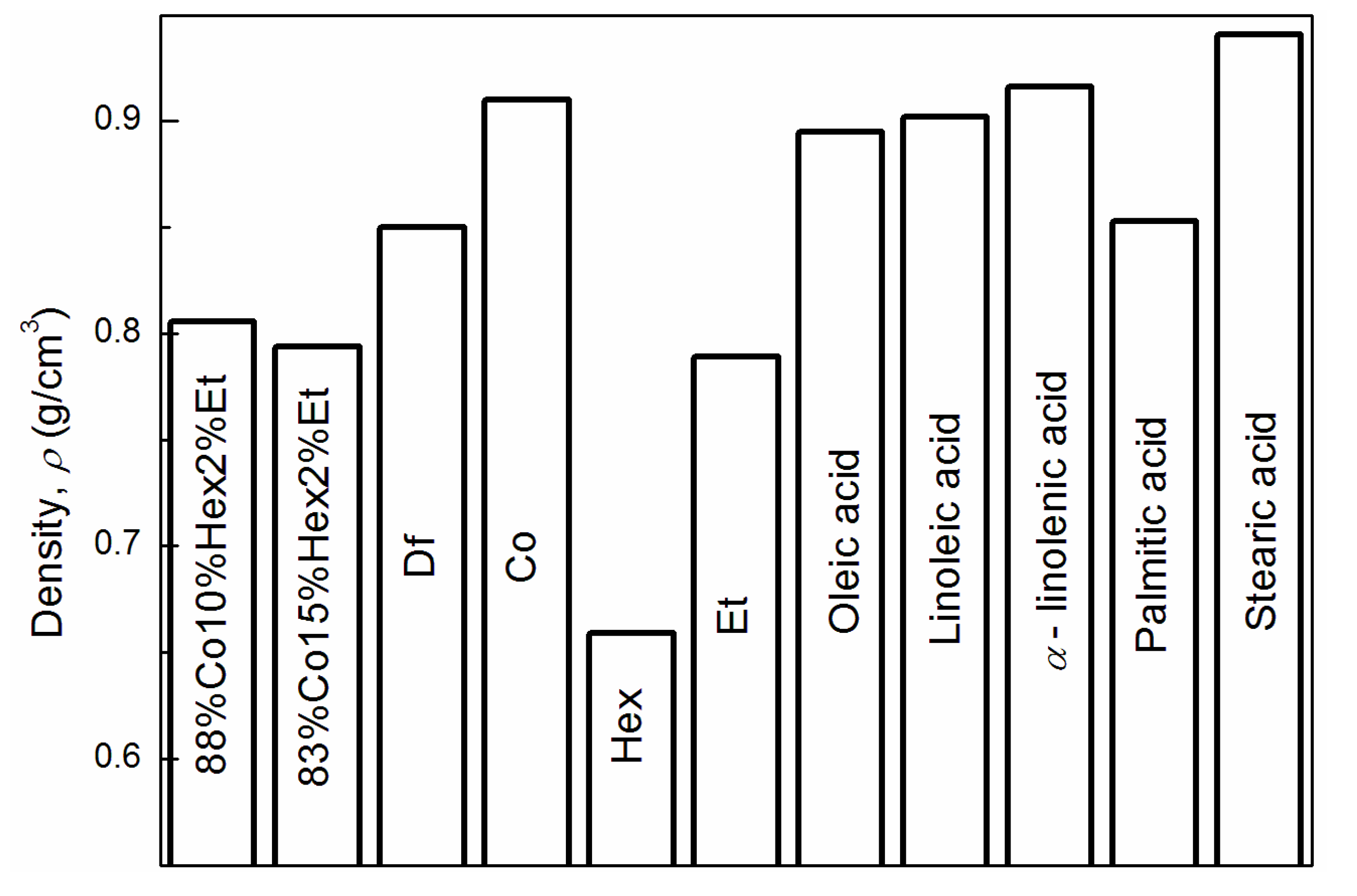
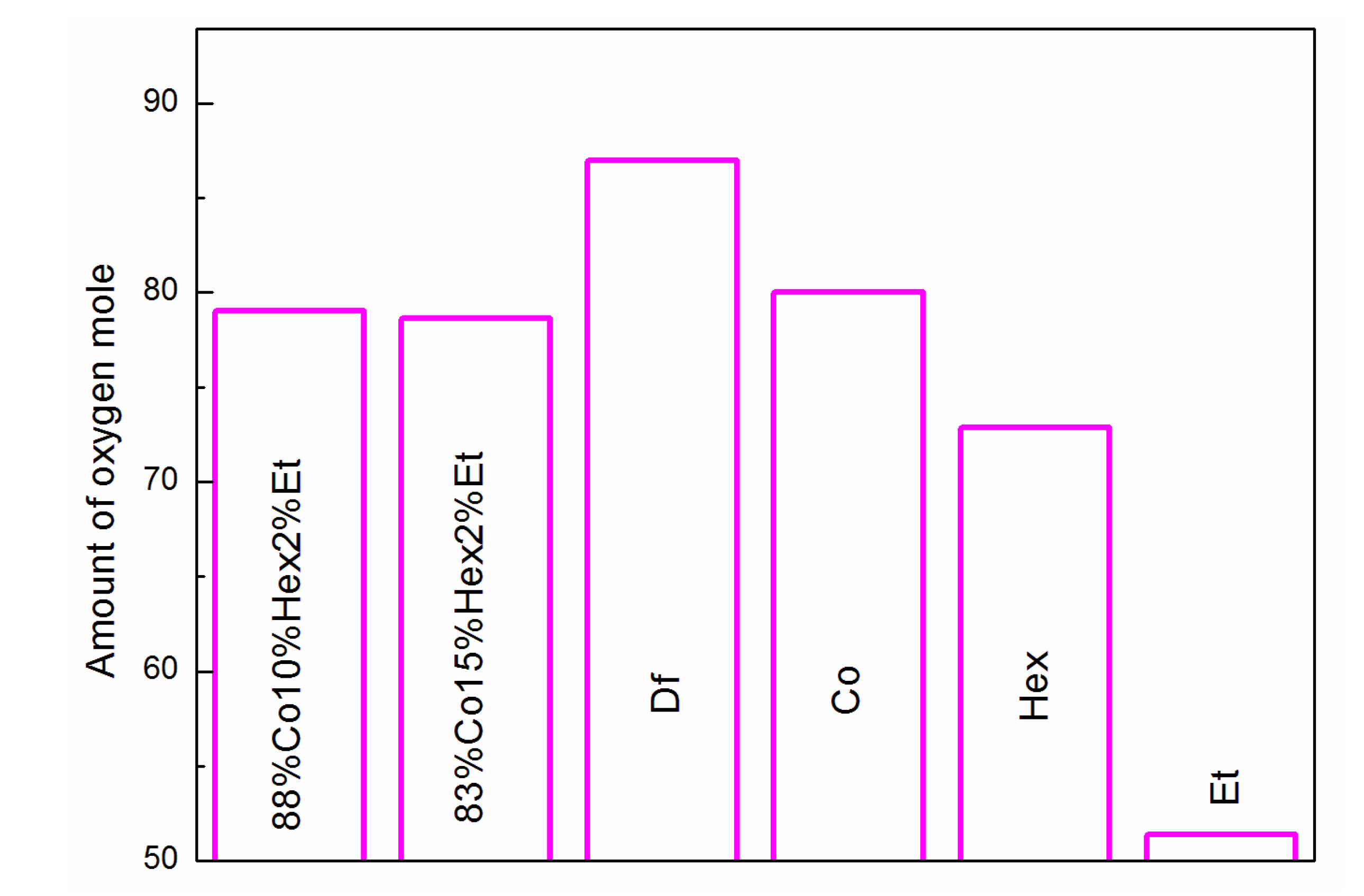
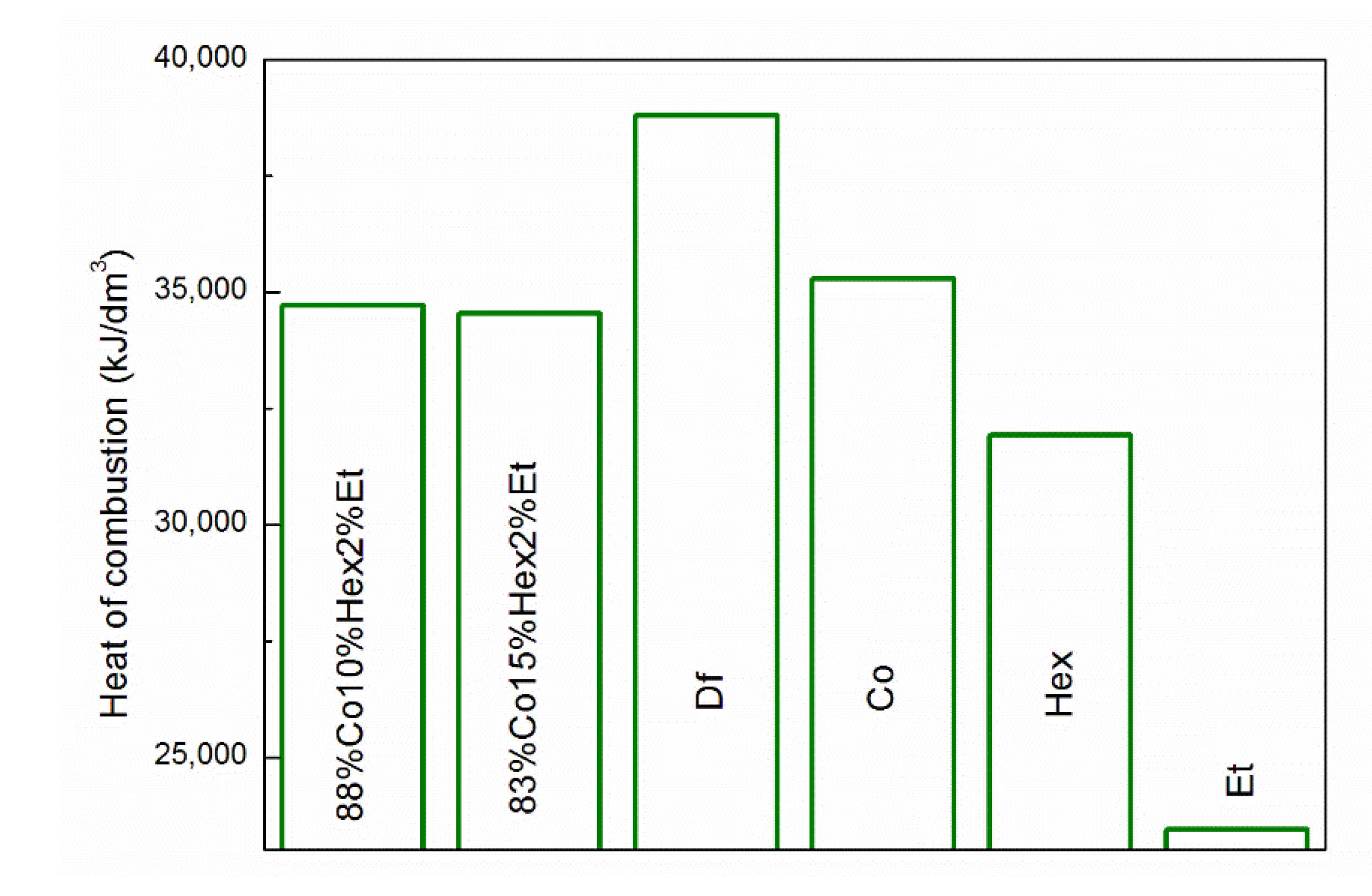
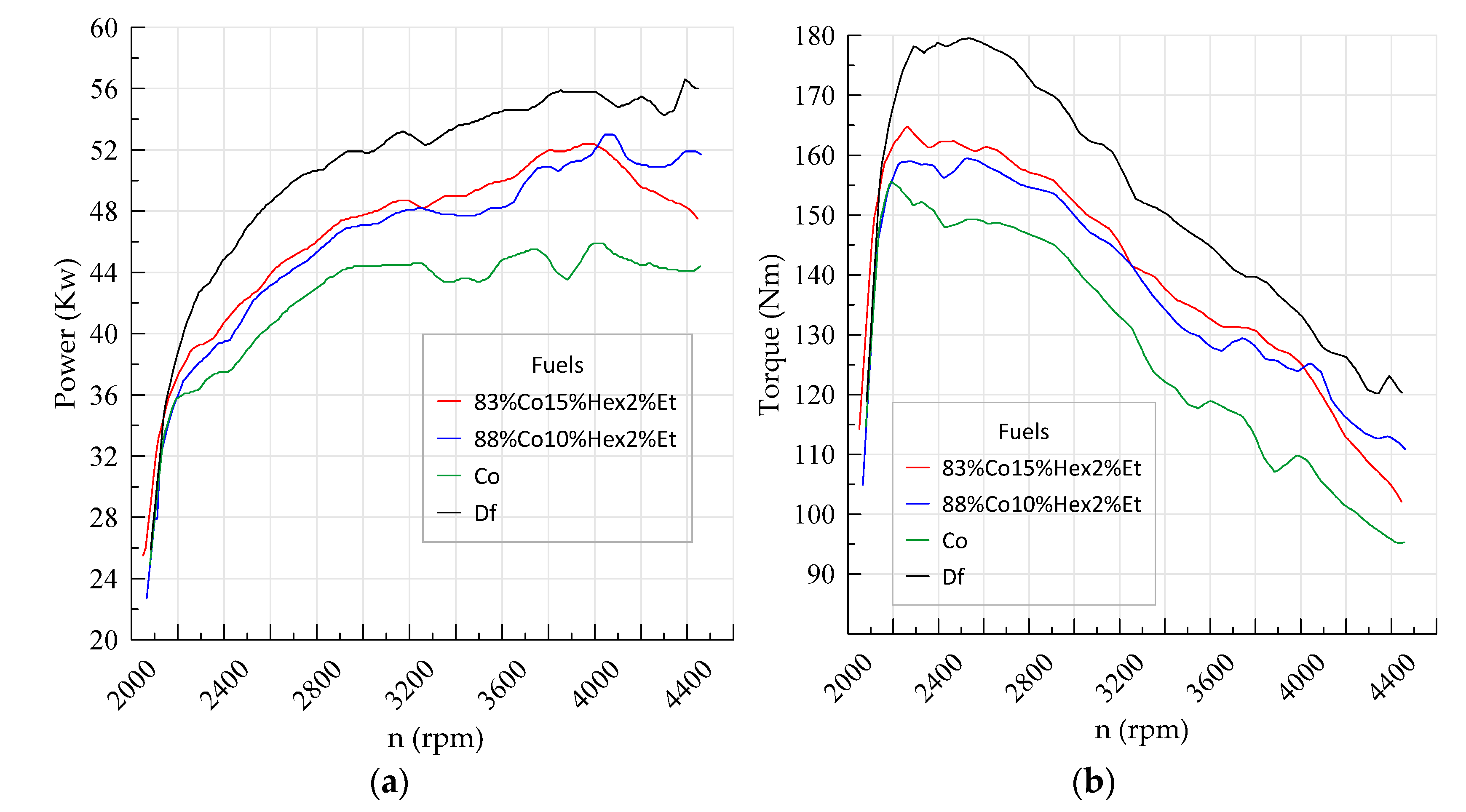
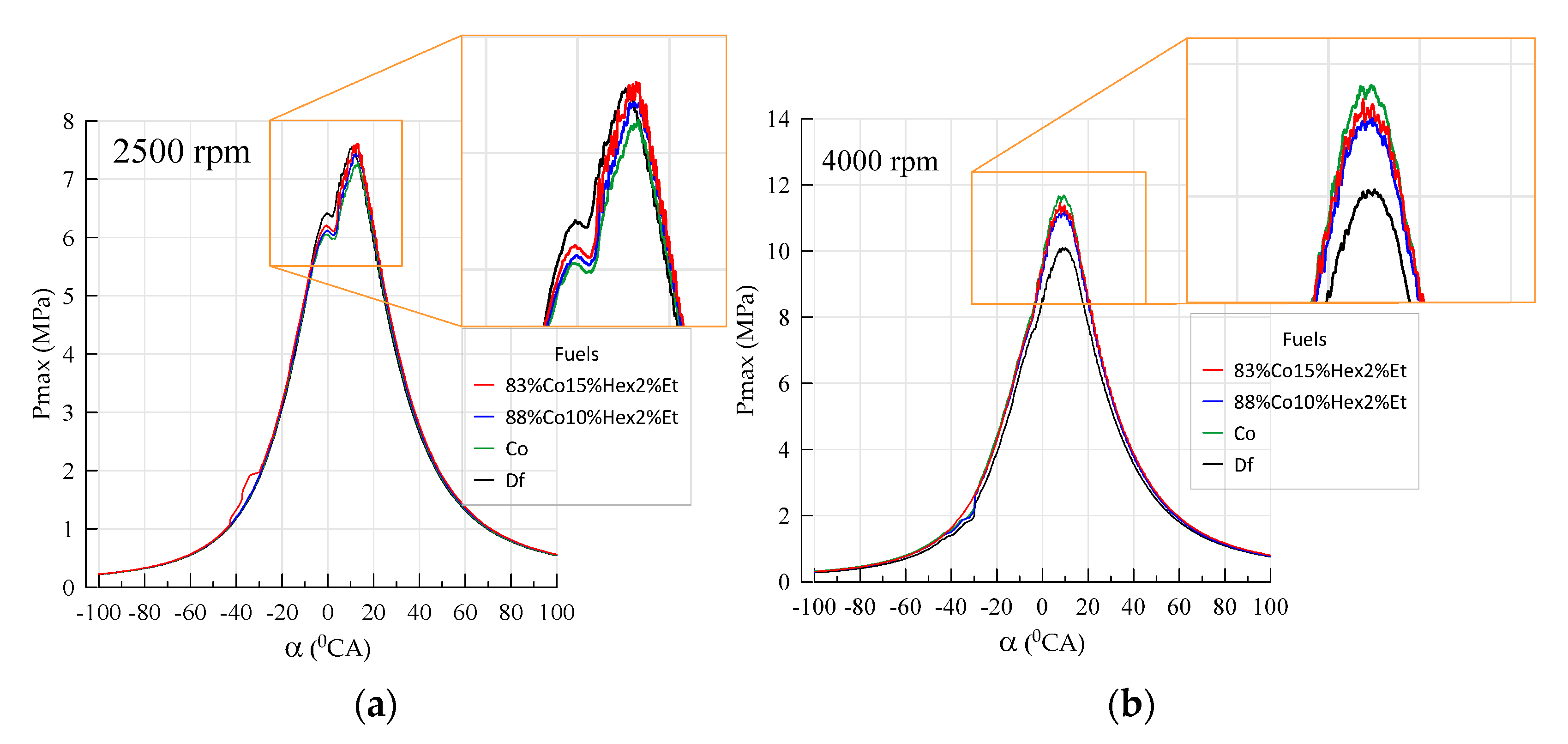
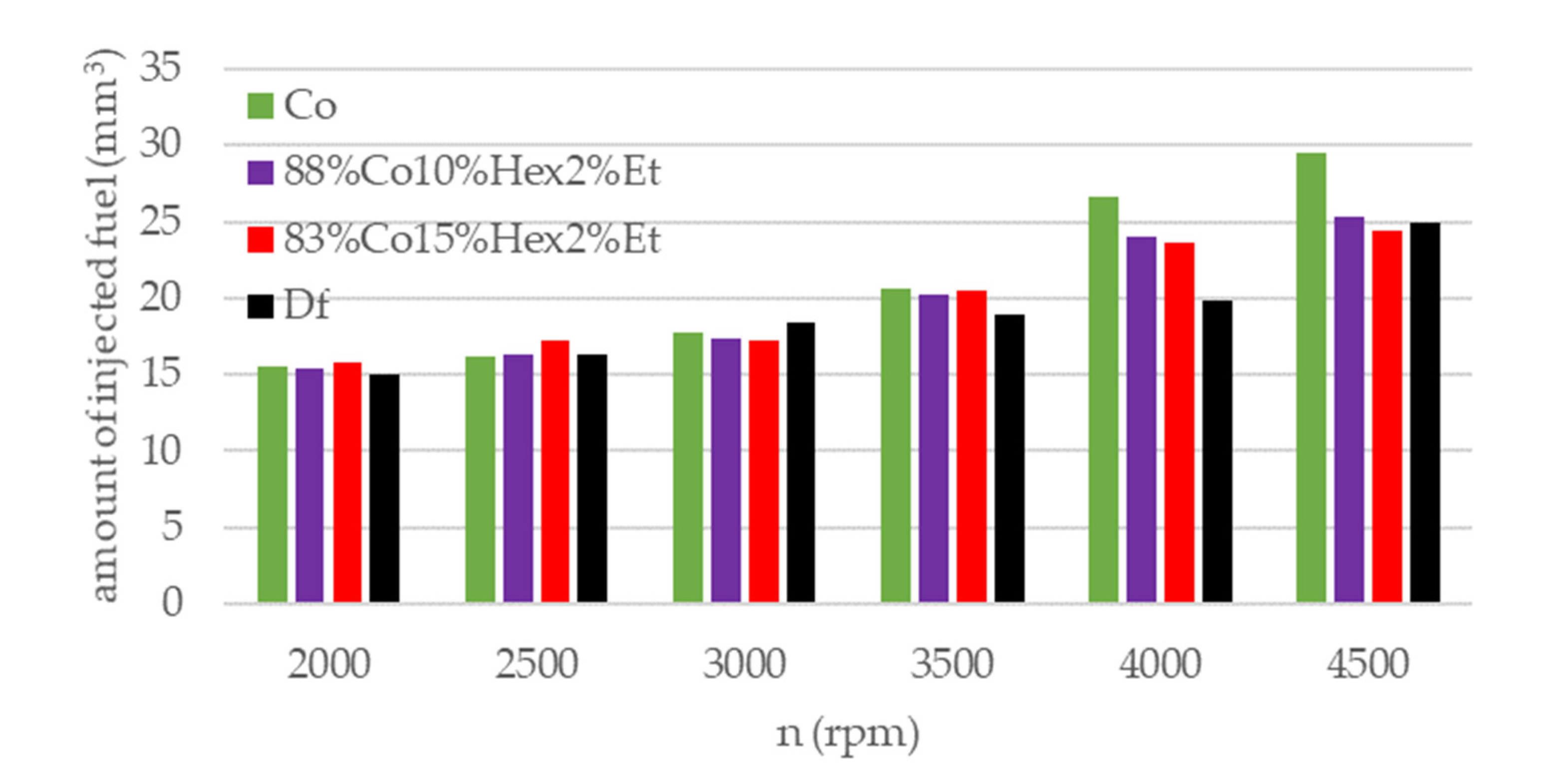
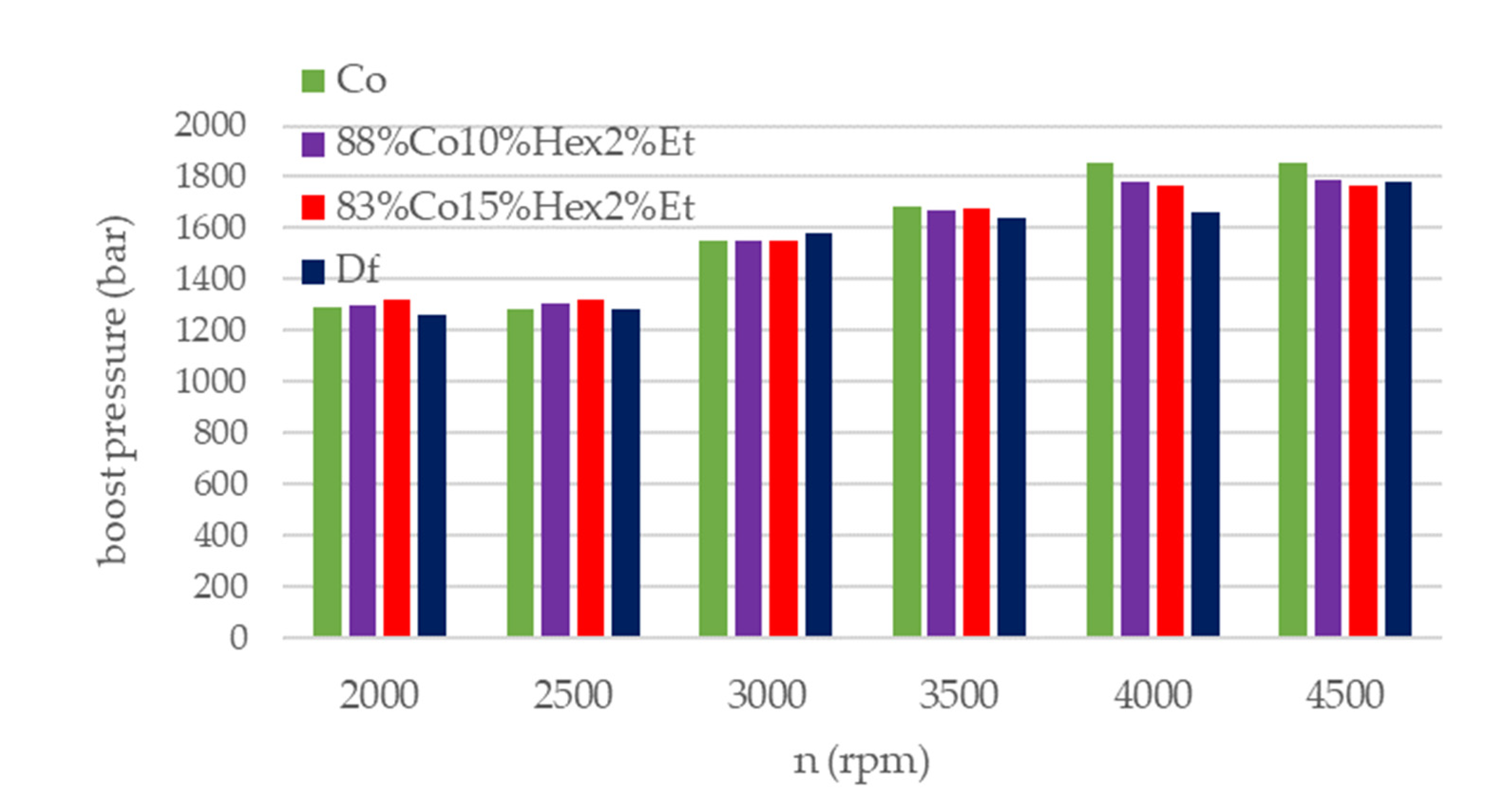
| Parameter | Df | 83%Co15%Hex2%Et | 88%Co10%Hex2%Et | Co |
|---|---|---|---|---|
| Power | 56.6 kW 4391 rpm | 52.4 kW 3962 rpm | 53.0 kW 4056 rpm | 45.9 kW 4024 rpm |
| Torque | 179.6 Nm 2235 rpm | 164.8 Nm 2264 rpm | 159.5 Nm 2527 rpm | 155.6 Nm 2191 rpm |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Longwic, R.; Sander, P.; Jańczuk, B.; Zdziennicka, A.; Szymczyk, K. Modification of Canola Oil Physicochemical Properties by Hexane and Ethanol with Regards of Its Application in Diesel Engine. Energies 2021, 14, 4469. https://doi.org/10.3390/en14154469
Longwic R, Sander P, Jańczuk B, Zdziennicka A, Szymczyk K. Modification of Canola Oil Physicochemical Properties by Hexane and Ethanol with Regards of Its Application in Diesel Engine. Energies. 2021; 14(15):4469. https://doi.org/10.3390/en14154469
Chicago/Turabian StyleLongwic, Rafał, Przemysław Sander, Bronisław Jańczuk, Anna Zdziennicka, and Katarzyna Szymczyk. 2021. "Modification of Canola Oil Physicochemical Properties by Hexane and Ethanol with Regards of Its Application in Diesel Engine" Energies 14, no. 15: 4469. https://doi.org/10.3390/en14154469
APA StyleLongwic, R., Sander, P., Jańczuk, B., Zdziennicka, A., & Szymczyk, K. (2021). Modification of Canola Oil Physicochemical Properties by Hexane and Ethanol with Regards of Its Application in Diesel Engine. Energies, 14(15), 4469. https://doi.org/10.3390/en14154469








