Decarbonization of Marine Fuels—The Future of Shipping
Abstract
:1. Introduction
2. Carbon Dioxide Emission from Shipping
- The Energy Efficiency Design Index (EEDI) for newbuilds mandating up to 30% improvement in design performance depending on ship type and size in comparison with 2013 (year of requirement introduction) [13];
- The Ship Energy Efficiency Management Plan (SEEMP) for all ships above 400 GT in operation [14]; and
- The Fuel Oil Consumption Data Collection System (DCS) mandating annual reporting of carbon dioxide emissions, and other activity data and ship particulars for all ships above 5000 GT.
- The Energy Efficiency Design Index for Existing Ships (EEXI) with expected entry into force on 1 January 2023;
- The Carbon Intensity Indicator (CII) (as AER (Annual Emission Rating) in grams of CO2 per deadweight-mile) with rating scheme from A to E calculated every year for all cargo and cruise ships above 5000 GT; and
- A strengthening of the SEEMP with mandatory content achieving the CII targets.
3. Carbon Dioxide Emission Coefficient for Marine and Alternative Fuels
4. Decarbonization Process of Marine Fuels
5. Transitional and Future Fuels in Shipping
5.1. Biofuels
5.2. Alcohols
5.3. Ammonia as a Remedy for the Decarbonization Process
5.4. Marine Gaseous Fuels—Dual or Tri Fuel Engines
5.5. Hydrogen—Marine Fuel of the Future
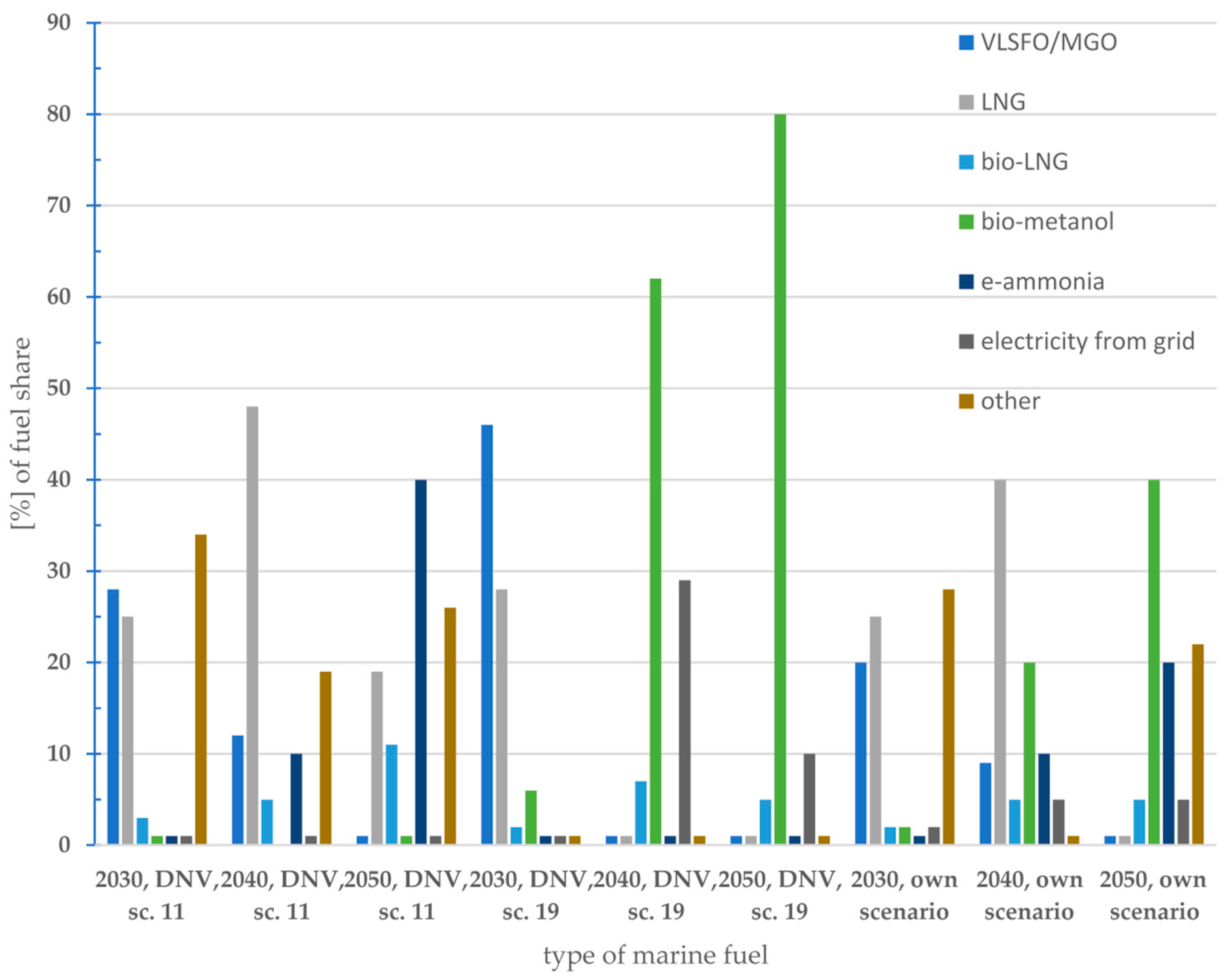
6. Possible Scenarios of Marine Fuel Usage
7. Discussion—What Type of Marine Fuel Will Be Used in the Future?
8. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Olmer, N.; Comer, B.; Roy, B.; Mao, X.; Rutherford, D. Greenhouse Gas Emission from Global Shipping 2013–2015, Detailed Methodology; The International Council on Clean Transportation: Washington, DC, USA, 2017. [Google Scholar]
- IMO MEPC 72/INF.5. Reduction of GHG from Ships. Understanding CO2 Emissions and Challenges in Assessing the Operational Efficiency for Ships; International Maritime Organization: London, UK, 2018. [Google Scholar]
- IMO (International Maritime Organization). 2019. Available online: http://www.imo.org/en/OurWork/Environment/PollutionPrevention/AirPollution/Pages/GHG-Emissions.aspx (accessed on 15 March 2021).
- IMO. IMO Action to Reduce Greenhouse Gas Emission from International Shipping; International Maritime Organization: London, UK, 2018. [Google Scholar]
- Hyungju, K.; Kwi, Y.K.; Tae-Hwan, J. A study on a necessity of integrated evaluation of alternative marine fuels. J. Int. Marit. Saf. Environ. Aff. Shipp. 2020, 4, 26–31. [Google Scholar] [CrossRef]
- Liu, M.; Li, C.; Koh, E.K.; Ang, Z.; Lam, J.S.L. Is methanol a future marine fuel for shipping? IOP Conf. Ser. J. Phys. 2019, 1357. [Google Scholar] [CrossRef]
- Zincir, B. A short review of ammonia as an alternative marine fuel for decarbonized maritime transportation. In Proceedings of the ICEESEN, Kayseri, Turkey, 19–21 November 2020. [Google Scholar]
- DNV. Bio Diesel: Emissions Depend on the Production Method; DNV GL: Bærum, Norway, 2018. [Google Scholar]
- Available online: http://www.ppmc-transport.org/battery-electric-car-ferry-in-norway/ (accessed on 19 May 2021).
- Global Warming Potential Values. Greenhouse Gas. Protocol. 2016. Available online: https://www.ghgprotocol.org/sites/default/files/ghgp/Global-Warming-Potential-Values%20%28Feb%2016%202016%29_1.pdf (accessed on 17 May 2021).
- EPA. Emission Factors for Greenhouse Gas Inventories. United States Environmental Protection Agency. 2020. Available online: https://www.epa.gov/sites/production/files/2020-04/documents/ghg-emission-factors-hub.pdf (accessed on 22 May 2021).
- Hodnebrog, O.; Dalsoren, S.B.; Hyhre, G. Lifetimes, direct and indirect radiative forcing, and global warming potentials of ethane (C2H6), propane (C3H8) and butane (C4H10). Atmos. Sci. Lett. 2018, 19, e804. [Google Scholar] [CrossRef]
- IMO Resolution MEPC.2015(63). Guidelines for Calculation of Reference Lines for Use with EEDI; International Maritime Organization: London, UK, 2012. [Google Scholar]
- IMO MEPC 59/24/Add.1, Annex 19. Guidance for the Development of a Ship Energy Efficiency Management Plan, SEEMP; International Maritime Organization: London, UK, 2012. [Google Scholar]
- Greene, S.; Jia, H.; Rubio-Domingo, G. Well-to-tank carbon emission from crude oil maritime transportation. Transp. Res. Part. D 2020, 88, 102587. [Google Scholar] [CrossRef]
- Comer, B.; Osipova, L. Accounting Well-To-Wake Carbon Dioxide Equivalent Emission in Maritime Transportation Climate Policies. The International Council on Clean Transportation, ICCT. 2021. Available online: https://theicct.org/publications/well-to-wake-co2-mar2021 (accessed on 17 May 2021).
- IMO MEPC 75/7/15. Reduction of GHG Emissions from Ships. Fourth IMO GHG Study 2020–Final Report; International Maritime Organization: London, UK, 2020. [Google Scholar]
- IMO, Marine Environment Protection Committee. Initial IMO Strategy on Reduction of GHG Emission from Ships; International Maritime Organization: London, UK, 2018. [Google Scholar]
- Roadmap to Decarbonizing European Shipping. Transport & Environment. 2018. Available online: https://www.transportenvironment.org/publications/roadmap-decarbonising-european-shipping (accessed on 21 May 2021).
- Reaching Zero Carbon Emissions from Shipping. ETC Energy Transitions Commission. 2018. Available online: https://www.ieta.org/resources/COP24/Misc%20Media%20Files/Dec7/SE16%20(3).pdf (accessed on 22 May 2021).
- Balcombe, P.; Brierley, J.; Lewis, C.; Skatvedt, L.; Speirs, J.; Hawkes, A.; Staffell, I. How to decarbonise international shipping: Options for fuels, technologies and policies. Energy Convers. Manag. 2019, 182, 72–88. [Google Scholar] [CrossRef]
- IMO (International Maritime Organization). 2018. Available online: http://www.imo.org/en/ourwork/environment/pollutionprevention/airpollution/pages/data-collection-system.aspx (accessed on 29 May 2021).
- Witkowski, K. Research of the effectiveness of selected methods of reducing toxic exhaust emissions of marine diesel engines. J. Mar. Sci. Eng. 2020, 8, 452. [Google Scholar] [CrossRef]
- Kass, M.; Abdullah, Z.; Biddy, M.J.; Drennan, C.; Haq, Z.; Hawkins, T.; Jones, S.; Holliday, J.; Longman, D.E.; Menter, S.; et al. Understanding the Opportunities of Biofuels for Marine Shipping. Oak Ridge National Laboratory. 2018. Available online: https://info.ornl.gov/sites/publications/Files/pub120597.pdf. (accessed on 14 May 2021).
- Hsieh, C.C.; Felby, C. Biofuels for the Marine Shipping Sector. IEA Bioenergy. 2017. Available online: https://www.ieabioenergy.com/wp-content/uploads/2018/02/Marine-biofuel-report-final-Oct-2017.pdf. (accessed on 20 May 2021).
- Andersson, K.; Salazar, C.M. Methanol as a Marine Fuel Report. 2015. Available online: https://www.methanol.org/wp-content/uploads/2018/03/FCBI-Methanol-Marine-Fuel-Report-Final-English.pdf (accessed on 14 May 2021).
- Engineering the Future Two-Stroke Green-Ammonia Engine. MAN Energy Solutions. 2019. Available online: https://fathom.world/wp-content/uploads/2020/05/engineeringthefuturetwostrokegreenammoniaengine1589339239488.pdf. (accessed on 14 May 2021).
- IMO CCC 7/INF8. Forecasting the Alternative Fuel. Ammonia; International Maritime Organization: London, UK, 2020. [Google Scholar]
- ABS. Ammonia as Marine Fuel, Sustainability Whitepaper; ABS: Spring, TX, USA, 2020. [Google Scholar]
- DNV. Ammonia as A Marine Fuel, Safety Handbook; DNV-GL: Bærum, Norway, 2020. [Google Scholar]
- Hansson, J.; Brynolf, S.; Fridell, E.; Lehtveer, M. The potential role of ammonia as marine fuel–based on energy system modelling and multi-criteria decision analysis. Sustainability 2020, 12, 3265. [Google Scholar] [CrossRef] [Green Version]
- DNV. Assessment of Selected Alternative Fuels and Technologies; DNV-GL Maritime: Bærum, Norway, 2019. [Google Scholar]
- IMO Resolution MSC 39(95). Adoption of the International Code for the Ships Using Gases Other Low-Flashpoint Fuels (IGF Code), Adopted 11 June 2015; International Maritime Organization: London, UK, 2015. [Google Scholar]
- DNV. LPG as a Marine Fuel; Group Technology & Research, DNV-GL: Bærum, Norway, 2017. [Google Scholar]
- IMO. International Convention for the Prevention of Pollution from Ships, Revised MARPOL Annex VI; International Maritime Organization: London, UK, 2008. [Google Scholar]
- Bouman, E.A.; Lindstad, E.; Rialland, A.I.; Strømman, A.H. State-of-the-art technologies, measures, and potential for reducing GHG emissions from shipping—A review. Transp. Res. Part D 2017, 52, 408–421. [Google Scholar] [CrossRef]
- Available online: http://alsafetydatasheets.com/download/se/Hydrogen_compressed-SE_ENG.pdf (accessed on 27 May 2021).
- Available online: https://www.airgas.com/msds/001033.pdf (accessed on 27 May 2021).
- Sadik-Zada, E.R.; Gatto, A. Energy security pathways in South East Europe: Diversification of the natural gas supplies, energy transition, and energy futures. In Economic to Energy Transition. Energy, Climate and the Environment; Mišík, M., Oravcová, V., Eds.; Palgrave Macmillan: Cham, Switzerland, 2021. [Google Scholar] [CrossRef]
- Lloyds Register Marine. Global Marine Fuel Trends 2030; Lloyd’s Register: London, UK, 2021. [Google Scholar]
- Available online: https://www.wfw.com/articles/alternative-fuels-what-does-the-future-hold-for-shipping/ (accessed on 5 July 2021).
- DNV. 2020 Fuels and Beyond; DNV: Bærum, Norway, 2020. [Google Scholar]
- DNV. Maritime Forecast to 2050, Energy Transition Outlook 2020; DNV: Bærum, Norway, 2020. [Google Scholar]
- DNV. Rising to the Challenge of Hydrogen Economy; DNV: Bærum, Norway, 2021. [Google Scholar]
- Winterthur Gas & Diesel Ltd. 2021. Available online: https://www.wingd.com/en/documents/general/brochures/wingd-low-speed-engines-booklet-2021.pdf/ (accessed on 28 May 2021).
- Bengtsson, S.; Andersson, K.; Fridell, E. A comparative life cycle assessment of marine fuels liquefied natural gas and three other fossil fuels. Proc. Inst. Mech. Eng. Part M J. Eng. Marit. Environ. 2011, 225, 97–110. [Google Scholar]
- Andersson, K.; Brynolf, S.; Hansson, J.; Grahn, M. Criteria and decision support for a sustainable choice of alternative marine fuels. Sustainability 2020, 12, 3623. [Google Scholar] [CrossRef]
| Type of Gas | GWP20 | GWP100 |
|---|---|---|
| Carbon dioxide CO2 | 1 | 1 |
| Carbon monoxide CO | 2.8 ÷ 10 1 | 1 ÷ 3 1 |
| Methane CH4 | 84 | 30 |
| Nitrous oxide N2O | 300 | 265 |
| Propane C3H8 | 9.5 | 3.3 |
| Butane C4H10 | 6.5 | 4 |
| Black Carbon BC | 4.47 | 1.055 ÷ 2.24 1 |
| Ammonia NH3 | 0 | 0 |
| Type of Fuel | Carbon Content (m/m) | Fuel Coefficient (cF) (kg CO2/kg of Fuel) 1 |
|---|---|---|
| Marine gas oil | 0.875 | 3.206 |
| Marine diesel oil | 0.875 | 3.206 |
| Light fuel oil | 0.86 | 3.151 |
| Marine heavy oil | 0.85 | 3.112 |
| Methane | 0.75 | 2.750 |
| Propane | 0.819 | 3.000 |
| Butane | 0.827 | 3.030 |
| Propylene | 0.857 | 3.141 |
| Biodiesel | 0.86 | 3.151 |
| Methanol | 0.375 | 1.375 |
| Ethanol | 0.522 | 1.913 |
| Dimethyl ether | 0.522 | 1.913 |
| Ammonia | 0 | 0 |
| Parameter/Type of Biofuel | Biodiesel | Renewable Diesel | Fatty acid Methyl Esters |
|---|---|---|---|
| Density (15 °C) (kg/dm3) | 0.88 | 0.78 | 0.765 |
| Kinetic viscosity (40 °C) (cSt) | 4 ÷ 6 | 2 ÷ 4 | 2 |
| Cetane number | 47 ÷ 65 | >70 | >70 |
| Sulfur content (mass%) | <0.0015 | <0.0005 | <0.1 |
| Lower Heating Value | 37.2 MJ/kg | 44.1 MJ/kg | 43 MJ/kg |
| Parameter | Methanol | Ethanol |
|---|---|---|
| Density (at 15 °C) | 794 kg/m3 | 789 kg/m3 |
| Lower Heating Value | 20 ÷ 23 MJ/kg | 27 ÷ 30 MJ/kg |
| Boiling point | 64.7 °C | 77 °C |
| Flash point | 12 °C | <10 °C |
| Lower and upper explosion limits | 6 ÷ 36.5% v/v | 3.2 ÷ 18.3% v/v |
| Water solubility | miscible | miscible |
| Kinetic viscosity at 20 °C | 0.77 cSt | 1.77 cSt |
| Parameter | Ammonia NH3 | Propane C3H8 |
|---|---|---|
| Boiling point at 0.1 MPa | −33.3 °C | −42.1 °C |
| Density, liquid | 682 kg/m3 | 493 kg/m3 |
| Lower Heating Value | 18.6 MJ/kg | 46.6 MJ/kg |
| Flammability limit ratio, (stoichiometric with air = 1) | 0.63 ÷ 1.4 | 0.51 ÷ 2.51 |
| Maximum burning velocity | 0.09 m/s | 0.43 m/s |
| Ignition temperature | 651 °C | 432 °C |
| Maximum adiabatic flame temperature | 1750 °C | 2020 °C |
| Range LFL–UFL | 15 ÷ 25% | 2.1 ÷ 9.5% |
| Cetane number | Very low | >34 |
| Parameter | Hydrogen | Methane (Natural Gas) |
|---|---|---|
| Gas density at normal conditions (ISO) | 0.0905 kg/m3 | 0.716 kg/m3 |
| Relative density, gas (air = 1) | 0.07 | 0.554 |
| Relative density, liquid (water = 1) | 0.071 | 0.44 ÷ 0.48 |
| Lower Heating Value | 119.96 MJ/kg | 50 MJ/kg |
| Critical temperature | −240 °C | −82.45 °C |
| Boiling point | −253 °C | −161.48 °C |
| Melting point | −259 °C | −187.6 °C |
| Flammability range | 4 ÷ 77% (v/v) | 5 ÷ 14% (v/v) |
| Maximum burning velocity | 2.91 m/s | 0.37 m/s |
| Autoignition temperature | 585 °C | 537 °C |
| UN | 1954 | 1971 |
| Other information | Burns with invisible flame, extremely flammable gas | Asphyxiant |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Herdzik, J. Decarbonization of Marine Fuels—The Future of Shipping. Energies 2021, 14, 4311. https://doi.org/10.3390/en14144311
Herdzik J. Decarbonization of Marine Fuels—The Future of Shipping. Energies. 2021; 14(14):4311. https://doi.org/10.3390/en14144311
Chicago/Turabian StyleHerdzik, Jerzy. 2021. "Decarbonization of Marine Fuels—The Future of Shipping" Energies 14, no. 14: 4311. https://doi.org/10.3390/en14144311
APA StyleHerdzik, J. (2021). Decarbonization of Marine Fuels—The Future of Shipping. Energies, 14(14), 4311. https://doi.org/10.3390/en14144311






