Determination of Nitrogen and Sulphur Mineralization in Batch and Semi-Continuous Anaerobic Digestion Using an Artificial Fiber Bag Technique
Abstract
:1. Introduction
2. Materials and Methods
2.1. Artificial Fiber Bag Preparation
2.2. Batch Anaerobic Digestion Bottle Setup
2.3. Continuous Reactor Setup
2.4. Artificial Fiber Bag Washing and Drying Procedure
2.5. Analysis
2.6. Determination of N and S Mineralization
2.7. Model Fitting and Kinetic Calculations
2.8. Continuous Experiment Feedstock Characteristics
3. Results and Discussion
3.1. Validating the Artificial Fiber Bag Technique by Batch Anaerobic Digestion
3.1.1. Comparing Batch Test Biogas Production
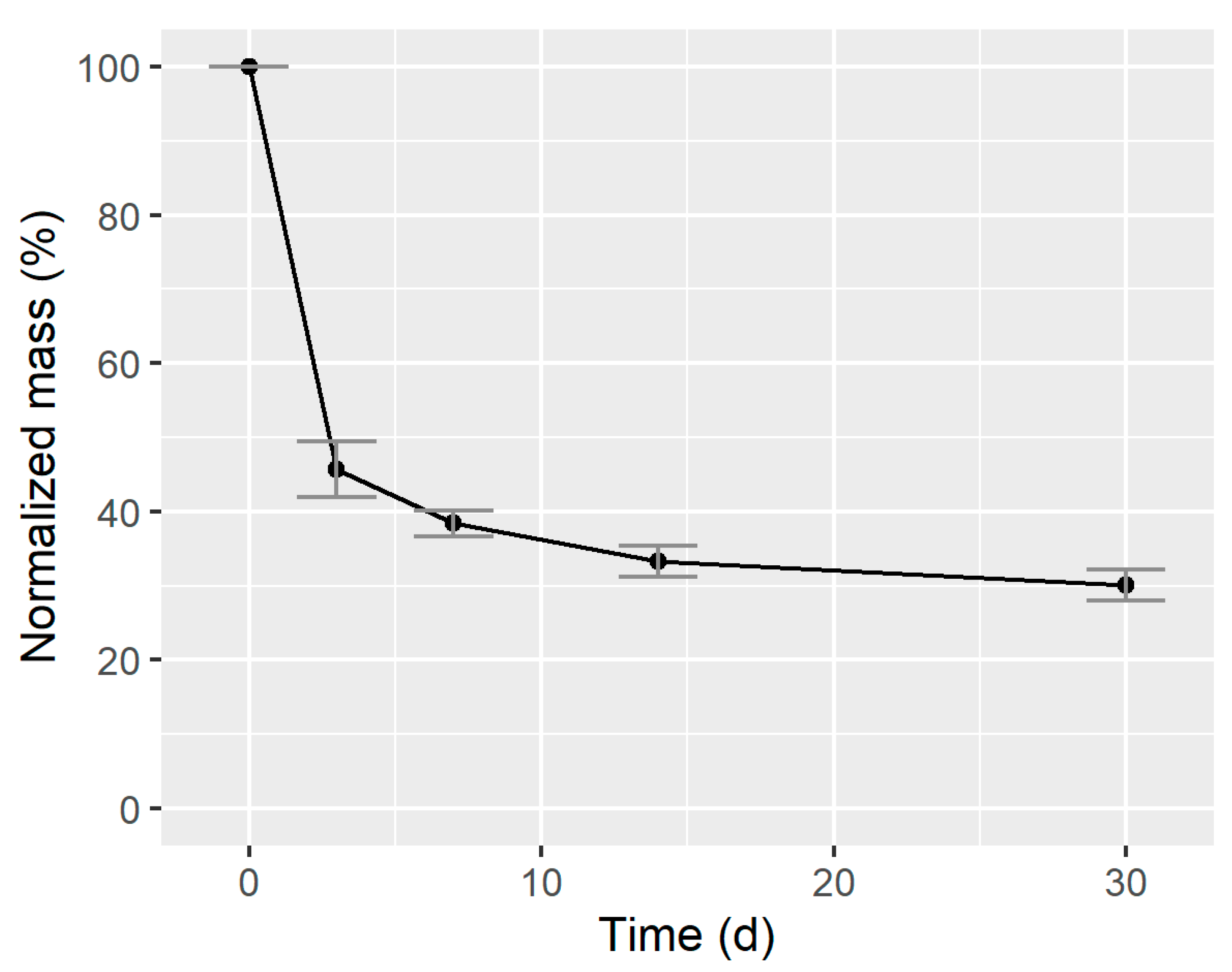
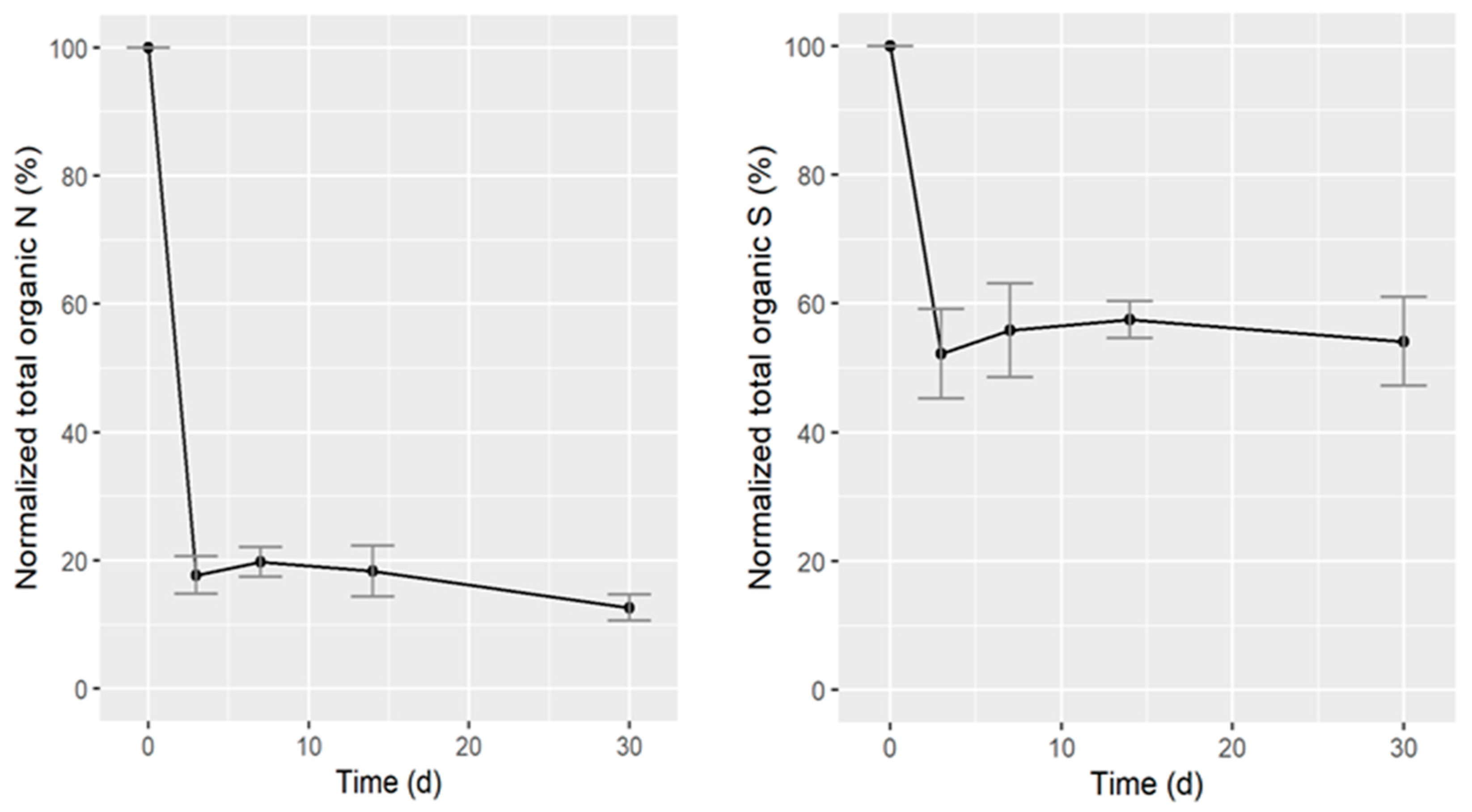
3.1.2. Mineralization in Batch Anaerobic Digestion
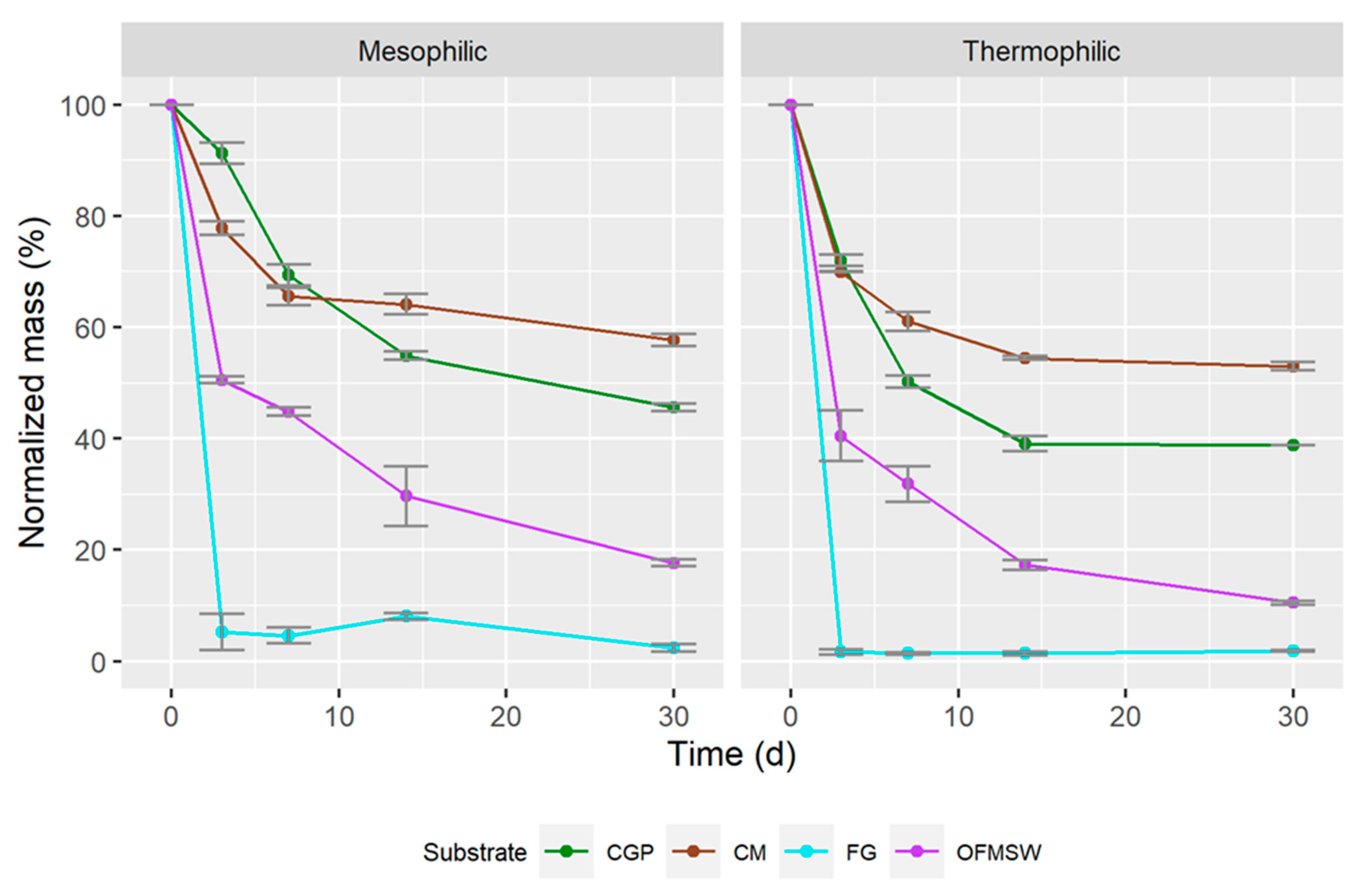
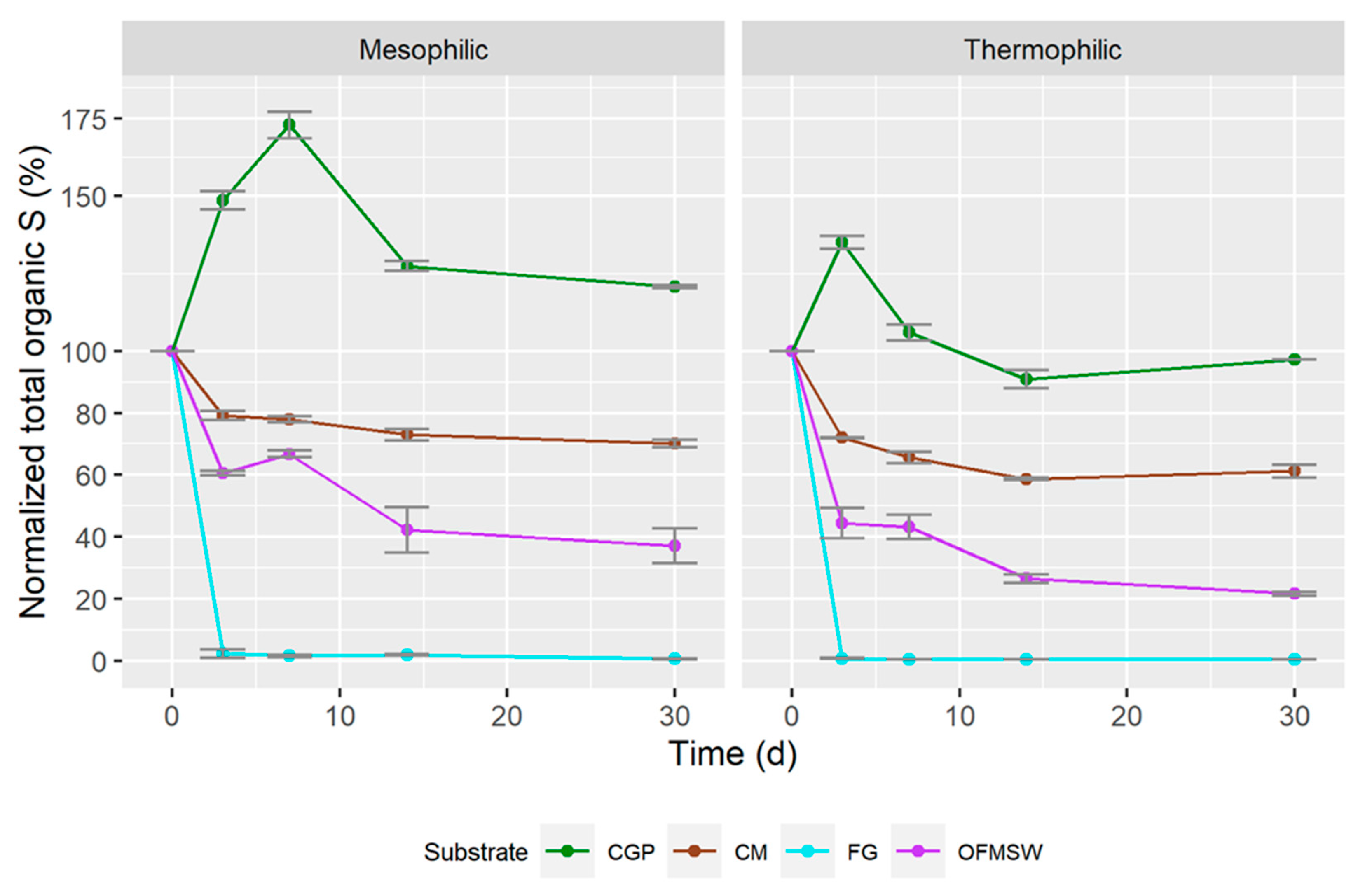
3.2. Semi-Continuous Anaerobic Digestion
3.3. Potential and Further Aspects
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- ACEA. Position Paper—Charging and Re-Fuelling Infrastructure Required for Heavy-Duty Vehicles. Automobile Manufacturers Association. 2020. Available online: https://www.acea.auto/publication/position-paper-charging-and-refuelling-infrastructure-required-for-heavyduty-vehicles/ (accessed on 25 November 2020).
- Blake, L.I.; Halim, F.A.; Gray, C.; Mair, R.; Manning, D.A.C.; Sallis, P.; Hutchinson, H.; Gray, N.D. Evaluating an anaerobic digestion (AD) feedstock derived from a novel non-source segregated municipal solid waste (MSW) product. Waste Manag. 2017, 59, 149–159. [Google Scholar] [CrossRef] [Green Version]
- Allegue, L.B.; Hinge, J. Biogas and Bio-Syngas Upgrading; Danish Technological Institute: Aarhus, Danmark, 2012. [Google Scholar]
- Díaz Vázquez, D.; Alvarado-Cummings, S.C.; Meza-Rodriguez, D.; Senés-Guerrero, C.; de Anda, J.; Gradilla-Hernández, M.S. Evaluation of Biogas Potential from Livestock Manures and Multicriteria Site Selection for Centralized Anaerobic Digester Systems: The Case of Jalisco, México. Sustainability 2020, 12, 3527. [Google Scholar] [CrossRef]
- EIP-AGRI Focus Group. Reducing Emissions from Cattle Farming; Final Report; European Commission: Brussels, Belgium, 2017. [Google Scholar]
- Ward, A.J.; Hobbs, P.J.; Holliman, P.J.; Jones, D.L. Optimisation of the anaerobic digestion of agricultural resources. Bioresour. Technol. 2008, 99, 7928–7940. [Google Scholar] [CrossRef]
- Paungfoo-Lonhienne, C.; Lonhienne, T.G.A.; Rentsch, D.; Robinson, N.; Christie, M.; Webb, R.I.; Gamage, H.K.; Carroll, B.J.; Schenk, P.M.; Schmidt, S. Plants can use protein as a nitrogen source without assistance from other organisms. Proc. Natl. Acad.Sci. USA 2008, 105, 4524–4529. [Google Scholar] [CrossRef] [Green Version]
- Olesen, J.E.; Møller, H.B.; Petersen, S.O.; Sørensen, P.; Nyord, T.; Sommer, S.G. Bæredygtig Biogas—Klima og Miljøeffekter af Biogasproduktion; 28 s.—DCA rapport nr. 175; Aarhus Universitet, DCA—Nationalt Center for Fødevarer og Jordbrug: Tjele, Danmark, 2020; Available online: https://dcapub.au.dk/djfpdf/DCArapport175.pdf (accessed on 2 December 2020).
- Yan, L.i.; Ye, J.; Zhang, P.; Xu, D.; Wu, Y.; Liu, J.; Zhang, H.; Fang, W.; Wang, B.; Zeng, G. Hydrogen sulfide formation control and microbial competition in batch anaerobic digestion of slaughterhouse wastewater sludge: Effect of initial sludge pH. Bioresour. Technol. 2018, 259, 67–74. [Google Scholar] [CrossRef]
- Ghoorchi, T.; Lund, P.; Larsen, M.; Hvelplund, T.; Hansen-Møller, J.; Weisbjerg, M.R. Assessment of the mobile bag method for estimation of in vivo starch digestibility. Animal 2013, 7, 265–271. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hafner, S.D.; Rennuit, C.; Olsen, P.J.; Pedersen, J.M. Quantification of leakage in batch biogas assays. Water Pract. Technol. 2018, 13, 52–61. [Google Scholar] [CrossRef]
- Hafner, S.D.; Rennuit, C.; Triolo, J.M.; Richards, B.K. Validation of a simple gravimetric method for measuring biogas production in laboratory experiments. Biomass Bioenerg. 2015, 83, 297–301. [Google Scholar] [CrossRef]
- Hafner, S.D.; Richards, B.K.; Astals, S.; Holliger, C.; Koch, K.; Weinrich, S. Calculation of Methane Production from Gravimetric Measurements. Standard BMP Methods Document 203, Version 1.1. 2020. Available online: https://raw.githubusercontent.com/sashahafner/BMP-methods/master/200_BMP_calculations.pdf (accessed on 12 March 2021).
- Hafner, S.D.; Astals, S.; Holliger, C.; Koch, K.; Weinrich, S. Calculation of Biochemical Methane Potential (BMP). Standard BMP Methods Document 200, Version 1.7. 2020. Available online: https://raw.githubusercontent.com/sashahafner/BMP-methods/master/203_gravimetric_calculations.pdf (accessed on 12 March 2021).
- Hafner, S.D.; Koch, K.; Carrere, H.; Astals, S.; Weinrich, S.; Rennuit, C. Software for Biogas Research: Tools for Measurement and Prediction of Methane Production. SoftwareX 2018, 7, 205–210. [Google Scholar] [CrossRef]
- Eriksen, J.; Sørensen, P.; Elsgaard, L. The fate of sulfate in acidified pig slurry during storage and following application to cropped soil. J. Environ. Qual. 2008, 37, 280–286. [Google Scholar] [CrossRef] [Green Version]
- APHA. Standard Methods for the Examination of Water and Wastewater, 21st ed.; APHA: Washington, DC, USA, 2005. [Google Scholar]
- Holliger, C.; Astals, S.; de Laclos, H.F.; Hafner, S.D.; Koch, K.; Weinrich, S. Towards a standardization of biomethane potential tests: A commentary. Water Sci. Technol. 2021, 83, 247–250. [Google Scholar] [CrossRef] [PubMed]
- Holliger, C.; de Laclos, H.F.; Hafner, S.D.; Koch, K.; Weinrich, S.; Astals, S.; Alves, M.; Andrade, D.; Angelidaki, I.; Appels, L.; et al. Requirements for Measurement of Biochemical Methane Potential (BMP). Standard BMP Methods Document 100, Version 1.9. 2021. Available online: https://www.dbfz.de/en/BMP (accessed on 12 March 2021).
- Li, P.; Li, W.; Sun, M.; Xu, X.; Zhang, B.; Sun, Y. Evaluation of Biochemical Methane Potential and Kinetics on the Anaerobic Digestion of Vegetable Crop Residues. Energies 2018, 12, 26. [Google Scholar] [CrossRef] [Green Version]
- Koch, K.; Hafner, S.D.; Weinrich, S.; Astals, S. Identification of Critical Problems in Biochemical Methane Potential (BMP) Tests from Methane Production Curves. Front. Environ. Sci. 2019. [Google Scholar] [CrossRef]
- Boyle, W.C. Energy recovery from sanitary landfills—A review. In Microbial Energy Conversion; Schlegel, H.G., Barnea, S., Eds.; Elsevier: Amsterdam, The Netherlands, 1977; pp. 119–138. [Google Scholar]
- Fog, E. Protein Feed from Clover Grass for Pigs and Poultry. Results from Danish Innovation Projects; Technical Report Presented at Agromania: Herning, Denmark, 27–30 November 2018. [Google Scholar]
- Meyer, A.K.P.; Ehimen, E.A.; Holm-Nielsen, J.B. Bioenergy production from roadside grass: A case study of the feasibility of using roadside grass for biogas production in Denmark. Resour. Conserv. Recycl. 2014, 93, 124–133. [Google Scholar] [CrossRef] [Green Version]
- Paul, S.; Onduru, D.; Wouters, B.; Gachimbi, L.; Zake, J.; Ebanyat, P.; Ergano, K.; Abduke, M.; van Keulen, H. Cattle Manure Management in East Africa: Review of Manure Quality and Nutrient Losses and Scenarios for Cattle and Manure Management; Wageningen UR Livestock Research, Technical Report No. 258; Staff Publications: Long Island City, NY, USA, 2009. [Google Scholar]
- Møller, H.B.; Moset, V.; Brask, M.; Weisbjerg, M.R. Feces composition and manure derived methane yield from dairy cows: Influence of diet with focus on fat supplementation and roughage type. Atmos. Environ. 2014, 94, 36–43. [Google Scholar] [CrossRef]
- ECOGI. Pre-Treatment of Biomass for Anaerobic Digestion; ETV Test Report, Project No.: 1005; ETA: Copenhagen, Danmark, 2015. [Google Scholar]
- Ward, A.J.; Feng, L.; Moset, V.; Moller, H.B. Estimation of methane yields in continuous biogas reactors using kinetic and mass flow models Estimation of Methane Yields in Continuous Biogas Reactors Using Kinetic and Mass Flow Models. Chem. Eng. Technol. 2018, 41, 761–767. [Google Scholar] [CrossRef]
- Park, J.; Park, S.; Kim, M. Anaerobic degradation of amino acids generated from the hydrolysis of sewage sludge. Environ. Technol. 2014, 35, 1133–1139. [Google Scholar] [CrossRef] [PubMed]
- Skiba, B.; Weisbjerg, M.R.; Hvelplund, T. Rumen and total intestinal tract digestibility of protein and amino acids from different roughages, determined in situ. J. Anim. Feed Sci. 1996, 5, 347–363. [Google Scholar] [CrossRef]
- Damborg, V.K.; Stødkilde, L.; Jensen, S.K.; Weisbjerg, M.R. Protein value and degradation characteristics of pulp fibre fractions from screw pressed grass, clover, and lucerne. Anim. Feed Sci. Technol. 2018, 244, 93–103. [Google Scholar] [CrossRef]
- Hvelplund, T.; Weisbjerg, M.R. In situ techniques for the estimation of protein degradability and postrumen availability. In Forage Evaluation in Ruminant Nutrition; Givens, D.I., Oven, E., Axford, R.F.E., Omed, H.M., Eds.; CAB International: Wallingford, UK, 2000; pp. 233–258. [Google Scholar]
- Massé, D.I.; Croteau, F.; Masse, L. The fate of crop nutrients during digestion of swine manure in psychrophilic anaerobic sequencing batch reactors. Biores. Technol. 2007, 98, 2819–2823. [Google Scholar] [CrossRef] [PubMed]
- He, R.; Yao, X.; Chen, M.; Ma, R.; Li, H.; Wang, C.; Ding, S. Conversion of sulfur compounds and microbial community in anaerobic treatment of fish and pork waste. Waste Manag. 2018, 76, 383–393. [Google Scholar] [CrossRef] [PubMed]
- Krämer, M.; Weisbjerg, M.R.; Lund, P.; Jensen, C.S.; Pedersen, M.G. Estimation of indigestible NDF in forages and concentrates from cell wall composition and organic matter digestibility. Anim. Feed Sci. Technol. 2012, 177, 40–51. [Google Scholar] [CrossRef]


| Number of Bottles | Measurement Days | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 2 | 3 | 5 | 7 | 9 | 14 | 20 | 30 | ||
| Inoculum control | 3 | M | M | M | M | M | M | M | M |
| Cellulose control | 3 | M | M | M | M | M | M | M | M |
| ISR = 2:1 AFB | 3 | M | M/O | ||||||
| ISR = 2:1 AFB | 3 | M | M | M | M/O | ||||
| ISR = 2:1 AFB | 3 | M | M | M | M | M | M/O | ||
| ISR = 2:1 AFB | 3 | M | M | M | M | M | M | M | M/O |
| ISR = 2:1 no AFB | 3 | M | M | M | M | M | M | M | M/O |
| ISR = 1:1 AFB | 3 | M | M | M | M | M | M | M | M/O |
| AFB only | 1 | M | M | M | M | M | M | M | M/O |
| Water control | 2 | M | M | M | M | M | M | M | M |
| Reactor 1 | Reactor 2 | |
|---|---|---|
| Condition | Mesophilic | Thermophilic |
| Temperature (°C) | 40 | 52 |
| Inoculum source | Maabjerg Bioenergy 1 | AU Foulum 2 |
| Inoculum pH | 8.26 ± 0.06 | 8.21 ± 0.03 |
| Inoculum alkalinity (g CaCO3eq L−1) | 15.28 ± 0.10 | 11.71 ± 0.06 |
| Inoculum total VFA (g L−1) | 1.52 ± 0.09 | 0.82 ± 0.06 |
| Inoculum NH4+-N (g L−1) | 3.97 ± 0.03 | 2.07 ± 0.15 |
| HRT (d) | 25 | 25 |
| Model Fit (Pearson R2) | B0 [mL gVS−1] | [d] | Rmax [mL gVS−1 d−1] | |
|---|---|---|---|---|
| No AFB 2:1 (rep 1) | 0.999 | 258 | 0.23 | 67.8 |
| No AFB 2:1 (rep 2) | 0.997 | 301 | 0.00 | 67.6 |
| No AFB 2:1 (rep 3) | 0.997 | 293 | 0.00 | 70.5 |
| No AFB 2:1 (mean data) | 0.998 | 279 | 0.00 | 65.9 |
| AFB 1:1 (rep 1) | 0.999 | 252 | 0.00 | 38.9 |
| AFB 1:1 (rep 2) | 0.999 | 270 | 0.00 | 42.7 |
| AFB 1:1 (rep 3) | 0.998 | 275 | 0.00 | 41.6 |
| AFB 1:1 (mean data) | 0.999 | 284 | 0.00 | 44.0 |
| AFB 2:1 (rep 1) | 0.999 | 279 | 0.07 | 65.8 |
| AFB 2:1 (rep 2) | 0.998 | 253 | 0.19 | 57.9 |
| AFB 2:1 (rep 3) | 0.999 | 254 | 0.08 | 58.9 |
| AFB 2:1 (mean data) | 0.999 | 279 | 0.11 | 64.8 |
| Substrate | TS (%) | VS (%) | Ash (%) | C (%) | H (%) | N (%) | S (%) | O b (%) | Crude Protein (%) | VFA (g L−1) | BMP, 63 d (mL gVS−1) | TBMP a (mL gVS−1) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CM | 6.7 | 5.2 | 1.5 | 43.8 | 5.5 | 2.6 | 0.6 | 46.0 | 15.9 | 4.9 | 195 ± 3 | 391 |
| CGP | 23.5 | 21.9 | 1.6 | 46.8 | 6.1 | 2.5 | 0.1 | 42.9 | 15.8 | na | 210 ± 7 | 448 |
| FG | 17.4 | 10.7 | 6.7 | 21.9 | 6.9 | 3.8 | 1.5 | 59.2 | 23.9 | 0.4–2.3 | 299 ± 17 | 176 |
| OFMSW | 12.7 | 10.8 | 1.9 | 48.1 | 7.1 | 2.7 | 0.2 | 40.0 | 17.0 | 2.7 | 415 ± 7 | 498 |
| Mesophilic | |||
| Total Mass (g) | Total N (g) | Total S (g) | |
| CGP T0 | 25.0 | 0.63 | 0.04 |
| CGP T30 | 11.4 | 0.38 | 0.05 |
| Reduction | 54.4% | 39.6% | −25% |
| CM T0 | 25.0 | 0.62 | 0.16 |
| CM T30 | 14.4 | 0.49 | 0.11 |
| Reduction | 42.4% | 21.0% | 31.3% |
| FG T0 | 50.0 | 1.78 | 0.78 |
| FG T30 | 1.20 | 0.02 | 0.04 |
| Reduction | 97.6% | 99.4% | 94.9% |
| OFMSW T0 | 25.0 | 0.63 | 0.07 |
| OFMSW T30 | 4.4 | 0.15 | 0.02 |
| Reduction | 82.4% | 76.2% | 63.0% |
| Thermophilic | |||
| Total Mass (g) | Total N (g) | Total S (g) | |
| CGP T0 | 25.0 | 0.63 | 0.04 |
| CGP T30 | 9.7 | 0.27 | 0.04 |
| Reduction | 61.2% | 57.6% | 2.8% (decimals) |
| CM T0 | 25.0 | 0.64 | 0.16 |
| CM T30 | 13.2 | 0.42 | 0.10 |
| Reduction | 47.2% | 34.4% | 37.5% |
| FG T0 | 50.0 | 1.82 | 0.80 |
| FG T30 | 0.9 | 0.01 | 0.00 |
| Reduction | 98.2% | 86.2% | 99.6% |
| OFMSW T0 | 25.0 | 0.65 | 0.07 |
| OFMSW T30 | 2.6 | 0.09 | 0.01 |
| Reduction | 89.6% | 86.2% | 79.9% |
| Mesophilic | |||||
| T0 | T3 | T7 | T14 | T30 | |
| CGP %N | 2.53 | 2.67 | 2.73 | 2.88 | 3.37 |
| CGP %S | 0.17 | 0.27 | 0.41 | 0.38 | 0.44 |
| CM %N | 2.55 | 3.01 | 3.18 | 3.27 | 3.43 |
| CM %S | 0.64 | 0.64 | 0.70 | 0.71 | 0.75 |
| FG %N | 3.83 | 2.72 | 2.20 | 1.96 | 1.61 |
| FG %S | 1.51 | 0.61 | 0.51 | 0.36 | 0.42 |
| OFMSW %N | 2.72 | 2.52 | 2.47 | 2.52 | 3.24 |
| OFMSW %S | 0.26 | 0.31 | 0.39 | 0.36 | 0.54 |
| Thermophilic | |||||
| T0 | T3 | T7 | T14 | T30 | |
| CGP %N | 2.53 | 2.87 | 3.14 | 2.60 | 2.79 |
| CGP %S | 0.17 | 0.31 | 0.35 | 0.38 | 0.42 |
| CM %N | 2.55 | 3.05 | 3.26 | 3.24 | 3.19 |
| CM %S | 0.64 | 0.64 | 0.68 | 0.68 | 0.74 |
| FG %N | 3.83 | 1.52 | 0.67 | 0.64 | 0.89 |
| FG %S | 1.51 | 0.63 | 0.38 | 0.40 | 0.35 |
| OFMSW %N | 2.72 | 2.21 | 2.24 | 2.59 | 3.24 |
| OFMSW %S | 0.26 | 0.29 | 0.36 | 0.40 | 0.55 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mortensen, J.R.; Ward, A.J.; Weisbjerg, M.R.; Hafner, S.D.; Møller, H.B. Determination of Nitrogen and Sulphur Mineralization in Batch and Semi-Continuous Anaerobic Digestion Using an Artificial Fiber Bag Technique. Energies 2021, 14, 4205. https://doi.org/10.3390/en14144205
Mortensen JR, Ward AJ, Weisbjerg MR, Hafner SD, Møller HB. Determination of Nitrogen and Sulphur Mineralization in Batch and Semi-Continuous Anaerobic Digestion Using an Artificial Fiber Bag Technique. Energies. 2021; 14(14):4205. https://doi.org/10.3390/en14144205
Chicago/Turabian StyleMortensen, Jacob Rosholm, Alastair James Ward, Martin Riis Weisbjerg, Sasha Daniel Hafner, and Henrik Bjarne Møller. 2021. "Determination of Nitrogen and Sulphur Mineralization in Batch and Semi-Continuous Anaerobic Digestion Using an Artificial Fiber Bag Technique" Energies 14, no. 14: 4205. https://doi.org/10.3390/en14144205






