Operating Limits for Ammonia Fuel Spark-Ignition Engine
Abstract
:1. Introduction
- Lean operation leads to high NOx (higher than with gasoline) but low unburnt NH3
- Rich operation leads to low NOx but too high unburnt NH3, unacceptable due to the smell and toxicity
- Increasing H2 quantity at the intake leads to a slightly higher NOx level, very low unburnt NH3 (due to a better combustion efficiency) but with some H2 content at the exhaust.
- First, as a function of different engine speeds, with small H2 quantities at atmospheric intake pressure
- Second to determine the lowest acceptable intake pressure, i.e., load conditions,
- And lastly, on a first evaluation of the possible dilution to limit pollutants for one particular condition (slight turbocharged conditions, fixed engine speed of 2000 rpm).
2. Experimental Methods
2.1. Experimental Set-Up
2.2. Investigated Conditions
3. Results
3.1. Operating Limits
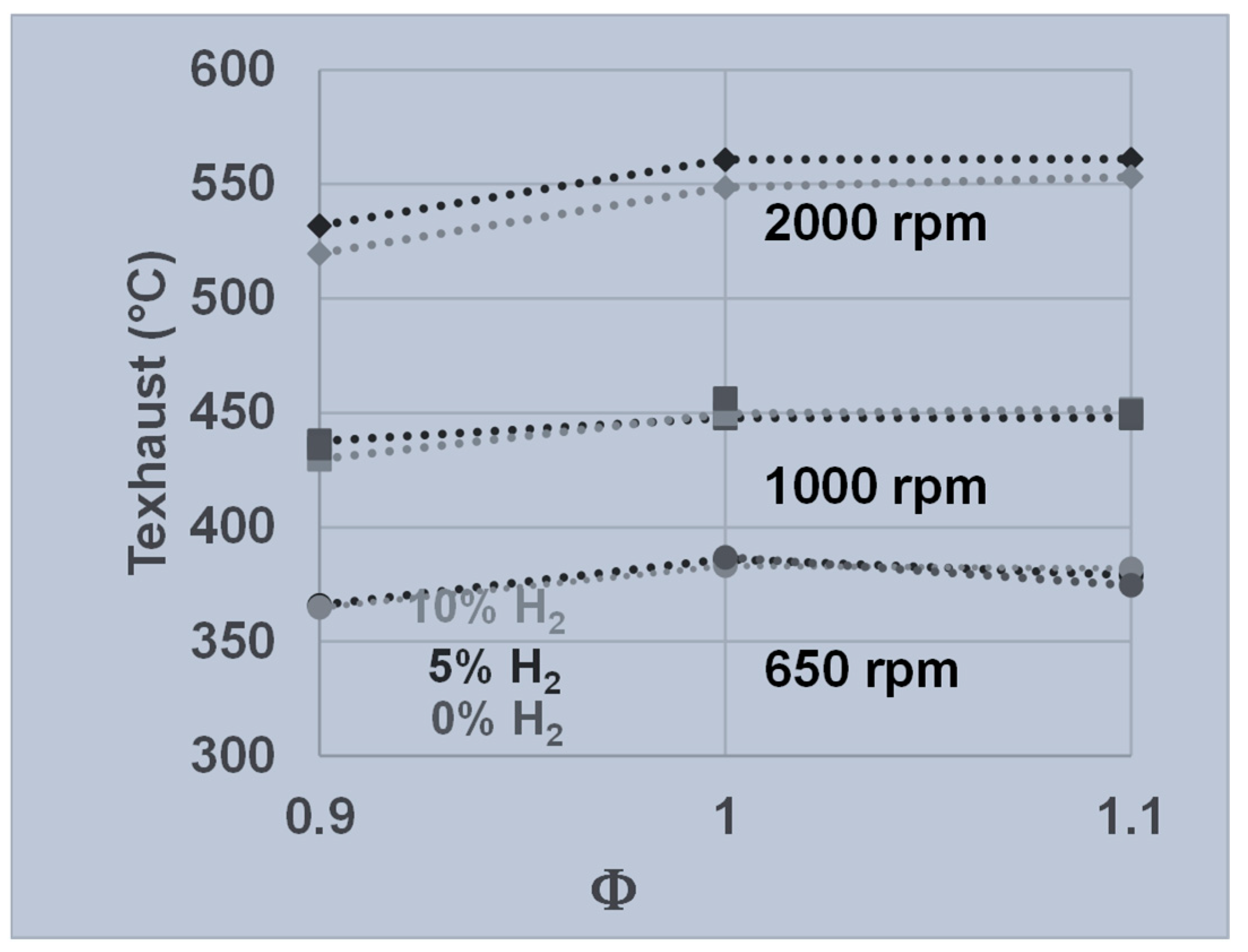
3.2. Engine Performance as a Function of Regime
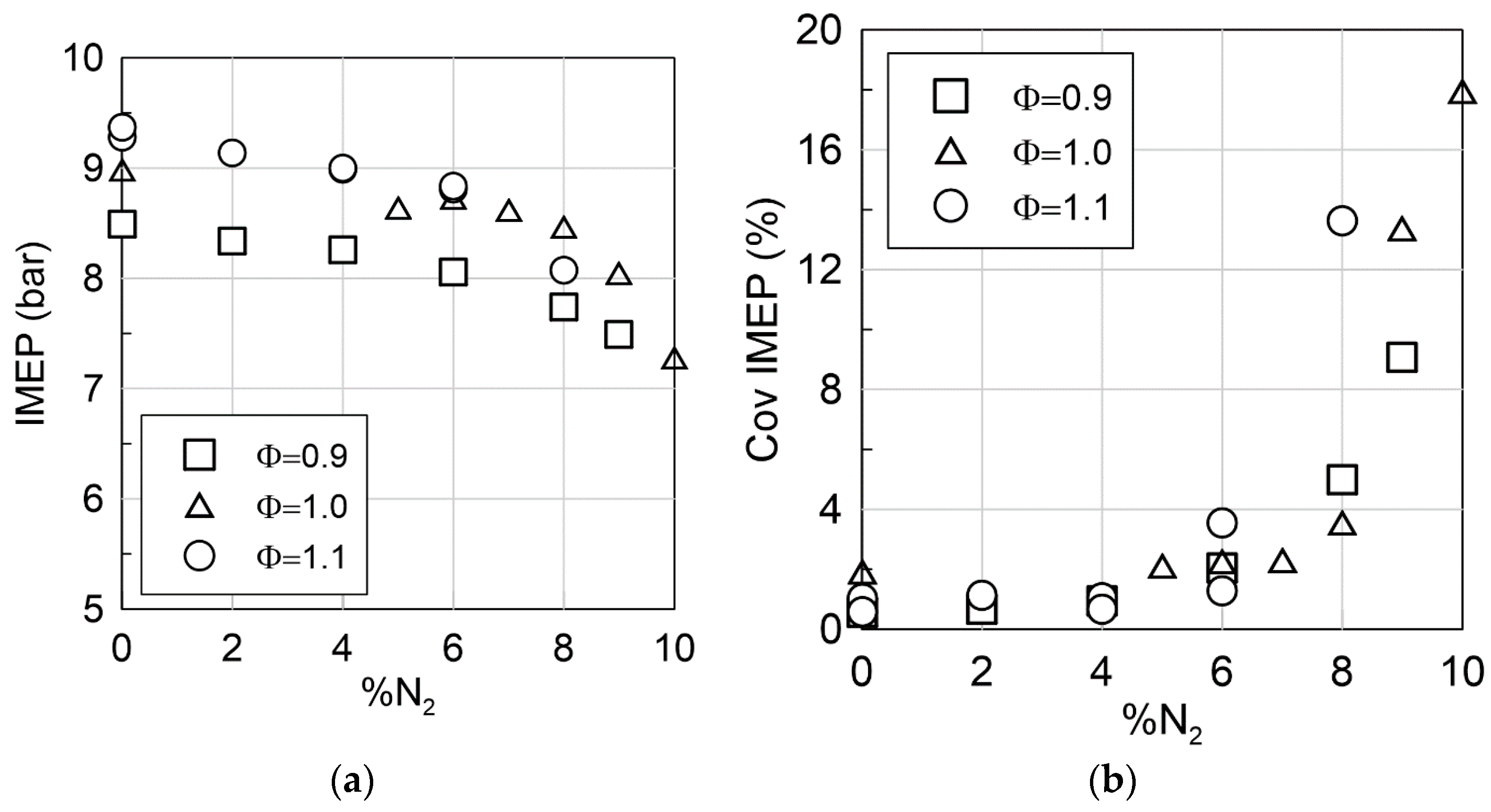
3.3. Dilution Opportunity @ 1500 rpm and Pin = 1.3 Bar
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Valera-Medina, A.; Xiao, H.; Owen-Jones, M.; David, W.I.F.; Bowen, P.J. Ammonia for power. Prog. Energy Combust. Sci. 2018, 69, 63–102. [Google Scholar] [CrossRef]
- Valera-Medina, A.; Amer-Hatem, F.; Azad, A.K.; Dedoussi, I.C.; de Joannon, M.; Fernandes, R.X.; Glarborg, P.; Hashemi, H.; He, X.; Mashruk, S.; et al. Review on Ammonia as a Potential Fuel: From Synthesis to Economics. Energy Fuels 2021, 35, 6964–7029. [Google Scholar] [CrossRef]
- AWijayanta, A.T.; Oda, T.; Purnomo, C.W.; Kashiwagi, T.; Aziz, M. Liquid hydrogen, methylcyclohexane, and ammonia as potential hydrogen storage: Comparison review. Int. J. Hydrog. Energy 2019, 44, 15026–15044. [Google Scholar] [CrossRef]
- Linstrom, P.J.; Mallard, W.G. NIST Chemistry WebBook, NIST Standard Reference Database Number 69, 20899; National Institute of Standards and Technology: Gaithersburg, MD, USA, 2018. [Google Scholar] [CrossRef]
- Mørch, C.; Bjerre, A.; Gøttrup, M.; Sorenson, S.; Schramm, J. Ammonia/hydrogen mixtures in an SI-engine: Engine performance and analysis of a proposed fuel system. Fuel 2011, 90, 854–864. [Google Scholar] [CrossRef]
- Bicer, Y.; Dincer, I. Life cycle assessment of ammonia utilization in city transportation and power generation. J. Clean. Prod. 2018, 170, 1594–1601. [Google Scholar] [CrossRef]
- Koch, E. Ammonia as a fuel for motor buses. J. Inst. Pet. 1949, 31, 21–32. [Google Scholar]
- Gray, J.T.; Dimitroff, E.; Meckel, N.T.; Quillian, R.D. Ammonia Fuel—Engine Compatibility and Combustion. SAE Tech. Pap. 1966. [Google Scholar] [CrossRef]
- Cornelius, W.; Huellmantel, L.W.; Mitchell, H.R. Ammonia as an Engine Fue. SAE Tech. Pap. 1965. [Google Scholar] [CrossRef]
- Starkman, E.S.; Newhall, H.K.; Sutton, R.; Maguire, T.; Farbar, L. Ammonia as a Spark Ignition Engine Fuel: Theory and Application. SAE Tech. Pap. 1966. [Google Scholar] [CrossRef]
- Starkman, E.S.; James, G.E.; Newhall, H.K. Ammonia as a Diesel Engine Fuel: Theory and Application. SAE Tech. Pap. 1967. [Google Scholar] [CrossRef]
- TPearsall, J.; Garabedian, C.G. Combustion of Anhydrous Ammonia in Diesel Engines. SAE Tech. Pap. 1967. [Google Scholar] [CrossRef]
- Sawyer, R.F.; Starkman, E.S.; Muzio, L.; Schmidt, W.L. Oxides of Nitrogen in the Combustion Products of an Ammonia Fueled Reciprocating Engine. SAE Tech. Pap. 1968. [Google Scholar] [CrossRef]
- Garabedian, C.; Johnson, J. The Theory of Operation of an Ammonia Burning Internal Combustion Engine, Report 634681; MI, USA, 1965; Available online: https://apps.dtic.mil/sti/citations/AD0634681 (accessed on 7 July 2021).
- Dimitriou, P.; Javaid, R. A review of ammonia as a compression ignition engine fuel. Int. J. Hydrog. Energy 2020, 45, 7098–7118. [Google Scholar] [CrossRef]
- Mounaïm-Rousselle, C.; Brequigny, P. Ammonia as Fuel for Low-Carbon Spark-Ignition Engines of Tomorrow’s Passenger Cars. Front. Mech. Eng. 2020, 6, 70. [Google Scholar] [CrossRef]
- Westlye, F.R.; Ivarsson, A.; Schramm, J. Experimental investigation of nitrogen based emissions from an ammonia fueled SI-engine. Fuel 2013, 111, 239–247. [Google Scholar] [CrossRef]
- Grannell, S.M.; Assanis, D.N.; Bohac, S.V.; Gillespie, D.E. The Fuel Mix Limits and Efficiency of a Stoichiometric, Ammonia, and Gasoline Dual Fueled Spark Ignition Engine. J. Eng. Gas Turbines Power 2008, 130, 042802. [Google Scholar] [CrossRef]
- Grannell, S.M.; Assanis, D.N.; Gillespie, D.E.; Bohac, S.V. Exhaust Emissions From a Stoichiometric, Ammonia and Gasoline Dual Fueled Spark Ignition Engine. In Proceedings of the ASME International Combustion Engine Division 2009 Spring Tech. Conf., Milwaukee, WI, USA, 3–6 May 2009. [Google Scholar] [CrossRef]
- Ryu, K.; Zacharakis-Jutz, G.E.; Kong, S.-C. Performance enhancement of ammonia-fueled engine by using dissociation catalyst for hydrogen generation. Int. J. Hydrog. Energy 2014, 39, 2390–2398. [Google Scholar] [CrossRef]
- Ryu, K.; Zacharakis-Jutz, G.E.; Kong, S.-C. Effects of gaseous ammonia direct injection on performance characteristics of a spark-ignition engine. Appl. Energy 2014, 116, 206–215. [Google Scholar] [CrossRef]
- Koike, M.; Miyagawa, H.; Suzuoki, T.; Ogasawara, K. Ammonia as a hydrogen energy carrier and its application to internal combustion engines. In Sustainable Vehicle Technologies; Warwickshire, G., Ed.; Woodhead Publishing: Cambridge, UK; pp. 61–70. [CrossRef]
- Lhuillier, C.; Brequigny, P.; Contino, F.; Mounaïm-Rousselle, C. Experimental study on ammonia/hydrogen/air combustion in spark ignition engine conditions. Fuel 2020, 269, 117448. [Google Scholar] [CrossRef]
- Lhuillier, C.; Brequigny, P.; Contino, F.; Rousselle, C. Performance and Emissions of an Ammonia-Fueled SI Engine with Hydrogen Enrichment. SAE Tech. Pap. 2019. [Google Scholar] [CrossRef]
- Lhuillier, C.; Brequigny, P.; Contino, F.; Rousselle, C. Combustion Characteristics of Ammonia in a Modern Spark-Ignition Engine. SAE Tech. Pap. 2019. [Google Scholar] [CrossRef]
- Woo, Y.; Jang, J.Y.; Lee, Y.J.; Kim, J.N. Recent progress on the Ammonia-Gasoline and the Ammonia-Diesel Dual Fueled Internal Combustion Engines in Korea. In Proceedings of the 11th Annual NH3 Fuel Conferences, Des Moines, IA, USA, 22 September 2014. [Google Scholar]
- Haputhanthri, S.O.; Maxwell, T.T.; Fleming, J.; Austin, C. Ammonia and Gasoline Fuel Blends for Spark Ignited Internal Combustion Engines. J. Energy Resour. Technol. 2015, 137, 062201. [Google Scholar] [CrossRef]
- Frigo, S.; Gentili, R. Analysis of the behaviour of a 4-stroke Si engine fuelled with ammonia and hydrogen. Int. J. Hydrog. Energy 2013, 38, 1607–1615. [Google Scholar] [CrossRef]
- Comotti, M.; Frigo, S. Hydrogen generation system for ammonia-hydrogen fuelled internal combustion engines. Int. J. Hydrog. Energy 2015, 40, 10673–10686. [Google Scholar] [CrossRef]
- Lhuillier, C.; Brequigny, P.; Lamoureux, N.; Contino, F.; Mounaïm-Rousselle, C. Experimental investigation on laminar burning velocities of ammonia/hydrogen/air mixtures at elevated temperatures. Fuel 2020, 263, 116653. [Google Scholar] [CrossRef] [Green Version]
- Heywood, J.B. Internal Combustion Engine Fundamentals, 2nd ed.; McGraw-Hill Education: New York, NY, USA, 2018; Available online: https://www.accessengineeringlibrary.com/content/book/9781260116106 (accessed on 7 July 2021).
- Francqueville, L.; Michel, J.-B. On the Effects of EGR on Spark-Ignited Gasoline Combustion at High Load. SAE Int. J. Engines 2014, 7, 1808–1823. [Google Scholar] [CrossRef]
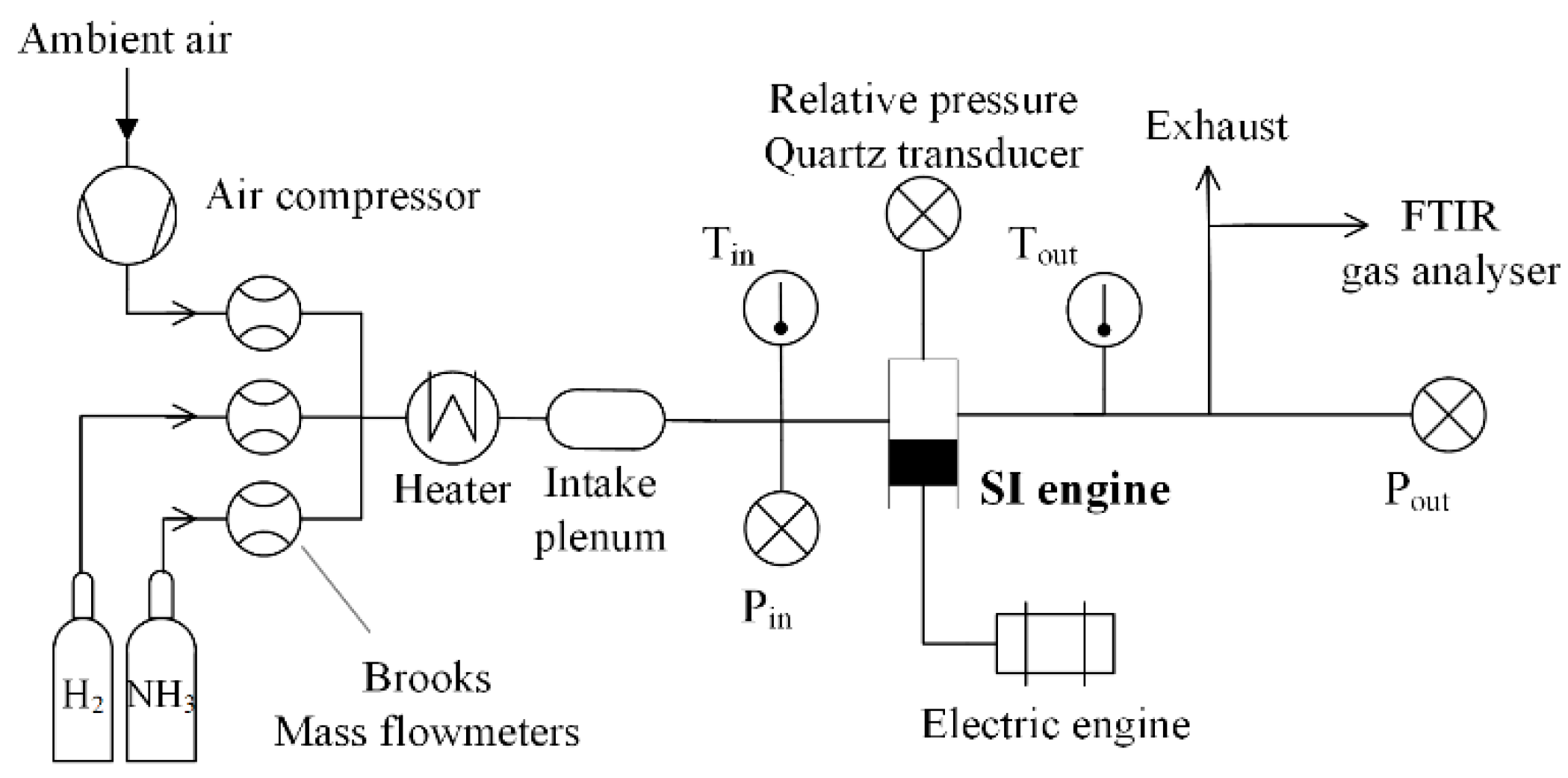

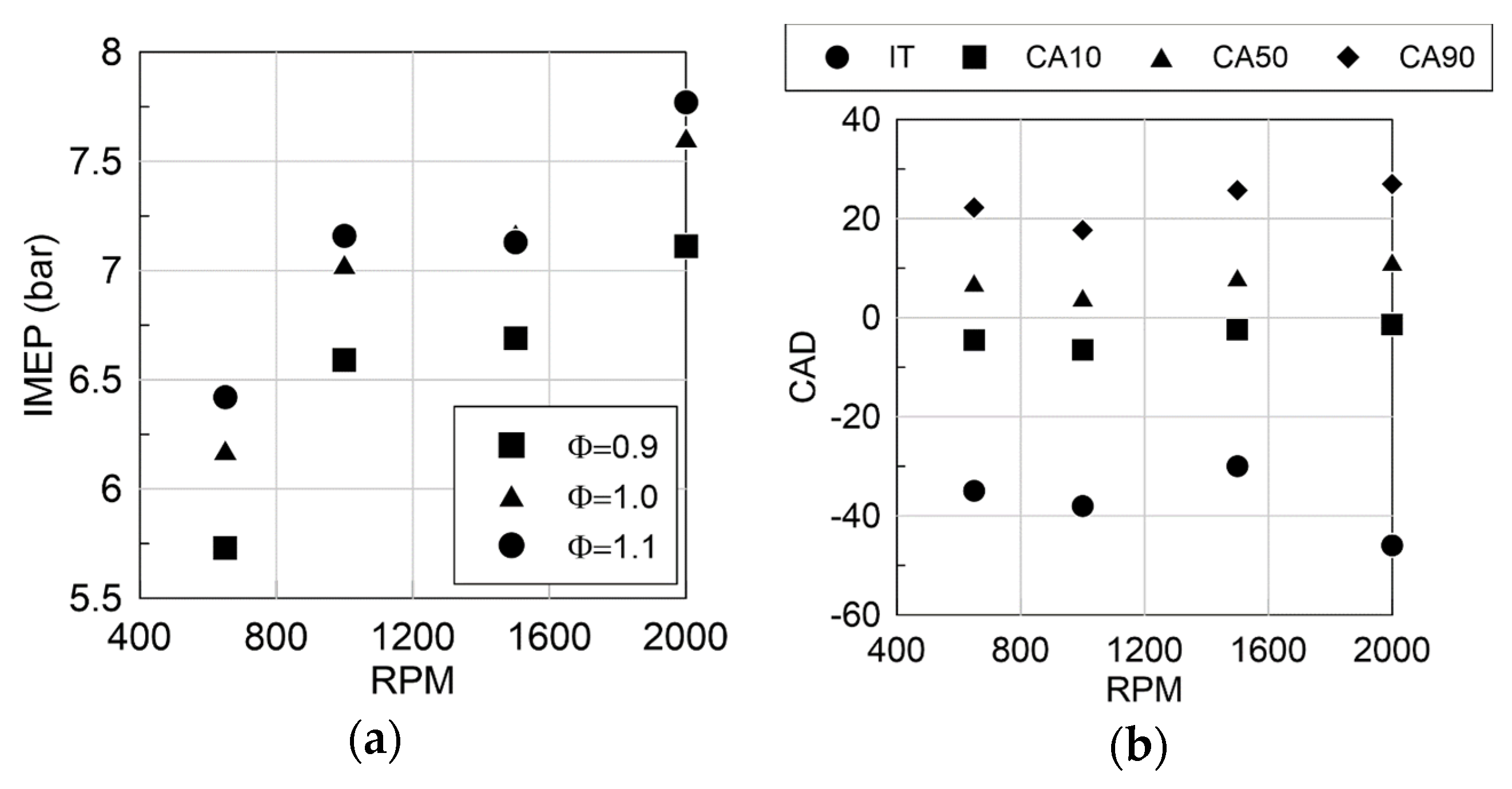
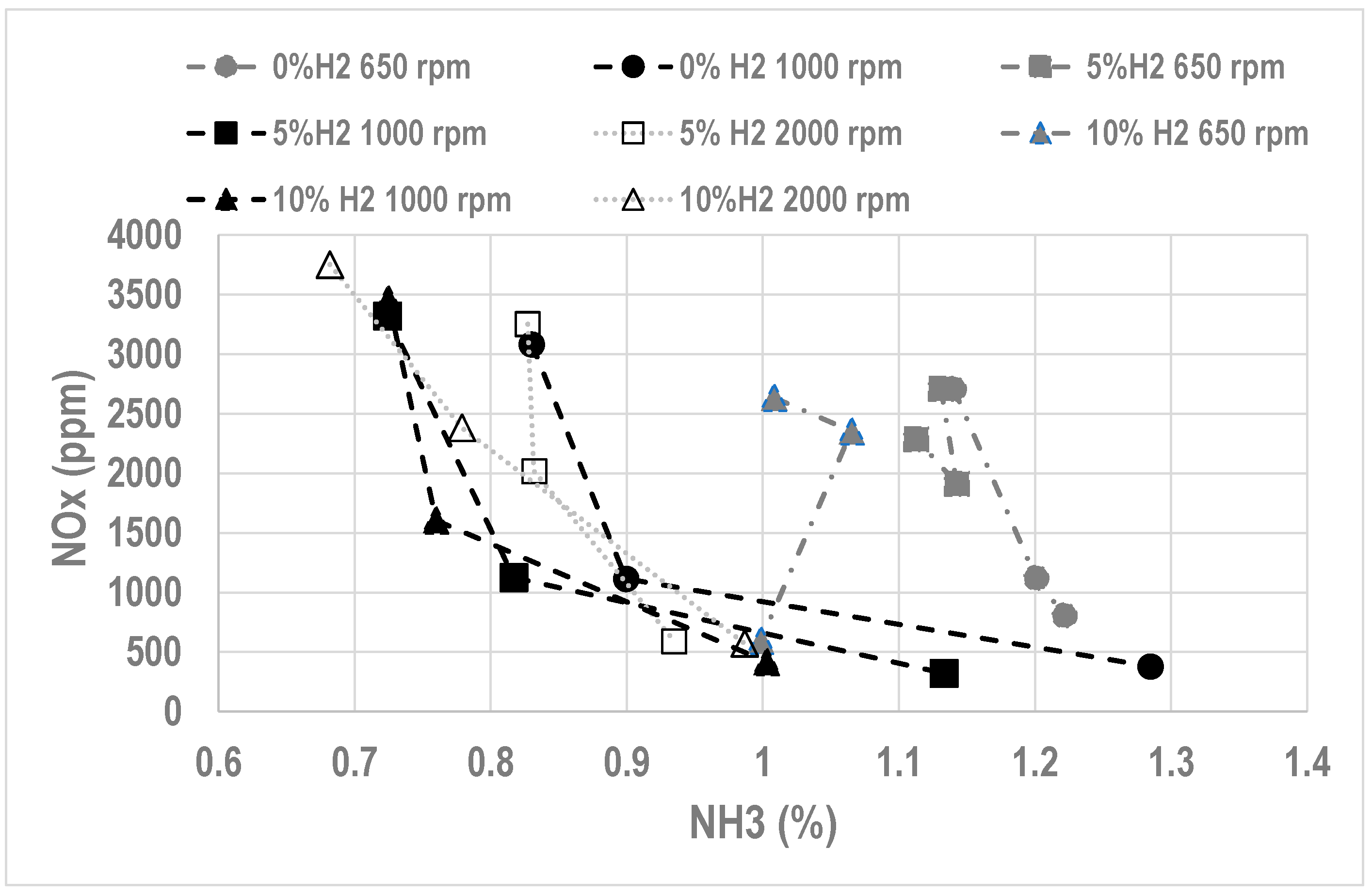

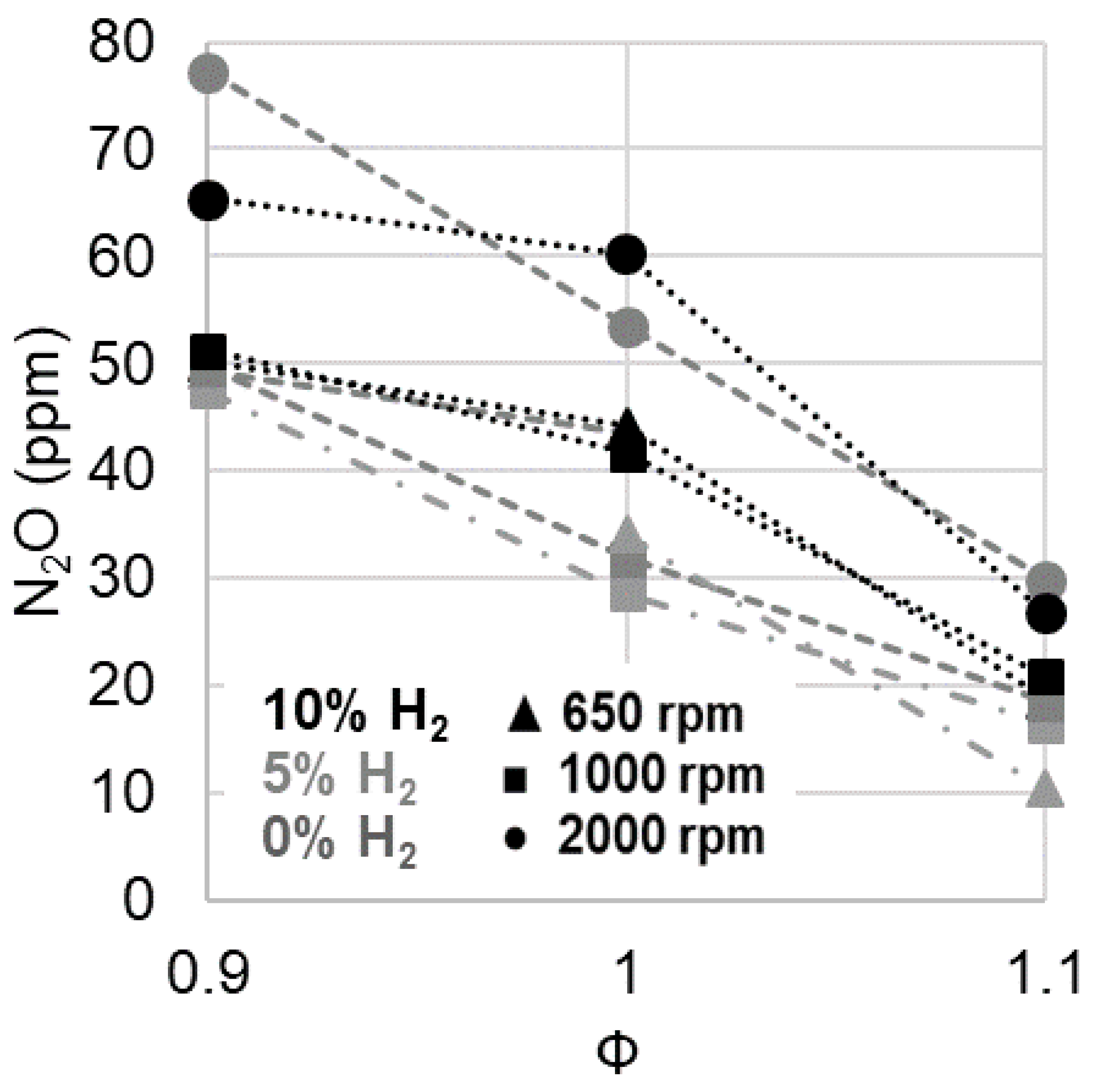


| Species | Ammonia | Methanol | Hydrogen | Methane | Gasoline |
|---|---|---|---|---|---|
| Formula | NH3 | CH3OH | H2 | CH4 | - |
| Storage | Liquid | Liquid | Compressed | Compressed | Liquid |
| Storage temperature (K) | 300 | 300 | 300 | 300 | 300 |
| Storage pressure (MPa) | 1.1 | 0.1 | 70 | 25 | 0.1 |
| density @ storage conditions (kg.m−3) | 600 | 784.6 | 39.1 | 187 | ~740 |
| FL in air (vol.%) | 15–28 | 6.7–36 | 4.7–75 | 5–15 | 0.6–8 |
| LBV @ stoichiometry (m.s−1) | 0.07 | 0.36 | 3.51 | 0.38 | 0.58 |
| Auto-ignition T (K) | 930 | 712 | 773–850 | 859 | 503 |
| Research Octane Number | 130 | 119 | >100 | 120 | 90–98 |
| LHV (MJ/kg) | 18.8 | 19.9 | 120 | 50 | 44.5 |
| Displaced Volume | 399.5 cm3 |
| Stroke | 85.8 mm |
| Bore | 77 mm |
| Connecting rod length | 138.5 mm |
| Compression ratio | 10.5:1 |
| Number of valves | 4 |
| Engine Speed (rpm) | 650; 1000; 1500; 2000 |
| Intake temperature (K) | 323 |
| Intake pressure (bar) | [0.6–1] 1.3 for dilution impact |
| H2 molar fraction in the fuel, | [0.0–0.10] |
| [0.9–1.1] | |
| %N2 dilution | Up to 8% of the total at the intake |
| 650 rpm | 1000 rpm | 1500 rpm | 2000 rpm | ||||||||||
| %H2 | |||||||||||||
| Φ | Pin (bar) | 0% | 5% | 10% | 0% | 5% | 10% | 0% | 5% | 10% | 0% | 5% | 10% |
| 0.9 | <=0.65 | X | X | X | X | ||||||||
| 0.8–0.85 | X | X | X | X | X | X | X | X | |||||
| 1 | X | X | X | X | X | X | X | X | X | X | |||
| 1 | <=0.65 | X | X | X | X | X | |||||||
| 0.8–0.85 | X | X | X | X | X | X | X | X | X | X | |||
| 1 | X | X | X | X | X | X | X | X | X | X | X | ||
| 1.1 | <=0.65 | X | X | X | X | X | |||||||
| 0.8–0.85 | X | X | X | X | X | X | X | X | X | X | |||
| 1 | X | X | X | X | X | X | X | X | X | X | X | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mounaïm-Rousselle, C.; Bréquigny, P.; Dumand, C.; Houillé, S. Operating Limits for Ammonia Fuel Spark-Ignition Engine. Energies 2021, 14, 4141. https://doi.org/10.3390/en14144141
Mounaïm-Rousselle C, Bréquigny P, Dumand C, Houillé S. Operating Limits for Ammonia Fuel Spark-Ignition Engine. Energies. 2021; 14(14):4141. https://doi.org/10.3390/en14144141
Chicago/Turabian StyleMounaïm-Rousselle, Christine, Pierre Bréquigny, Clément Dumand, and Sébastien Houillé. 2021. "Operating Limits for Ammonia Fuel Spark-Ignition Engine" Energies 14, no. 14: 4141. https://doi.org/10.3390/en14144141
APA StyleMounaïm-Rousselle, C., Bréquigny, P., Dumand, C., & Houillé, S. (2021). Operating Limits for Ammonia Fuel Spark-Ignition Engine. Energies, 14(14), 4141. https://doi.org/10.3390/en14144141





